Abstract
Background:
Vaginal/uterine rhabdomyosarcoma (VU RMS) is one of the most favorable RMS sites. To determine the optimal therapy, the experience of four cooperative groups (Children's Oncology Group [COG], International Society of Pediatric Oncology (SIOP) Malignant Mesenchymal Tumor Group [MMT], Italian Cooperative Soft Tissue Sarcoma Group [ICG], and European pediatric Soft tissue sarcoma Study Group [EpSSG]) was analyzed.
Procedure:
From 1981 to 2009, 237 patients were identified. Median age (years) at diagnosis differed by tumor location; it was 1.9 for vagina (n = 160), 2.7 for uterus corpus (n = 26), and 13.5 for uterus cervix (n = 51). Twenty-eight percent of patients received radiation therapy (RT) as part of primary therapy (23% COG, 27% MMT, 46% ICG, and 42% EpSSG), with significant differences in the use of brachytherapy between the cooperative groups (23% COG, 76% MMT, 64% ICG, and 88% EpSSG).
Results:
Ten-year event-free (EFS) and overall survival (OS) were 74% (95% CI, 67–79%) and 92% (95% CI, 88–96%), respectively. In univariate analysis, OS was inferior for patients with uterine RMS and for those with regional lymph node involvement. Although EFS was slightly lower in patients without initial RT (71% without RT vs. 81% with RT; P = 0.08), there was no difference in OS (94% without RT vs. 89% with RT; P = 0.18). Local control using brachytherapy was excellent (93%). Fifty-one (51.5%) of the 99 survivors with known primary therapy and treatment for relapse were cured with chemotherapy with or without conservative surgery.
Conclusions:
About half of all patients with VU RMS can be cured without systematic RT or radical surgery. When RT is indicated, modalities that limit sequelae should be considered, such as brachytherapy.
Keywords: brachytherapy, children, genital tract, radiotherapy, rhabdomyosarcoma, vagina
1. INTRODUCTION
Rhabdomyosarcoma (RMS) of the female genital tract is rare, accounting for 4% of RMS cases overall and 10% of cases in females.1 It is one of the most favorable sites with a 5-year overall survival (OS) rate greater than 90%2–4; but, because of its specific location, it poses unique challenges for local tumor control.
To limit late effects resulting from treatment and to preserve organ function, the approach to local treatment has undergone radical change over the last 30 years.2,3,5–8 In the International Society of Pediatric Oncology (SIOP) Malignant Mesenchymal Tumor Group (MMT) studies, no local treatment was performed in patients in whom complete remission (CR) was achieved with primary chemotherapy, while for others the strategy evolved from radical surgery and/or external-beam radiotherapy (EBRT) to a more conservative multidisciplinary approach including limited surgical intervention and, when feasible, radiation therapy (RT) with intracavitary brachytherapy (BT). This approach resulted in an 18% local failure rate and a 5-year OS of 91%.2 This conservative approach became the current strategy for patients treated within the European pediatric Soft Tissue Sarcoma Group (EpSSG) protocol and was initially adopted by the Children's Oncology group (COG) in 1997. However, in 2011, COG reported a high local failure rate in patients with noncompletely resected vaginal RMS, using a similar response-based approach that could delay or eliminate RT.3 Notably, among the 14 patients treated on COG ARST0331 with a lower dose of cyclophosphamide, the 2-year cumulative incidence of local recurrence was 43% for vaginal RMS with gross residual disease prior to chemotherapy (Intergroup Rhabdomyosarcoma Studies (IRS) group III) and no regional lymph node involvement (N0). Following this analysis, COG recommended a reversion to systematic RT for nonresected female genital RMS.
To determine the optimal therapy, taking into account the total burden of local therapy and chemotherapy, the experience of four collaborative groups (COG, SIOP MMT, Italian Cooperative Soft Tissue Sarcoma Group (ICG), and EpSSG) was shared at an international workshop. The primary purpose of the workshop was to explore the impact of RT on local failure rate and OS when used as part of primary treatment, and to assess the use of BT between the different cooperative groups.
2. PATIENTS AND METHODS
2.1. Patient population
Analyses were performed on the data derived from 13 studies from the four cooperative groups (IRS-III,9 IRS-IVp,10 IRS-IV,11 COG D9602,12 COG D9803,13 COG ARST0331,14 MMT84,15 MMT89,16 MMT95,17 RMS79,18 RMS88,18 RMS96,18 and RMS200519). The population consisted of 237 females, aged 0–21 years, with nonmetastatic RMS of the genital tract (excluding vulva), treated between 1981 and 2009 (Table 1 ). All patients had histological confirmation of RMS, and all were more than 2 years beyond the date of last treatment. Central pathology review was not repeated for this study. FOXO1 fusion status was not available for the majority of patients and was not analyzed in this report.
TABLE 1.
Characteristics of the 237 females and survival rates by prognostic variables
| Variables | No. (%) | 10-year EFS % | Log-rank test (P) | 10-year OS % | Log-rank test (P) | |
|---|---|---|---|---|---|---|
| Total | 237 | 74 | 92 | |||
| Age (years) | <1 | 25(11) | 68 | 0.4 | 82 | 0.39 |
| 1–10 | 157(66) | 73 | 93 | |||
| ≥10 | 55(23) | 77 | 96 | |||
| Site of the primary tumor* | Vagina | 160 (68) | 74 | 0.37 | 94 | 0.05 |
| Cervix | 51 (22) | 81 | 100 | |||
| Uterus | 26(11) | 73 | 87 | |||
| Tumor status | T1 | 177 (75) | 74 | 0.89 | 94 | 0.22 |
| T2 | 53(22) | 73 | 91 | |||
| Unknown | 7(3) | |||||
| Tumor size | <5 cm | 132 (56) | 71 | 0.44 | 94 | 0.31 |
| >5 cm | 93 (39) | 77 | 92 | |||
| Unknown | 12(5) | |||||
| Lymph node involvement | N0 | 209 (88) | 74 | 0.15 | 94 | 0.001 |
| N1 | 11(5) | 48 | 73 | |||
| Unknown | 17(7) | |||||
| Pathology | Nonalveolar RMS | 231 (97) | 74 | 0.19 | 93 | 0.3 |
| Alveolar RMS | 6(3) | 50 | 83 | |||
| Clinical group | I | 33 (14) | 79 | 0.46 | 97 | 0.63 |
| IIa | 39 (17) | 72 | 85 | |||
| IIb | 1 | 72 | 92 | |||
| III | 159(67) | |||||
| Unknown | 5(2) | |||||
| First-line radiation | No | 171 (72) | 71 | 0.08 | 94 | 0.15 |
| Yes | 66 (28) | 81 | 89 | |||
| Cooperative groups | COG | 132 | 72 | 0.54 | 92 | 0.6 |
| SIOPMMT | 62 | 74 | 90 | |||
| ICG | 24 | 83 | 96 | |||
| EpSSG | 19 | 73 | 100 | |||
| Periods of treatment | 1981–1994 | 99 (42) | 75 | 0.89 | 89 | 0.11 |
| 1995 onward | 138 (58) | 72 | 96 |
LN, lymph node; PT, primary tumor; T1, Tumor localized to the organ or tissue of origin; T2, Tumor extending beyond the tissue of origin to involve one or more adjacent tissues; Clinical groups are defined in Supplemental Table S1.
