Abstract
Systemic and organ-confined inflammation has been associated with cancer development and progression. Resistin, initially described as an adipocyte-derived cytokine in mice, is mostly expressed by the macrophages in humans. It has potent pro-inflammatory properties, and its elevated serum levels are detected in cancer patients. Aberrant expression of resistin receptors is also reported in several malignancies and associated with aggressive clinicopathological features. Several lines of evidence demonstrate that resistin, acting through its different receptors, promotes tumor growth, metastasis, and chemoresistance by influencing a variety of cellular phenotypes as well as by modulating the tumor microenvironment. Racially disparate expression of resistin has also attracted much interest, considering prevalent cancer health disparities. This review discusses the aberrant expression of resistin and its receptors, its diverse downstream signaling and impact on tumor growth, metastasis, angiogenesis, and therapy resistance to support its clinical exploitation in biomarker and therapeutic development.
Keywords: Resistin, inflammation, cancer, cytokine, tumor microenvironment
1. Introduction
Cancer remains the second most common cause of death in the United States and worldwide [1, 2]. According to an estimate by the American Cancer Society, about 1,806,950 new cancer cases and nearly 606,520 cancer-related deaths are projected for this year in the United States [3]. Cancer is a complex disease driven through a variety of mechanisms. In many cases, its asymptomatic progression results in the late diagnosis and limiting therapeutic options. Further, the genetically advanced nature of cancer cells and intra-tumoral heterogeneity lead to the failure of most therapies. These challenges enforce the need for a better understanding of tumor biology and characterization of associated molecular factors that drive cancer development, aggressive progression, and therapeutic resistance.
Inflammation has been intricately linked with cancer either as a cause or consequence [4, 5]. An inflammatory milieu supports sustenance and accumulation of genomic aberrations and thus the initiation and progression of cancer [6, 7]. In general, inflammation is a protective arm of the immune system that protects us from external threats and helps in the healing of the damaged tissue. However, unresolved chronic inflammation, induced by abnormal immune reactions, infections that perpetuate, or conditions such as obesity, can support the process of carcinogenesis. The relevant epidemiological and clinical data exists to suggest that chronic inflammation leads to the development of cancer and is associated with aggravated clinicopathological features [7–9]. Indeed, about 25% of all cancers are etiologically linked to chronic inflammation and infection [10]. Obesity has also been associated with several types of cancer [11, 12]. Excessive accumulation of macronutrients in the adipose tissues causes the release of inflammatory mediators and thus creates a pro-inflammatory tumor supportive environment. Similarly, Helicobacter pylori infection induces pro-inflammatory gene expression leading to chronic gastritis and peptic ulceration and promotes gastric carcinogenesis [13]. Chronic inflammation is mediated through a variety of cytokines and hormones, which are also implicated in cell proliferation, apoptosis resistance, invasion, angiogenesis, and metastasis [10]. Indeed, tumor microenvironment (TME) contains a variety of non-tumor cells, including the immune cells (resident and infiltrated) that support tumor development by producing growth factors and cytokines [14–16].
In recent years, several reports have published that describe multi-faceted roles of resistin influencing almost all the critical hallmarks of cancer. Resistin is an inflammatory cytokine mostly expressed by the macrophages in humans, although it was initially described as an adipocyte-derived cytokine in mice [17]. It has potent pro-inflammatory properties and is shown to promote cancer progression either directly or indirectly [18–21]. Elevated levels of resistin were observed in the serum of obese individuals [22, 23]. Also, a considerable amount of (experimental and epidemiological) evidence suggests an association between increased resistin levels and obesity-associated malignancies [18, 24]. However, the mechanistic link of this association is not clearly understood. In this review article, we discuss the aberrant expression of resistin and its receptors in cancer, diverse downstream signaling of resistin and its impact on tumor growth, survival, metastasis, angiogenesis, and therapy resistance in multiple malignancies.
