Graphical abstract
Keywords: Bio-based surfactants, Cleansing plants, Conservation, Local knowledge, Saponins
Abstract
The purpose of this study is to access the existing awareness of nearly forgotten Thai detergent plants by the use of chemometrics tool. A Northern Thai forest dependent community was chosen as it played vital role on knowledge retaining of plant utilisations. For initial perception, ethnobotanical survey was conducted to determine usage of plants by the community. Then the utilised plant parts were screened for phytochemicals and their relationships with the defined cleansing terms (viz., shampoo, scrub, detergent, soap, scent and spiritual) were analysed by Principal Component Analysis (PCA). From the results, the most cited plants as known, used and found were Acacia concinna, Clitoria ternetea, Oryza sativa and Citrus hystrix. Biometric analyses advised that knowledge of detergent plant utilisation was well preserved at all age ranges and it was not variable with genders. Cluster analysis described that term ‘spiritual’ was not narrated with cleansing properties. For phytochemical analysis, plant extracts showed positive variable of bioactive ingredients and the main compounds in the extracts was saponins. These findings confirmed that the knowledge of indigenous plant utilisation was reserved by the forest dependent community and the information is beneficial toward local plant conservation movement.
1. Introduction
Traditionally ecological knowledge represents the human experience acquired over thousands of years from the direct contact with the environment (Berkes, 1993, Srithi et al., 2012, Turner et al., 2000). Anthropogenic pressures such as invasion of cash crops and expansion of urbanisation have threatened the richness of local plant species and their biological complexities. Moreover, the knowledge of indigenous plant utilisation is at risk of loss due to the limit biodiversity as well as the replacement of chemical-synthesis products. By using ethno-informatic documentation, the traditional knowledge could be well preserved and reinforce the better understanding between local communities and plants, which is, in turn, essential to support biodiversity conservation (Almeida et al., 2014, Hoekou et al., 2016, Nankaya et al., 2019, Srithi et al., 2012). It is commonly concured that forest dependent community plays an important role for retaining knowledge of natural resource utilisation and possess as front-liner for the assessment of loss of traditionally ecological knowledge (Stibig et al., 2014).
Thai people have historically reaped the benefits of native detergent plants with cleansing properties over hundreds of years. These group of plants contain main bioactive compounds which are saponins, known as bio-based surfactants (Bouillon, 1996, Kregiel et al., 2017, Oleszek and Hamed, 2010, Osbourn, 1996). Need for these valuable active ingredients has ramped-up along with the urge for the replacement of chemical synthesis that is harm our environment, however, the knowledge of Thai local plants used for cleansing purposes is being declined (Wisetkomolmat et al., 2019). Moreover, scientific evidences regarding the perception of local detergent plants as well as terms to describe detergent properties are somewhat confusing (Inta et al., 2013). Wisetkomolmat et al. (2019) documented the list of plants utilised as for cleaning purposes by Thai ethnic groups. Nonetheless, the analyses regarding to their physiochemical functionalities, particulary to describe detergent properties have yet been elucidated anywhere.
The multivariate Principal Component Analysis or PCA is the most fundamental method used in chemometric (Esbensen and Geladi, 2009). This method reduces data dimension by geometrical projection analogy and visually cluster the variants using principal components. For this instance, this tool has been used in many fields, including the biochemistry and biology (Lever et al., 2017, Roessner et al., 2011). To fill the gaps mentioned above, the valuably ethno-informatic data from a forest dependent community as well as the presence phytochemicals from the collected plant species were gathered. Taking the advantage of chemometrics, we hoped to be able to fully comprehend the status of the current knowledge and terms used to describe our local detergent plants. The result from our study could validate urge for local plant conservation driven by existing social knowledge.
2. Materials and methods
2.1. Study area
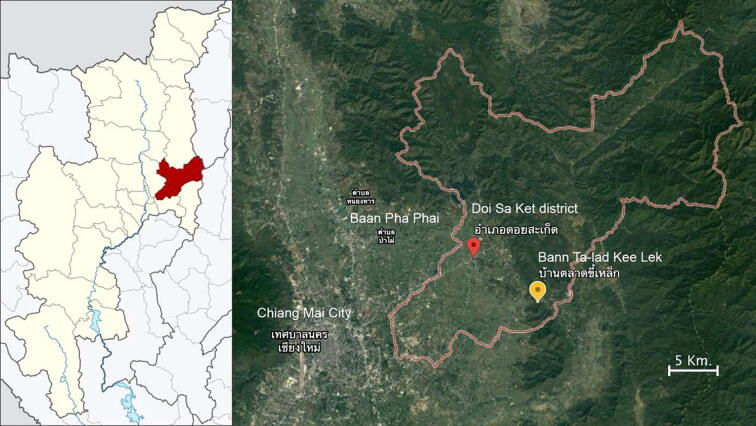
The forest dependent community chosen was located in Doi Sa Ket district (location: 18.8407128, 99.199082), ~ 50 km out-skirt to the North of the urban Chiang Mai city, Thailand (Fig. 1). According to population data in 2018, the district had an estimated total number of 73,500 habitants. The majority of the residents are largely subsistent famers with some engagement in agroforest system. Inter-connecting between Chiang Mai urban city and the forest, the community has resided and employed the benefits of natural resources from the forest reserve restored by the Huai Hong Khrai Royal Development Study Centre (HHKC), for almost 40 years. The forest represents one of the highly fertile areas and riches with local plant biodiversity.
Fig. 1.
Study site in the forest dependent community chosen located in Doi Sa Ket district, Chiang Mai, Thailand.
2.2. Bio- and ethno-informatic data collection
The general terms used to describe cleansing purposes including; ‘shampoo’, ‘scrub’, ‘detergent’, ‘soap’, ‘scent’ and ‘spiritual cleansing’ were generated from our previous study and also list of the plants used for same purposes in Northern Thai areas from various ethnobotanical documents (Wisetkomolmat et al., 2019) (Table 1). The total of 68 interviewees were randomly selected based on the length of time that they resided in this community (>20 years). They also represented a single race. After a brief explanation of the study objectives, a consent was informed and all participants were asked to sign a consent form certifying their agreements (Dos Santos et al., 2009, Hoekou et al., 2016, Monteiro et al., 2014). The ethnobotanical surveys were conducted between January to May 2019. All of personal identification data was eliminated after analysed. Prior to conducting the survey, semi-structured questionnaire was constructed in which it was composed of two parts (Martin, 2010). The first part was interviewee general information (age and gender) and the second part was consisting of (i) general questions that included a binary respond type (yes/no) asking if they recognised the plants and their local names from the prepared pictures, seen/found these plants in the area and utilised those plants (ii) an open interview of plant utilisation in cleansing purposes (viz., plant part used and how they used them). The binary data was used to calculate degree of recognition (%DR), degree of found (%DF), and utilisation value in general (%UV) by using number of times a particular species was mentioned divided by total number of interviewees and multiply by 100 (Faruque et al., 2018). For, the use value of the specific species used in cleansing purposes (UVs) was according to Monteiro et al., 2014, Hoffman and Gallaher, 2007 which is calculated from the following equation;
| (1) |
Table 1.