For survival analysis according to primary site (i.e. vagina, uterus, and cervix), therapy-related deaths were not considered since 3/5 occurred in the uterus.
2.2. Treatment
Surgery was considered radical when involving organ ablation or impairing urogenital function (such as in cases when hysterectomy, total vaginectomy, or cystectomy were performed). Partial vaginectomy, partial or total excision of the cervix, and trachelectomy were considered conservative procedures. Patients with group I nonalveolar RMS did not receive RT in any of the studies. Details regarding chemotherapy, surgery, and RT in each cooperative group are shown in Table 2 and Supplementary Material S1.
TABLE 2.
Details of primary therapy by cooperative group
| Total | COG | SIOP | ICG | EpSSG | |
|---|---|---|---|---|---|
| No. of Patients | 237 | 132 | 62 | 24 | 19 |
| Chemotherapy regimen | |||||
| VA (%) | 37 (16) | 26 (20)d | 5 (8) | 6 (25) | 0 |
| Alkylating agent-based (%) (cumulative cyclophosphamide equivalent dose per protocol in g/m2)22a | 177 (75) | 106 (80)d
IRS-III: 24.4 IRS-IV/IVp: 24.6 D9602: 28.6 D9803: 30.8 ARST0331: 4.8 |
46 (74) MMT84:8.8–14.6 MMT89:5.8–8.8 MMT95:8.8–13.2 |
8 (33) RMS96: 13.2 |
17 (89) RMS2005: 7.3–13.2 |
| Doxorubicin-based (%) | 22 (9) | 0d | 10 (16) | 10 (42) | 2 (11) |
| Surgerye | |||||
| Total surgery (%) | 139 (59) | 77 (58) | 36 (58) | 14 (58) | 12 (64) |
| Conservative (%) | 96 (41) | 48 (36) | 26 (42) | 12 (50) | 10 (53) |
| Tumorectomy | 63 | 32 | 17 | 8 | 6 |
| Partial vaginectomy | 17 | 6 | 5 | 4 | 2 |
| Partial cervix excision | 11 | 8 | 2 | 0 | 1 |
| Total cervix excision/trachelectomy | 4 | 2 | 1 | 0 | 1 |
| Partial cystectomy | 1 | 0 | 1 | 0 | 0 |
| Radical (%) | 43 (18) | 29 (22) | 10 (16) | 2 (8) | 2 (11) |
| Hysterectomy | 37 | 25c | 8 | 2 | 2 |
| Total vaginectomy | 4 | 3 | 1 | 0 | 0 |
| Cystectomy | 1 | 0 | 1b | 0 | 0 |
| Pelvic exenteration | 1 | 1 | 0 | 0 | 0 |
| Radiotherapye | |||||
| No. of patients irradiated (%) | 66 (28) | 30 (23) | 17 (27) | 11 (46) | 8 (42) |
| BT (%) | 28 (44) | 4 (14) | 12 (71) | 5 (45) | 7 (88) |
| EBRT (%) | 30 (47) | 21 (75) | 4 (24) | 4 (36) | 1 (12) |
| BT with EBRT (%) | 6 (9) | 3 (11) | 1 (5) | 2 (18) | 0 |
BT, brachytherapy, EBRT: External beam radiotherapy.
The following chemotherapy regimens were considered alkylating agent-based regimens: VAC (vincristine, dactinomycin, and cyclophosphamide), VAI/IVA (vincristine, dactinomycin, and ifosfamide), VIE (vincristine, dactinomycin, and etoposide) or doxorubicin-based regimens: “6 drugs” (IVA plus carboplatin, epirubicin, and etoposide), VAIA (IVA plus doxorubicin), VACA (VAC plus doxorubicin), and CAV (cyclophosphamide, dactinomycin, and doxorubicin).
Cumulative equivalent dose of cyclophosphamide was calculated according to the model of Green et al (2014).22 In the SIOP MMT protocol, the total number of courses of ifosfamide varied according to the quality of initial resection (group II or III) and the response to chemotherapy. In the RMS2005 protocol, the total number of courses of ifosfamide varied according to RT realization.
with total vaginectomy
2 with total vaginectomy
for COG patients, chemotherapy group was considered on an intention to treat basis
Missingdata: modality of surgery unknown (n = 2), modality of radiotherapy unknown (n = 2).
2.3. IRSG/COG studies
In IRS-III9, patients with group II tumors (see Supplementary Table S1 for the Intergroup Rhabdomyosarcoma Study Group (IRSG) surgical-pathologic grouping system) received 41.4 Gy beginning at week 2. Patients with group III tumors did not receive RT if in CR following chemotherapy and second-look surgery, but received 45–55 Gy if not in CR.
In IRS-IV11, IV pilot10, and D980313, systematic RT was given at week 9. If EBRT was performed rather than BT, patients with gross residual disease in IRS-IV were randomized between 50.4 Gy in a conventional daily schedule and 59.4 Gy in a hyperfractionated schedule.