2. Resistin and its downstream signaling
Resistin is a member of the resistin-like molecule family (RELMs) that includes three additional proteins, RELM-α, RELM-β, RELM-y [25]. The name ‘resistin’ was introduced in 2001 to describe a novel adipocyte-derived molecule that resisted insulin action and promoted glucose tolerance in rodents [26]. In another more recent study, resistin was associated with lung inflammation and named as ‘Found in Inflammatory Zone 3’ (FIZZ3) protein [27, 28]. Resistin is almost exclusively expressed in white adipocytes in mice, whereas macrophages are the primary source in humans [29, 30]. The gene encoding for resistin is localized on chromosome19p13.3 and 8A1 in humans and mice, respectively [31]. The genomic organization is more or less conserved between the two species except for an extra intron of 2279 nucleotides in mice following the stop codon. This intronic region contains several regulatory sites that could have a possible role in the different expression patterns of both the resistin [32]. Resistin shares 59 % homology at the protein level between human and mouse forms [17, 32]. Mouse resistin is an 11 kilo Dalton (kDa) polypeptide, which is synthesized as a 114 amino acid (aa) precursor consisting of a 20 aa signal sequence and a 94 aa mature segment [31]. On the other hand, human resistin is a 12.5 kDa polypeptide with 108 aa mature segment [31]. Resistin contains a 10–11-cysteine-rich motif at the carboxyl-terminal (C-terminal) in both mice and humans that promotes the assembly of the globular domain through the formation of disulfide bonds [33]. This C-terminal globular head domain is the receptor-binding region, which is linked with the N-terminal or tail domain by a flexible neck domain [33, 34] (Figure 1).
Figure 1.
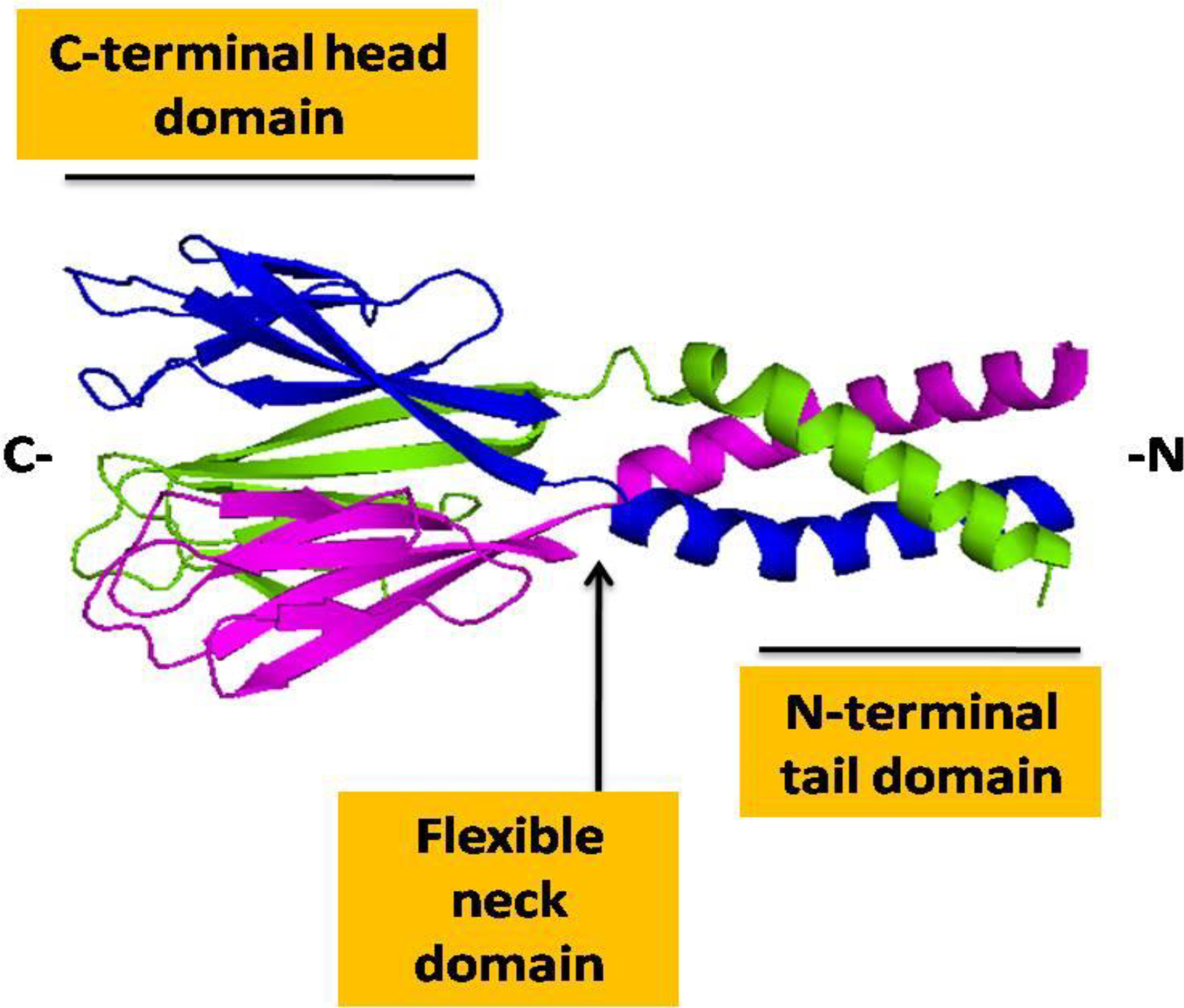
Structure of human resistin: Cartoon view of trimeric structure of resistin showing C-terminal globular domain containing antiparallel β-sheets and N-terminal α-helical domain linked by a flexible neck domain. The structure was predicted using the SWIS-MODEL program (https://swissmodel.expasy.org) [37] and drawn using PyMoL software.
