Abstract
In major ischemic stroke caused by a large artery occlusion, neuronal loss varies considerably across individuals without revascularization. This study aims to identify which patient characteristics are most highly associated with this variability. Demographic and clinical information were retrospectively collected on a registry of 878 patients. Imaging biomarkers including Alberta Stroke Program Early CT score, noncontrast head computed tomography infarct volume, perfusion computed tomography infarct core and penumbra, occlusion site, collateral score, and recanalization status were evaluated on the baseline and early follow-up computed tomography images. Infarct growth rates were calculated by dividing infarct volumes by the time elapsed between the computed tomography scan and the symptom onset. Collateral score was graded into four levels (0, 1, 2, and 3) in comparison with the normal side. Correlation of perfusion computed tomography and noncontrast head computed tomography infarct volumes and infarct growth rates were estimated with the nonparametric Spearman’s rank correlation. Conditional inference trees were used to identify the clinical and imaging biomarkers that were most highly associated with the infarct growth rate and modified Rankin Scale at 90 days. Two hundred and thirty-two patients met the inclusion criteria for this study. The median infarct growth rates for perfusion computed tomography and noncontrast head computed tomography were 11.2 and 6.2 ml/log(min) in logarithmic model, and 18.9 and 10.4 ml/h in linear model, respectively. Noncontrast head computed tomography and perfusion computed tomography infarct volumes and infarct growth rates were significantly correlated (rho 0.53; P < 0.001). Collateral status was the strongest predictor for infarct growth rates. For collateral 0, the perfusion computed tomography and noncontrast head computed tomography infarct growth rate were 31.56 and 16.86 ml/log(min), respectively. Patients who had collateral >0 and penumbra volumes>92 ml had the lowest predicted perfusion computed tomography infarct growth rates (6.61 ml/log(min)). Collateral status was closely related to the diversity of infarct growth rates, poor collaterals were associated with a faster infarct growth rates and vice versa.
Keywords: Ischemic stroke, computed tomography, perfusion imaging, collateral
Introduction
In the setting of an ischemic stroke caused by a large artery occlusion, an average patient is estimated to lose 1.9 million neurons, 13.8 billion synapses, and 12 km of axonal fibers every minute in the absence of revascularization.1 These figures were calculated with the hypothesis of linear infarct growth, using an average volume of infarct, dividing it by the average time from symptom onset, and then calculating how many neurons, axons, and synapses are included in this volume. A study using MRI but this time involving multiple patients found that the numbers listed above are correct on average, but that individual losses are quite varied.2 In clinical practice, patients with the same site and degree of occlusion in the anterior circulation often demonstrate penumbra of different extent and different rates of transformation of this penumbra into infarct.
This diversity of infarct growth pattern supporting the growing evidence in favor of more individualized brain tissue-based treatment decisions as opposed to decisions based exclusively on time since symptom onset.3 Recently, the DEFUSE 3 study demonstrated endovascular therapy along with standard treatment can extend the treatment window for acute ischemic stroke up to 16 h, and a slower infarct growth rate (IGR) in some patients might be one of the contributing factors to the longer therapeutic window.4 Multiple factors have been studied regarding the infarct growth, including genetic, demographic, clinical, and radiologic factors.5 In the era of precision medicine, improved understanding of the mechanisms underlying the diversity of the IGR will influence clinical decision making, allowing for personalized treatment decisions and improved patient outcome. In the present study, we aimed at presenting the variability of IGR using CT in acute stroke patients with large artery occlusion and identifying the possible imaging and clinical biomarkers associated with this variability.
Methods
Study population
This retrospective observational study was approved by both our institutional review board. All of the data were collected between January 2008 and June 2016 at the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland. From the registry of 878 patients, the enrollment should meet the following criteria:
(1) Age≥18 yr; (2) complete clinical data including time from symptom onset; (3) time from symptom onset to baseline CT study < 24 h; (4) baseline imaging work-up including a noncontrast head CT (NCT), perfusion CT (PCT), and CTA (CT angiography); (5) PCT coverage at least 8 cm; (6) acute infarct in the MCA territory with presence of M1 occlusion with/without ICA/M2 involvement; (7) no subacute/chronic/remote infarct and no hemorrhage after review of the baseline NCT; (8) early recanalization images (<72 h).
Clinical data
We collected demographic information including age, sex, and time from symptom onset to baseline imaging for each patient included in our study. Systemic blood pressure and glucose values were recorded at presentation. The following elements of medical history were also collected: hypertension (HT), diabetes mellitus (DM), hyperlipidemia, atrial fibrillation (AF), coronary artery disease (CAD), smoking status, and statin treatment. Smoking status was categorized as never smoked, active smoker or stopped for less than two years, and stopped smoking for more than two years. Functional outcome was assessed by the modified Rankin Scale (mRS) at 90 days.
