Abstract
Background:
During the past decade, a trend has been observed in the United States toward initiation of chronic dialysis at higher levels of estimated glomerular filtration rate. This likely reflects secular trends in the composition of the dialysis population and a tendency toward initiation of dialysis earlier in the course of kidney disease.
Methods:
The goal of this study was to generate model-based estimates of the magnitude of changes in the timing of dialysis initiation between 1997 and 2007. We used information from a national registry for end-stage renal disease on estimated glomerular filtration rate at initiation among patients who received their first chronic dialysis treatment in 1997 or 2007. We used information regarding predialysis estimated glomerular filtration rate slope from an integrated health care system.
Results:
After accounting for changes in the characteristics of new US dialysis patients from 1997 to 2007, we estimate that chronic dialysis was initiated a mean of 147 days earlier (95% confidence interval, 134–160) in the later compared with the earlier year. Differences in timing were consistent across a range of patient subgroups but were most pronounced for those aged 75 years or older; the mean difference in timing in that subgroup was 233 days (95% confidence interval, 206–267).
Conclusions:
Chronic dialysis appears to have been initiated substantially earlier in the course of kidney disease in 2007 compared with 1997. In the absence of strong evidence to suggest that earlier initiation of chronic dialysis is beneficial, these findings call for careful evaluation of contemporary dialysis initiation practices in the United States.
Chronic dialysis is an intensive and costly therapy used to treat advanced kidney disease. During the past decade, a trend has been observed toward initiation of chronic dialysis at progressively higher levels of estimated glomerular filtration rate (eGFR).1–4 Because Egfr at dialysis initiation often is higher for older patients and for those with a greater burden of comorbidity, an upward trend in eGFR at initiation may reflect changes in the composition of the dialysis population over time.2,5 However, it is also likely that such trends reflect changes in practice, where by chronic dialysis now is initiated earlier in the course of chronic kidney disease compared with previous years.1 We designed a study to evaluate the latter possibility and to estimate the magnitude of changes in the timing of dialysis initiation.
METHODS
OVERVIEW
The goal of this study was to generate model-based estimates of changes in timing of dialysis initiation between 1997 and 2007. We used information on trends in eGFR at initiation from the United States Renal Data System (USRDS),6 a national registry of end-stage renal disease. However, the relationship between trends in eGFR at initiation and timing of dialysis initiation depends on the predialysis eGFR slope (Figure 1), which is not available in USRDS data sources. Therefore, we estimated predialysis eGFR slope for USRDS patients using serial creatinine measures from Group Health Cooperative, a mixed-model nonprofit health care system in Seattle, Washington.
