Sclerotic chronic graft-versus-host disease (cGVHD) appears in 20% of cGVHD patients within 3 years of transplant.1 Skin sclerosis severely impairs quality of life and responds slowly to therapy.1 The current gold standard for grading cGVHD sclerosis, the NIH skin score, is coarse and subjective,2 motivating calls for more reproducible measures.3
The Myoton is a handheld device (Figure S1) that extracts soft tissue biomechanical parameters through a brief mechanical impulse.4 Previously, we reported that sclerotic cGVHD patients and healthy controls have significant differences in dynamic stiffness measurements.5 However, whether changes in dynamic stiffness correspond to clinical perception of skin cGVHD response to treatment has not been assessed.
In this study, we evaluated the Myoton’s ability to numerically monitor sclerosis over time and correlated these measurements with clinical disease progression and response. Ten sclerotic cGVHD patients and two cGVHD patients with no skin manifestations were followed (Table S1). Skin dynamic stiffness (N/m), characterized by the tissue resistance to deformation by an external force,4 was evaluated using the MyotonPRO device per our previously published protocol5 between April 2017 and March 2020 during routine clinic visits. For each body region, irrespective of sclerosis, we measured the skin overlying the middle of the region’s named muscle on both sides of the body (Figure S2). However, body regions selected at each session varied according to patient time constraints, comfort, and clinician perception of sentinel sites to monitor. We calculated each session’s average stiffness across only the sites that were also measured bilaterally in all other sessions for that subject (Figure S2). Simple linear regression was performed on these averages to assess the trend over time (N/m/month) for that patient. Later, clinical notes were reviewed from enrollment to one month after the last measurement to classify provider perception of skin disease into progressive disease (PD), stable disease (SD), and partial response (PR). Response type was determined from provider’s designation of PD/SD/PR or descriptions of skin improvement/worsening. Borderline cases were resolved by discussing with two oncologists (TK and MB) familiar with the patients. Providers were blinded to Myoton measurements.
All 12 subjects had at least three (up to fifteen) measurement sessions, over a median of five months (Figure S2). Three patients (patients 1, 2, 7) had PR, two (8, 9) had PD, and five (3, 4, 5, 6, 10) had SD. Patient 7 had a reduction in NIH skin score from 3 to 2; no other patients had changes in skin score. Two cGVHD patients (11, 12) had no skin involvement at any point during the study or in two months of subsequent follow-up.
Time courses are shown in Figure 1A (representative) and Figure S3 (all subjects). Both PD patients (8, 9) corresponded to positive outlier slopes (Figure 1B) in dynamic stiffness (18 N/m/month; 36 N/m/month). Of the three PR patients, patients 1 and 2 had negative outlier slopes (−148 N/m/month; −40 N/m/month), while patient 7’s regression slope was near zero (3 N/m/month). Of note, patient 7 had pitting edema affecting bilateral lower extremities and trunk at the start of the study that improved following diuresis, overlapping with the clinical documentation of cGVHD skin improvement. Given that edema has been associated with lower soft tissue stiffness,6 this opposing effect may account for the stable dynamic stiffness values. Importantly, while four of five patients with clinical changes in disease status demonstrated significant trends in dynamic stiffness consistent with the clinical perception of change, only one (patient 7) had a change in NIH skin score (Table S1). Additionally, we observed that the three PR patients had lower dynamic stiffness values at study baseline than the two PD patients. While the sample size limits generalizability, the findings encourage further longitudinal study into whether quantitatively measured skin stiffness could indicate response.
Figure 1. Average dynamic stiffness measurements and linear regression slopes.