Imaging data
PCT series were scanned after the NCT and before the CTA. Each PCT series involved successive gantry rotations performed in cine mode or shuttle mode during intravenous administration of one or two boluses of 40–50 ml of iodinated contrast material at an injection rate of 4–5 ml/s (Omnipaque 350 mg/ml, 80–120 kVp, 75–200 mA s). After another 90–120 ml single bolus intravenous contrast injection, thin sliced source CTA images were acquired with a Z-axis coverage from aortic arch up to the vertex (120 kVp, 260 mA s).
Image interpretation and analysis
Alberta Stroke Program Early CT (ASPECT) score was assessed on the NCT. Also, the NCT hypodensity was delineated manually and the area calculated on each slice, and then multiplied by the slice thickness to determine baseline NCT infarct volume.
PCT datasets were processed using Philips Brain Perfusion software (version 6.0.0 Philips Medical Systems, Cleveland, OH, USA). PCT infarct core and penumbra were determined using validated MTT and CBV thresholds published in the literature (PCT ischemic core: MTT > 145% of the contralateral side values and CBV <2.0 ml/100 g; PCT penumbra: MTT > 145% of the contralateral side values and CBV ≥2.0 ml/100 g).6
CTA images were reconstructed in axial, coronal, and sagittal maximum intensity projection (MIP). The occlusion site and collateral status were evaluated on the MIP images. According to the previously reported scoring system,7 the collaterals were graded into four levels in comparison with the normal side: 0, 1, 2, and 3 (0 = absent collaterals; 1 = collaterals filling ≤ 50% of the occluded territory; 2 = 50% < collaterals filling <100% of the occluded territory; 3 = collaterals filling 100% of the occluded territory). Recanalization status was assessed by comparing the baseline CTA and early follow-up vascular imaging.
Statistical analysis
We first considered IGR to have a logarithmic relationship with time since symptom onset. The IGR for both PCT and NCT was defined by infarct volume (ml) at baseline imaging divided by the logarithm of minutes from symptom onset to baseline CT scan (volume/log(min)). We also assessed the IGR in a linear model using the baseline volume divided by the hours from symptom onset to baseline CT scan (volume/h). Since infarct volume and IGR do not have a normal distribution, we used the nonparametric Spearman’s rank correlation to calculate and test the correlation between PCT and NCT infarct volumes and growth rates. Summary statistics included median and interquartile range (IQR) for continuous characteristics, and number and percentage for categorical characteristics. All analyses were completed using the R environment for statistical computing.8
In order to identify patient characteristics associated with the IGR and outcomes of mRS > 2 at 90 days, we used univariate analyses and conditional inference trees. For continuous variables, we used t-tests or Mann–Whitney U-test depending on the distribution of the data. We used Fisher’s exact tests for categorical variables. Decision trees are a natural choice when predictive characteristics are highly correlated and interactions among characteristics are also of interest. Traditional tree-based algorithms, like CART,9 can suffer from overfitting as well as bias toward selecting covariates with a larger number of possible splits.10,11 Conditional inference trees avoid overfitting by the use of robust permutation tests to calculate p-values and ensure that the right sized tree is grown, without the need for cross-validation, and appropriately measure the association between the response and covariates, even when covariates have different scales.12 We used the party package 713 in R 68 for the conditional inference trees. The algorithm recursively tests the global null hypothesis of independence between the response and the covariates and when rejected, selects the covariate with the strongest association to the response. The algorithm stops growing the tree when the global null hypothesis cannot be rejected at the Bonferroni-corrected 0.05 level; all reported p-values were adjusted for multiple testing with the Bonferroni correction.
For all outcomes, we included the following characteristics as possible predictors: age, sex, HT status, diabetes status, glucose level (mg/dl), hyperlipidemia status, statin use, AF, CAD, smoking status (never smoked, prior smoker or stopped > 2 years, current smoker or stopped < 2 years), occlusion site (M1 only, M1 and M2, ICA and M1, ICA and M1 and M2), and penumbra volume (ml) (Table 1).
Table 1.