In COG D960212 and COG ARST0331,14 delayed resections and biopsies to assess response for vaginal RMS were postponed until week 28 (D9602) or 24 (ARST0331) for patients with Group IIA or III vaginal RMS without clinical involvement of lymph nodes (N0). RT was omitted if the patient was in CR after chemotherapy with or without surgery. For patients with group III vaginal primaries with clinical involvement of lymph nodes (N1), RT to primary tumor and nodes was to start at week 12 on D9602 and at week 13 on ARST0331. Patients with RMS of the uterus or cervix did not receive RT if a hysterectomy had been performed.
2.4. ICG studies
The local therapy strategy was similar in all ICG studies. RT began at weeks 13–14 and the dose was defined according to chemotherapy response or the extent of resection at delayed surgery, if performed. Patients with genital tumors achieving clinical (RMS79) or histologically confirmed (RMS 88 and 96) CR were not intended to receive RT. The dose given was 40–45 Gy in patients with microscopic residual disease and 50–55 Gy in those with gross residual disease.
2.5. SIOP studies
In all three SIOP studies, patients who achieved a CR with chemotherapy with or without surgery did not receive additional local therapy as part of first-line treatment. Local therapy was given only to the patients who had residual macroscopic or histologically proven microscopic disease after six courses of chemotherapy (week 17).
2.6. EpSSG study
Local treatment, if indicated, was administered at week 13. Patients with favorable age (<10 years) and tumor ≤ 5 cm at diagnosis, who had achieved clinical CR after the three initial vincristine, dactinomycin, and ifosfamide (IVA or VAI) courses received six additional IVA courses without RT (option A), or if CR was obtained after secondary surgery with histological CR (option B). Patients with unfavorable features (age ≥ 10 years and/or tumor size > 5 cm) achieving CR, and all those only in partial remission received systematic RT.
2.7. Modality of radiotherapy
In each cooperative group, endocavitary with or without interstitial BT was encouraged when RT was indicated but there were significant differences regarding its use between the individual groups.5,6,20 The usual dose was 60–65 Gy. The initial tumor extent was included in the target BT volume for patients treated before 1990. In most cases after 1990, only the residual tumor after chemotherapy was treated. The modality of EBRT was not registered for the purpose of this analysis. However, since the study period ranged from 1981 to 2009, it is likely that external radiation was mainly done with photons.
2.8. Statistical analysis
Statistical analyses were performed at Gustave Roussy in Villejuif, France, using a general database management system. Survival curves were calculated by the method of Kaplan–Meier. Survival was calculated from the date of the start of treatment to the time of the last follow-up or death. Event-free survival (EFS) was calculated from the date of the start of treatment to the date of first event, such as failure to achieve CR, relapse, or death from any cause. Local control was defined as disappearance of all clinical and radiological signs of disease or as stable residual radiographic images for 6 months after completion of treatment. The date of the main analysis was March 2013, providing a minimum potential follow-up of 45 months from the last date of study entry.
3. RESULTS
3.1. Patient characteristics
The initial characteristics of the 237 patients are presented in Table 1 and in Supplementary Table S2 according to cooperative group. Median age at diagnosis was 2.4 years (range, 1 month to 20 years) but differed with tumor location; the median age of females with vaginal and uterine tumors was 1.9 years (range, 1 month to 19.7 years) and 2.7 years (range, 6 months to 20 years), respectively, whereas in patients with cervical tumors, the median age at diagnosis was 13.5 years (range, 3 months to 20.4 years). The primary tumor originated in the vagina in 160 patients (68%), in the cervix in 51 patients (21%), and in the uterus in 26 patients (11%). Two hundred and thirty one (97%) patients had nonalveolar histology; the botryoid variant was the most common (n = 154, 65%). Eleven patients (5%) had clinical or radiologic involvement of regional lymph nodes (N1). Median follow-up of survivors was 6.8 years (range, 2–17 years).
3.2. Primary treatment
Details of primary therapy by cooperative group are available in Table 2. At the time of diagnosis, 33 patients underwent initial complete resection without nodal involvement, one underwent complete resection with nodal involvement, 39 had microscopically incomplete resection, and 159 had macroscopic residual disease or only a biopsy. Initial surgery was done before chemotherapy in 80 patients (34%) (including seven patients with macroscopic residue). Initial complete excision rates varied according to tumor site (22/38 cervix [one hysterectomy], 4/8 uterus [all with hysterectomy], and 7/34 vagina [all conservative surgery]). In total, eight patients underwent initial hysterectomy, including three with incomplete tumor resection.
All except one patient (physician decision) received chemotherapy; 37 (16%) received vincristine–dactinomycin (VA) only, 177 (75%) an alkylating agent-based regimen (vincristine, dactinomycin, and cyclophosphamide [VAC] or IVA), and 22 (9%) a doxorubicin-based regimen. The cumulative dose of alkylating agent and duration of therapy varied among cooperative groups and protocols.
Overall, surgery (at diagnosis and/or after chemotherapy) was performed in 139 patients (59%). Surgery was conservative in 96 patients (41% of all patients) and radical in 43 (18%). Primary tumor location was the main factor associated with the frequency of radical surgery (12/26 [46%] for patients with uterus corpus vs. 25/160 [16%], and 6/51 [12%] for those with vagina and uterus cervix, respectively).
Altogether, 66 patients (28%) were irradiated during their initial treatment, but this proportion varied between the different cooperative groups; 30/132 (23%), 17/62 (27%), 11/24 (46%), and 8/19 (42%) patients were irradiated in COG, SIOP, ICG, and EpSSG studies, respectively. Indications for RT varied among studies and cooperative groups (Section 2). Patient age and extent of surgery (conservative vs. radical) did not influence the proportion of those receiving RT. In contrast, tumor site influenced the use of RT; 3/51 (6%), 7/26 (27%), and 56/160 (35%) patients with RMS of the cervix, uterus, or vagina, respectively, were irradiated. Endocavitary with or without interstitial BT was encouraged when RT was indicated but there were significant differences regarding its use between cooperative groups. Overall, 34 (52%) of all irradiated patients had BT (six also had EBRT); this included 7/30 patients (23%) in the COG studies, 13/17 patients (76%) in the SIOP studies, 7/11 patients (64%) in the ICG studies, and 7/8 patients (88%) in the EpSSG study. All except three patients who had BT had vaginal RMS. Among the irradiated cohort, 44 (68%) were younger than 3 years of age and 22 (50%) of these received BT. Two of the 28 females who had BT only experienced isolated local failure (7%) (both EpSSG patients). Details of primary therapy by primary tumor site are available in Supplementary Table S3.