Figure 1.
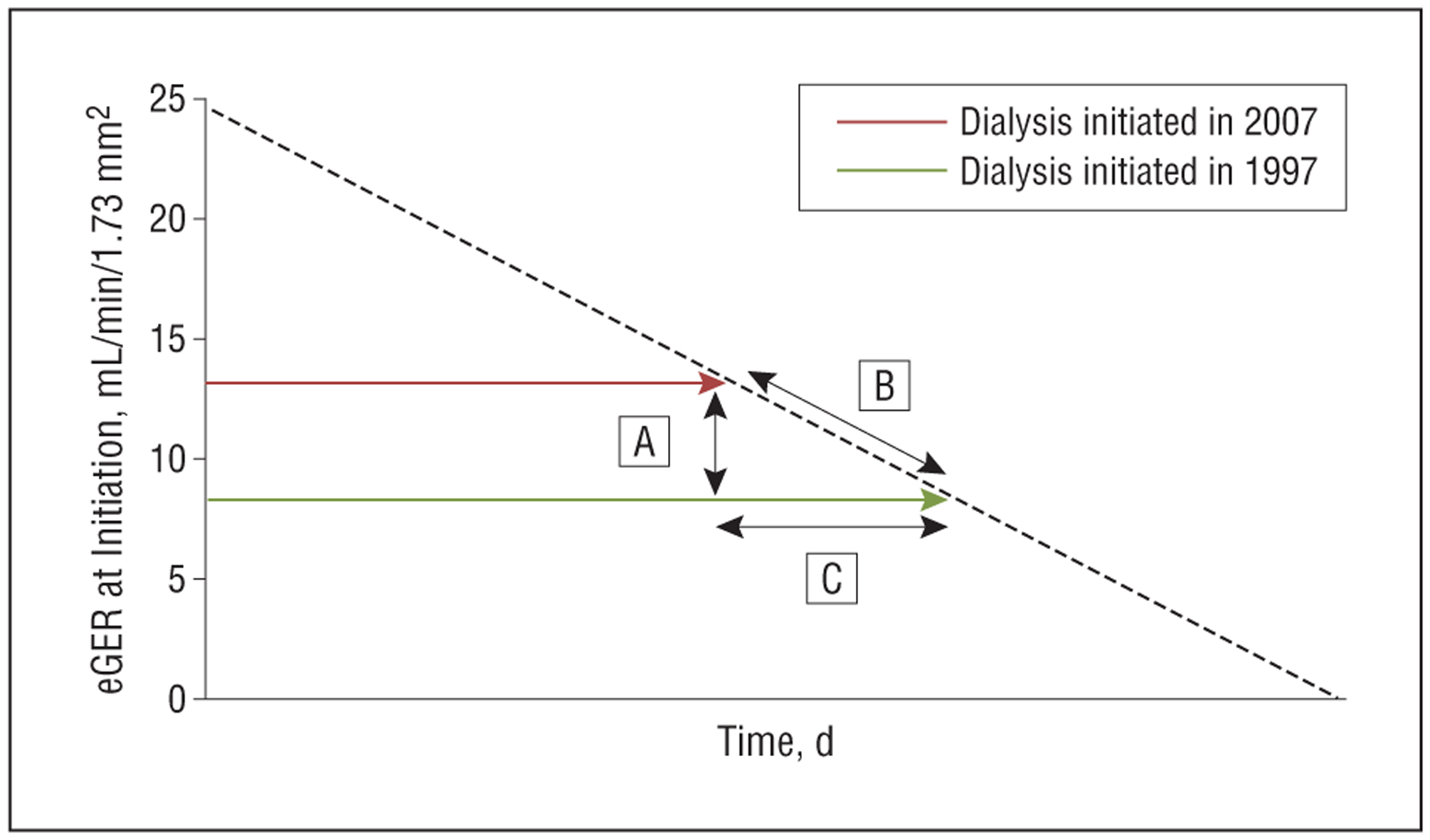
Schematic to illustrate the relationship between level of renal function at dialysis initiation, loss of renal function prior to initiation, and timing of initiation in a hypothetical patient. A, Difference between each patient’s level of renal function at initiation of dialysis in 2007 and a prediction of the level of renal function at which he or she would have initiated dialysis based on 1997 practice (estimated glomerular filtration rate [eGFR]2007−eGFR1997). B, Prediction of how rapidly each patient was losing renal function prior to dialysis initiation (chronic outpatient eGFR slope before dialysis initiation [SLOPEeGFR]). C, A (difference in eGFR in mL/min/1.73 m2)/B (rate of eGFR loss in mL/min/1.73 m2/day)=number of days earlier patients initiated dialysis in 2007 compared with when they would have initiated dialysis based on 1997 practice.
USRDS PATIENTS AND DATA SOURCES
The USRDS contained data for 185 677 patients (aged 18–100 years) who had initiated chronic dialysis in 1997 (n=78 904) or 2007 (n=106 773) and had not received a renal transplant. We excluded 5394 patients (2.9%) with missing information regarding demographic characteristics, comorbid conditions, and/or eGFR at dialysis initiation, yielding an analytic cohort of 75 572 patients who had initiated dialysis in 1997 (1997 USRDS cohort) and 104 711 patients who had initiated dialysis in 2007 (2007 USRDS cohort).Demographic characteristics and comorbid conditions at the time of dialysis initiation were ascertained from USRDS sources and included age, race (black vs nonblack), sex, diabetes, vascular disease (defined as coronary artery disease, peripheral arterial disease, or stroke), and congestive heart failure.
GROUP HEALTH COOPERATIVE PATIENTS AND DATA SOURCES
Using a comprehensive electronic medical record system developed by the nephrology specialty clinic at Group Health Cooperative, we identified 748 patients who had initiated chronic dialysis between January 1, 1997, and August 31, 2007; had information regarding demographic characteristics; and had not received a renal transplant. We selected an eGFR of less than 25 mL/min/1.73 m2 as the upper threshold for slope calculation for the primary analysis to provide a summary measure of chronic eGFR slope during the course of advanced kidney disease but before dialysis initiation. We excluded 82 patients (11.0%)with fewer than 3 serum creatinine (SCr) measurements after their first eGFR measurement less than 25 mL/min/1.73 m2, leaving an analytic cohort of 666 patients. We used the Group Health Research Institute data warehouse, a comprehensive source of administrative and clinical data for Group Health Cooperative patients, to ascertain predialysis SCr measurements, demographic characteristics, and comorbid conditions for cohort patients. The abbreviated Modification of Diet in Renal Disease equation was used to calculate eGFR based on SCr, age, race, and sex.