Clinical and imaging characteristics
| Clinical and imaging biomarkers | N (%) | Biomarkers for prediction | ||
|---|---|---|---|---|
| PCT IGR | NCT IGR | mRS | ||
| Clinical characteristics Total number of patients | 232 | |||
| Age in years (median [IQR]) | 69.5 [58.8, 78.0] | + | + | + |
| Female (%) | 111 (47.8) | + | + | + |
| Hypertension (%) | 137 (59.1) | + | + | + |
| Diabetes mellitus (%) | 44 (19.0) | + | + | + |
| Glucose (mg/dl) (median [IQR]) | 6.7 [5.9, 7.8] | + | + | + |
| Hyperlipidemia (%) | 160 (69.0) | + | + | + |
| Statin use (%) | 57 (24.6) | + | + | + |
| Atrial fibrillation (%) | 102 (44.0) | + | + | + |
| NIHSS | 17 (12.21) | + | + | + |
| Cardiovascular (%) | 26 (11.2) | + | + | + |
| Smoking status (%) | + | + | + | |
| Never smoked | 139 (59.9) | |||
| Active smoker or stopped < 2 years ago | 62 (26.7) | |||
| Stopped smoking > 2 years ago | 31 (13.4) | |||
| Imaging biomarkers Time since symptom onset (h) (median [IQR]) | 2.5 [1.8, 4.5] | |||
| ASPECT score (median [IQR]) | 7.0 [5.0, 8.0] | + | ||
| NCT infarct volume (ml) (median [IQR]) | 32.2 [11.5, 59.8] | + | ||
| PCT infarct volume (ml) (median [IQR]) | 58.3 [25.0, 110.0] | + | ||
| Penumbra volume(ml) (median [IQR]) | 119.85 [78.67, 160.2] | + | + | + |
| Occlusion site (%) | + | + | + | |
| ICA and M1 and M2 | 55 (23.7) | |||
| ICA and M1 | 25 (10.8) | |||
| M1 and M2 | 104 (44.8) | |||
| M1 only | 48 (20.7) | |||
| Collateral (%) | + | + | ||
| Absent collaterals | 26 (11.2) | |||
| Collaterals filling < 50% of occluded territory | 102 (44.0) | |||
| 50% ≤ collaterals filling < 100% occluded territory | 72 (31.0) | |||
| Collaterals filling 100% of occluded territory | 32 (13.8) | |||
| Recanalization (%) | 146 (62.9) | + | ||
ASPECT: Alberta Stroke Program Early CT; ICA: Internal Carotid Artery; IGR: infarct growth rate; IQR: interquartile range; mRS: modified Rankin Scale; NCT: noncontrast head CT; NIHSS: National Institutes of Health Stroke Scale; PCT: perfusion CT.
In addition to these characteristics, we included collateral score (0, 1, 2, 3) as possible predictors of NCT and PCT IGRs; ASPECT score was also included as a possible predictor of PCT IGR. For mRS > 2 at 90 days, we included PCT and NCT IGRs, and PCT and NCT infarct volume. To better understand predictors of mRS at 90 days in those that recanalized and did not recanalize, we also separately evaluated possible predictors of mRS > 2 at 90 days in patients in each of these groups (Table 1).
Results
Study population
From our registry of 878 patients, 232 patients met the inclusion criteria for this study (Supplemental Figure 1). The study population included 121 males and 111 females, ages 25–94 years (median 69.5 years with IQR 58.8, 78.0) (Table 1). More than half (137/232, 59.1%) of the patients had arterial HT, 19% (44/232) had DM, 69% (160/232) had hyperlipidemia, 24.6% (57/232) received statins, 11.2% (26/232) had cardiovascular disease, and 26.7% (62/232) were active smokers or had stopped smoking for less than two years. For imaging biomarkers, the median ASPECT score was 7 (IQR 5.0, 8.0). Occlusion site percentages were 23.7% (55/232) for ICA+M1+M2, 10.8% (25/232) for ICA+M1, 44.8% (104/232) for ICA+M2, and 20.7% (48/232) for M1 only. The distribution of collateral score from 0 to 3 was 11.2% (26/232), 44% (102/232), 31% (72/232), and 13.8% (32/232), respectively (Table 1). A total of 62.9% (146/232) patients showed early recanalization.
Patterns of infarct growth
The median time from symptom onset to the CT scan was 2.5 h (IQR 1.8, 4.5); the median NCT and PCT infarct volumes were 32.2 ml (IQR 11.5, 59.8) and 58.3 ml (IQR 25.0, 110.0), respectively (Table 1). PCT and NCT infarct volumes in multiple time windows are summarized in the supplemental Table 1. In the vast majority of patients, the NCT infarct volumes were lower than the PCT infarct volumes, as expected; in 166/232 (71.6%) patients, the NCT infarct volumes were less than 90% of PCT infarct volumes.