3.3. Remission, survival, and relapse
CR was achieved in 225 patients (95%). Recurrence occurred in 56 patients (24%). Median time to relapse/progression was 18 months (range, 25 days to 11 years), with three relapses occurring beyond 5 years from diagnosis. Fifty (89%) experienced a local relapse (including two patients with local plus regional nodal relapse), two had isolated nodal relapse, and three patients developed distant metastases (one relapse site unknown). Among the 50 local recurrences, 42 (84%) occurred in nonirradiated patients (42/171; 25%) and eight occurred after RT (8/66; 12%). Eight out of the 50 patients who experienced local relapse died of disease (four relapsed after initial RT). The rate of local relapse did not vary significantly between the cooperative groups (31/132 [23%], 12/62 [19%], 3/24 [12.5%], and 4/19 [21%] in COG, SIOP, ICG, and EpSSG studies, respectively [P = 0.23]).
One hundred and thirty-four females (57%) achieved CR with chemotherapy alone after biopsy (n = 63) or with chemotherapy and conservative surgery (n = 71, including one female treated with surgery only). Among them, 32 experienced local relapse (24%), two had metastatic relapse, and two died of treatment-related toxicity during initial therapy. The rate of tumor control varied according to primary therapy; 38/63 (60%) and 60/71 (85%) of patients who had achieved CR with chemotherapy only and chemotherapy and conservative surgery, respectively, remained in continuous first CR. The rate of tumor control also varied among tumor locations: 38/43 (88%), 6/8 (75%), and 58/83 (70%) of patients with uterus cervix, uterus corpus, and vagina RMS, respectively, who had achieved CR with chemotherapy with or without conservative surgery, remained in first local CR.
At the time of analysis, the median follow-up of survivors was 8 years. The 10-year actuarial EFS was 74% (95% CI, 67–79%) and OS was 92% (95% CI, 88–96%) for all patients (Figure 1), with no difference between the cooperative groups (Table 1). A total of 221 patients were alive, of whom 176 were in first remission and 45 were in subsequent remission after relapse. In total, 16 patients died, of whom one never achieved CR, nine died after relapse, five died from treatment-related causes during initial therapy, and one died from a secondary osteosarcoma. Three out of the five toxic deaths occurred between 1986 and 1988, in young females with a median age of 21 months (exact causes of death were not available for this analysis). Figure 2 shows the EFS and OS according to the use of RT as part of primary treatment. While EFS was slightly decreased in patients without initial RT (71% without RT vs. 81% with RT; P = 0.08), there was no difference in OS (94% without RT vs. 89% with RT; P = 0.12).
FIGURE 1.
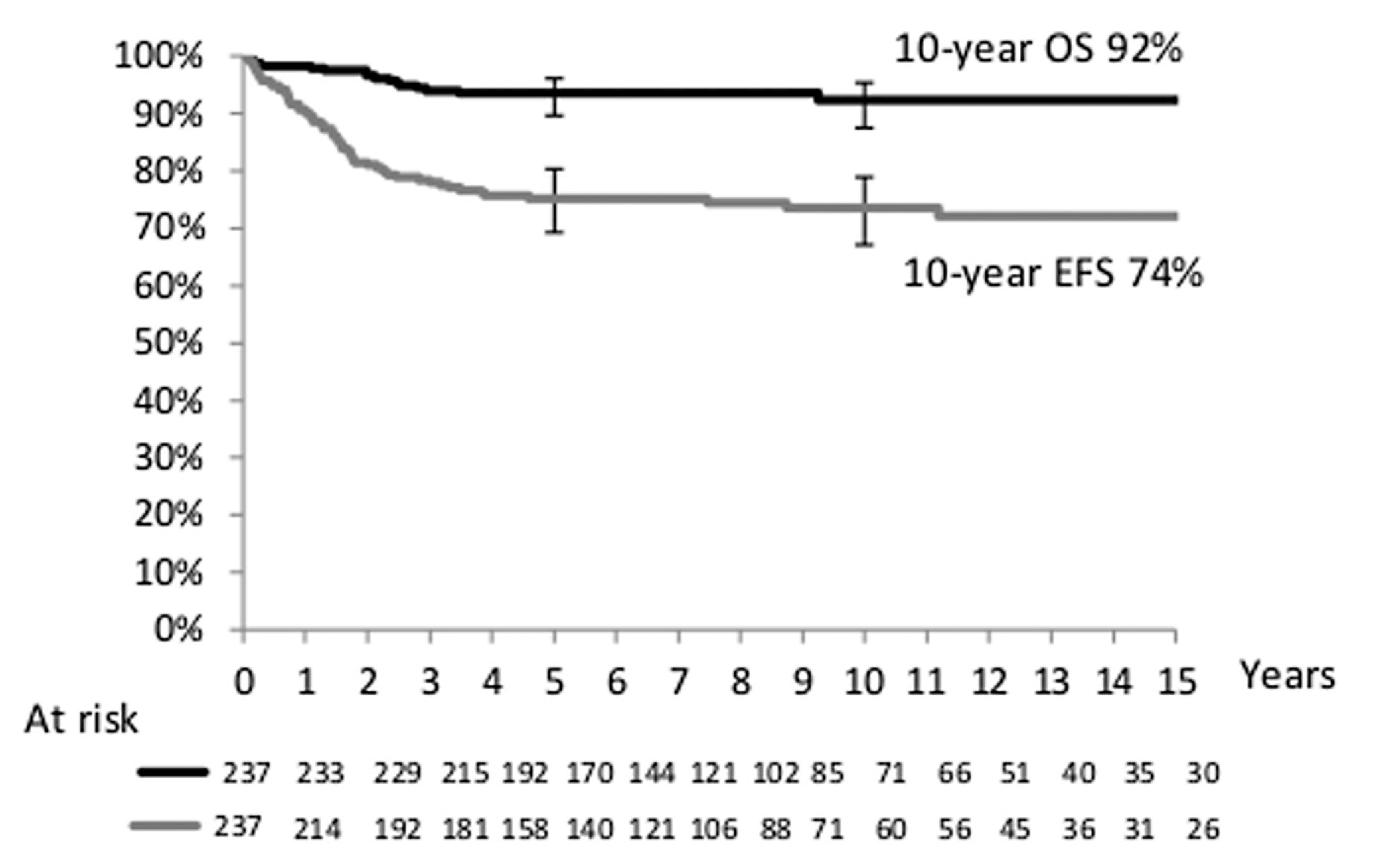
Overall survival and event-free survival for the whole population
FIGURE 2.
