Abstract
Purpose of review
Severe Acute Respiratory Syndrome Coronavirus 2 presents as symptomatic COVID-19 disease in susceptible patients. Severe pediatric COVID-19 disease is rare, limiting potential data accumulation on associated respiratory failure in children. Pediatric intensivists and pulmonologists managing COVID-19 patients look to adult guidelines and pediatric-specific consensus statements to guide management. The purpose of this article is to review the current literature and recommended strategies for escalation of non-invasive and invasive respiratory support for acute respiratory failure associated with COVID-19 disease in children.
Recent Findings
There are no prospective studies comparing COVID-19 treatment strategies in children. Adult and pediatric ventilation management interim guidance is based on evidence-based guidelines in non-COVID acute respiratory distress syndrome, with considerations of 1) non-invasive positive pressure ventilation versus high-flow nasal cannula and 2) high versus lower PEEP strategies related to lung compliance and potential lung recruitability.
Summary
Management of acute respiratory failure from COVID-19 requires individualized titration of non-invasive and invasive ventilation modalities with consideration of preserved or compromised pulmonary compliance. Research regarding best practices in management of pediatric severe COVID-19 with respiratory failure is lacking and is acutely needed as the pandemic surges and vaccination of the pediatric population will be delayed compared to adults.
Keywords: COVID-19, respiratory failure, mechanical ventilation, non-invasive ventilation, pediatric acute respiratory distress syndrome
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection resulting in coronavirus disease 2019 (COVID-19) continues to sweep through the world’s vulnerable populations, generally sparing children the worst outcomes [1–3]. Cumulative CDC data on COVID-19 incidence in pediatric patients aged 0–24 years from March to December 2020 reported that 2.5% required hospitalization and 0.8% required admission to an ICU [3]. Early reports on hospitalized COVID-19 pediatric patients indicated that 5.8% required invasive mechanical ventilation, 3.9% were managed with non-invasive positive pressure ventilation (CPAP or BiPAP), 2.4% with HFNC, and 2% met criteria for acute respiratory distress syndrome (ARDS) [2]. Of children with acute COVID-19 disease admitted to pediatric ICUs in North America, 38% required invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO) was required rarely, and mortality was low (4%) [1]. Early in the pandemic, adult COVID-19 management guidelines favored an early intubation strategy given risks of aerosolization with modes of non-invasive ventilation. As more safety data has become available, adult guidelines now allow for a more conservative approach with high flow nasal cannula (HFNC) and self-proning when appropriate [4–6].
Ultimately, there is no definitive pediatric evidence to support one mode of respiratory support over another for severe COVID-19 disease, and it is reasonable to consider that pediatric respiratory illness may be distinct from adult respiratory illness related to differences in the maturation of the alveoli, airways and respiratory musculature [7]. In that setting, pediatric intensivists and pulmonologists should utilize lung protective ventilation strategies, routinely applied in pediatric acute respiratory distress syndrome (PARDS) patients, to titrate support for each child’s individual respiratory mechanics [8, 9].
Pathophysiology and Impairment of Gas Exchange
COVID-19 disease is an acute viral illness associated with diffuse alveolar damage and pulmonary endothelial disease that can lead to clinical respiratory failure [10]. SARS-CoV-2 requires angiotensin-converting enzyme 2 (ACE2) receptors for virion entry into susceptible cells [11]. ACE2 receptors are extensively expressed on type II pneumocytes and infection and damage of these surfactant-producing cells can result in significant lung damage with alveolar collapse and widespread atelectasis leading to impaired gas exchange and profound hypoxemia [12, 13]. Pulmonary capillary endothelial cells are also susceptible to SARS-CoV-2 infection and a compromised epithelial-endothelial barrier results in early capillary leak and alveolar influx of inflammatory immune cells [14]. Persistent inflammation leads to increased vascular permeability, pulmonary edema, alveolar interstitial thickening and hyaline membrane formation that, along with microvascular thrombosis, compromises gas exchange [14, 15]. Microvascular thrombi can be caused by direct viral injury to the endovascular tissue and the resultant host immune response and highly active coagulation and inflammatory cascades result in endotheliitis [16]. Pulmonary vasoconstriction driven by hypoxemia likely contributes to increased dead space and is exacerbated by microvascular thrombi which further impairs gas exchange [17]. Together, these insults on both sides of the alveolar-capillary interface results in compromised gas exchange through a mixed disease process of intrapulmonary shunting, disruption of V/Q matching, and increased pulmonary dead space [18].