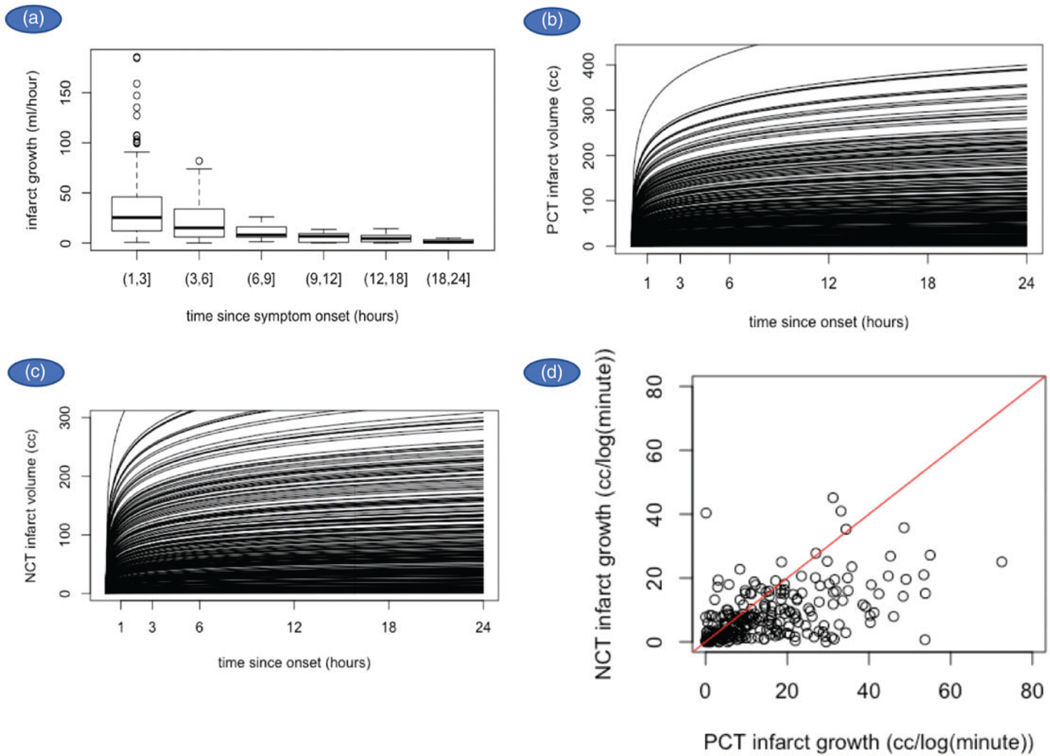
Based on the computed IGR, the expected curve for each individual was plotted (Figure 1(a) to (c)). The median IGR for PCT and NCT was 11.2 ml/log(min) with an IQR of 17.1, and 6.2 ml/log(min) with an IQR of 9.6 (Table 2), respectively. NCT and PCT infarct volumes and IGR were significantly correlated; Spearman’s rho = 0.52 (p < 0.001) for infarct volume and Spearman’s rho = 0.53 (p < 0.001) for IGR (Figure 1(d)). To compare with the results from other articles, we also calculated PCT and NCT IGR assuming a linear relationship with time. The median IGR for PCT was 18.9 ml/h with an IQR of 34.1. The median IGR for NCT was 10.4 ml/h with an IQR of 19.0 (Table 2).
Figure 1.
(a) Correlation of PCT infarct volume and time since symptom onset demonstrated that infarct volumes are higher in patients who seek treatment within 3 h and decrease sharply with longer onset times. (b, c) Logarithmic relationship of IGRs for both PCT and NCT, using the infarcts volumes observed on PCT and NCT. (d) NCT and PCT infarct volumes and IGRs were significantly correlated.
Table 2.