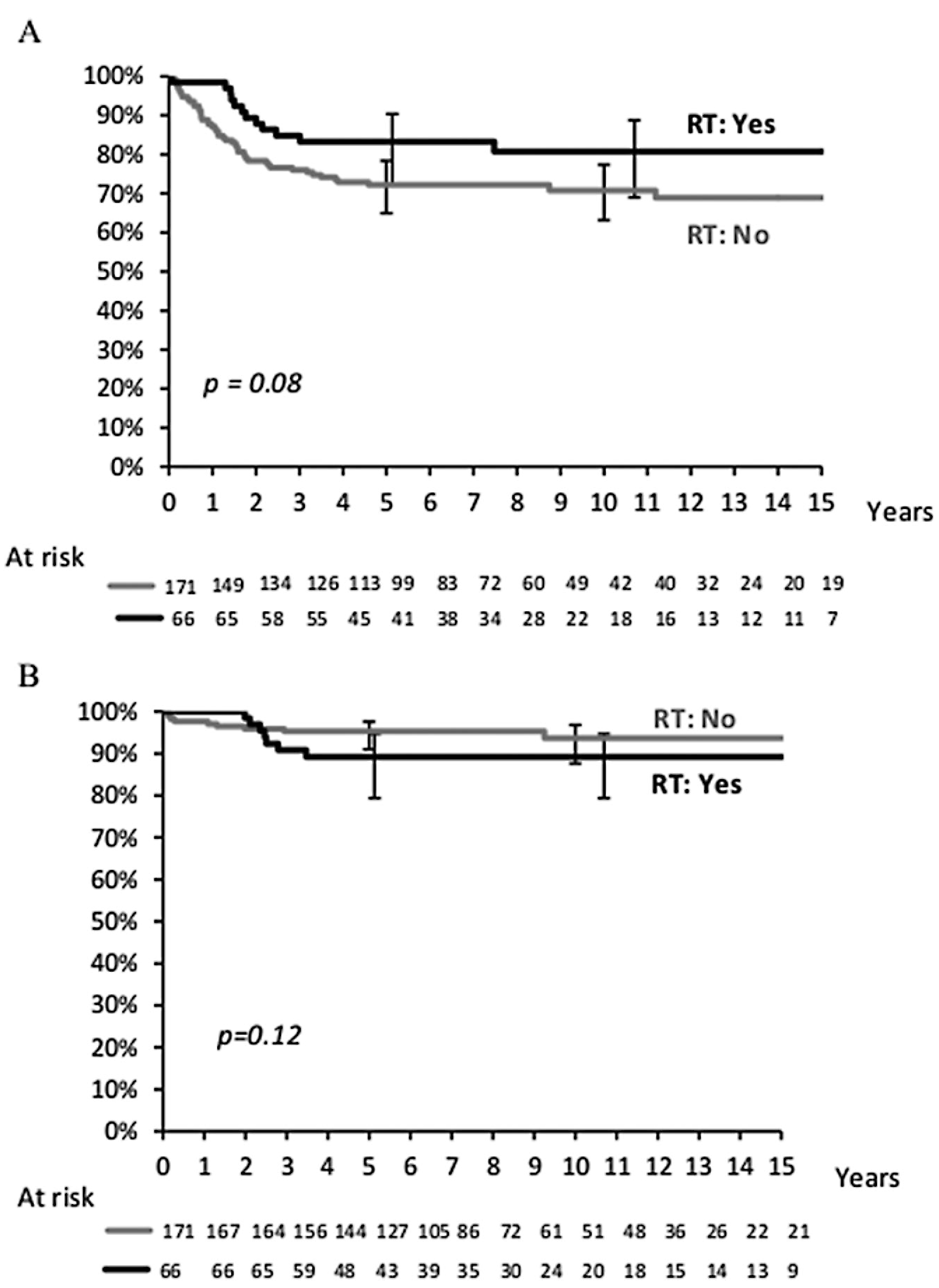
(A) Event-free survival and (B) overall survival according to initial radiotherapy
3.4. Burden of therapy in surviving patients
Among the 221 surviving patients, 137 had been initially treated with chemotherapy with or without biopsy only, or conservative surgery. Among them, 99 (72%) were in first CR and 38 were alive after relapse. Data for treatment received for relapse were not available for females treated in the COG studies. For those included in the three European group studies, data were available both for initial treatment and for the treatment of any relapse (Table 3). Summation of local therapy given as part of initial treatment and for the treatment of relapse indicated that 51.5% of females were cured with chemotherapy with or without conservative surgery and 26% were treated with chemotherapy and BT with or without conservative surgery, while only 22% had been cured with radical surgery, EBRT, or with unknown RT details. Additionally, among the 14 European females who relapsed after initial chemotherapy with or without conservative surgery and are cured (all except one who died of secondary malignancy), second local therapies were as follows: chemotherapy only (n = 2), BT (with or without conservative surgery) (n = 4), EBRT (n = 3), mutilating surgery with RT (n = 2), and RT only (no details regarding RT modality, n = 2).
TABLE 3.
