Abstract
Objectives:
To use three-dimensional (3D) mirroring and surface-to-surface techniques to determine any differences in mandibular functional unit shape and morphology between the crossbite side and non-crossbite side in adult patients with posterior unilateral crossbite who had not received any corrective treatment for malocclusion.
Materials and Methods:
Cone-beam computed tomography (CBCT) records from 24 consecutive adult white patients (mean age, 27.5 years; range 22.6–39.7 years; 14 women and 10 men) seeking treatment for maxillary transverse deficiency were assessed in this study. The control group comprised CBCT scans from age- and sex-matched patients. Segmentation masks were generated to obtain 3D surface mesh models of the mandibles and analyze the six skeletal functional units, which were further analyzed with reverse engineering software.
Results:
Statistically significant differences in the mean surface distance when comparing the study sample and the control sample were found at the condylar process, mandibular ramus, angular process (P ≤ .0001), and alveolar process (P ≤ .01); no statistically significant differences were found for the coronoid process, the chin, and the mandibular body (P ≥ .5).
Conclusions:
The condylar, angular, and alveolar processes plus the mandibular ramus appear to play a more dominant role than did the body, the coronoid, and the chin units in the asymmetry of the mandible in patients with unilateral crossbite.
Keywords: Mandible, Crossbite, CBCT, Surface-to-surface matching
INTRODUCTION
Unilateral posterior crossbite (PUXB) is one of the most prevalent malocclusions and is reported to occur in 8% to 22% of patients.1,2 The most common form, in children and adolescents, is a unilateral presentation with a functional shift of the mandible toward the crossbite side.
Due to the mandibular functional lateral shift toward the crossbite side, there is asymmetric activity of the masticatory muscles and asymmetrically positioned condyles.3,4 These effects are believed to influence normal mandibular growth, leading gradually to permanent mandibular asymmetry.3,5,6 However, the extent to which untreated PUXB affects the craniofacial structures has not been fully defined.7–10 In this respect, the belief that untreated PUXB leads to skeletal asymmetry of the mandible was not supported by some studies7,8 but was sustained by another one.9 These conflicting results and opinions, however, were from previous investigations that involved two-dimensional (2D) image analysis with its inherent limitations. More recently, some investigations assessed mandibular asymmetry in children and adolescents with crossbite using cone-beam computed tomography (CBCT)–derived three-dimensional (3D) images.11–13 Findings from those studies included 3D hemimandibular and ramal body volumes, and point-to-point 2D linear measurements.
Currently, 3D surface-to-surface matching allows observation of even the smallest morphologic differences in bones obtained from CBCT scans. Thus, use of this reverse engineering technology could help to clarify if asymmetric growth of the mandible occurred in patients with PUXB.
According to the functional matrix theory,14 mandibular development and growth are the sum of independent growth of each mandibular functional unit (condylar process, coronoid process, angular process, alveolar process, body, and chin). Therefore, the purpose of this study was to evaluate any differences in the shape and morphology of each mandibular functional unit on CBCT scans from adult patients with PUXB. The following null hypothesis was tested: there are no significant differences in the shape and morphology of the functional units of the mandible between the crossbite and non-crossbite sides of the same patient compared with a control sample.
MATERIALS AND METHODS
The study sample comprised CBCT records from 24 adult white patients (mean age, 27.5 years; range 22.6–39.7 years; 14 women and 10 men) seeking treatment by surgically assisted rapid maxillary expansion (SARME) who had been referred (between November 2016 and January 2018) to a private X-ray practice specializing in CBCT. The Institutional Review Board of the School of Dentistry, Catania University, approved the study.
The sample size was determined by a pilot study using data obtained from the first patients analyzed in both groups. The power analysis (DSS Research, Washington, Arlington, VA 22201) indicated that data from 24 participants would yield a confidence level of 95% and a beta error level of 25%, making it sufficient to determine statistically significant differences.
The inclusion criteria for the study group were maxillary transverse deficiency and PUXB involving at least the molar and the premolars. The exclusion criteria were (1) presence of dentofacial deformities and/or severe facial asymmetry, (2) unerupted lower third molars, (3) mandibular functional shift, (4) congenital craniofacial syndrome, (5) signs or symptoms of temporomandibular joint disorder, and (6) previous orthodontic, prosthodontic, and maxillofacial surgery treatments.