Adults with COVID-19 pneumonia and severe hypoxemia generally fulfill the Berlin criteria for acute respiratory distress syndrome (ARDS) [19], yet many patients have preserved ability to exchange CO2 and near-normal pulmonary compliance [20]. Two subphenotypes of adult COVID-19 ARDS distinguished by pulmonary compliance and lung recruitability have been proposed [20, 21]. Evaluating pulmonary compliance, where compliance equals the change in lung volume over the change in pressure, can help stratify COVID-19 ARDS patients by clinical subphenotype and potentially inform ventilator management strategies. The proposed “H-type” adult COVID-19 ARDS subphenotype is characterized by high elastance and low compliance with extensive pulmonary infiltrates and higher potential lung recruitability where a higher PEEP strategy to recruit lung may be beneficial [20, 21]. The proposed “L-type” adult COVID-19 phenotype is characterized by low elastance and high compliance with less prominent parenchymal disease and low recruitability and a lower PEEP strategy may mitigate risk of overdistention [20, 21]. Given that ARDS is a common endpoint definition for a heterogeneous group of disease processes, these physiologic subphenotypes are not likely to be unique to COVID-19 ARDS. The phenotypic pattern of preserved pulmonary compliance combined with profoundly impaired gas exchange is also evident in a subset of non-COVID-19 ARDS patients [22]. These clinical ARDS subphenotypes are frameworks to consider when determining initial or dynamic PEEP titration to ensure adequate lung expansion for optimal gas exchange while avoiding hemodynamic insult.
Assessing respiratory disease severity in children with COVID-19
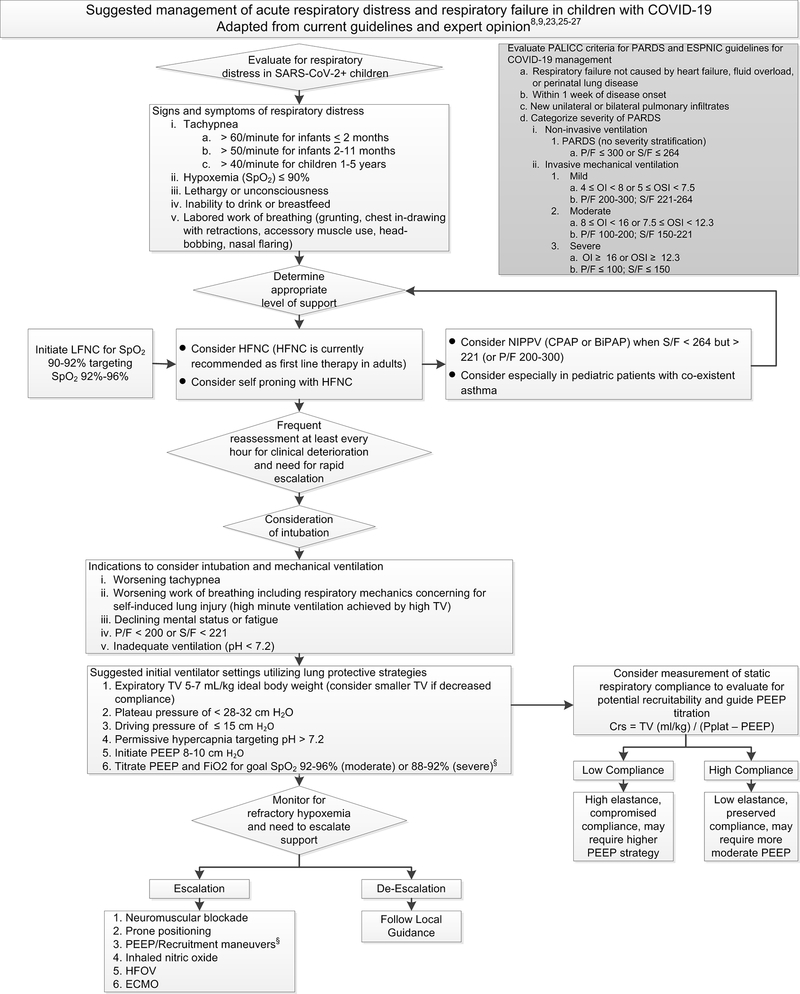
Children diagnosed with COVID-19 should be evaluated carefully and serially for evidence of respiratory distress or failure (Figure 1). The international Pediatric Acute Lung Injury Consensus Conference (PALICC) has defined criteria for pediatric ARDS (PARDS) and the PALICC criteria are applicable for diagnosing COVID-19 related ARDS in children and stratifying severity of hypoxemia (Figure 1) [9, 23, 24]. Guidelines for the management of acute respiratory failure with COVID-19 are largely focused on the adult population [4–6]. The European Society of Pediatric and Neonatal Intensive Care (ESPNIC) recently published a consensus statement providing recommendations for the care of critically ill children with COVID-19 [25] and an international collaboration of pediatric intensivists developed ICU guidelines for pediatric COVID-19 patients [26]. The ESPNIC and COVID-19 PICU Guidelines are consistent with PALICC and Pediatric Mechanical Ventilation Consensus Conference (PEMVECC) expert opinion recommendations on ventilation strategies for PARDS [8, 9, 27].