STATISTICAL ANALYSIS
The goal of our analysis was to estimate the time difference between when each 2007 USRDS patient had initiated dialysis and when he or she would have initiated dialysis based on 1997 practice (Figure 1, part C). To estimate this hypothetical quantity, we developed model-based predictions of 2 quantities: the eGFR at which each 2007 patient would have initiated dialysis based on 1997 practice (eGFR1997) and each patient’s chronic outpatient eGFR slope before dialysis initiation (SLOPEeGFR). Using these predicted measures, along with each patient’s actual eGFR at initiation of dialysis in 2007 (eGFR2007), the difference in timing from 1997 to 2007 was estimated for each patient as (eGFR2007−eGFR1997)/SLOPEeGFR. The models used to predict eGFR1997 and SLOPEeGFR are described in the next 2 subsections of the text. Because we compared the eGFR at initiation in patients who started dialysis in 2007 with that of similar patients who started dialysis in 1997, estimates of differences in timing between 1997 and 2007 derived from these models are not the result of changes in measured patient characteristics during this period.
eGFR1997 MODEL
The eGFR1997 model was a linear regression estimated among members of the 1997 USRDS cohort of the association of eGFR at dialysis initiation with demographic characteristics, individual comorbid conditions, and number of comorbid conditions. The variables for this model then were used to predict eGFR1997 in the 2007 USRDS cohort (an estimate of the eGFR at which patients who initiated dialysis in 2007 would have initiated dialysis based on 1997 practice).
SLOPEeGFR MODEL
The SLOPEeGFR model was a longitudinal mixed model of the trajectory of eGFR overtime prior to dialysis initiation among Group Health Cooperative patients. The dependent variable was the outpatient eGFR for patient i at time t from each patient’s first eGFR less than 25 mL/min/1.73 m2 until the time of dialysis initiation. All outpatient SCr measurements recorded during this time period were included in the model. The model included fixed effects for each covariate in the eGFR1997 model, time, interaction terms between time and each covariate, and random components for the intercept and time terms. The model thus assumes that trajectories are linear overtime, with systematic and random components to the subject-specific intercepts and slopes. Graphical diagnostics suggested that this linearity assumption was largely consistent with the data and that any departure from linearity was not strong enough to measurably affect the results. Model variables then were applied to the 2007 USRDS cohort to predict each patient’s predialysis eGFR slope (SLOPEeGFR). Confidence