The patients were age- and gender-matched with 24 subjects (10 men and 14 women, mean age 25.8, range 21.3–36.8 years) who served as the control group. The inclusion and exclusion criteria were the same as for the study group except for the absence of PUXB.
All CBCT images were taken with the NewTom 3G device (QR SRL, Verona, Italy; 110 kV, 6.19 mAs, 0.25 mm voxel size, and 8-mm aluminum filtration). The scans were deidentified to protect patient confidentiality. All the data sets were exported and converted using Digital Imaging and Communications in Medicine (DICOM), as previously validated and described.15–17 Briefly, to obtain 3D surface mesh models of the mandibles and analyze the skeletal functional units, a segmentation mask was generated (Figure 1). After segmentation, a 3D graphical rendering of each mandible was obtained (Mimics Research, version 19.0.0.347, Materialise NV, Liege, Belgium). Each rendered mandible was then mirrored by arbitrarily converting the image orientation from right–left, anterior–posterior and inferior–superior to left–right, anterior–posterior and inferior–superior.
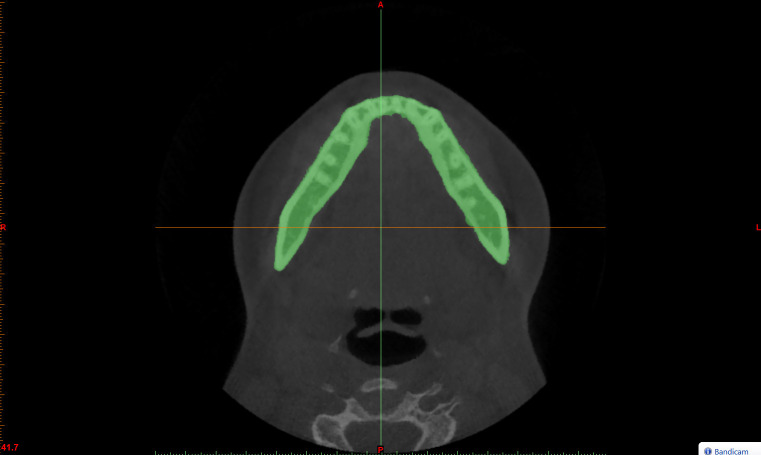
Figure 1.
The segmentation mask is manually selected in the axial view of the CBCT patient scans using the Threshold function of the Materialise Mimics software.
The original and the mirrored mandibular images were then registered, at first, on four points: the apical tip of the mandibular lingula, and the geometric center of mental foramina, both respectively, for the right and left sides (Figure 2). Thereafter, landmarks (Table 1 and Figure 2A) that enabled delimitation of each mandibular functional unit were identified on the axial, coronal, and sagittal views and then on the reconstructed 3D image (Figure 3). Subsequently, each functional unit underwent deviation analysis18 (Geomagic Control X, version 2017.0.0, 3D Systems, Santa Clara, CA 95054, USA)) (Figure 4). This was complemented by visualization of the 3D color-coded maps set with a range of tolerance of 0.50 mm (Figure 5). After the deviation analysis, percentages of all the distance values within the tolerance range were calculated.
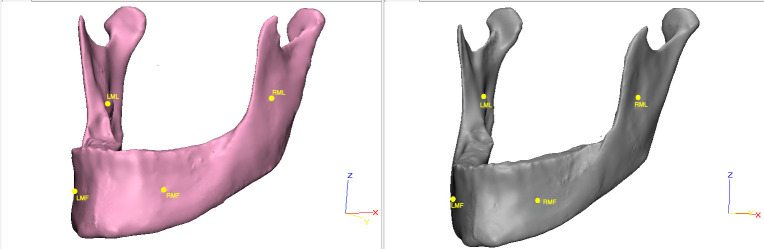
Figure 2.
First registration of mandibular images on the apical tip of the mandibular lingula and the geometric center of mental foramina/ LMF indicates left mental foramina; LML, left mandibular lingula; RMF, right mental foramina; LML, left mandibular lingual.
Table 1.