Detergent plants used by a Huai Hong Khrai reserved forest independent community.
| S. No. | Family name | Plant scientific name | Local name | Part used as Detergent | UVs in Cleansing purposes (Preparation) | Degree of recognition (%) | Degree of found (%) | UV in general (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Acoraceae | Acorus gramineus Aiton. | Waan Nam Lek | Leaf | 0.029 (Mashed in water as detergent and gave a scent) | 42.6 | 45.6 | 29.4 |
| 2 | Arecaceae | Calamus sp. | Waai | Leaf | – | 94.1 | 89.7 | 61.8 |
| 3 | Burseraceae | Garuga pinnata Roxb. | Ta Kram | Leaf | 0.059 (Mashed and used as shampoo) | 10.3 | 20.6 | 8.8 |
| 4 | Cucurbitaceae | Luffa cylindrica Roem. | Buab Khom | Fruit | 0.735 (Fibre of dried fruit is used as similar to cleansing sponge) | 97.1 | 94.1 | 91.2 |
| 5 | Cucurbitaceae | Trichosanthes cucumerina Linn. | Buab Ngoo | Fruit | 0.162 (Fibre of dried fruit is used as similar to cleansing sponge) | 82.4 | 82.8 | 67.6 |
| 6 | Dilleniaceae | Dillenia paroiflora Griff. | San Hing | Fruit | – | 8.8 | 20.6 | 2.9 |
| 7 | Euphorbiaceae | Antidesma acidum Retz. | Mao Sroi | Leaf | – | 79.4 | 77.9 | 64.7 |
| 8 | Euphorbiaceae | Homonoia riparia Lour. | Khrai-Naam | Leaf | 0.162 (Detergent for hand wash) | 54.4 | 58.8 | 20.6 |
| 9 | Euphorbiaceae | Flueggea virosa (Willd.) Voigt | Kang Pla Kao | Stem | 0.015 (Mashed and soaked in water used as shampoo) | 22.1 | 30.9 | 13.2 |
| 10 | Fabaceae | Acacia concinna (Willd.) D.C. | Som Poi | Fruit | 1.029 (Dried fruit is used for spiritual cleansing during Thai new year by soaking in water) | 100 | 98.5 | 94.1 |
| 11 | Fabaceae | Clitoria ternetea L. | An-chan | Flower | 0.765 (Mashed and mixed with soap or shampoo) | 100 | 100 | 97.1 |
| 12 | Hypoxidaceae | Curculigo latifolia Dryand. ex. W.T. Aiton | Wan Sak Lek | Tuber | – | 30.9 | 39.7 | 7.4 |
| 13 | Lauraceae | Litsea glutinosa (Lour.) C.B. Rob. | Mhee | Leaf | 0.456 (Leaves are mashed and soaked in water to be used as shampoo ingredients) | 52.9 | 54.4 | 35.3 |
| 14 | Oxalidaceae | Oxalis corniculate L. | Som Kob | Leaf | 0.162 (Used to polish silver wares) | 76.5 | 80.9 | 58.8 |
| 15 | Pedaliaceae | Sesamum orientale L. | Nga | Leaf | 0.015 (Used as scrub) | 97.1 | 97.1 | 92.6 |
| 16 | Poaceae | Oryza sativa L. | Kao, Kaw | Pericarp | 0.500 (Pericarp is used as similar to cleansing sponge or mixed in soap and shampoo) | 98.5 | 100 | 97.1 |
| 17 | Rutaceae | Citrus hystrix D.C. | Ma Krud | Fruit | 1.853 (Mashed and soaked in water used as shampoo or detergent) | 98.5 | 100 | 100 |
| 18 | Salicaceae | Salix tetrasperma Roxb. | Khrai Bok | Pericarp | 0.015 (Used as detergent) | 20.6 | 22.1 | 11.8 |
| 19 | Sapindaceae | Aesculus assamica Griff. | Ma Niang Nam | Leaf | 0.059 (Used as shampoo) | 23.5 | 25.0 | 13.2 |
| 20 | Oxalidaceae | Sapindus rarak D.C. | Ma Kham Di Khwai | Pericarp | 0.559 (Mashed and soaked in water used for cleansing clothes) | 63.2 | 64.7 | 51.5 |
| 21 | Solanaceae | Solanum erianthum D. Don | Dap Yang | Leaf | – | 25.0 | 27.9 | 11.8 |
| 22 | Malvaceae | Microcos tomentosa Sm. | Phlap-phla | Leaf | 0.015 (Mashed in water used as shampoo) | 11.8 | 10.3 | 5.9 |
| 23 | Vitaceae | Cissus modeccoides Var. Karri | Som La Op | Leaf and stem | – | 2.9 | 7.4 | 1.5 |
| 24 | Rutaceae | Cissus repen Lamk. | Tao Kan | Stem | – | 17.6 | 26.5 | 10.3 |
| 25 | Zingiberaceae | Amomum biflorum Jack. | Wan Sao Long | Rhizome | 0.015 (Mashed in water used as shampoo) | 27.9 | 30.9 | 10.3 |
Where, UVs = species use value, UR = number of times a particular species was mentioned using in cleansing purposes, Ni = total number of informants interviewed for each species (s).
2.3. Phytochemical screening
Plant sampling for phytochemical screening was achieved by village survey trips with head of the community (Male, 55 years old) who had intensive knowledge of plants in this forest dependent community for>30 years. Only plant species from the lists that were found, either in the market or by domestication or in their natural habitats were taken from at least three adult plants and the materials corresponding to the utilised plant part for each of detergent plant species were also collected (Ahumada-Santos et al., 2013). Plant specimens were prepared according to Faruque et al. (2018) and plant species confirmation was done by comparison the specimen with those deposited at plant collection library of the ethnobotanical laboratory, Department of Biology, Faculty of Science, Chiang Mai University (CMU). The specimens were assigned number and stored at Plant Bioactive Compound Herbarium (BACH), Department of Plant and Soil Science, Faculty of Agriculture, CMU (The assigned voucher specimen numbers are given in Table 2).
Table 2.
Pre-screening of bioactive compounds and total saponins content in collected plants used in cleansing purposes.
| Scientific name (voucher no.) | Part used | Solvent | Alk | Gly | Ster | Sap | Ter | Tan | TSC (mg saponin/ g sample) |
|---|---|---|---|---|---|---|---|---|---|
| C. hystrix (JW001) | Fruit | Methanol | + | ++ | + | + | ++ | + | 0.46 ± 0.09 |
| Water | + | ++ | ++ | + | ++ | + | 0.24 ± 0.01 | ||
| S. rarak (JW002) | Pericarp | Methanol | – | – | – | +++ | – | – | 2.66 ± 0.58 |
| Water | – | – | – | +++ | + | + | 1.88 ± 0.01 | ||
| A. acidum (JW003) | Leaf | Methanol | – | ++ | ++ | + | ++ | +++ | 0.22 ± 0.03 |
| Water | – | – | + | + | + | ++ | 0.15 ± 0.01 | ||
| Calamus sp. (JW004) | Leaf | Methanol | – | – | ++ | + | + | +++ | 0.49 ± 0.00 |
| Water | ++ | +++ | + | + | – | ++ | 0.41 ± 0.03 | ||
| H. riparia (JW005) | Leaf | Methanol | – | ++ | +++ | + | ++ | +++ | 0.33 ± 0.02 |
| Water | – | + | + | ++ | ++ | +++ | 0.18 ± 0.01 | ||
| A. concinna (JW006) | Fruit | Methanol | – | +++ | +++ | +++ | – | – | 1.15 ± 0.04 |
| Water | +++ | +++ | ++ | + | +++ | – | 0.52 ± 0.02 | ||
| L. glutinosa (JW007) | Leaf | Methanol | – | + | – | ++ | ++ | +++ | 0.52 ± 0.02 |
| Water | +++ | ++ | + | ++ | ++ | ++ | 0.33 ± 0.04 | ||
| A. biflorum (JW008) | Rhizome | Methanol | – | – | + | + | ++ | – | 0.17 ± 0.03 |
| Water | – | + | ++ | + | + | – | 0.18 ± 0.01 |
Symbol (+++) indicates presence in high concentration, Symbol (++) indicates presence in moderate concentration, Symbol (+) indicates presence in trace concentration, and (-) indicates absence of the respective phytochemical.