PCT and NCT IGR of logarithmic (ml/log(min)) and linear (ml/h) model of our study in comparison with DWI (Diffusion-weighted Imaging) results derived from Dr González study.2
| Mean | Range | Median | IQR | |
|---|---|---|---|---|
| PCT ml/log(min) | 15.2 | 72.5 | 11.2 | 17.1 |
| PCT ml/h | 36.1 | 368.0 | 18.9 | 34.1 |
| NCT ml/log(min) | 8.3 | 45.2 | 6.2 | 9.6 |
| NCT ml/h | 17.5 | 137.9 | 10.4 | 19.0 |
| DWI (retrospective) ml/h | 23 | 170 | - | - |
| DWI (prospective) ml/h | 12 | 77 | - | - |
| DWI (witnessed onset) ml/h | 12 | 68 | - | - |
Clinical and imaging biomarkers associated with PCT and NCT IGRs
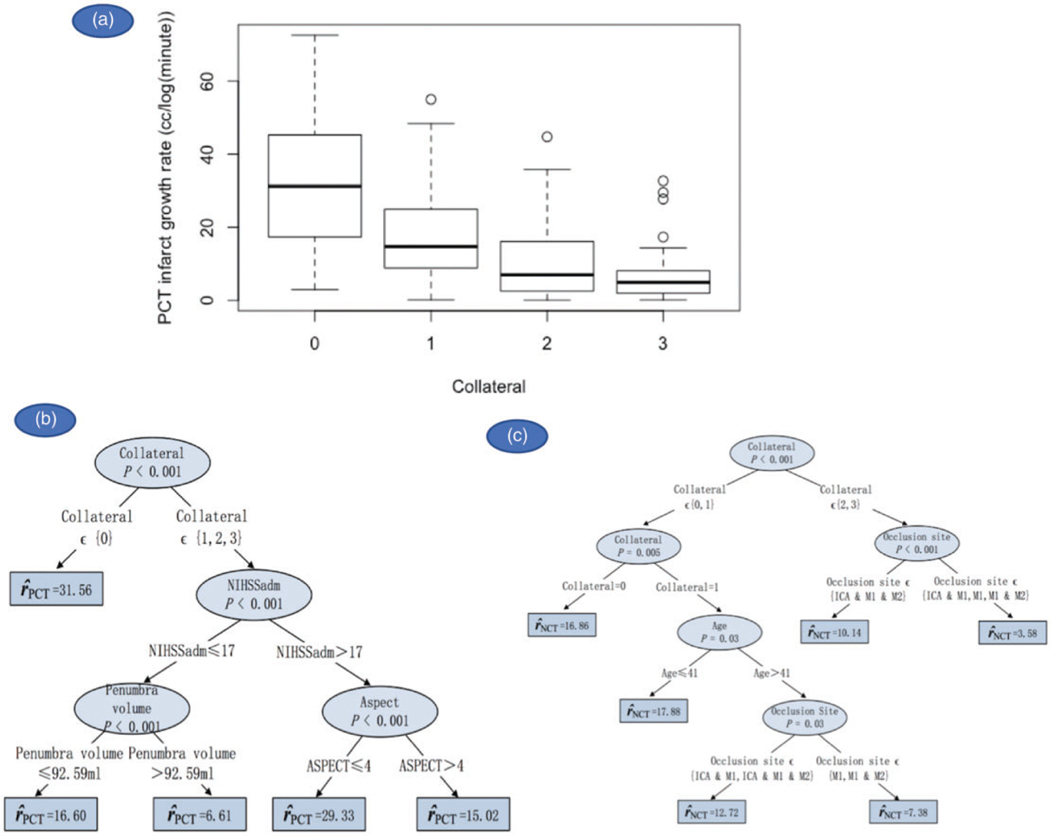
Univariate analyses showed that ASPECT score, penumbra volume, occlusion site, and NIHSS were strong predictors for PCT IGR. Collateral status was a relatively weak predictor of PCT IGR with a p value of 0.057. NIHSS, collateral status, and occlusion site were predictive of NCT IGR (supplemental Table 2). In the multivariate stage, conditional inference trees demonstrated that, among all of the clinical and imaging biomarkers, collateral status was the strongest predictor of PCT and NCT IGRs (Figures 2(a) to (c) and 3). Patients with no collaterals were predicted to have the highest PCT IGR (31.56 ml/log(min)) followed by patients who had a higher NIHSS score at admission (>17) and an ASPECT score of 4 or less (29.33 ml/log(min)). Comparatively, patients who had collateral>0 and penumbra volumes>92 ml had the lowest predicted PCT IGR (6.61 ml/log(min)). Similarly, patients with no collaterals or collateral = 1 but younger than 42 years had the highest predicted NCT IGR (16.86 and 17.88 ml/log(min), respectively). After collateral status and age, occlusion site predicted NCT IGR; patients who had collaterals = 2 or 3 and M1 occlusions (with/without M2/ICA) had the lowest predicted NCT IGR (3.58 ml/log(min)). Interestingly, patients who had collateral = 1 but who were older than 41 years and had no occlusion in ICA also had a low predicted NCT IGR (7.38 ml/log(min)).
Figure 2.
(a) Boxplot showing median and IQR of PCT IGR for each collateral status. (b, c) Clinical and imaging biomarkers predictive of PCT and NCT IGRs.