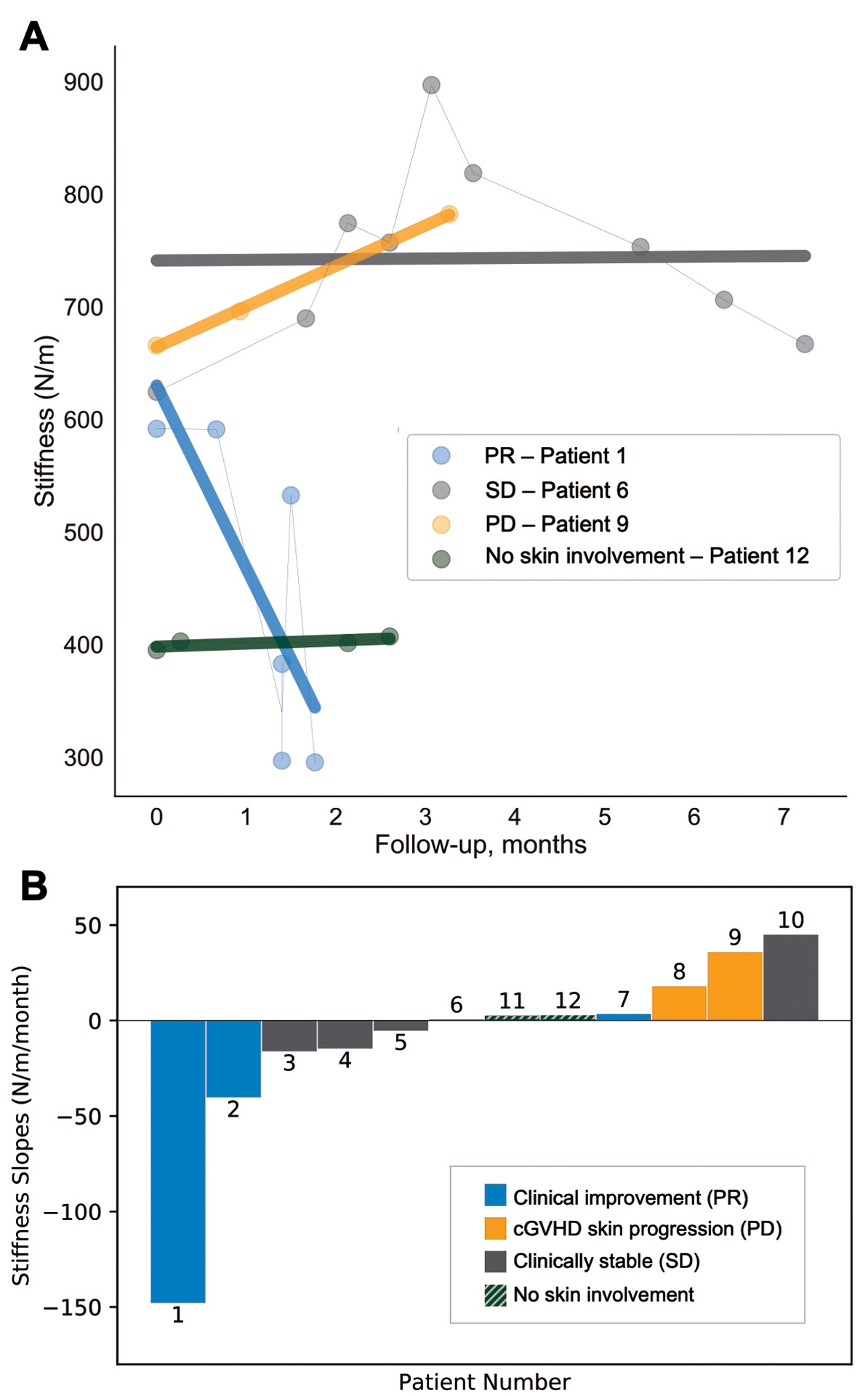
(A) Dynamic stiffness measurements (N/m) over time of a representative sclerotic cGVHD patient from each clinically-determined response type: partial response (PR), stable disease (SD), and progressive disease (PD), and one cGVHD patient with no skin symptoms. Simple linear regression lines are shown in bold. (B) Regression slopes of dynamic stiffness measurements over time in N/m/month for ten sclerotic cGVHD patients (patients 1–10) and two cGVHD patients without skin manifestations (patients 11, 12).
Among five SD patients, two had outlier trends in dynamic stiffness: patient 6 exhibited a clear upward trend followed by a steady downtrend (Figure 1A) and patient 10 had a positive outlier slope (45 N/m/month). Mitchell et al.7 demonstrated that interrater variability of body surface area (BSA) scoring increases with increasing extent of non-moveable sclerosis. Given that patient 6 has severe sclerosis involving >50% BSA, clinical assessment may lack the sensitivity required to detect subtle changes in her disease. Patient 10 had a short follow-up period, which limits the ability to assess response both clinically and by Myoton.
The remaining three SD patients (3, 4, 5) and the two cGVHD patients without skin manifestations (11, 12) had near zero regression slopes, consistent with clinical perception of stable status. Of note, patient 3 was receiving extracorporeal photopheresis (ECP) on a treatment schedule of 2 consecutive days every 4 weeks during the study. The second day dynamic stiffness measurements were consistently less than the previous day (Figure S3). We speculate that the short-term reduction in dynamic stiffness between consecutive ECP days may be related to fluid shifts. To our knowledge, there has been no longitudinal study evaluating the relationship between therapies that affect water balance (including diuretics, ECP) and skin biomechanical properties.