Total local therapy in European patients alive without disease (in first or subsequent remission) by primary site
| Total | Vagina | Cervix | Uterus | |
|---|---|---|---|---|
| No. of patients | 105 | 71 | 9 | 25 |
| No. of patients alive in remission | 99 | 67 | 7 | 25 |
| Treatment | ||||
| Chemotherapy only | 18 | 14 | 1 | 3 |
| Chemotherapy with conservative surgery | 33 | 11 | 2 | 20a |
| Total treated without significant local therapy (%) | 51 (52) | 25 (36) | 3 (43) | 23 (92) |
| Brachytherapy with or without conservative surgery (%) | 26 (26) | 25 (37) | 2 | |
| EBRT with or without conservative surgery | 8 | 4 | 1 | 1 |
| Radiotherapy in relapse without details | 2 | 3 | ||
| Radical surgery with or without RT | 12 | 10 | 1 | 1 |
| Total treated with significant local therapy (%) | 48 (48) | 42 (63) | 4 (57) | 2 (8) |
BT, brachytherapy; EBRT, External beam radiotherapy.
One patient was treated with surgery only without chemotherapy.
3.5. Prognostic factors
Analysis of 10-year EFS and OS rates by prognostic variables are shown in Table 1 for the whole population. OS differed by regional lymph node involvement (P = 0.001) and primary site, with RMS of the uterus having the worst outcome (P = 0.05) (Figure 3). Among the 11 females with regional lymph node involvement (seven vagina and four uterus), local relapse occurred in four, resulting in two deaths from disease and one from treatment-related toxicity. Age, tumor size, tumor invasiveness, clinical group, cooperative group, and era of therapy were not associated with EFS or OS. While histologic subtype did not significantly affect outcome, 3/6 patients with alveolar RMS relapsed (one local, one nodal, and one metastatic). Importantly, there was no statistical difference in OS between patients who did not receive RT as part of their initial therapy and those who did (OS: 94% without RT vs. 89% with RT), although EFS was slightly decreased in patients without initial RT (EFS, 71% vs. 81% with RT; P = 0.08).
FIGURE 3.
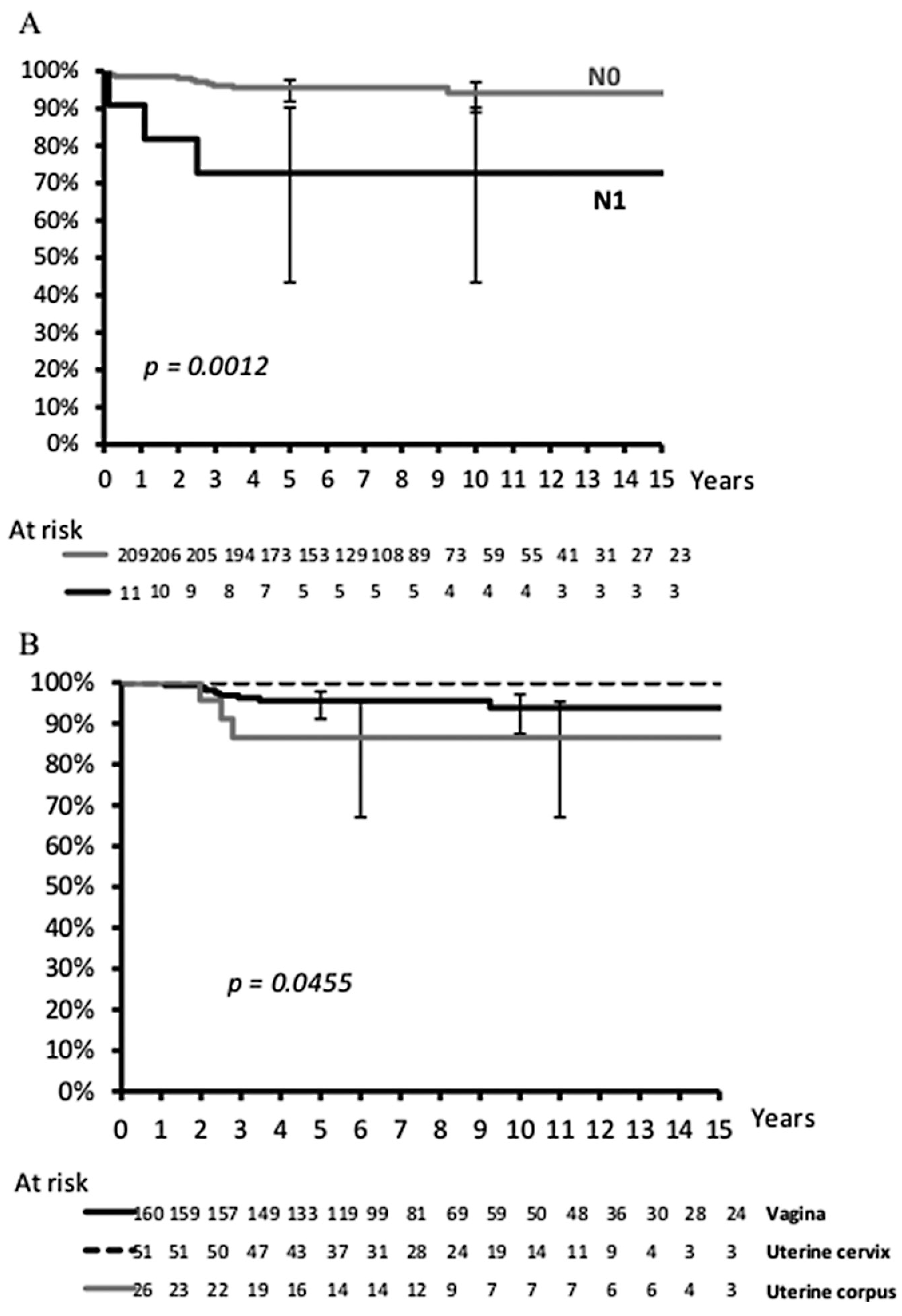
Overall survival according to lymph node status (A) and primary site (B)
4. DISCUSSION
Females with vaginal/uterine (VU) RMS have an excellent prognosis. The results of this analysis of 237 patients with localized VURMS show a 10-year survival of 92%. While EFS was slightly decreased in patients without initial RT, there was no difference in OS. Only uterine primary tumor site and regional lymph node involvement were associated with inferior OS. Importantly, age and tumor size were not prognostic. Overall, about half of the patients with VU RMS were cured without systematic RT or radical surgery. Moreover, in patients who achieved CR with chemotherapy with or without conservative surgery, the chance of prolonged local remission remained high (73%).