Abbreviations: Alk: Alkaloids, Gly: Glycosides, Ster: Steroids, Sap: Saponins, Ter: Terpenoids, Tan: Tannins, TSC: Total saponins content.
The values are means ± standard deviations (n = 3). Saponin content in the samples is expressed as mg of diosgenin equivalents.
The utilised parts were separated and dried at 35 °C in a hot-air oven, then ground into fine powder using an electric grinder. The powder (10 g) of each plant was extracted by maceration with either 100 mL of methanol or water at room temperature (25 ± 2 ℃) (Inalegwu and Sodipo, 2015). After 12 h, the solvents were filtered through Whatman No.1 filter paper and re-extracted the residue with the same solvent trice. Combined supernatants were evaporated by a rotary evaporator to obtain a crude extract, kept in flask and stored at 4 ℃. The preliminary qualitative phytochemical analysis was carried out to identify types of the secondary metabolites viz. alkaloids, glycosides, steroids, saponins, terpenoids, and tannins in the crude plant extracts. All of the evaluation was done by direct visual observation and the content was estimated based on a relative scale of absent and presence with low (+), medium (++) and high (+++) (Ahumada-Santos et al., 2013, Sommano et al., 2016).
2.4. Determination of total saponin content
The total saponin content was determined by the method of Makkar et al. (2007) with slight modification. Briefly, 50 µL of plant extract was mixed with 2.5 mL sulfuric acid 72% (v/v) and 0.1 mL, 8% vanillin solution in ethanol. The mixtures were incubated in a water bath at 60 °C for 10 min and then cooled with cold water. The absorbance of sample was measured at 544 nm using SPECTROstar Nano Microplate Reader (BMG LABTECH, Ortenberg, Germany). Diosgenin was used as the reference standard with saponin content expressed as mg diosgenin equivalents per gram of dried sample powder (mg DE/g DW). The final quantification of the phytochemicals is the value of mean ± SD of three measurements.
2.5. Ethical aspect
This research had minimal risk and was not clinical trial that may harm to all participants. All participants were not vulnerable to the work conducted. The author (Jiratchaya Wisetkomolmat) had attended the Human Research Ethics training conducted by Chiang Mai University Research Ethic Committee and understood the entire ethical procedure. All procedures performed in this study involving human participants were in accordance with the ethical standards (Belmont Report) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard. Informed consent was obtained from all individual participants included in the study.
2.6. Chemometric analysis
Statistical analysis based on the use of chemometric PCA was used to investigate the correlation between people traditional knowledge and plant use characteristics using Matlab software (The MathWorks, Inc., ver. 9.7, USA). In this research study, a total of seventeen variables including ‘Age’, ‘Gender’, ‘DR’, ‘DF’, ‘UV’, ‘Soap’, ‘Shampoo’, ‘Scrub’, ‘Detergent’, ‘Spiritual cleansing’, ‘Scent’, ‘Alk’, ‘Gly’, ‘Ster’, ‘Sap’, ‘Ter’ and ‘Tan’ were used in the analysis.
3. Results
3.1. Demographical characteristics
The total of 68 local informants in the study area was interviewed, and the majority of the respondents was female (72%) with the age ranged between 21 and 93 years. The population comprised of 4 age-groups (<40 = 30.88%, 51–60 = 32.35%, 61–70 = 20.58%, >71 = 16.17%) as shown in Fig. 2. The dominant age range of the correspondents was between 51 and 60 years old and the average age was 53 ± 17.5 years old.
Fig. 2.
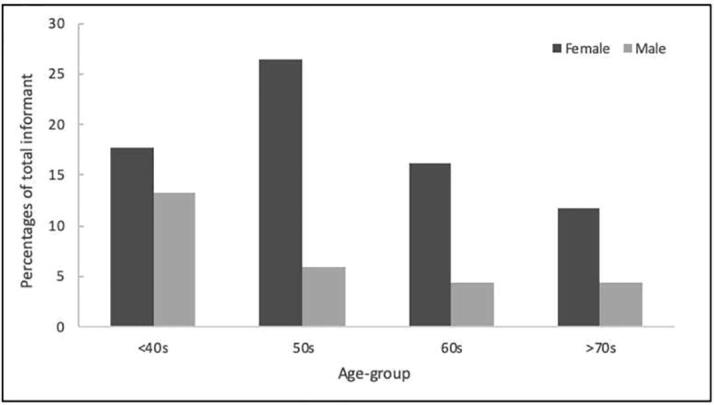
Demographical characteristics.
3.2. Plant recognition, visibility and utilisation
Ethnobotanical data derived from the interview is illustrated in Table 1. Within the list of 25 documented species from 21 families, the most cited as known was Acacia concinna (plant no. 10; soap pod) and Clitoria ternetea (plant no. 11; butterfly pea) (100% DR), followed by Oryza sativa (plant no. 16; rice) Citrus hystrix (plant no. 17; kaffir lime) (98.5% DR) and Luffa cylindrica (plant no. 4) and Sesamun oriental (plant no. 15) with 97.1% DR. The results also described that Cissus modeccoides (plant no. 23), Dillenia paroiflora (plant no. 6) and Garuga pinnata (plant no. 3) were of low DR (2.9%, 8.8% and 10.3% respectively). Plants that had been mentioned as highly found were C. ternetea (plant no. 11), O. sativa (plant no. 16) and C. hystrix (plant no. 17) (100% DF). Following the same trend, kaffir lime was the most utilised in general (100% UV), followed by butterfly pea and rice (97.1% UV) and the third place was A. concinna. The less utilised plants were C. modeccoides, D. paroiflora and G. pinnata (1.5%, 2.9% and 8.8% UV respectively). The UVs in the cleaning purposes also showed that A. concinna (1.029) and C. hystrix (1.853) were commonly used and the first one was known traditionally for spiritual cleansing particularly at Thai new year. For those of high UVs values (~0.5), viz. S. rarak, O. sativa, L. glutinosa and C. ternetea were of shampoo or soap ingredients. It is worthy to note that fibre from the dried fruit of both species in the Cucurbitaceae (UVs ~ 0.2–0.7) was also utilised in cleansing purposes.