We provide one of the largest longitudinal quantitative studies of sclerotic cGVHD to date. Dynamic stiffness, measured longitudinally with the MyotonPRO, is able to identify clinically important changes in disease. We observed high inter-session variability in patients’ measurements. Given the Myoton has demonstrated high inter-rater reliability in healthy volunteers and sclerotic cGVHD patients,8,9 the discrepancy may relate to day-to-day variations affecting stiffness, such as hydration10 and time of day11. Additionally, while our protocol was developed for a standard exam table,5 some measurements in this study were performed in a reclining infusion chair due to space limitations. This could alter the contact between Myoton and the skin surface, as well as the contributions from underlying relaxed or tense muscles.
Our analysis was limited by the variations in site selection between sessions and hence the ability to consistently compare only the common sites across sessions. Anterior tibialis, which has been shown to be a region with relatively lower reproducibility,8 was one of only two sites used in the analysis for patient 1 and patient 2. Limited number of measurement sites exacerbates variability related to measurement error, and thus requires an examination of multiple data points to assess for trend. The use of different combinations of sites between patients also limits inter-subject dynamic stiffness comparisons, as tissue stiffness varies between sites.5,6 Future studies could avoid this limitation by consenting patients to the necessary increased session duration (~15 minutes for the full body 20-site protocol5) to follow the full standardized protocol using all pre-determined measurement sites. Some patients also had relatively short follow-up or few measurement sessions. Serial measurements over several months are needed to accurately trend changes in skin dynamic stiffness. Lastly, clinical response type was determined through retrospective review of clinical notes, which may be less reliable than the provider’s real-time designation of response would have been with a study-specific clinical response-form.
This preliminary study encourages the use of Myoton for tracking the clinical course of sclerotic cGVHD. A larger study with longer follow-up and standardized measurement protocol is necessary to assess the accuracy of skin biomechanical measurements as a longitudinal biomarker. Eventually, this method could complement the qualitative maneuvers that clinicians currently perform to assess sclerosis and enhance clinical disease management.
Supplementary Material
Acknowledgments
This work was supported by Career Development Award Number IK2 CX001785 from the United States Department of Veterans Affairs Clinical Science R&D Service and NIH K12 CA090625. The authors are grateful for the patients and volunteers who participated in this study.
Footnotes
Competing Interests:
The authors declare no competing financial interests. A.V. is the inventor of the Myoton but has no financial interest in the device.
References
- 1.Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013;121(25):5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowen EW. Decoding skin involvement in chronic graft-vs-host disease. JAMA Dermatol. 2019;155(7):777–778. [DOI] [PubMed] [Google Scholar]
- 4.Vain A, inventor; Myoton AS, assignee. Device and method for real-time measurement of parameters of mechanical stress state and biomechanical properties of soft biological tissue. United States patent application US 13/977,873. 2011. [Google Scholar]
- 5.Chen F, Dellalana LE, Gandelman JS, Vain A, Jagasia MH, Tkaczyk ER. Non-invasive measurement of sclerosis in cutaneous cGVHD patients with the handheld device Myoton: a cross-sectional study. Bone Marrow Transplant. 2019;54(4):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mridha M, Odman S. Characterization of subcutaneous edema by mechanical impedance measurements. Journal of Investigative Dermatology. 1985;85(6):575–8. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell SA, Jacobsohn D, Thormann Powers KE, Carpenter PA, Flowers ME, Cowen EW, et al. A multicenter pilot evaluation of the National Institutes of Health chronic graft-versus-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant. 2011;17(11):1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellalana LE, Chen F, Vain A, Gandelman JS, Põldemaa M, Chen H, et al. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Skin Res Technol. 2019;25(3):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Wang L, Vain A, Ssempijja Y, Dellalana L, Zhang K, et al. Interobserver Reproducibility of the Myoton and Durometer Devices to Measure Skin Stiffness and Hardness in Chronic Cutaneous Graft-Versus-Host Disease Patients. Blood 2019; 134 (Supplement_1): 4515. [Google Scholar]
- 10.Palma L, Marques LT, Bujan J, Rodrigues LM. Dietary water affects human skin hydration and biomechanics. Clin Cosmet Investig Dermatol. 2015;8:413–421. Published 2015 Aug 3. doi: 10.2147/CCID.S86822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukahara K, Takema Y, Moriwaki S, Fujimura T, Imokawa G. Dermal fluid translocation is an important determinant of the diurnal variation in human skin thickness. Br J Dermatol. 2001;145:590–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