intervals (CIs) for mean differences in timing were derived from 1000 bootstrapped replicates of the variables from the eGFR1997 and SLOPEeGFRmodels.7Analyseswereconductedusingstatisticalsoftware (Stata, version 10.1; StataCorp LP, College Station, Texas; and R, version 2.10.1; RFoundation, Vienna, Austria).The study was approved by the institutional review boards at the Group Health Research Institute and the University of Washington, Seattle.
SENSITIVITY ANALYSES
We conducted 3 sensitivity and subgroup analyses. First, we repeated the primary analysis among patients with at least 10 (rather than 3) SCr measurements to account for the possibility that eGFR slope estimates may be less accurate for patients with fewer SCr measures. Second, we repeated the primary analysis among patients who had initiated dialysis in the outpatient setting to account for the possibility that outpatient SCr measurements may provide incomplete information regarding predialysis eGFR slope in patients who initiate dialysis in the hospital in the setting of an acute kidney injury. We were able to generate these estimates only for USRDS patients with Medicare coverage at the onset of end-stage renal disease because we did not have information on the site of initiation for other USRDS patients. Third, we repeated the primary analysis using eGFR slope estimates calculated from eGFR thresholds closer to the time of dialysis initiation (ie, <20 mL/min/1.73 m2 and <15 mL/min/1.73 m2) to account for possible variation in eGFR slope during the course of advanced kidney disease.
RESULTS
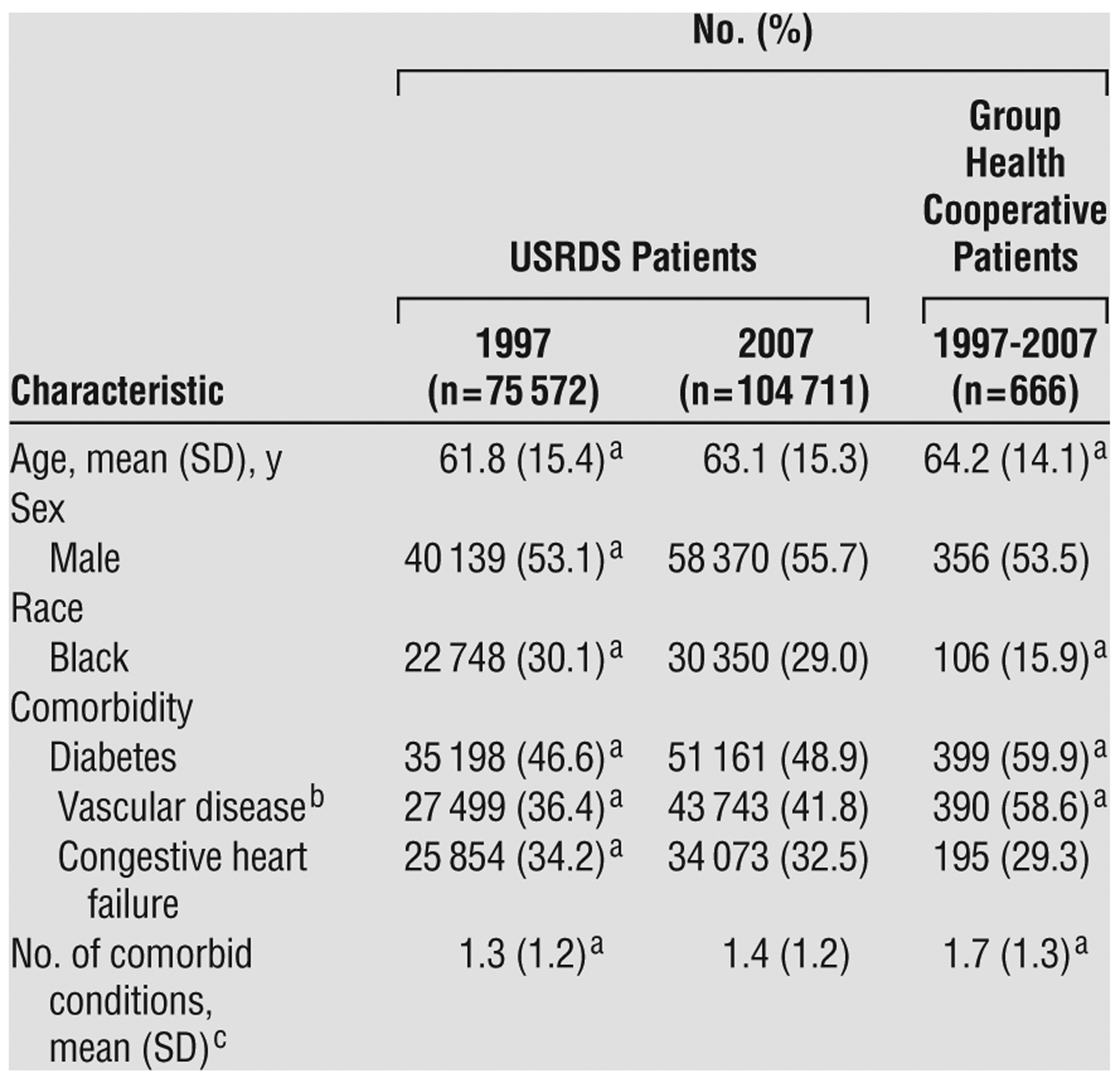
Compared with USRDS patients who initiated dialysis in 1997, those who initiated dialysis in 2007 were, on average, older and had a higher prevalence of diabetes and vascular disease (Table 1). The mean (SD) eGFR at initiation for USRDS patients was 10.8 (4.9) mL/min/1.73 m2 in 2007 compared with 8.1 (3.8) mL/min/1.73 m2 in 1997 (P<.001). The absolute change in eGFR over this 10-year period was slightly higher for patients aged 75 and older and for those with congestive heart failure but otherwise was similar across patient subgroups.
Table 1.
Characteristics of Study Cohorts
|
Abbreviation: USRDS, United States Renal Data System.
P < .05 for comparisons with USRDS patients who had initiated dialysis in 2007, based on t test or χ2 test results.
Defined as the presence of coronary or peripheral vascular disease or cerebrovascular disease (ie, stroke).
Defined as the total number of the above-listed comorbid conditions present in each patient.
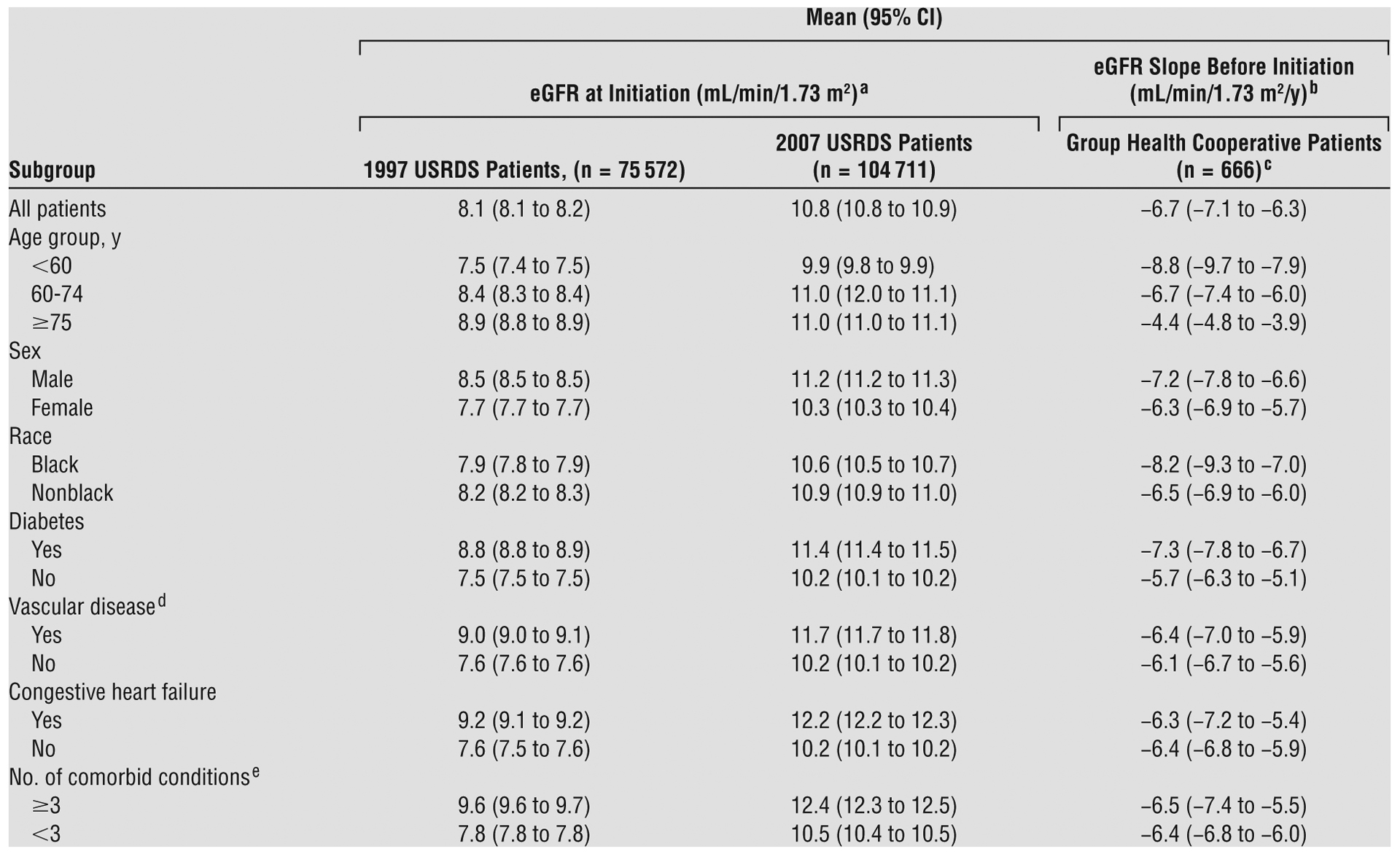