The result also indicated that the plants belonging to family Fabaceae had the most cited as being recognised, visible and utilised, followed by the families Rutacea and Poaceae, while the Vitaceae was less. For plant part used as cleansing purposes, the leaf was the most utilised part, followed by fruit and the pericarp was exploitable (viz., S. rarak and rice). Butterfly pea was the only plant type that its flower was claimed to be used as ingredient in detergent.
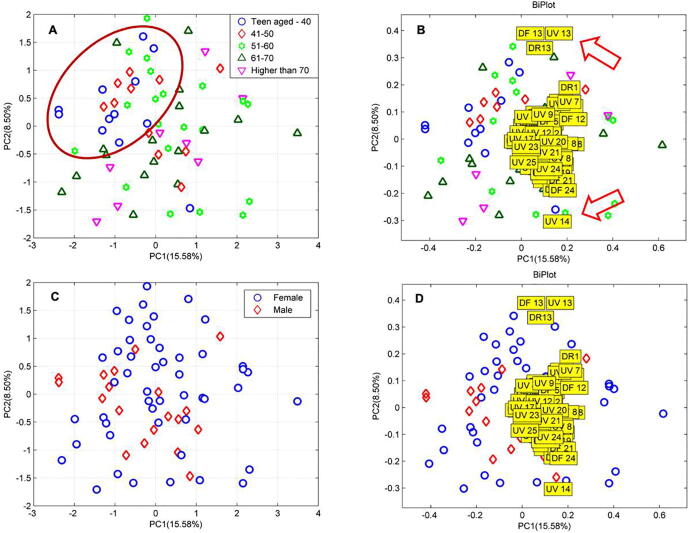
3.3. Biometric analyses of detergent plant knowledge
Relationship between ages or genders of the villagers and knowledge of 25 detergent plants was analysed using the multivariate PCA in Fig. 3. In Fig. 3A, all interviewees seemed to scatter across the PCA space that accounted in PC1 15.58% and PC2 8.50%. However, it is possible to observe the cluster of the people (ages under 40 s) on the top left of the main group meaning that there could have the similarity of the traditional knowledge. From the bi-plotted shown in Fig. 3B, factors DF, UV and DR of L. glutinosa (plant no. 13) and UV of O. corniculate (plant no. 14) separated from the other plants. However, when the samples were labeled according to the gender as shown in Fig. 3C and 3D, the clusters of the gender could not be seen.
Fig. 3.
Principal Component Analysis scoring of ages groups (A), gender (C) and biplot acclimations (B, D) with 25 detergent plants knowledge as defined by degree of recognition (DR), degree of finding (DF) and utilisation value in general (UV). Lists of plants are detailed according to the serial numbers listed in Table 1.
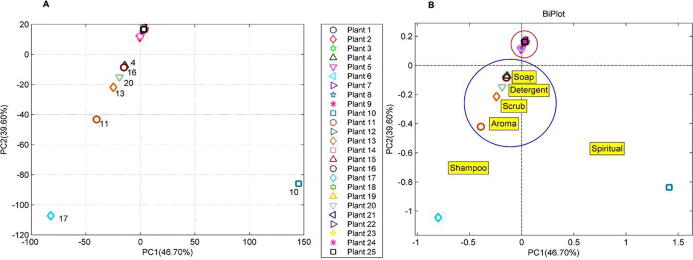
3.4. Detergent plant definition by knowledge
Terms used to describe cleansing proposes was categorised into 6 definitions as following; shampoo, scrub, detergent, soap, scent and spiritual cleansing. The relationship between such definitions and the 25 plant species (see Table 1) was illustrated in Fig. 4A. The PCA score plot (with PC1 46.70% and PC2 39.60%) illustrated that these plants can be assembled into 4 clusters. The results from the biplot analysis advised that A. concinna (plant no. 10) and C. hystrix (plant no. 17) were different from the others. Biplot cluster analysis in Fig. 4B suggested that most of the plants located in the red cycle while the rest of the plants allied with cleansing terms viz., ‘soap’, ‘detergent’, ‘scrub’ and ‘aroma’, were projected closely to each other. In addition, C. hystrix (plant no. 17) which projected far from the rest, had relationship with the term ‘shampoo’ more than other plants and A. concinna (plant no. 10) was distinct from the rest because of the term ‘spiritual cleansing’.
Fig. 4.
Biplot acclimations between detergent definitions and shampoo, scrub, detergent, soap, scent and spiritual. Lists of plants are detailed according to the serial numbers listed in Table 1.
3.5. Phytochemical screening
Based on the survey, only eight detergent plants out of 25 species from plant list in Table 1 were collected as followed C. hystrix, S. rarak, A. acidum, Calamus sp., H. riparia, A. concinna, L. glutinosa, O. corniculate, A. gramineus and A. biflorum. Crude methanolic and water extracts gave positive for saponins when analysed the phytochemical screening by froth test and vanillin-sulfuric test (Table 2). However, alkaloids were detected only in water extract of Calamus sp., A. concinna and L. glutinosa. The water extract of L. glutinosa showed the highest variety of secondary metabolites. A. concinna showed the highest positive presence of phytochemicals (+++). Total saponin content showed significant variations within the plant samples and had the highest content in methanol and water solvents, ranged between 0.17 and 2.66 and 0.15–1.88 mg DE/g extract, respectively. S. rarak illustrated the highest amount of saponin content in methanol extract (2.66 mg DE/g extract), followed by A. concinna (1.15 mg DE/g extract) and the less was A. acidum (0.15 mg DE/g extract) (Fig. 5).
Fig. 5.
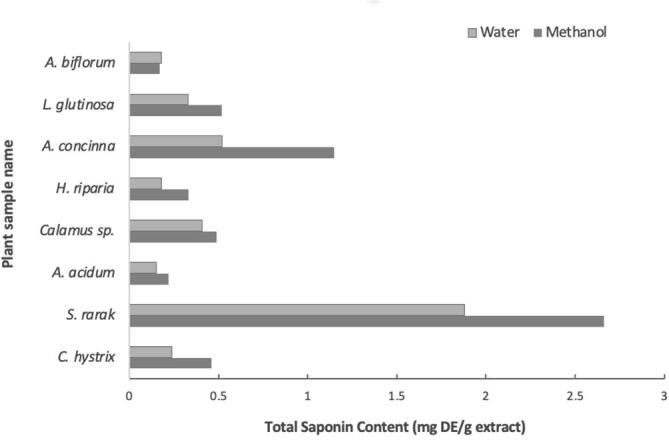
Total saponin contents in collected plants used in cleansing purposes.