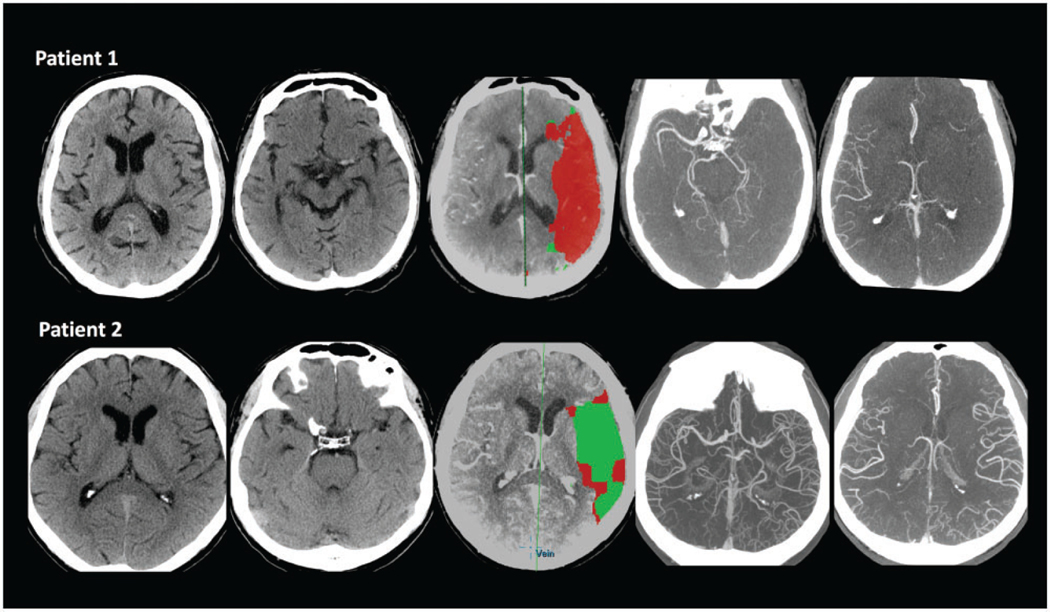
Figure 3.
Different IGRs in two patients. Patient 1: 81-year-old male patient. Time from symptom onset was 1.25 h. ASPECT score was 5 with left ICA and M1, M2 occlusion, and collateral score was 1. PCT showed a large area infarction which was 198.73 ml with penumbra 92.65 ml. The PCT IGR was 159 ml/h and 105.99 ml/log(min). Patient 2: 71-year-old male patient. Time from symptom onset was 14 h. ASPECT score was 9 with left ICA and M1 occlusion, and collateral score was 3. PCT showed a small infarct volume, which was 7.5 ml with penumbra 107.7 ml. The PCT IGR was 0.54 ml/h and 2.56 ml/log(min).
Prediction of clinical outcome
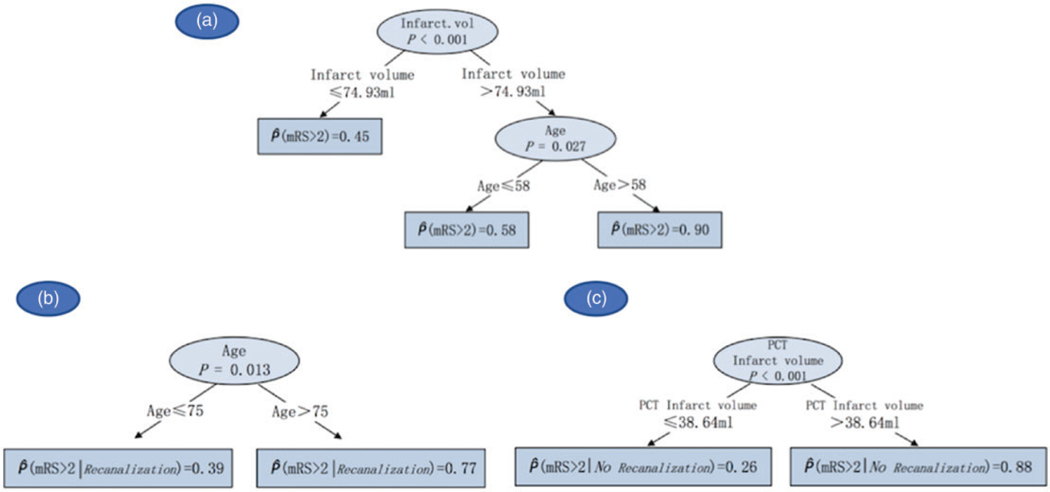
The distribution of mRS scores at 90 days in our study population is summarized in Supplemental Table 3. Univariate analyses showed that age, NIHSS, penumbra volume, and occlusion site were predictors for mRS. In the multivariate stage, conditional inference trees demonstrated that patients with PCT infarct volumes>75 ml were predicted to have a mRS > 2 at 90 days, which was exacerbated if the patient was older than 58 years (Figure 4(a)). To better understand how recanalization affected this outcome, we repeated the analysis in patients who did and did not recanalize. In recanalized group, patients who were older than 75 years were more likely to experience worse outcomes (mRS > 2) at 90 days (Figure 4(b)); while for those who did not recanalize, PCT infarct volumes>38.6 ml was predictive of mRS>2 (Figure 4(c)).