Landmarks on the Three-Dimensional Rendered Mandible From Cone-Beam Computed Tomography Scans.
| Landmark |
Definition |
| Sg | Deepest point on the sigmoid notch. |
| Li | Most superior point of the mandibular lingual. |
| B point (Supramentale) | Most concave point on mandibular symphysis. |
| Me (Menton) | Lowest point on the mandibular symphysis. |
| Me 1.5 | A point on the mandibular plane located 1,5 cm posterior to the Menton point. |
| Go (Gonion) | A point on the bony contour constructed by bisecting the angle formed by the intersection of the mandibular plane and ramus of mandible. |
| Go 1.0 | A point on the line that bisects the mandibular angle into two angles of the same degree and located 1 cm above the Go point. |
| Go-r 2.0 | A point on the posterior bony contour of the mandibular ramus drawn 2.0 cm from the gonion point. |
| Go-b 2.0 | A point on the inferior bony contour of the mandibular body drawn 2 cm mesially to the gonion point. |
| Rm | A point located on the retromolar trigone located where the line that bisects the gonial angle meets the outer bony portion of the trigone. |
Figure 3.
To delineate the Co and the Cr, a perpendicular line to Sg-Li passing through Sg is drawn. This line represents the inferior border of the Co and Cr functional units. The Ra is bounded by the perpendicular to Sg-Li at the top and by two lines at the bottom: the Go1.0-Gor2.0 and the Go1.0-Gob2.0 lines, respectively. The Ap-Ga is delimited at the top by the Go1.0-Gor2.0 and the Go1.0-Gob2.0 lines. The Mb is bounded posteriorly by Go1.0-Rm and Go1.0-Gob2, anteriorly Mb is delimited by a line drawn through point B and Me1.5. The Cp is delineated posteriorly by the line drawn through point B and Me1.5. The Ap is delimitated posterior by a line Go1.0-Rm and inferiorly by the line Go1.0-B (A). The functional mandibular units are then separated (B–D). AP, indicates alveolar process; Ap-Ga, angular process; Co, condylar process; Cp, chin process; Cr, coronoid process; Ra, mandibular ramus; Mb, mandibular body.
Figure 4.
The mandibular functional units from the crossbite side and non-crossbite side of SS and from the right and left side of CS are superimposed using the “best fit” algorithm (A–G).
Figure 5.
3D deviation analysis is carried out using the surface-to-surface technique. A scale bar is shown on the right side. Green color shows the range of tolerance (0.5 mm), red and blue show, respectively, the minimum and maximum deviation values (A–G).
To minimize random error and systematic errors, landmark detection was performed by a single examiner with 15 years of research experience on CBCT (R.L.) and who was calibrated previously.
To assess intrarater repeatability, digital casts from the study group and models from the control group were measured again, by the same operator after a washout period of 4 weeks (T2).
Statistical Analysis
All measurements were recorded in a Microsoft Excel 2016 spreadsheet (Microsoft, Redmond, Wash) and analyzed using SPSS version 24 Statistics software (IBM Corporation, Armonk, NY). Intraexaminer repeatability of landmark locations was assessed using an intraclass correlation coefficient (ICC). The Kolmogorov–Smirnov test was used to test the normality of the data. As all the data were normally distributed with homogeneous variance, parametric tests were used. Thus, mesh value percentages obtained by deviation analysis from the study group and control group were compared using an independent t-test. P values ≤.05 were considered statistically significant.
RESULTS
The ICC values the examiner obtained for landmarking and matching showed that the sets of recordings were highly correlated (ICC values ranged from 93.4% to 97.2%). The variability in matching percentages obtained for each functional unit ranged from 45.01% to 90.96% for the study group and from 59.89% to 91.05% for the control sample (Table 2). The lowest percentage of matching recorded between the crossbite side and non-crossbite side in the study group and between the right and left sides in the control group was at the alveolar process.
Table 2.
Surface-to-Surface Analysisa,b
| Total |
% Co |
% Ra |
% Ap |
% Cr |
% Ap-Ga |
% Cp |
% Mb |
|
| Study group | 40 | 65.86* | 75.81* | 45.01* | 90.96 NS | 75.91** | 83.55 NS | 82.96 NS |
| Control group | 30 | 79.95 | 83.47 | 59.89 | 91.05 | 81.22 | 83.78 | 81.86 |
Mean matching percentage obtained for each functional mandibular unit in the study and control groups. P values based on independent Student t test obtained by comparing the mean matching percentage of each functional unit from control sample and study sample.