Compared with 2007 USRDS cohort patients, Group Health Cooperative patients (who had initiated dialysis between 1997 and 2007) were slightly older and less likely to be black, with a similar sex distribution, a higher prevalence of diabetes and vascular disease, a similar prevalence of congestive heart failure, and more comorbid conditions (Table 1). Mean eGFR before dialysis initiation for Group Health Cooperative patients was 8.2 (4.7) mL/min/1.73 m2, ranging from 6.8 (3.7) mL/min/1.73 m2 for those who had initiated dialysis in 1997 to 9.9 (5.9) mL/min/1.73 m2 for those who had initiated dialysis in 2007. Mean predialysis eGFR slope was −6.7 mL/min/1.73 m2 per year (95% CI, −7.1 to −6.3) (Table 2). Loss of eGFR occurred more rapidly in younger vs older patients, in black vs nonblack patients, and in patients with vs those without diabetes but otherwise was similar across patient subgroups.
Table 2.
Reported eGFR at Dialysis Initiation Among USRDS Patients and Measured eGFR Slope Before Initiation Among Group Health Cooperative Patients
|
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; USRDS, United States Renal Data System.
P < .05 for all eGFR comparisons between 1997 and 2007 USRDS cohorts, using t test or χ2 test results.
Unadjusted eGFR slope estimates were obtained using a linear mixed model that included only time as a covariate.
The eGFR slope among Group Health Cooperative patients was calculated using a total of 16 302 serum creatinine measurements. Each patient had a median of 19 outpatient serum creatinine measurements (25th–75th percentile range, 12–32 measurements) after the first eGFR value <25 mL/min/1.73 m2. These measurements were obtained during a median of 1.9 y before dialysis initiation (25th–75th percentile range, 0.9–3.4 y). The most recent outpatient serum creatinine measurement was obtained a median of 7 d before initiation (25th–75th percentile, 3–14 d).
Defined as the presence of coronary or peripheral vascular disease or cerebrovascular disease (ie, stroke).
Defined as the total number of the comorbid conditions present in each patient.
CHANGES IN TIMING OF DIALYSIS INITIATION FROM 1997 TO 2007 AMONG USRDS PATIENTS
We estimated that, in 2007, patients initiated dialysis a mean of 147 days earlier (95% CI, 134–160) in the course of their kidney disease than would have been the case for comparable patients in 1997 (Figure 2). Across patient subgroups, mean change in timing of dialysis initiation between 1997 and 2007 ranged from a minimum of 95 days earlier (95% CI, 86–105) for those younger than 60 to a maximum of 233 days earlier (206–267) for those 75 and older. In sensitivity analyses, differences in timing from 1997 to 2007 were similar to or larger than those observed in the primary analysis (Table 3).
Figure 2.