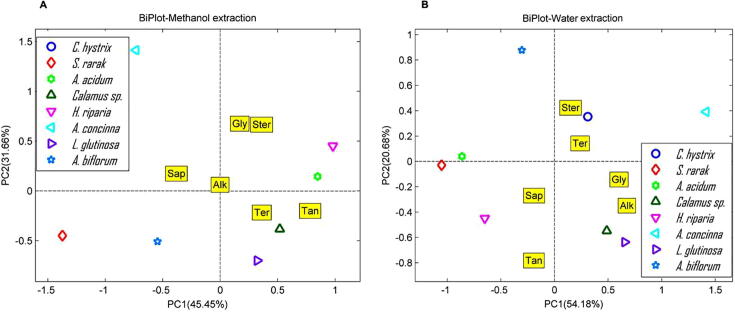
3.6. Detergent plant definition by phytochemicals
From Fig. 6, the different solvents used for chemical extraction affected the content of six chemicals. According to methanol extraction (Fig. 6A), the principal component analysis of the analysed detergent plants showed their degree of correlation with respect to their phytochemicals and surfactant components in methanol extractions. The first principal component depicted 45.45% of the total variation in which Calamus sp. and L. glutinosa correlated positively with ‘Ter’ and ‘Tan’. ‘Gly’ and ‘Ster’ were found to be solely dominated by H. riparia and A. acidum. From the PCA analysis the PC2 exhibited 31.66% of variability having positively correlation with Sap. This accounted for higher cleansing properties of A. concinna that scored heavily for this particular component. The species S. rarak and A. biflorum were found to be divorced of any phytochemicals and thus correlated negatively with the phytochemical components. This might account for their lowest detergent properties and cleansing action.
Fig. 6.
Biplot acclimations between detergent plants and the chemical contents extracted by methanol and water.
Fig. 6B shows the performance of each plant according to the chemical contents, extracted by water. Based on the position of A. biflorum which located far from the others, might refer that it had ‘Ster’ higher than other plants. In addition, both Calamus sp. and L. glutinosa also had ‘Alk’ higher than the rests while S. rarak and A. acidum had ‘Sap’ and ‘Tan’ as well as H. riparia but H. riparia had higher content of ‘Tan’.
4. Discussion
The loss of local plants and the associated knowledge have generally negative impacts on the local communities who rely on these resources. Factors influences the declining perception of local plant utilisation by indigenous people include, but of course not limited to, the introduction of western medicine, urbanisation, land degradation (Nankaya et al., 2019), modernisation (Siriphon, 2006) and increasing of environmental changes (Turner et al., 2000) as well as the lack of propagation techniques (Nandwani et al., 2008, Wisetkomolmat et al., 2020). In this study, we access the status of the existing knowledge of the 25 Thai local detergent plants. The forest dependent community in the Northern Thai was chosen as they exploit the true relationship between human being and the forest.
Data from the survey agrees with literatures described the common plants used for the purpose of cleansing in Thai culture are butterfly pea, fruit of kafir lime and rice (Kumar et al., 2012, Pasukamonset et al., 2016). Butterfly pea is also known by several common names including blue pea or anchan in Thai. It is a highly palatable forage legume from the Fabaceae family. In Asia, young shoots, leaves, flowers and tender pods are eaten as vegetable. Due to its attractive blueish flowers they are often crushed and filtered through cloth. The juice is applied on the scalp to promote hair growth (Gomez and Kalamani, 2003, Kumar et al., 2012, Mukherjee et al., 2008). Our result showed that Ma-kruut (C. hystrix; Rutaceae) or kafir lime had the highest of UVs (1.853). Ma-kruut is very common in every household as an ingredient in Asian dishes including those of Thailand, Sri Lanka and Myanmar. The essential oil from the skin of the fruit and juice is used as shampoo to defecate lice in children (Panthong et al., 2013, Subhadrabandhu, 2001). Yabesh et al. (2014) reported high UVs of C. hystrix (2.00) which was used by local traditional healers in India for anti-dandruff and hair loss. Also report of Silalahi (2019), UVs = 4.08, it was used as steam-bathing materials by the people in Indonesia. In Thailand fresh kafir lime fruits are squeezed and mixed with coconut oil for hair conditioner and to promote hair growth (Kumar et al., 2012). The fruit skin was traditionally used among the Malaysians for shampoo and also the fruit juice is rubbed onto the skin to soften or mixed with bath water (Abirami et al., 2014). Thai people consume rice in every meal and all parts of rice gain are deployed (Chongchorhor and Kabmala, 2017). For the Northern Thai, glutenous rice grains required over-night soaking with clean water before steaming. The by-product of this process enriched with nutrients and good bacteria culture is known for good skin cleanser and shampoo (Marto et al., 2018).
Indigenous people have employed the benefits from the forest environment thereby developed ways of culture and knowledge linked to their environment based on an accumulation of knowledge about forest ecology and ecosystem biodiversity (Siriphon, 2006, Visweswari et al., 2013). Based on plant recognition, utilisation and visibility results in general, plants that are often diverse utilised are tended to be domesticated and therefore are generally found in the villager home gardens such as C. ternetea, C. hystrix and O. sativa which have the highest percentages of citation. Normally, the majority of plant found in many home gardens are edible plants used in many purposes including for food and medicine (Panyadee et al., 2019). More result illustrated that in this forest community, few plant species such as S. rarak were used and were not grown or available in the village. This indicates that the villagers had knowledge of plants from areas beyond their territory (Rijal, 2011). Similar with the results of Faruque et al. (2018) found that some species had the highest frequency of citation but not found in the village because those were collected by traditional healers from the wild. The results also illustrated that the community lacked knowledges of some plant species. To this Turner et al. (2000) urgued that the knowledge of plants is also culturally exemplified and could reflex vastly to the traditional knowledge of the community. Traditional knowledge of plant uses has accumulated and has been passed on from ancestors to the later generations by spoken word and by life style (Inta et al., 2013).
In other cultural complexes, the selection of wild plants was governed primarily by availability of the plants in the local environment (Pala et al., 2019, Raj et al., 2018). Many ethnic groups rely on wild-collected plants for food and many other purposes from birth to death for examples; some indigenous plants are used in aroma therapy and as baths, detergents such as fruit of Dillenia indica as well as during the spiritual rituals such as Bambusa sp., Nymphaea lotus, Gnetum gnemon, Rhynchotechum ellipticum and Musa × paradesiaca (Phangchopi et al., 2014). In previous days, S. rarak has been traditionally used as natural detergent, laundry detergent and shampoo by people in many areas of the world. The saponins found in fruits are responsible for detergent properties (Tmáková et al., 2016).
From biometric analyses of detergent plant knowledge in Fig. 3 shown that knowledge of plant uses may not thoroughly distributed with people in all age groups and local people with younger ages appeared to have a less extensive utilisation knowledge of this group of plant (Nandwani et al., 2008, Turner et al., 2000). Our result, however interestingly indicate that L. glutinosa has been recognised, visible, and utilised by the younger generations. Therefore, in this particular community, the knowledge of detergent plants is persistent. Even though they had not used them, but they could at least distinguish or experience the uses of these plants in other ways. It is known by Thai people that the mucilaginous leaves of L. glutinosa are used in combination with kafir lime and flower of butterfly pea for Thai traditional shampoo (Panyadee et al., 2016, Wisetkomolmat et al., 2019). Gender was also not significantly correlated to knowledge of plant utilisation (Lara Reimers et al., 2019).