Ap indicates alveolar process; Ap-Go, angular process at gonion ; Co, condylar process; Cp, chin process; Cr, coronoid process; Mb, mandibular body; NS, not significant; Ra, mandibular ramus.
P ≤ .0001; ** P ≤ .01.
Of the 7 anatomic areas measured, the condylar process, mandibular ramus, angular process at gonion (P ≤ .0001), and alveolar process (P ≤ .01) showed statistically significant differences in mean surface distance when comparing the study sample and the control sample. The greatest differences between the two samples were obtained for the condylar process with a mean difference of 14.09 percentage points.
There was no statistically significant difference in the mean surface distance measurements between the study group and control group for the coronoid process, the chin, and the mandibular body (P ≥ .05) (Table 2).
DISCUSSION
In this study, a recently developed 3D technique was used to evaluate mandibular functional unit shape and morphology. The investigation was carried out in adults with PUXB who had not received corrective treatment for malocclusion in order to evaluate long-term effects of this malocclusion on mandibular growth. The image-analysis procedure used in this investigation included the construction of 3D models from CBCT scans. Then, the surface-to-surface matching method was used to calculate the distances between the 3D superimposed surfaces. This latter tool, calculated thousands of color-coded point-to-point comparisons (surface distances in millimeters) between 3D models. This, in turn, allowed quantification of differences in shape and morphology.15 No previous study has used this new digital technology to compare and evaluate each functional mandibular unit.
According to the functional matrix theory, each unit is affected by the surrounding functional matrix,14 and the overall mandibular growth is a sum of the independent growth of each unit.19 Findings obtained from the current study found statistically significant differences between the two sides of the mandible of the same patients with PUXB in comparison to the control group. The highest differences were observed at the condylar process, at the angular process, and at the alveolar process units, with less at the coronoid process and nearly none at the body of the mandible and chin.
These results may be explained by previous studies that found asymmetric activity of masticatory muscles and differences in condyle position within the fossa between the crossbite side and non-crossbite side in patients with PUXB.4,20–24 So far, it was claimed that optimal masticatory muscle force during growth was necessary for normal mandibular growth25 and that masticatory muscle function was a determinant of bone quality in the growing mandible.21 In this respect, previous studies demonstrated that mandibular asymmetry can result after experimental unilateral muscle removal or after resection.23,24 Asymmetric postural and functional activity of the masticatory muscles has been recorded by surface electromyography measurements in patients with PUXB.20 This asymmetric activity causes a thinner masseter muscle on the crossbite side.4,22 Additionally, bone formation and chondrogenesis in the condylar cartilage were observed after unilateral masseter muscle resection26; this in turn seemed to determine mandibular asymmetry.
Thus, it may be speculated that asymmetric muscle activity is transferred to the mandibular bone, causing a regional mandibular asymmetry located especially in the area of muscle insertions. After the subsequent adaptation of the neuromusculature to the acquired new mandibular position, asymmetric mandibular growth can occur.
Regarding the condylar process, previous studies found that the mandible was rotated posteriorly on the crossbite side when related to the cranial floor27 and that the condyles on the crossbite side were positioned relatively more superiorly and posteriorly in the glenoid fossa than those on the non-crossbite side. Therefore, displacement of the mandible seems to be compensated through increased growth of the contralateral condyle, reduced growth of the ipsilateral condyle, corresponding surface remodeling in the articular fossae, or a combination of these factors.27 After temporomandibular joint bone remodeling, the condyles become more symmetrically positioned in their fossae, and mandibular midline deviation toward the crossbite side might persist due to long-term adaptive changes.7
The findings of the current study suggested that positional asymmetry produced a mild mandibular regional asymmetry especially affecting the condyle, the mandibular angle, the alveolar processes. and the mandibular ramus.3 The belief that an untreated unilateral crossbite can lead to skeletal asymmetry of the mandible was supported by this study. Interestingly, not every mandibular functional unit was involved to the same extent, with the condyle, angular, and alveolar processes being the anatomic areas showing the greatest variation in shape and morphology.