The risk of local failure after chemotherapy with or without surgery varied with primary site. RMS of the cervix, characterized by older age at diagnosis, had the highest local tumor control rate (86%), perhaps due to the feasibility of conservative but complete surgical resection, whereas RMS of the uterus and vagina had lower rates of definitive local control (~70%) after primary therapy. Moreover, primary site was an important factor influencing the requirement for radical surgery with about half of those with RMS of the uterus having hysterectomy (7/12 hysterectomy as initial surgery) while only a minority (16% and 12%) of vagina and uterus cervix RMS had radical surgery. Additionally, the tumor site also influenced the use of RT with 6%, 22%, and 35% of patients with RMS of the cervix, uterus, and vagina, respectively, receiving RT as part of primary treatment. Finally, although the primary site did not influence EFS, the chance of curing RMS of the uterus after relapse was lower when compared with the other tumor locations (OS: 87% for RMS of the uterus vs. 94% and 100% for RMS of the vagina or cervix, respectively, P = 0.05). Thus, primary tumor site influenced not only the outcome but also the burden of therapy.
COG recently reported21 an increased risk of recurrence with the reduction of cyclophosphamide dose for patients with subset 2 low-risk RMS, including group III female VU, analyzed in the present workshop. Indeed, therapy on ARST0331 for group III VU RMS included four cycles of VAC (total cumulative cyclophosphamide dose of 4.8 g/m2) followed by 12 cycles of VA over 46 weeks. Patients with group II or III tumors received RT, except for females with group III vaginal RMS enrolled from 2004 to 2009 who achieved a CR with chemotherapy with or without surgical resection. The 3-year failure-free survival rate was 57% for females with VU embryonal RMS (21 patients) and 77% for all other patients (45 patients) (P = 0.02). With a median follow-up of 3.5 years, there were eight recurrences among 15 females (53.3%) with group III genital tract tumors who did not receive RT on ARST0331 (total cumulative cyclophosphamide 4.8 g/m2). In contrast with a median follow-up of 5.6 years, there were two recurrences among 14 females (14.2%) with group III genital tract tumors who did not receive RT on D9602 (total cumulative cyclophosphamide 28.6 g/m2).3 Thus, although the numbers are small, the elimination of RT after CR with chemotherapy with or without surgery, in combination with reduced cyclophosphamide, likely contributed to the suboptimal tumor local control. In addition, the omission of RT in females treated in the European groups who achieved CR and received a total ifosfamide dose of at least 24 g/m2 (inthemorerecent European trials [MMT95, ICG RMS96, and EpSSG RMS2005], the total ifosfamide dose varied from 36 to 54 g/m2) was associated with a limited rate of local failure in this series (19/105; 18%). Although the cyclophosphamide equivalent dose22 of ifosfamide is not determined in terms of drug efficacy in RMS, it appears that MMT, ICG, and EpSSG trials used higher doses of alkylating agents than the COG ARST0331 trial.
The incidence and management of long-term consequences of treatment are a constant challenge in childhood cancers. In VU RMS, along with the well-known effect of RT, major long-term complications of local therapy can potentially impair genitourinary and digestive functions, sexuality, and fertility, and may have psychological or quality-of-life consequences. When RT is indicated, BT has the advantage of affecting a smaller tissue volume than EBRT, thereby restricting the amount of healthy tissue affected and potentially reducing later growth and functional impairment. Moreover, BT can also be regarded as an alternative to radical surgery when vaginectomy might be needed and permits the administration of higher doses of RT up to 60–65 Gy.20,23 In a recent study, Levy et al.24 reported the long term toxicity of BT in 42 females treated for childhood genital tract tumors. The 15-year actuarial incidence rate of grade 3–4 late effects was 51%; the most common late toxicities of any grade were gynecological (47%) and the most common of all grade 3–4 toxicities affected the urinary tract (53%). However, long-term effects decreased very significantly with the treatment period and the BT volume. Indeed, before 1990, the target BT volume encompassed the initial tumor extent while after 1990, only the residual tumor was irradiated. In the 14 patients who completed the item on sexual function, sexual activity was regular for 12 patients. In 34 adults at the time of the evaluation, four patients had given birth to a child.
By comparison, in a retrospective series of childhood pelvic RMS treated between 1962 and 1996, Spunt et al.25 described late effects in 24/26 patients (23 of whom had grade 3/4 late effects). Endocrine (77%) and gastrointestinal (69%) were the most frequent complications. The majority of the patients (73%) received external radiotherapy, which was correlated with the rate of toxicity. The use of proton therapy may decrease the incidence of long-term complications. A systematic review on quality of life of patients treated with protons (including adults) showed favorable results, especially in pediatric cancers.26 Leiser et al. assessed quality of life in children treated with protons for RMS. None of urogenital RMS presented ≥= grade 3 toxicity. However, only 10 patients with urogenital RMS were included in the study with a median follow-up of 55 months.27
Finally, in the current series of 237 VU RMS, 52% of irradiated patients had BT with a good local control, with only two local failures out of 28 females who underwent BT only during initial therapy. Additionally, BT appears to be an effective approach at relapse with 4/5 females treated with second-line BT achieving prolonged second CR.
The results of this study confirm the strong possibility of cure for females with RMS of the genital tract with an approach designed to limit the use of local therapy. The challenge for the future must be to identify the characteristics shown by patients who can be safely treated in this manner without a significant risk of relapse, and to ensure that RT is delivered to the remaining patients in a manner that is both effective and offers the lowest risk of severe late sequelae.28
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to all children and parents that participated in the trials, and all the pathologists and investigators.