Figure 1. Suggested management of acute respiratory distress and respiratory failure in children with COVID-19.
Abbreviations: PARDS – pediatric acute respiratory distress syndrome, SpO2 – oxygen saturation, LFNC – low flow nasal cannula, HFNC – high flow nasal cannula, NIPPV – non-invasive positive pressure ventilation, CPAP – continuous positive airway pressure, BiPAP – bilevel positive airway pressure, S/F – SpO2/FiO2 ratio, P/F – PaO2/FiO2 ratio, TV – tidal volume, HFOV – high frequency oscillatory ventilation, ECMO – extracorporeal membrane oxygenation, Crs – respiratory system compliance, VT– tidal volume, Pplat – plateau pressure. OI – oxygenation index = [(FiO2 × mean airway pressure)/PaO2]. OSI – oxygenation saturation index = [(FiO2 × mean airway pressure)/SpO2]. § Evaluate closely for deleterious changes in hemodynamics with all ventilator pressure titrations.
In the following sections, we review and compile recommendations for escalation of respiratory support for critically ill pediatric COVID-19 patients (Figure 1) with reference to available pediatric and adult literature and guidelines.
Supplemental oxygen and high flow nasal cannula (HFNC)
For children with COVID-19 and hypoxemia (SpO2 ≤ 90%), supplemental oxygen should be initiated via nasal cannula and titrated to target SpO2 92–96% [25, 26]. If hypoxemia persists, oxygen via simple facemask may be initiated [26]. There are not clear recommendations on escalation to non-rebreather facemasks in pediatric patients. In adult COVID-19 patients in whom conventional oxygen therapy is insufficient to achieve oxygenation goals, guidelines recommend humidified HFNC over non-invasive positive pressure ventilation (NIPPV) for escalation of support [4–6]. HFNC has been shown to be more effective than standard oxygen supplementation or NIPPV in adults with non-COVID-19 acute hypoxemic respiratory failure in achieving lower rates of intubation and lower mortality [28–30]. In adults with COVID-19, HFNC may reduce the need for intubation and invasive mechanical ventilation [31] and can be utilized in lower resource settings [32]. Awake self-prone positioning combined with HFNC may be an effective non-invasive strategy for hypoxemia in adults with COVID-19 [33–35]. Physiologic mechanisms by which HFNC provides benefit are likely multifactorial with contributions of anatomic dead space washout with reduced minute ventilation and decreased work of breathing, increased alveolar PaO2 via reduced entrainment of ambient air, and possibly improved dynamic compliance and V/Q matching [36, 37].