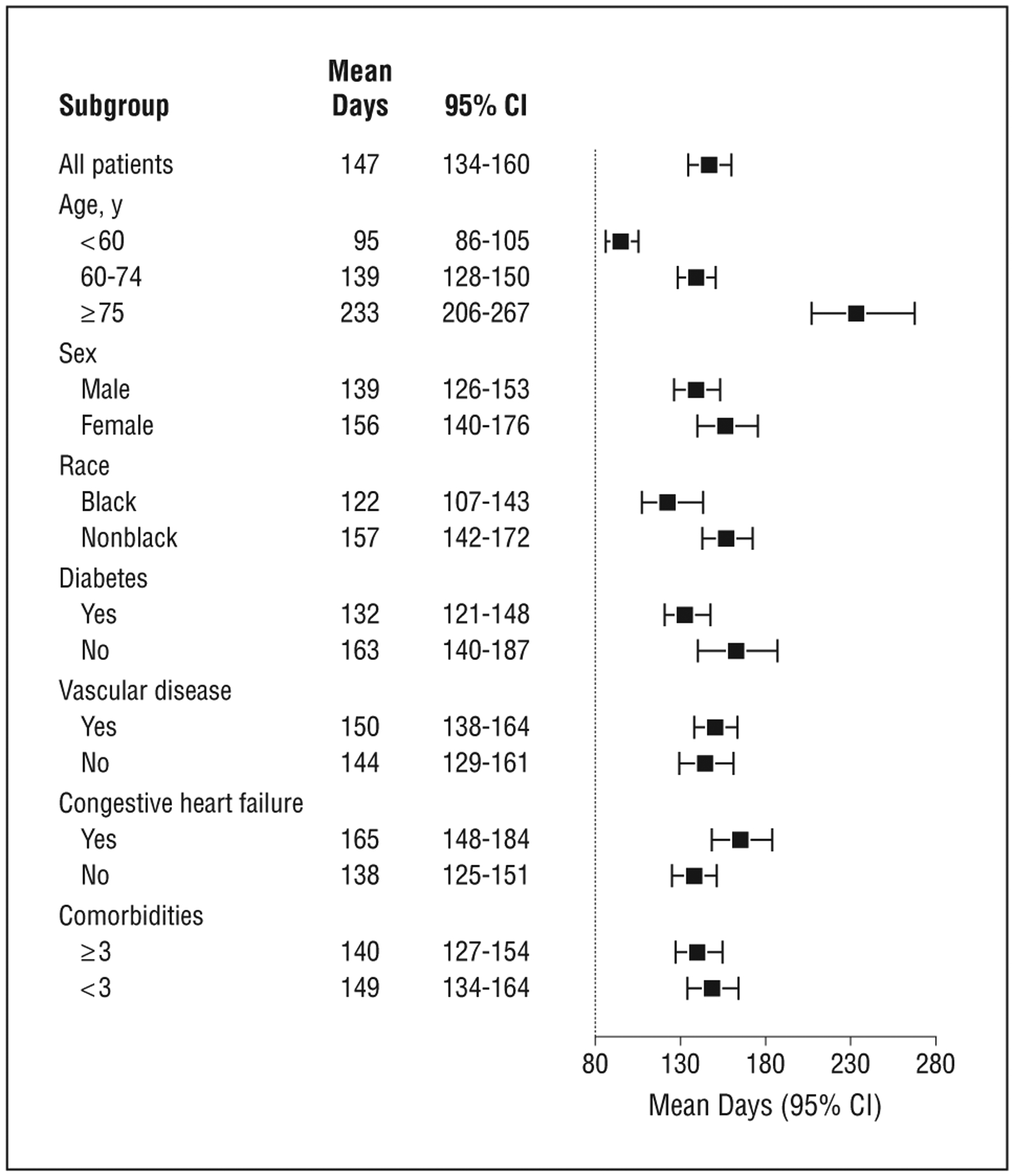
Mean number of days earlier that patients initiated dialysis in 2007 compared with 1997.
Table 3.
Mean Difference in Timing of Initiation of Chronic Dialysis in 2007 Compared With 1997: Results of Sensitivity and Subgroup Analyses

|
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
Defined as the presence of coronary or peripheral vascular disease or cerebrovascular disease (ie, stroke).
Defined as the total number of comorbid conditions present in each patient.
COMMENT
We estimate that in 2007, US patients had initiated chronic dialysis almost 5 months earlier in the course of advanced kidney disease compared with patients who had initiated dialysis in 1997. This difference in timing is not explained by changes in measured patient characteristics and most likely reflects a shift in dialysis initiation practices during this time period.
Few chronic outpatient treatment regimens are as intensive or as costly as dialysis. Outpatient center hemodialysis, the dominant modality in the United States, typically involves 3 treatments per week, each lasting 3 or more hours. In 2007, Medicare composite payments for hemodialysis conducted in outpatient centers were approximately $230 per treatment.8 The mean difference in timing of 147 days reported herein translates into approximately 63 additional hemodialysis treatments during 189 or more hours of treatment, which would translate into approximately $14 490 in additional payments for dialysis for each patient, or more than $1.5 billion if extrapolated to all cohort patients who initiated dialysis in 2007.