Grant sponsor: National Cancer Institute, Grant numbers:U10CA098543, U10CA098413, Grant sponsor:PHRC-K (2004)
Abbreviations:
- BT
brachytherapy
- COG
Children's Oncology Group
- CR
complete remission
- EBRT
external-beam radiotherapy
- EFS
event-free survival
- EpSSG
European pediatric Soft Tissue Sarcoma Group
- ICG
Italian Cooperative Soft Tissue Sarcoma Group
- MMT
Mesenchymal Tumor Group
- OS
overall survival
- RMS
rhabdomyosarcoma
- RT
radiation therapy
- VU
vaginal/uterine
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Kirsch CH, Goodman M, Esiashvili N. Outcome of female pediatric patients diagnosed with genital tract rhabdomyosarcoma based on analysis of cases registered in SEER database between 1973 and 2006. Am J Clin Oncol. 2014;37:47–50. [DOI] [PubMed] [Google Scholar]
- 2.Martelli H, Oberlin O, Rey A, et al. Conservative treatment for females with nonmetastatic rhabdomyosarcoma of the genital tract: a report from the Study Committee of the International Society of Pediatric Oncology. J Clin Oncol. 1999;17:2117–2122. [DOI] [PubMed] [Google Scholar]
- 3.Walterhouse DO, Meza JL, Breneman JC, et al. Local control and outcome in children with localized vaginal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma committee of the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arndt CA, Donaldson SS, Anderson JR, et al. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract?. Cancer. 2001;91:2454–2468. [PubMed] [Google Scholar]
- 5.Gerbaulet AP, Esche BA, Haie CM, Castaigne D, Flamant F, Chassagne D. Conservative treatment for lower gynecological tract malignancies in children and adolescents: the Institut Gustave-Roussy experience. Int J Radiat Oncol Biol Phys. 1989;17:655–658. [DOI] [PubMed] [Google Scholar]
- 6.Flamant F, Gerbaulet A, Nihoul-Fekete C, Valteau-Couanet D, Chassagne D, Lemerle J. Long-term sequelae of conservative treatment by surgery, brachytherapy, and chemotherapy for vulval and vaginal rhabdomyosarcoma in children. J Clin Oncol. 1990;8:1847–1853. [DOI] [PubMed] [Google Scholar]
- 7.Corpron CA, Andrassy RJ, Hays DM, et al. Conservative management of uterine pediatric rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study III and IV pilot. J Pediatr Surg. 1995;30:942–944. [DOI] [PubMed] [Google Scholar]
- 8.Nasioudis D, Alevizakos M, Chapman-Davis E, Witkin SS, Holcomb K. Rhabdomyosarcoma of the lower female genital tract: an analysis of 144 cases. Arch Gynecol Obstet. 2017;296:327–334. [DOI] [PubMed] [Google Scholar]
- 9.Crist W, Gehan EA, Ragab AH, et al. The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. [DOI] [PubMed] [Google Scholar]
- 10.Arndt CAS, Hawkins DS, Meyer WH, Sencer SF, Neglia JP, Anderson JR. Comparison of results of a pilot study of alternating vincristine/doxorubicin/cyclophosphamide and etoposide/ifosfamide with IRS-IV in intermediate risk rhabdomyosarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;50:33–36. [DOI] [PubMed] [Google Scholar]
- 11.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. [DOI] [PubMed] [Google Scholar]
- 12.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group Study D9803. J Clin Oncol. 2009;27:5182–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2014;32:3547–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flamant F, Rodary C, Rey A, et al. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence. Results of the second study of the International Society of Pediatric Oncology: MMT84. Eur J Cancer. 1998;34:1050–1062. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MCG, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Pediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. [DOI] [PubMed] [Google Scholar]
- 17.Oberlin O, Rey A, Sanchez de Toledo J, et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: long-term results from the International Society of Pediatric Oncology MMT95 study. J Clin Oncol. 2012;30:2457–2465. [DOI] [PubMed] [Google Scholar]
- 18.Bisogno G, De Rossi C, Gamboa Y, et al. Improved survival for children with parameningeal rhabdomyosarcoma: results from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008;50:1154–1158. [DOI] [PubMed] [Google Scholar]
- 19.Bisogno G, De Salvo GL, Bergeron C, et al. The role of doxorubicin in the treatment of rhabdomyosarcoma: preliminary results from the EPSSG RMS2005 randomized trial. Pediatr Blood Cancer. 2014;61:S133–S134. [Google Scholar]
- 20.Magné N, Oberlin O, Martelli H, Gerbaulet A, Chassagne D, Haie-Meder C. Vulval and vaginal rhabdomyosarcoma in children: update and reappraisal of Institut Gustave Roussy brachytherapy experience. Int J Radiat Oncol Biol Phys. 2008;72:878–883. [DOI] [PubMed] [Google Scholar]
- 21.Walterhouse DO, Pappo AS, Meza JL, et al. Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Cancer. 2017;123:2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magné N, Haie-Meder C. Brachytherapy for genital-tract rhabdomyosarcomas in females: technical aspects, reports, and perspectives. Lancet Oncol. 2007;8:725–729. [DOI] [PubMed] [Google Scholar]
- 24.Levy A, Martelli H, Fayech C, et al. Late toxicity of brachytherapy after female genital tract tumors treated during childhood: prospective evaluation with a long-term follow-up. Radiother Oncol. 2015;117:206–212. [DOI] [PubMed] [Google Scholar]
- 25.Spunt SL, Sweeney TA, Hudson MM, Billups CA, Krasin MJ, Hester AL. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005;23:7143–7151. [DOI] [PubMed] [Google Scholar]
- 26.Verma V, Simone CB, Mishra MV. Quality of life and patient-reported outcomes following proton radiation therapy: a systematic review. J Natl Cancer Inst. 2018;110:djx208. 10.1093/jnci/djx208. [DOI] [PubMed] [Google Scholar]
- 27.Leiser D, Calaminus G, Malyapa R, et al. Tumour control and quality of life in children with rhabdomyosarcoma treated with pencil beam scaning proton therapy. Radiother Oncol. 2016;120:163–168. [DOI] [PubMed] [Google Scholar]
- 28.Oberlin O, Rey A, Anderson JA, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment—results of an international workshop. J Clin Oncol. 2001;19:197–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