Children with COVID-19 with persistent hypoxemia or labored work of breathing despite supplemental oxygen can be escalated to humidified HFNC, particularly if SpO2/FiO2 ratio is maintained > 264 [25, 26]. For children with SpO2/FiO2 < 264 but > 221, ESPNIC guidelines recommend NIPPV rather than HFNC [25]. Pediatric practitioners are versed in the use of HFNC for children with viral bronchiolitis [38, 39] and HFNC reduces work of breathing in pediatric acute respiratory failure [40]. Vigilant monitoring for clinical deterioration is imperative when escalating respiratory support in infants and children and the clinical response to a trial of non-invasive respiratory support should be determined within ~60 minutes [25].
Non-invasive positive pressure ventilation: CPAP and BiPAP
Current adult guidelines generally recommend the use of HFNC over NIPPV for worsening COVID-19 associated hypoxemia unless HFNC is unavailable or if intubation is not within the patient’s goals of care [4–6]. Contraindications to the safe use of NIPPV in children and adults include altered mental status, risk of aspiration, vomiting, significant secretions, or severe respiratory distress and high work of breathing. Pediatric COVID-19 patients with progressive or persistently increased work of breathing and hypoxemia can be escalated to NIPPV (CPAP or BiPAP) [25, 26]. Recommendations differ as to whether NIPPV or HFNC should be the first line approach, but for the pediatric patient whose SpO2/FiO2 is < 264 but > 221 (or PaO2 / FiO2 200–300) or where HFNC is unavailable, use of NIPPV can be considered [25, 26]. The use of NIPPV remains appropriate per standard of care treatment algorithms for status asthmaticus in pediatric asthma patients with SARS-CoV-2 [41, 42]. Helmet type non-invasive interfaces are preferable if available given reduced risk of aerosolized droplet dispersion [25]. Care must be taken to observe the spontaneously breathing patient for signs of significant work of breathing and large tidal volumes that could result in patient self-inflicted lung injury and may contribute to non-invasive ventilation failure [43, 44]. Pediatric data on self-inflicted lung injury is insufficient to determine the relevance of this pathophysiology in children.
Signs of success with non-invasive ventilation strategies include improvement in oxygenation, reduction in respiratory rate, and diminished work of breathing. Evaluating for clinical improvement after initiation of NIPPV in a child should occur rapidly (~60 minutes), and intubation should be pursued if non-invasive support fails to achieve target SpO2 92–96% and FiO2 < 60% [25]. Failure of non-invasive ventilation in non-COVID-19 PARDS is strongly correlated with degree of hypoxemia with 50% of patients with PaO2 / FiO2 ratio < 100 on NIPPV requiring intubation within 24 hours [45].
Indications for intubation of pediatric COVID-19 patients
In the event of progressive respiratory distress or inadequate gas exchange despite HFNC or NIPPV support, intubation and mechanical ventilation should be initiated. Guidelines are clear that intubation should not be delayed in pediatric COVID-19 patients with clinical deterioration and evidence of severe respiratory distress, high work of breathing and compromised respiratory mechanics, moderate to severe hypoxemia (PaO2/FiO2 < 200 or SpO2/FiO2 < 221), inadequate ventilation (pH <7.2) or lethargy and evidence of progressive fatigue (Figure 1) [25, 26]. A higher threshold for intubation is noted to be a reasonable approach in patients with isolated hypoxemia with non-labored work of breathing and non-injurious respiratory mechanics [25].
Conventional Mechanical Ventilation
A lung protective ventilation strategy targeting expiratory tidal volume 5–7 ml/kg ideal body weight, inspiratory plateau pressure < 28–32 cm H2O, driving pressure < 15 cm H2O, and permissive hypercapnia (pH > 7.2) is recommended (Figure 1) [25]. Lower tidal volumes (3–6 ml/kg) may be necessary for patients with impaired compliance [9, 25, 27]. Measurements of static respiratory system compliance may facilitate distinguishing patients with preserved versus impaired compliance [46] and can inform PEEP management. Considering the proposed clinical subphenotypes of adult COVID-19 ARDS patients with preserved compliance (“L type”, low elastance) versus those with low compliance (“H type”, high elastance) [20, 21] may also guide ventilator management in pediatric patients [25]. It should be noted, however, that there is no data from prospective clinical trials to support that subphenotype classification predicts treatment response or improves outcomes. Many experts conclude that tailored ventilation strategies based on subphenotypes is premature and advocate for applying evidence-based lung protective ventilation protocols developed in the pre-COVID-19 era to COVID-19 ARDS patients as standard of care [47–49].