It seems unlikely, based on available evidence, that such a large increase in treatment intensity and costs has been balanced by an equal or larger treatment benefit. Most observational studies4,9–13 have reported higher rather than lower mortality in patients who initiate chronic dialysis at higher levels of kidney function, and 2 recent randomized trials14,15 failed to show a benefit of earlier dialysis initiation. Brunori and colleagues14 randomized 112 Italian patients older than 70 years with an eGFR between 5 and 7 mL/min/1.73 m2 to receive dialysis or a supplemented very-low-protein diet and found no statistically significant difference in survival during a median of 26.5 months. The Initiating Dialysis Early and Late trial15 randomized 828 adults in Australia and New Zealand with an eGFR between 10 and 15 mL/min/1.73 m2 to initiate dialysis at an eGFR of 10 to 14 mL/min/1.73 m2 (early start) vs 5 to 7 mL/min/1.73 m2 (late start). No benefit was observed with earlier initiation of dialysis for a wide range of outcomes, including death, cardiovascular and infectious events, treatment complications, and quality of life; also, dialysis costs were higher.16 It is not clear whether the results of these trials can be generalized to the US dialysis population; however, they certainly support the need for careful evaluation of contemporary dialysis initiation practices in this country.17
Several factors may have contributed to the alterations in US dialysis initiation practices during the past decade. First, opinion-based clinical practice guidelines17–21 have endorsed successively higher threshold levels of renal function as being appropriate for dialysis initiation, particularly in the presence of malnutrition. Second, beginning in 2002, US practice guidelines22,23 have favored the use of SCr-based equations such as the Modification of Diet in Renal Disease and Cockroft-Gault equations vs measures based on 24-hour urine collection. In concert with more wide-spread use of automated eGFR reporting, newer equations, such as the Modification of Diet in Renal Disease equation, are increasingly favored over the Cockroft-Gault equation. Because each of these methods for estimating renal function can yield different results in the same individual, these changes clearly have potential to affect dialysis initiation decisions for some patients.24,25 Also, it is possible that the current classification scheme for chronic kidney disease inadvertently may have affected initiation practices by conflating an eGFR less than 15 mL/min/1.73 m2 with dialysis (both are classified as stage 5 chronic kidney disease or renal failure).22,23 Finally, Medicare, which is the primary payer for chronic dialysis in this country, now provides significantly less oversight regarding the level of renal function among new dialysis patients compared with earlier years.26,27
Compared with other groups examined, it appears that older patients disproportionately have been affected by changes in the timing of dialysis initiation. More pronounced differences in timing among older patients appear to reflect a larger temporal change in eGFR at initiation and slower loss of eGFR before initiation. The latter finding is consistent with reports28–30 of stable or very slow rates of eGFR decline among older patients with advanced kidney disease, a finding that may reflect slower loss of true GFR and/or greater concurrent loss of muscle mass at older ages.31 Regardless of the underlying explanation, we estimate that patients aged 75 and older initiated dialysis almost 8 months earlier in 2007 compared with 1997. Because mortality rates among older adults with advanced kidney disease are high,29 these results suggest that members of this group spent a substantially greater fraction of their limited remaining life undergoing dialysis in 2007 compared with 1997 and that some may never have initiated dialysis during the earlier period. Although older patients were not well represented in the Initiating Dialysis Early and Late trial, it seems unlikely that these individuals would gain any more benefit from earlier initiation of dialysis than would their younger counterparts. Observational data suggest that older patients receiving dialysis, particularly those with a high burden of comorbidity, are less likely than younger, healthier patients to derive a survival benefit from dialysis compared with more conservative therapy.32
The following assumptions and limitations should be considered when interpreting our results. First, we assumed that rates of decline in eGFR among Group Health Cooperative patients could be extrapolated to USRDS patients who had initiated dialysis in 2007. An important advantage of using Group Health Cooperative data to estimate this quantity is that most patients in this system who begin chronic dialysis have multiple outpatient SCr measurements available for slope estimation. However, it is possible that our results may not be generalizable to groups with in the USRDS population who are not represented in the Group Health Cooperative (eg, patients without health insurance coverage). Second, detailed information regarding the circumstances of dialysis initiation (eg, indications and inpatient SCr measurements) was not available for either cohort, precluding a detailed understanding of how practices have changed. Finally, although we controlled for all variables that could be ascertained with reasonable reliability and completeness across data sources and time periods, it is possible that our analyses did not completely adjust for changes in patient characteristics between 1997 and 2007, particularly given that USRDS data pertaining to comorbid conditions are lacking in sensitivity.33
Our results suggest that patients may be initiating chronic dialysis at a substantially earlier point in the course of advanced kidney disease. The dearth of evidence available to suggest that this practice is beneficial and the sizable potential effect on treatment burden and costs support the need for careful evaluation of contemporary dialysis initiation practices in this country.