Figure 4.
Clinical and imaging parameters predictive of patient outcome at three months (mRS>2) in all patients (a), recanalized (b), and not recanalized patients (c).
Discussion
This work demonstrates that collaterals are the strongest factor contributing to the variability of IGR. A strong collateral network is associated with a slow IGR. This is in agreement with previous studies showing that a better collateral score was associated with smaller infarcts and slower IGR.14–20 In patients with no collaterals, the IGR could reach 31.56 ml/log(min). In patients with collaterals>0, the IGR could be as low as 6.61 ml/log(min). In the emergency setting, the size of the infarct on the baseline NCT, combined with the time from symptom onset, may provide information on the collateral status in individual patients.
In addition, we found that arterial occlusions limited to a short segment (e.g. M1 or M2) were associated with a slower infarct growth compared to arterial occlusions involving a longer segment (e.g. ICA+M1+M2). This is also in agreement with published literature,21 and may be explained by the fact that a longer segment arterial occlusion may lower the possibility of the brain tissue to recruit enough collaterals to perfuse the large ischemic area resulting from the longer arterial occlusion.
In experimental animal models, studies have shown that the IGR pattern is typically logarithmic.22–25 The infarct core grows rapidly on the first few hours and then trend to a plateau. In humans, the IGR pattern and speed are not well known because it is hard to study sequentially, but the commonly accepted concept is a similar logarithmic growth. In one study which involved serial MRIs over the first few days after stroke onset, the infarct grew rapidly in the first few hours until the initial MRI scan.26 Subsequently, more than 80% patients with MCA occlusion were shown to have stable perfusion/diffusion mismatch and little diffusion growth during the first 24 h following the initial MRI scan.26
In order to compare our results with those of a previous study, we also calculated the IGR using a linear model; the mean rate for PCT was 36.1 ml/h, and the mean rate for NCT was 17.5 ml/h. In a previous study,2 which used DWI as an imaging biomarker, assuming a linear IGR, the IGR was 23 ml/h in a retrospective group, and 12 ml/h in a prospective group. Similar average growth rates and mean infarct volumes were found in ours and this study (Table 2 and Supplemental Table 1). Similar trends in terms of the IGR evolution could be observed as well (Table 2 and Supplemental Table 1), including a peak reached at 3–6 h, followed by a slower growth, further suggesting that infarcts grow logarithmically along with time.
Our study also demonstrated that age was the only demographic factor influencing the IGR. Younger patients had faster IGR, while older patients had slower IGR. Some studies reported that natural aging had a negative effect on collaterals and long-term outcomes. Hypothesized underling mechanisms include age-related endothelial dysfunction inducing nitric oxide synthesis reduction, vessel stiffness, and collateral rarefaction.27 Also, the IGR and tissue fate are not only determined by the vascular supply but also by the tissue’s tolerance to ischemia, which is termed ischemic preconditioning. Studies had demonstrated that patients with multiple TIAs develop a resistance against severe strokes.28 The better tolerance of older patients to ischemia may explain our results.
Early prediction of patient outcome is essential in guiding clinical management.29–31 To this end, we evaluated patient characteristics predictive of modified Rankin score at 90 days, and only age and baseline infarct volume predicted patient outcome. We tried to explore but could not confirm the predictive value of IGR for clinical outcome. The possible underlying reason would probably be explained in two aspects, first, the IGR is dynamic, second, it is not an independent variable, and can be influenced by collateral status, the tolerance of brain tissue to hypoxia, etc. Collaterals fluctuate, and around half patients imaged within 1 h showed diminished collaterals up to 14.5% when imaged 12–24 h later.32 The unforeseen fluctuation of collateral flow over time highlights the difficulties in accurately predicting infarct growth based on the severity of the baseline perfusion lesion.33 We still lack complete understanding of the evolution of infarct growth, which may need more evidence pathophysiologically to help interpret the internal connections between collaterals, infarct growth, and outcome.
We acknowledge several limitations to our study. First, our study was retrospective in nature. Second, the collaterals were evaluated on single-phase CTA images in this study. The updated PCT scanning techniques made it feasible to extract multi-phase CTA images from PCT dataset. The ultimate approach to assess the natural history of infarct evolution would best be assessed on serial imaging. However, obtaining serial imaging in acute stroke patients is logistically challenging. Lastly, the approach we used in this study does not apply to patients with wakeup stroke or unknown time since symptom onset.