One drawback of this study was the small sample size. However, calculation of the sample size was obtained by a power analysis to ensure adequate power to detect significant differences. Additionally, despite the small sample size, the differences in shape and morphology were so clear that they were statistically significant. Nevertheless, studies with larger samples of subjects are warranted to uncover the full range of biological variability in PUXB.
CONCLUSIONS
The null hypothesis that there are no differences in the mandibular functional unit shape and morphology between adult subjects with and without PUXB was rejected.
The condylar, angular, and alveolar processes, plus the mandibular ramus, are the functional units showing the lowest percentage of matching symmetry. Therefore, they appear to play a more dominant role in the asymmetry of the mandible in unilateral posterior crossbite patients than did the body, the coronoid, and chin units.
REFERENCES
- 1.Kutin G, Hawes RR. Posterior cross-bites in the deciduous and mixed dentitions. Am J Orthod. 1969;56:491–504. doi: 10.1016/0002-9416(69)90210-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhu JF, Crevoisier R, King DL, Henry R, Mills CM. Posterior crossbites in children. Compend Contin Educ Dent. 1996;17:1051–1054. 1056, 1058. [PubMed] [Google Scholar]
- 3.Pinto AS, Buschang PH, Throckmorton GS, P Chen. Morphological and positional asymmetries of young children with functional unilateral posterior crossbite. Am J Orthod Dentofacial Orthop. 2001;120:513–520. doi: 10.1067/mod.2001.118627a. [DOI] [PubMed] [Google Scholar]
- 4.Tsanidis N, Antonarakis GS, Kiliaridis S. Functional changes after early treatment of unilateral posterior cross-bite associated with mandibular shift: a systematic review. J Oral Rehabil. 2016;43:59–68. doi: 10.1111/joor.12335. [DOI] [PubMed] [Google Scholar]
- 5.Primozic J, Perinetti G, Richmond S, Ovsenik M. Three-dimensional evaluation of facial asymmetry in association with unilateral functional crossbite in the primary, early, and late mixed dentition phases. Angle Orthod. 2013;83:253–258. doi: 10.2319/041012-299.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thilander B, Lennartsson B. A study of children with unilateral posterior crossbite, treated and untreated, in the deciduous dentition–occlusal and skeletal characteristics of significance in predicting the long-term outcome. J Orofac Orthop. 2002;63:371–383. doi: 10.1007/s00056-002-0210-6. [DOI] [PubMed] [Google Scholar]
- 7.Langberg BJ, Arai K, Miner RM. Transverse skeletal and dental asymmetry in adults with unilateral lingual posterior crossbite. Am J Orthod Dentofacial Orthop. 2005;127:6–15. doi: 10.1016/j.ajodo.2003.10.044. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 8.O'Byrn BL, Sadowsky C, Schneider B, BeGole EA. An evaluation of mandibular asymmetry in adults with unilateral posterior crossbite. Am J Orthod Dentofacial Orthop. 1995;107:394–400. doi: 10.1016/s0889-5406(95)70092-7. [DOI] [PubMed] [Google Scholar]
- 9.Takada J, Miyamoto JJ, Yokota T, Ono T, Moriyama K. Comparison of the mandibular hinge axis in adult patients with facial asymmetry with and without posterior unilateral crossbite. Eur J Orthod. 2015;37:22–27. doi: 10.1093/ejo/cju009. [DOI] [PubMed] [Google Scholar]
- 10.Iodice G, Danzi G, Cimino R, Paduano S, Michelotti A. Association between posterior crossbite, skeletal, and muscle asymmetry: a systematic review. Eur J Orthod. 2016;38:638–651. doi: 10.1093/ejo/cjw003. [DOI] [PubMed] [Google Scholar]
- 11.Halicioglu K, Celikoglu M, Yavuz I, Sekerci AE, Buyuk SK. An evaluation of condylar and ramal vertical asymmetry in adolescents with unilateral and bilateral posterior crossbite using cone beam computed tomography (CBCT) Aust Orthod J. 2014;30:11–18. [PubMed] [Google Scholar]
- 12.Illipronti-Filho E, Fantini SM, Chilvarquer I. Evaluation of mandibular condyles in children with unilateral posterior crossbite. Braz Oral Res. 2015;29:49. doi: 10.1590/1807-3107BOR-2015.vol29.0049. [DOI] [PubMed] [Google Scholar]