Financial Disclosure:
Dr O’Hare serves as the principal investigator for an interagency agreement between the Centers for Disease Control and Prevention and the Veterans Affairs Puget Sound Healthcare System. Within the past year, she has received royalties from UpToDate, Inc. Dr Choi was supported by grant K23DK080645 and Dr Kurella Tamura was supported by grant K23AG028952 (a Paul B. Beeson Career Development Award) from the American Federation for Aging Research and the National Institute on Aging. Dr Larson has received research support from the National Institutes of Health; royalties from UpToDate, Inc; Springer Publishing Company; and Elsevier BV; and speaker honoraria from universities and other nonprofit organizations.
Funding/Support:
This study was supported by Beeson Career Development Award K23AG28980 (principal investigator: Dr O’Hare).
Role of the Sponsor:
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Additional Contributions:
We thank Peggy Tobin, BA, of the Group Health Center for Health Studies for providing project coordination for this study and Cristin Weekley, BA, at the Department of Veterans Affairs Medical Center, San Francisco office for preparing Figure 2.
Footnotes
Publisher's Disclaimer: Disclaimer: The study was conducted at the University of Washington and Group Health Research Institute, Seattle, Washington, and does not represent the opinion of the US Renal Data System or the Department of Veterans Affairs.
REFERENCES
- 1.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76(3):257–261. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3): 177–183. [DOI] [PubMed] [Google Scholar]
- 3.Knauf F, Aronson PS. ESRD as a window into America’s cost crisis in health care. J Am Soc Nephrol. 2009;20(10):2093–2097. [DOI] [PubMed] [Google Scholar]
- 4.Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mau L-W, Liu J, Qiu Y, et al. Trends in patient characteristics and first-year medical costs of older incident hemodialysis patients, 1995–2005. Am J Kidney Dis. 2010;55(3):549–557. [DOI] [PubMed] [Google Scholar]
- 6.The United States Renal Data System. http://www.usrds.org. Accessed July 12, 2010.
- 7.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36–48. [Google Scholar]
- 8.Report to the Congress: Medicare payment policy. 2007. Medical Payment Advisory Commission Web site. http://www.medpac.gov/documents/Mar07_EntireReport.pdf. Accessed October 18, 2010.
- 9.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125–2132. [DOI] [PubMed] [Google Scholar]
- 10.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009;24(10):3186–3192. [DOI] [PubMed] [Google Scholar]
- 11.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171(5):396–403. [DOI] [PubMed] [Google Scholar]
- 12.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887–896. [DOI] [PubMed] [Google Scholar]
- 13.Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305–2312. [DOI] [PubMed] [Google Scholar]
- 14.Brunori G, Viola BF, Parrinello G, et al. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis. 2007;49(5):569–580. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Cooper BA, Li JJ, et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011;57(5):707–715. [DOI] [PubMed] [Google Scholar]
- 17.Johansen KL. Time to rethink the timing of dialysis initiation. Arch Intern Med. 2011;171(5):382–383. [DOI] [PubMed] [Google Scholar]
- 18.Peritoneal Dialysis Adequacy Work Group. Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. Am J Kidney Dis. 2006;48(suppl 1):S98–S129. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. NKF-DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. Am J Kidney Dis. 1997;30(3)(suppl 2):S67–S136. [DOI] [PubMed] [Google Scholar]
- 20.II: NKF-K/DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy: Update 2000. Am J Kidney Dis. 2001;37(1)(suppl 1):S65–S136. [DOI] [PubMed] [Google Scholar]
- 21.I: NKF-K/DOQI clinical practice guidelines for hemodialysis adequacy: Update 2000. Am J Kidney Dis. 2001;37(1)(suppl 1):S7–S64. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(2)(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Balk E, et al. ; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. [DOI] [PubMed] [Google Scholar]
- 24.Fehrman-Ekholm I, Skeppholm L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol. 2004;38(1):73–77. [DOI] [PubMed] [Google Scholar]
- 25.Gill J, Malyuk R, Djurdjev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group—a cautionary tale. Nephrol Dial Transplant. 2007; 22(10):2894–2899. [DOI] [PubMed] [Google Scholar]
- 26.1995 Version of Centers for Medicare and Medicaid Services 2728 form. United States Renal Data System Web site. http://www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed July 9, 2010.
- 27.2005 Version of the Centers for Medicare and Medicaid Services 2728 Form. United States Renal Data System Web site. http://www.usrds.org/2008/rg/forms/03_2728_2005.pdf. Accessed July 12, 2010.
- 28.Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52(4):661–671. [DOI] [PubMed] [Google Scholar]
- 29.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. [DOI] [PubMed] [Google Scholar]
- 30.O’Hare AM, Bertenthal D, Walter LC, et al. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int. 2007;71(6):555–561. [DOI] [PubMed] [Google Scholar]
- 31.Grootendorst DC, Michels WM, Richardson JD, et al. ; for the NeDOSAD Study Group. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26(6):1932–1937. [DOI] [PubMed] [Google Scholar]
- 32.Murtagh FEM, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? a comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(7):1955–1962. [DOI] [PubMed] [Google Scholar]
- 33.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study: Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000; 11(3):520–529. [DOI] [PubMed] [Google Scholar]