In the study of the terms used, we purposed that ‘detergent’ should be defined only for soap, shampoo, scrub, scent and detergent but not for spiritual cleansing. Wisetkomolmat et al., 2019, Basu et al., 2015 explained that when using plants as detergents, they should be able to remove the outer layer of grease in which the dirt is embedded. For scientific term, detergents are amphipathic molecules that exhibit unique properties in aqueous solutions, in which they spontaneously form spherical micellar structures in addition to lowering the surface tension of their solutions (Bhat et al., 2011, Niven, 1955, Seddon et al., 2004). Soap, in another hand, is the major product of saponification between triglyceride and lye solution (Atolani et al., 2016) while, foam is formed in aqueous solution through the dispersion of air bubbles in water surface stabilised by the surface-active agents such as surfactants, proteins, polymers or macromolecule (Chen et al., 2017, Tadros, 2006).
Plants contains phytochemicals that are generally bioactive. Among those, the alkaloids are substances produced by plants to protect themselves against the aggression from other organisms and are medicinal proven to be beneficial for pain relief (Debnath et al., 2018). Glycosides and phytosteroids are extracted from plants include some pharmacologically important products such as cardiac aglycones (Goncharov et al., 2016, Heftmann, 1975). Tannins are a class of complex biomolecules of polyphenolic nature synthesised by a large variety of plants, in which they are used as mordents, medical astringents, antipredation or pesticide agents (Hassan et al., 2015, Izawa et al., 2010, Patachia and Croitoru, 2016). Saponins are amphipathic glycosides grouped having one or more hydrophilic glycoside moieties combined with a lipophilic triterpene derivative. These compounds are traditionally used as natural surfactant (Böttcher and Drusch, 2016, Goncharov et al., 2016, Kregiel et al., 2017), called bio-based surfactant (Tmáková et al., 2016, Wisetkomolmat et al., 2020). Due to the presence of a lipid‐soluble aglycone and water‐soluble sugar chain, natural saponins own surfactant properties that are foam formation (with liquid‐gaseous phases) (Hassan et al., 2015), an emulator effect (with liquid‐liquid phases) and dispersion abilities (with liquid‐solid phases). In the aqueous solution, saponin molecules align themselves vertically on the surface with their hydrophobic ends faced away from the water (Almutairi and Ali, 2015). This has the effect of reducing the surface tension of the water, causing bubbles or foamability (Böttcher and Drusch, 2016, Kregiel et al., 2017).
The results of phytochemical screening showed that water extract of L. glutinosa leaf had the high variety of secondary metabolites. The total saponin content, S. rarak showed the highest amount of saponin content followed by A. concinna and L. glutinosa. This is in agreement with the reports of Pradeepa et al., 2013, Gulzar et al., 2015, Mandal et al., 2000 who advised that the extract from leaves of L. glutinosa contained alkaloids, steroids, flavonoids, terpenoids, tannins glycosides and saponins. The results of Devi and Meera (2010) suggested that methanolic extracts of L. glutinosa confined high saponin and alkaloid content that had significant antioxidant, anti-inflammatory, antispasmodic, emollient and possessed wound healing and antibacterial activity. Pradeepa et al. (2013) added that the significant effect of the phytochemical extracts of L. glutinosa is due to the presence of a single active constituent in higher levels or due to the combined effect of more than one phytoconstituents.
5. Conclusion
In conclusion, traditional ecologically knowledge is rapidly declined, owing to cultural change and urbanisation. The present study showed that forest dependent community was vitally important group of people to reserve the knowledge of indigenous plant utilisation. From the preliminary investigation, the use of chemometric combined with ethno-informatic data had led to the understanding of the relationships between the phytochemicals and plant usage as cleansing purposes. The research outcome could support the awareness regarding the role of the community to conserve, protect and promote the dissemination of knowledge for local Thai detergent plants.
CRediT authorship contribution statement
Jiratchaya Wisetkomolmat: Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Project administration. Angkhana Inta: Methodology, Writing - review & editing, Supervision. Chanida Krongchai: Software, Data curation. Sila Kittiwachana: Methodology, Software, Writing - review & editing, Supervision. Kittisak Jantanasakulwong: Funding acquisition. Pornchai Rachtanapun: Funding acquisition. Sarana Rose Sommano: Conceptualization, Methodology, Data curation, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research project was supported by TSRI and was partially supported by Chiang Mai University. We also would like to thanks Huai Hong Krai Royal Development Study Centre.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abirami A., Nagarani G., Siddhuraju P. The medicinal and nutritional role of underutilized citrus fruit-Citrus hystrix (kaffir lime): A review. Drug Invention Today. 2014;6(1):1–5. [Google Scholar]
- Ahumada-Santos Y.P., Montes-Avila J., De Jesús Uribe-Beltrán M., Díaz-Camacho S.P., López-Angulo G., Vega-Aviña R., López-Valenzuela J.Á., Heredia J.B., Delgado-Vargas F. Chemical characterization, antioxidant and antibacterial activities of six agave species from sinaloa, mexico. Industrial Crops and Products. 2013;49:143–149. doi: 10.1016/j.indcrop.2013.04.050. [DOI] [Google Scholar]
- Almeida M.Z., Léda P.H., Da Silva M.Q., Pinto A., Lisboa M., Guedes M.L.M., Peixoto A.L. Species with medicinal and mystical-religious uses in são francisco do conde, bahia, brazil: A contribution to the selection of species for introduction into the local unified health system. Revista Brasileira de Farmacognosia. 2014;24(2):171–184. doi: 10.1016/j.bjp.2014.04.006. [DOI] [Google Scholar]
- Almutairi M.S., Ali M. Direct detection of saponins in crude extracts of soapnuts by ftir. Natural product research. 2015;29(13):1271–1275. doi: 10.1080/14786419.2014.992345. [DOI] [PubMed] [Google Scholar]
- Atolani O., Olabiyi E.T., Issa A.A., Azeez H.T., Onoja E.G., Ibrahim S.O., Zubair M.F., Oguntoye O.S., Olatunji G.A. Green synthesis and characterisation of natural antiseptic soaps from the oils of underutilised tropical seed. Sustainable Chemistry and Pharmacy. 2016;4:32–39. doi: 10.1016/j.scp.2016.07.006. [DOI] [Google Scholar]
- Basu A., Basu S., Bandyopadhyay S., Chowdhury R. Optimization of evaporative extraction of natural emulsifier cum surfactant from Sapindus mukorossi—characterization and cost analysis. Industrial Crops and Products. 2015;77:920–931. doi: 10.1016/j.indcrop.2015.10.006. [DOI] [Google Scholar]
- Berkes, F. 1993. Traditional ecological knowledge in perspective. Traditional ecological knowledge: Concepts and cases 1.