- 13.Veli I, Uysal T, Ozer T, Ucar FI, Eruz M. Mandibular asymmetry in unilateral and bilateral posterior crossbite patients using cone-beam computed tomography. Angle Orthod. 2011;81:966–974. doi: 10.2319/022011-122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss ML, Rankow RM. The role of the functional matrix in mandibular growth. Angle Orthod. 1968;38:95–103. doi: 10.1043/0003-3219(1968)038<0095:TROTFM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.AlHadidi A, Cevidanes LH, Mol A, Ludlow J, Styner M. Comparison of two methods for quantitative assessment of mandibular asymmetry using cone beam computed tomography image volumes. Dentomaxillofac Radiol. 2011;40:351–357. doi: 10.1259/dmfr/13993523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forst D, Nijjar S, Flores-Mir C, Carey J, Secanell M, Lagravere M. Comparison of in vivo 3D cone-beam computed tomography tooth volume measurement protocols. Prog Orthod. 2014;15:69. doi: 10.1186/s40510-014-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonardi R, Muraglie S, Crimi S, Pirroni M, Musumeci G, Perrotta R. Morphology of palatally displaced canines and adjacent teeth, a 3-D evaluation from cone-beam computed tomographic images. BMC Oral Health. 2018;18:156. doi: 10.1186/s12903-018-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonardi R, Muraglie S, Rugieri M, Barbato E. Three-dimensional evaluation on digital casts of morphologic maxillary teeth symmetry, in patients with unilateral palatally displaced canines. Am J Orthod Dentofacial Orthop. 2019;155:339–46. doi: 10.1016/j.ajodo.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Precious D, Delaire J. Balanced facial growth: a schematic interpretation. Oral Surg Oral Med Oral Pathol. 1987;63:637–644. doi: 10.1016/0030-4220(87)90360-4. [DOI] [PubMed] [Google Scholar]
- 20.Alarcon JA, Martin C, Palma JC. Effect of unilateral posterior crossbite on the electromyographic activity of human masticatory muscles. Am J Orthod Dentofacial Orthop. 2000;118:328–334. doi: 10.1067/mod.2000.103252. [DOI] [PubMed] [Google Scholar]
- 21.Bresin A, Kiliaridis S, Strid KG. Effect of masticatory function on the internal bone structure in the mandible of the growing rat. Eur J Oral Sci. 1999;107:35–44. doi: 10.1046/j.0909-8836.1999.eos107107.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiliaridis S, Mahboubi PH, Raadsheer MC, Katsaros C. Ultrasonographic thickness of the masseter muscle in growing individuals with unilateral crossbite. Angle Orthod. 2007;77:607–611. doi: 10.2319/101105-360. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues L, Traina AA, Nakamai LF, Luz JG. Effects of the unilateral removal and dissection of the masseter muscle on the facial growth of young rats. Braz Oral Res. 2009;23:89–95. doi: 10.1590/s1806-83242009000100015. [DOI] [PubMed] [Google Scholar]
- 24.Sarnat BG. Craniofacial change and non-change after experimental surgery in young and adult animals. Angle Orthod. 1988;58:321–342. doi: 10.1043/0003-3219(1988)058<0321:CCANAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Monje F, Delgado E, Navarro MJ, Miralles C, Alonso del Hoyo JR. Changes in the temporomandibular joint caused by the vertical facial pattern. Study on an experimental model. J Craniomaxillofac Surg. 1994;22:361–370. doi: 10.1016/s1010-5182(05)80118-0. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki M, Yonemitsu I, Takei M, Kure-Hattori I, Ono T. The imbalance of masticatory muscle activity affects the asymmetric growth of condylar cartilage and subchondral bone in rats. Arch Oral Biol. 2016;63:22–31. doi: 10.1016/j.archoralbio.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Nerder PH, Bakke M, Solow B. The functional shift of the mandible in unilateral posterior crossbite and the adaptation of the temporomandibular joints: a pilot study. Eur J Orthod. 1999;21:155–166. doi: 10.1093/ejo/21.2.155. [DOI] [PubMed] [Google Scholar]