ESPNEC recommend initiating PEEP at 8–10cm H2O and titrating PEEP and FiO2 according to the ARDSNet “low PEEP/FiO2 grid” [50, 51] to maintain SpO2 92–96% for moderate or 88–92% for severe PARDS [25]. Patients with preserved lung compliance may require lower PEEP (and higher PEEP may be injurious given risk of overdistension and volutrauma) whereas patients with impaired compliance may benefit from higher PEEP to improve lung recruitment [20, 21, 52]. Optimal application of PEEP for recruitment of the injured lung must balance prevention of both atelectrauma and volutrauma while weighing improvements in oxygenation and lung recruitment against deleterious effects on hemodynamics [53]. Individualized titration of PEEP with stepwise trials of increasing and decreasing PEEP to optimize oxygenation and lung recruitment while closely monitoring for and avoiding hemodynamic compromise is warranted [8, 9, 20, 21, 25–27].
Ancillary supportive measures and advanced modes of ventilation
Neuromuscular blockade is advised for ~48 hours in patients with profound hypoxemia (PaO2 / FiO2 < 150, Oxygenation index (OI) ≥ 16 or Oxygenation Saturation Index (OSI) ≥ 10) to mitigate patient/ventilator dysynchrony and to avoid high plateau pressures [25]. Prone positioning can be considered for refractory hypoxemia despite PEEP titration [25] – see also “TITLE” by Dr. Peter Mourani in this edition. A trial of inhaled nitric oxide (iNO) could be considered if refractory hypoxemia is presumed related to hypoxic pulmonary vasoconstriction, increased dead space fraction, and V/Q mismatch [25], but improved oxygenation with iNO in PARDS has not been shown to reduce mortality [54]. High frequency oscillatory ventilation (HFOV) could be considered for the patient requiring high ventilator pressures or when hypoxemia is refractory to PEEP, but mitigating aerosol generation by HFOV by adding a filter to the expiratory limb would be required [25]. Alternate ventilation modes (e.g. APRV) should be considered with caution unless pediatric providers are experienced with the mode. Support with extracorporeal membrane oxygenation (ECMO) may be considered in available centers for pediatric COVID-19 patients without contraindications with refractory hypoxemia despite maximal medical management [25, 26, 55].
Aerosol generation with different modes of ventilatory support
A challenging aspect of caring for patients with severe COVID-19 disease has been mitigating the risk of transmission of SARS-CoV-2 to providers during aerosol generating procedures (AGPs). Which procedures and supports are considered AGPs varies amongst hospitals, but commonly include HFNC, CPAP, BiPAP, bag-valve-mask ventilation, intubation, extubation, bronchoscopy, open tracheal suctioning, and chest compressions. Transmission of SARS-CoV-2 occurs primarily through spread of respiratory droplets (particles > 5–10 μm in diameter) but AGPs can generate smaller aerosol particles (< 5–10 μm in diameter) that may remain suspended in air and more easily dispersed [56]. Quantifying the risk of aerosol transmission should include considerations of both the amount of aerosol particles generated and the distance of dispersion. Respiratory droplet dispersion distance is determined by both the force of expiration (e.g. coughing) and the mechanical support device and filtration system utilized [57]. In healthy adults, the amount of aerosol particles generated from the respiratory tract may be equivalent between low flow oxygen, HFNC, and BiPAP [58]. CPAP and BiPAP, however, disperse particles over a further distance than low flow oxygen or HFNC, but utilization of oronasal interfaces or helmet devices can significantly mitigate droplet dispersion during NIPPV (reviewed in [57]). Notably, HFNC may not increase aerosol dispersion more than low flow oxygen as initially feared [57, 59] and a surgical mask placed over HFNC could be an effective strategy to mitigate droplet dispersion in COVID-19 [57, 60]. Comparative data are lacking in children who are generally managed on lower flow rates of HFNC and lower CPAP/BiPAP pressures, which could influence both aerosol generation and dispersion.