In conclusion, our study demonstrated that collateral status was closely related to the variability of IGR: poor collateral status was associated with a faster IGR while good collateral status was associated with slow IGR.
Supplementary Material
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Saver JL. Time is brain – quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 2.Hakimelahi R, Vachha BA, Copen WA, et al. Time and diffusion lesion size in major anterior circulation ischemic strokes. Stroke 2014; 45: 2936–2941. [DOI] [PubMed] [Google Scholar]
- 3.Hill MD, Goyal M, Demchuk AM and Fisher M. Ischemic stroke tissue-window in the new era of endovascular treatment. Stroke 2015; 46: 2332–2334. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha M and Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017; 48: 2621–2627. [DOI] [PubMed] [Google Scholar]
- 6.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979–985. [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Dillon WP, Liu S, Adler F, Smith WS and Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007; 61: 533–543. [DOI] [PubMed] [Google Scholar]
- 8.RC T. R: a language and environment for statistical computing Vienna: R Foundation for Statistical Computing. [Google Scholar]
- 9.Breiman L, Friedman JH, Olshen RA, et al. Classification and regression trees. New York: Champman & Hall/CRC, 1984. [Google Scholar]
- 10.Hothorn T, Hornik K and Zeileis A. Ctree: conditional inference trees. The Comprehensive R Archive Network, 2015. [Google Scholar]
- 11.Mingers J. Expert systems – rule induction with statistical data. J Oper Res Soc 1987; 38: 8. [Google Scholar]
- 12.Hothorn T, Hornik K and Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 2006; 15(3): 651–674. [Google Scholar]
- 13.Hothorn T, Hornik K, Zeileis A. party: A Laboratory for Recursive Partytioning. 2006. [http://CRAN.R-project.org/].
- 14.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B and Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005; 26: 1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 16.Souza LC, Yoo AJ, Chaudhry ZA, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol 2012; 33: 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon E, Lefevre PH, Thouant P, Osseby GV, Ricolfi F and Chavent A. Collateral circulation in acute stroke: assessing methods and impact: a literature review. J Neuroradiol 2014; 41: 97–107. [DOI] [PubMed] [Google Scholar]
- 18.Fanou EM, Knight J, Aviv RI, et al. Effect of collaterals on clinical presentation, baseline imaging, complications, and outcome in acute stroke. AJNR Am J Neuroradiol 2015; 36: 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Baumgarten L, Thierfelder KM, Beyer SE, et al. Early CT perfusion mismatch in acute stroke is not time-dependent but relies on collateralization grade. Neuroradiology 2016; 58: 357–365. [DOI] [PubMed] [Google Scholar]
- 20.Yeo LL, Paliwal P, Low AF, et al. How temporal evolution of intracranial collaterals in acute stroke affects clinical outcomes. Neurology 2016; 86: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009; 30: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann-Haefelin T, Kastrup A, de Crespigny A, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke 2000; 31: 1965–1972; discussion 1972–1963. [DOI] [PubMed] [Google Scholar]
- 23.Bardutzky J, Shen Q, Bouley J, Sotak CH, Duong TQ and Fisher M. Perfusion and diffusion imaging in acute focal cerebral ischemia: temporal vs. spatial resolution. Brain Res 2005; 1043: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markus R, Reutens DC, Kazui S, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke 2003; 34: 2646–2652. [DOI] [PubMed] [Google Scholar]
- 25.Christoforidis GA, Vakil P, Ansari SA, Dehkordi FH and Carroll TJ. Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am J Neuroradiol 2017; 38: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez RG, Hakimelahi R, Schaefer PW, Roccatagliata L, Sorensen AG and Singhal AB. Stability of large diffusion/perfusion mismatch in anterior circulation strokes for 4 or more hours. BMC Neurol 2010; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon BK, Smith EE, Coutts SB, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol 2013; 74: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke 2004; 35: 616–621. [DOI] [PubMed] [Google Scholar]
- 29.Eilaghi A, Brooks J, d’Esterre C, et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology 2013; 269: 240–248. [DOI] [PubMed] [Google Scholar]
- 30.Dankbaar JW, Horsch AD, van den Hoven AF, et al. Prediction of clinical outcome after acute ischemic stroke: the value of repeated noncontrast computed tomography, computed tomographic angiography, and computed tomographic perfusion. Stroke 2017; 48: 2593–2596. [DOI] [PubMed] [Google Scholar]
- 31.van Seeters T, Biessels GJ, Kappelle LJ, et al. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis 2015; 40: 258–269. [DOI] [PubMed] [Google Scholar]
- 32.Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009; 40: 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.