- Bhat R., Prajna P., Menezez V.P., Shetty P. Antimicrobial activities of soap and detergents. Advanced Biomedical Research. 2011;2(2):52–62. [Google Scholar]
- Böttcher S., Drusch S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophysics. 2016;11(1):91–100. doi: 10.1007/s11483-015-9420-5. [DOI] [Google Scholar]
- Bouillon C. Shampoos. Clinics in dermatology. 1996;14(1):113–121. doi: 10.1016/0738-081x(95)00118-y. [DOI] [PubMed] [Google Scholar]
- Chen X.-W., Yang D.-X., Zou Y., Yang X.-Q. Stabilization and functionalization of aqueous foams by quillaja saponin-coated nanodroplets. Food Research International. 2017;99:679–687. doi: 10.1016/j.foodres.2017.06.045. [DOI] [PubMed] [Google Scholar]
- Chongchorhor C., Kabmala M. Using facet analytico-synthetic method for organizing Thai's indigenous rice knowledge. Kasetsart Journal of Social Sciences. 2017 doi: 10.1016/j.kjss.2017.09.003. [DOI] [Google Scholar]
- Debnath B., Singh W.S., Das M., Goswami S., Singh M.K., Maiti D., Manna K. Role of plant alkaloids on human health: A review of biological activities. Materials today chemistry. 2018;9:56–72. [Google Scholar]
- Devi P., Meera R. Study of antioxdant, antiinflammatory and woundhealing activity of extracts of Litsea glutinosa. Journal of Pharmaceutical Sciences and Research. 2010;2(3):155. [Google Scholar]
- Dos Santos L.L., Ramos M.A., Da Silva S.I., De Sales M.F., De Albuquerque U.P. Caatinga ethnobotany: Anthropogenic landscape modification and useful species in Brazil’s semi-arid Northeast. Economic Botany. 2009;63(4):363. doi: 10.1007/s12231-009-9094-3. [DOI] [Google Scholar]
- Esbensen K.H., Geladi P. Principal Component Analysis: Concept, Geometrical Interpretation, Mathematical Background, Algorithms, History, Practice. In: Brown S.D., Tauler R., Walczak B., editors. Comprehensive Chemometrics, 211–226. Elsevier; Oxford: 2009. [Google Scholar]
- Faruque M.O., Uddin S.B., Barlow J.W., Hu S., Dong S., Cai Q., Li X., Hu X. Quantitative ethnobotany of medicinal plants used by indigenous communities in the Bandarban district of Bangladesh. Frontiers in pharmacology. 2018;9:40. doi: 10.3389/fphar.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S.M., Kalamani A. Butterfly pea (Clitoria ternatea): A nutritive multipurpose forage legume for the tropics-an overview. Pakistan Journal of Nutrition. 2003;2(6):374–379. doi: 10.3923/pjn.2003.374.379. [DOI] [Google Scholar]
- Goncharov, N., E. Maevsky, N. Voitenko, A. Novozhilov, I. Kubasov, R. Jenkins and P. Avdonin. 2016. Nutraceuticals in sports activities and fatigue. In Nutraceuticals, 177-88: Elsevier.
- Gulzar H., Chowdhury M., Alam M.N., Alam S., Shakil M., Khan S., Rahman M., Mazumdar M. Phytochemical screening, antimicrobial and anticancerous activities of two different plant extracts. Journal of Medicinal Plants Studies. 2015;3(6):76–81. [Google Scholar]
- Hassan A., Achakzai P., Nangyal H. Detection and estimation of alkaloids, saponins and tannins in herbs of quetta baluchistan. American-Eurasian Journal of Agricultural & Environmental Sciences. 2015;15(6):985–990. [Google Scholar]
- Heftmann E. Functions of steroids in plants. Phytochemistry. 1975;14(4):891–901. [Google Scholar]
- Hoekou Y.P., Tchacondo T., Karou S.D., Koudouvo K., Atakpama W., Pissang P., Gbogbo A.K., Woegan A.Y., Batawila K., Akpagana K. Ethnobotanical study of latex plants in the maritime region of togo. Pharmacognosy research. 2016;8(2):128. doi: 10.4103/0974-8490.175613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Gallaher T. Importance indices in ethnobotany. Ethnobotany Research and Applications. 2007;5:201–218. doi: 10.17348/era.5.0.201-218. [DOI] [Google Scholar]
- Inalegwu B., Sodipo O. Antimicrobial and foam forming activities of extracts and purified saponins of leaves of Tephrosia vogelii. European Journal of Experimental Biology. 2015;5(5):49–53. [Google Scholar]
- Inta A., Trisonthi P., Trisonthi C. Analysis of traditional knowledge in medicinal plants used by Yuan in Thailand. Journal of Ethnopharmacology. 2013;149(1):344–351. doi: 10.1016/j.jep.2013.06.047. [DOI] [PubMed] [Google Scholar]
- Izawa, K., Y. Amino, M. Kohmura, Y. Ueda and M. Kuroda. 2010. Human–environment interactions–taste. http://doi.org/10.1016/B978-008045382-8.00108-8.
- Kregiel D., Berlowska J., Witonska I., Antolak H., Proestos C., Babic M., Babic L., Zhang B. Saponin-based, biological-active surfactants from plants. Application and characterization of surfactants. 2017;183–205 doi: 10.5772/68062. [DOI] [Google Scholar]
- Kumar N., Rungseevijitprapa W., Narkkhong N.-A., Suttajit M., Chaiyasut C. 5α-reductase inhibition and hair growth promotion of some thai plants traditionally used for hair treatment. Journal of Ethnopharmacology. 2012;139(3):765–771. doi: 10.1016/j.jep.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Lara Reimers E.A., Lara Reimers D.J., Chaloupkova P., Zepeda Del Valle J.M., Milella L., Russo D. An ethnobotanical survey of medicinal plants used in papantla, veracruz, mexico. Plants. 2019;8(8):246. doi: 10.3390/plants8080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever J., Krzywinski M., Altman N. Principal component analysis. Nature Methods. 2017;14(7):641–642. [Google Scholar]
- Makkar H.P., Siddhuraju P., Becker K. Plant secondary metabolites. Springer; 2007. Saponins; pp. 93–100. [DOI] [PubMed] [Google Scholar]
- Mandal S.C., Kumar C.A., Majumder A., Majumder R., Maity B. Antibacterial activity of Litsea glutinosa bark. Fitoterapia. 2000;71(4):439–441. doi: 10.1016/s0367-326x(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Martin G.J. Earthscan Publications; 2010. Ethnobotany: A methods manual (people and plants conservation series) [Google Scholar]
- Marto J., Neves Â., Gonçalves L.M., Pinto P., Almeida C., Simões S. Rice water: A traditional ingredient with anti-aging efficacy. Cosmetics. 2018;5(2):26. doi: 10.3390/cosmetics5020026. [DOI] [Google Scholar]
- Monteiro J.M., De Souza J.S., Neto E.M.L., Scopel K., Trindade E.F. Does total tannin content explain the use value of spontaneous medicinal plants from the brazilian semi-arid region? Revista Brasileira de Farmacognosia. 2014;24(2):116–123. doi: 10.1016/j.bjp.2014.02.001. [DOI] [Google Scholar]
- Mukherjee P.K., Kumar V., Kumar N.S., Heinrich M. The ayurvedic medicine clitoria ternatea—from traditional use to scientific assessment. Journal of Ethnopharmacology. 2008;120(3):291–301. doi: 10.1016/j.jep.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Nandwani D., Calvo J.A., Tenorio J., Calvo F., Manglona L. Medicinal plants and traditional knowledge in the northern mariana islands. Journal of Applied Biosciences. 2008;8(2):323–330. [Google Scholar]
- Nankaya J., Gichuki N., Lukhoba C., Balslev H. Sustainability of the loita maasai childrens’ ethnomedicinal knowledge. Sustainability. 2019;11(19):5530. doi: 10.3390/su11195530. [DOI] [Google Scholar]
- Niven Jr, W.W. 1955. Industrial detergency. Industrial detergency.