Intubation of COVID-19 patients is ideally performed in a negative pressure room by an expert in airway management utilizing maximal PPE, video laryngoscopy, cuffed endotracheal tube, and avoidance of positive pressure to minimize respiratory droplet dispersion [61]. Best patient care practices should be followed at individual hospitals to keep both healthcare providers and patients safe from exposure to infectious particles during AGPs [62] and frameworks to safely address airway complications in mechanically ventilated COVID-19 patients can be helpful tools [63].
Conclusion
Management of COVID-19 associated respiratory failure in pediatric patients requires a multidisciplinary team of pediatric intensivists, pulmonologists, respiratory therapists and expert nurses to monitor and escalate respiratory support rapidly. The phenotypes of COVID-19 associated lung disease in children appear to be on the well-described spectrum of classic PARDS [9]. Utilizing evidence-based PARDS criteria and management guidelines, including adjusting ventilation strategy for dynamic respiratory mechanics, should guide escalation of respiratory support for severe pediatric COVID-19 disease. As data and experience in managing children with COVID-19 is expected to accumulate over time as the pandemic persists, pediatric clinicians would be prudent to continue proactive and ongoing updates to their hospital’s approach to the care of the child with COVID-19 disease as the collective knowledge advances.
Key Points.
Severe pediatric COVID-19 disease is relatively uncommon, but some children develop respiratory failure requiring escalation of respiratory support.
Severe COVID-19 compromises gas exchange through V/Q mismatch, increased pulmonary dead space, and intrapulmonary shunting.
High flow nasal cannula or non-invasive support with CPAP or BiPAP can be trialed with close monitoring for clinical deterioration and need for rapid escalation.
Mechanical ventilation strategies for children with COVID-19 should follow lung protective ventilation guidelines for pediatric acute respiratory distress syndrome.
Aerosol generation should be minimized while providing adequate respiratory support.
Acknowledgements
The authors thank Dr. John Arnold, Dr. Jeffrey Burns, and Dr. David Cornfield for expert guidance and review of the manuscript; the International COVID-19 PICU Collaborative, in particular content expertise on ventilator management by Dr. Robinder Khemani, Dr. Peter Rimensberger, and Dr. Heidi Flori; Brigham and Women’s Hospital COVID-19 clinical decision-making tool (www.covidprotocols.org) for reference; and Dimple Mirchandani for assistance with manuscript preparation.
Financial support and sponsorship
MGD has received research support from the National Institutes of Health and the Department of Anesthesiology, Critical Care and Pain Medicine at Boston Children’s Hospital.
Footnotes
Conflicts of interest
None
References
- 1.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatrics. 2020;174(9):868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69(32):1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leidman E, Duca LM, Omura JD, et al. COVID-19 Trends Among Persons Aged 0–24 Years - United States, March 1-December 12, 2020. MMWR Morb Mortal Wkly Rep 2021;70(3):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med 2020;48(6):e440–e469* Society of Critical Care Medicine / Surviving Sepsis Campaign COVID-19 Guidelines for Critically Ill Adults
- 5.World Health Organization. (2020). Clinical Management of COVID-19: interim guidance, 27 May 2020. Geneva: World Health Organization. Available from: https://apps.who.int/iris/handle/10665/332196 Accessed: 2/2/2021*WHO Guidelines for Clinical Management of COVID-19
- 6.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health; Available from: https://www.covid19treatmentguidelines.nih.gov/ Accessed 2/2/2021*NIH Treatment Guidelines for COVID-19
- 7.West J. Respiratory Physiology—The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 8.Kneyber MCJ, de Luca D, Calderini E, et al. on behalf of the section Respiratory Failure of the European Society for Paediatric and Neonatal Intensive Care. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med 2017;43(12):1764–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric Acute Respiratory Distress Syndrome: Consensus Recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16(5):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batah SS, Fabro AT. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respiratory Medicine. 2021;176:106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med 2020;48(9):e799–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). JAMA. 2020;324(8):782–793. [DOI] [PubMed] [Google Scholar]
- 15.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nature reviews Disease primers. 2019;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price LC, Mccabe C, Garfield B, et al. Thrombosis and COVID-19 pneumonia: the clot thickens! European Respiratory Journal. 2020;56(1):2001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigatello L, Pesenti A. Respiratory Physiology for the Anesthesiologist. Anesthesiology. 2019;130(6):1064–1077. [DOI] [PubMed] [Google Scholar]
- 19.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 20.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329–2330.*Proposed clinical subphenotypes of COVID-19 ARDS adult patients
- 21.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46(6):1099–1102.*Proposed clinical subphenotypes of COVID-19 ARDS adult patients
- 22.Panwar R, Madotto F, Laffey JG, et al. Compliance Phenotypes in Early Acute Respiratory Distress Syndrome before the COVID-19 Pandemic. Am J Respir Crit Care Med 2020;202(9):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khemani RG, Smith LS, Zimmerman JJ, et al. for the Pediatric Acute Lung Injury Consensus Conference Group. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence, and Epidemiology: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16:S23–S40. [DOI] [PubMed] [Google Scholar]
- 24.Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am J Respir Crit Care Med. 2016;193(1):52–59. [DOI] [PubMed] [Google Scholar]
- 25.Rimensberger PC, Kneyber MCJ, Deep A, et al. Caring for Critically Ill Children With Suspected or Proven Coronavirus Disease 2019 Infection: Recommendations by the Scientific Sections’ Collaborative of the European Society of Pediatric and Neonatal Intensive Care. Pediatric Critical Care Medicine. 2021;22(1):56–67.**ESPNIC consensus statement recommendations for the care of critically ill children with COVID-19
- 26.Kache S, Chisti MJ, Gumbo F, et al. COVID-19 PICU guidelines: for high- and limited-resource settings. Pediatric Research. 2020;88(5):705–716.**International pediatric ICU expert guidelines for ICU management of pediatric COVID-19 patients.
- 27.Rimensberger PC, Cheifetz IM, for the Pediatric Acute Lung Injury Consensus Conference Group. Ventilatory Support in Children With Pediatric Acute Respiratory Distress Syndrome. Pediatric Critical Care Medicine. 2015;16:S51–S60. [DOI] [PubMed] [Google Scholar]
- 28.Frat J-P, Thille AW, Mercat A, et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. New England Journal of Medicine. 2015;372(23):2185–2196. [DOI] [PubMed] [Google Scholar]
- 29.Ferreyro BL, Angriman F, Munshi L, et al. Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure. JAMA. 2020;324(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Y-N, Luo J, Yu H, et al. The effect of high-flow nasal cannula in reducing the mortality and the rate of endotracheal intubation when used before mechanical ventilation compared with conventional oxygen therapy and noninvasive positive pressure ventilation. A systematic review and meta-analysis. The American Journal of Emergency Medicine. 2018;36(2):226–233. [DOI] [PubMed] [Google Scholar]
- 31.Demoule A, Vieillard Baron A, Darmon M, et al. High-Flow Nasal Cannula in Critically III Patients with Severe COVID-19. Am J Respir Crit Care Med 2020;202(7):1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine. 2020;28:100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Despres C, Brunin Y, Berthier F, et al. Prone positioning combined with high-flow nasal or conventional oxygen therapy in severe Covid-19 patients. Critical Care. 2020;24:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu G-W, Liao Y-X, Li Q-Y, et al. Prone positioning in high-flow nasal cannula for COVID-19 patients with severe hypoxemia: a pilot study. Annals of Translational Medicine. 2020;8(9):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q, Wang T, Qin X, et al. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Critical Care. 2020;24:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goligher EC, Slutsky AS. Not Just Oxygen? Mechanisms of Benefit from High-Flow Nasal Cannula in Hypoxemic Respiratory Failure. Am J Respir Crit Care Med 2017;195(9):1128–1131. [DOI] [PubMed] [Google Scholar]
- 37.Mauri T, Turrini C, Eronia N, et al. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. [DOI] [PubMed] [Google Scholar]
- 38.Franklin D, Babl FE, Schlapbach LJ, et al. A Randomized Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis. New England Journal of Medicine. 2018;378(12):1121–1131. [DOI] [PubMed] [Google Scholar]
- 39.Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. The Lancet. 2017;389(10072):930–939. [DOI] [PubMed] [Google Scholar]