- Oleszek W., Hamed A. Saponin-based surfactants. Surfactants from renewable resources. 2010;239 doi: 10.1002/9780470686607.ch12. [DOI] [Google Scholar]
- Osbourn A. Saponins and plant defence—a soap story. Trends in plant science. 1996;1(1):4–9. doi: 10.1016/S1360-1385(96)80016-1. [DOI] [Google Scholar]
- Pala N.A., Sarkar B.C., Shukla G., Chettri N., Deb S., Bhat J.A., Chakravarty S. Floristic composition and utilization of ethnomedicinal plant species in home gardens of the eastern himalaya. Journal of Ethnobiology and Ethnomedicine. 2019;15(1):14. doi: 10.1186/s13002-019-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthong K., Srisud Y., Rukachaisirikul V., Hutadilok-Towatana N., Voravuthikunchai S.P., Tewtrakul S. Benzene, coumarin and quinolinone derivatives from roots of citrus hystrix. Phytochemistry. 2013;88:79–84. doi: 10.1016/j.phytochem.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Panyadee P., Balslev H., Wangpakapattanawong P., Inta A. Woody plant diversity in urban homegardens in northern thailand. Economic Botany. 2016;70(3):285–302. doi: 10.1007/s12231-016-9348-9. [DOI] [Google Scholar]
- Panyadee P., Balslev H., Wangpakapattanawong P., Inta A. Medicinal plants in homegardens of four ethnic groups in Thailand. Journal of ethnopharmacology. 2019;239 doi: 10.1016/j.jep.2019.111927. [DOI] [PubMed] [Google Scholar]
- Pasukamonset P., Kwon O., Adisakwattana S. Alginate-based encapsulation of polyphenols from clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food hydrocolloids. 2016;61:772–779. doi: 10.1016/j.foodhyd.2016.06.039. [DOI] [Google Scholar]
- Patachia S., Croitoru C. Woodhead Publishing; 2016. Biopolymers and biotech admixtures for eco-efficient construction materials. [Google Scholar]
- Phangchopi U., Tamuli A., Teron R. Inventory of wild food plants in marat longri wildlife sanctuary in assam, india. Pleione. 2014;8(2):331–343. [Google Scholar]
- Pradeepa K., Krishna V., Santosh K., Girish K.K. Antinociceptive property of leaves extract of litsea glutinosa. Asian Journal of Pharmaceutical and Clinical Research. 2013;6(1):182–184. [Google Scholar]
- Raj A.J., Biswakarma S., Pala N.A., Shukla G., Kumar M., Chakravarty S., Bussmann R.W. Indigenous uses of ethnomedicinal plants among forest-dependent communities of northern bengal, india. Journal of ethnobiology and ethnomedicine. 2018;14(1):8. doi: 10.1186/s13002-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijal A. Surviving on knowledge: Ethnobotany of chepang community from mid-hills of nepal. Ethnobotany Research and Applications. 2011;9:181–215. doi: 10.17348/era.9.0.181-215. [DOI] [Google Scholar]
- Roessner U., Nahid A., Chapman B., Hunter A., Bellgard M. Metabolomics – The Combination of Analytical Biochemistry, Biology, and Informatics. In: Moo-Young M., editor. Comprehensive Biotechnology (Second Edition), 447–459. Academic Press; Burlington: 2011. [Google Scholar]
- Seddon A.M., Curnow P., Booth P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2004;1666(1–2):105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Silalahi M. An ethnobotanical study of traditional steam-bathing by the Batak people of North Sumatra, Indonesia. Pacific Conservation Biology. 2019;25(3):266–282. [Google Scholar]
- Siriphon A. Local knowledge, dynamism and the politics of struggle: A case study of the hmong in northern thailand. Journal of Southeast Asian Studies. 2006;37(1):65–81. doi: 10.1017/S002246340500041X. [DOI] [Google Scholar]
- Sommano S., Sirikum P., Suksathan R. Phytochemical screening and ethnobotanical record of some medicinal plants found in huai hong krai royal development study centre, Chiang Mai Thailand. Medicinal Plants-International Journal of Phytomedicines and Related Industries. 2016;8(3):213–218. doi: 10.5958/0975-6892.2016.00025.3. [DOI] [Google Scholar]
- Srithi K., Trisonthi C., Wangpakapattanawong P., Balslev H. Medicinal plants used in hmong women's healthcare in northern thailand. Journal of Ethnopharmacology. 2012;139(1):119–135. doi: 10.1016/j.jep.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Stibig H.-J., Achard F., Carboni S., Rasi R., Miettinen J. Change in tropical forest cover of southeast asia from 1990 to 2010. Biogeosciences. 2014;11(2):247. doi: 10.5194/bg-11-247-2014. [DOI] [Google Scholar]
- Subhadrabandhu S. Under-utilized tropical fruits of thailand. RAP Publication (FAO) 2001 [Google Scholar]
- Tadros T.F. John Wiley & Sons; 2006. Applied surfactants: Principles and applications. [Google Scholar]
- Tmáková L., Sekretár S., Schmidt Š. Plant-derived surfactants as an alternative to synthetic surfactants: Surface and antioxidant activities. Chemical Papers. 2016;70(2):188–196. doi: 10.1515/chempap-2015-0200. [DOI] [Google Scholar]
- Turner N.J., Ignace M.B., Ignace R. Traditional ecological knowledge and wisdom of aboriginal peoples in british columbia. Ecological applications. 2000;10(5):1275–1287. doi: 10.1890/1051-0761(2000)010[1275:TEKAWO]2.0.CO;2. [DOI] [Google Scholar]
- Visweswari G., Christopher R., Rajendra W. Phytochemical screening of active secondary metabolites present in withania somnifera root: Role in traditional medicine. International journal of pharmaceutical sciences and research. 2013;4(7):2770. doi: 10.13040/IJPSR.0975-8232.4(7).2770-76. [DOI] [Google Scholar]
- Wisetkomolmat J., Suppakittpaisarn P., Sommano S.R. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources. 2019;8(1):10. doi: 10.3390/resources8010010. [DOI] [Google Scholar]
- Wisetkomolmat J., Suksathan R., Puangpradab R., Kunasakdakul K., Jantanasakulwong K., Rachtanapun P., Sommano S.R. Natural surfactant saponin from tissue of Litsea glutinosa and its alternative sustainable production. Plants. 2020;9(11):1521. doi: 10.3390/plants9111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabesh J.M., Prabhu S., Vijayakumar S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. Journal of ethnopharmacology. 2014;154(3):774–789. doi: 10.1016/j.jep.2014.05.004. [DOI] [PubMed] [Google Scholar]
Further Reading
- Ismail A.M., Mohamed E.A., Marghany M.R., Abdel-Motaal F.F., Abdel-Farid I.B., El-Sayed M.A. Preliminary phytochemical screening, plant growth inhibition and antimicrobial activity studies of Faidherbia albida legume extracts. Journal of the Saudi Society of Agricultural Sciences. 2016;15(2):112–117. doi: 10.1016/j.jssas.2014.06.002. [DOI] [Google Scholar]