- 40.Rubin S, Ghuman A, Deakers T, et al. Effort of breathing in children receiving high-flow nasal cannula. Pediatric Critical Care Medicine. 2014;15(1):1–6. [DOI] [PubMed] [Google Scholar]
- 41.Abrams EM, Sinha I, Fernandes RM, et al. Pediatric asthma and COVID-19: The known, the unknown, and the controversial. Pediatric Pulmonology. 2020;55(12):3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Q, Xing Y, Shi L, et al. Coinfection and Other Clinical Characteristics of COVID-19 in Children. Pediatrics. 2020;146(1):e20200961. [DOI] [PubMed] [Google Scholar]
- 43.Brochard L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: Yes. Intensive Care Medicine. 2017;43(2):250–252. [DOI] [PubMed] [Google Scholar]
- 44.Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of Noninvasive Ventilation for De Novo Acute Hypoxemic Respiratory Failure. Critical Care Medicine. 2016;44(2):282–290. [DOI] [PubMed] [Google Scholar]
- 45.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. The Lancet Respiratory medicine. 2019;7(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newth CJ, Stretton M, Deakers TW, et al. Assessment of pulmonary function in the early phase of ARDS in pediatric patients. Pediatric Pulmonology. 1997;23(3):169–175. [DOI] [PubMed] [Google Scholar]
- 47.Bos LDJ, Sinha P, Dickson RP. The perils of premature phenotyping in COVID-19: a call for caution. The European respiratory journal. 2020;56:2001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardin CC. Novel Phenotypes in Respiratory Failure: Same As It Ever Was. Am J Respir Crit Care Med 2020;202(9):1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mart MF, Ely EW. Coronavirus Disease 2019 Acute Respiratory Distress Syndrome: Guideline-Driven Care Should Be Our Natural Reflex. Crit Care Med 2020;48(12):1835–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England journal of medicine. 2000;342(18):1301–1308. [DOI] [PubMed] [Google Scholar]
- 51.Khemani RG, Parvathaneni K, Yehya N, et al. Positive End-Expiratory Pressure Lower Than the ARDS Network Protocol Is Associated with Higher Pediatric Acute Respiratory Distress Syndrome Mortality. Am J Respir Crit Care Med 2018;198(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020;201(10):1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L, Marini JJ, Quintel M. Recruiting the Acutely Injured Lung: How and Why? Am J Respir Crit Care Med 2020;201(2):130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamburro RF, Kneyber MCJ, for the Pediatric Acute Lung Injury Consensus Conference Group. Pulmonary Specific Ancillary Treatment for Pediatric Acute Respiratory Distress Syndrome: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16:S61–S72. [DOI] [PubMed] [Google Scholar]
- 55.Shekar K, Badulak J, Peek G, et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: A Consensus Document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO Journal. 2020;66(7):707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. (2014). Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. Geneva: World Health Organization. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24983124 Accessed: 2/2/2021 [PubMed] [Google Scholar]
- 57.Leasa D, Cameron P, Honarmand K, et al. Knowledge translation tools to guide care of non-intubated patients with acute respiratory illness during the COVID-19 Pandemic. Crit Care. 2021;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaeckle NT, Lee J, Park Y, et al. Aerosol Generation from the Respiratory Tract with Various Modes of Oxygen Delivery. Am J Respir Crit Care Med 2020;202(8):1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. European Respiratory Journal. 2020;55(5):2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PloS one. 2012;7(12):e50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matava CT, Kovatsis PG, Lee JK, et al. Pediatric Airway Management in COVID-19 Patients: Consensus Guidelines From the Society for Pediatric Anesthesia’s Pediatric Difficult Intubation Collaborative and the Canadian Pediatric Anesthesia Society. Anesthesia & Analgesia. 2020;131(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schünemann HJ, Khabsa J, Solo K, et al. Ventilation Techniques and Risk for Transmission of Coronavirus Disease, Including COVID-19. Annals of Internal Medicine. 2020;173(3):204–216.*Review article highlighting differences in aerosol generation and risk of transmission of COVID-19 with various modes of ventilation
- 63.Ginestra JC, Atkins J, Mikkelsen M, et al. The I-READI Quality and Safety Framework: A Health System’s Response to Airway Complications in Mechanically Ventilated Patients with Covid-19. NEJM Catalyst. 2021;2(1). [Google Scholar]