To date, four distinct functional receptors have been identified for resistin, namely adenylyl cyclase-associated protein 1 (CAP1), toll-like receptor 4 (TLR4), an isoform of decorin (ΔDCN), and receptor tyrosine kinase-like orphan receptor 1 (ROR1). Resistin exhibits differential binding affinity towards these receptors leading to the activation of diverse cell signaling pathways (Figure 2). CAP1 and TLR4 have been the most studied resistin receptors. CAP1 is an actin-binding protein consisting of 475 aa with a carboxy-terminal domain that binds to actin, a central proline-rich domain, and an amino-terminal domain of the unknown function [35, 36]. CAP1 was first identified as a resistin receptor in human monocytes, where it mediated inflammatory actions [34].
Figure 2. Resistin signaling pathways in cancer.

Resistin binding to the transmembrane Toll-like receptor 4 (TLR4) and adenylyl cyclase-associated protein 1 (CAP1) receptors activates several signaling pathways leading to the cancer cell proliferation, invasion, migration, angiogenesis, apoptosis inhibition, and therapy resistance. Akt: Protein Kinase B; cAMP: Cyclic adenosine 3′,5′-monophosphate; EGFR: Epidermal growth factor receptor; ERK: extracellular-signal-regulated kinase; IRAK: Interleukin-1 receptor-associated kinase; IRF: Interferon Regulatory Factor; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; mTOR: mammalian target of rapamycin; MyD88: Myeloid differentiation primary response 88; NF-κβ: nuclear factor κβ; PKA: Protein kinase A; PKC: Protein kinase C; PI3K: phosphoinositide 3-kinase; p70 S6K: 70-kDa ribosomal protein S6 kinase; ROR1: Receptor Tyrosine Kinase Like Orphan Receptor; SP-1: specificity protein-1(transcription factor); SRC: Proto-oncogene tyrosine-protein kinase; STAT 3: transducer and activator of transcription 3; TIRAP: TIR Domain Containing Adaptor Protein; TRAF6:TNF receptor-associated factor 6; TRAM: TRIF-related adaptor molecule; TRIF: TIR-domain-containing adapter-inducing interferon-β.
Binding of resistin to the CAP1 upregulated intracellular cAMP concentration, enhanced Protein Kinase A (PKA) activity, and promoted NF-κβ -mediated transcription of inflammatory cytokines (IL-6, TNF-α, and IL-1B). Another study in mouse liver cells demonstrated that CAP1 mediated resistin-induced expression of genes involved in insulin resistance, inflammation, and apoptosis [38]. We also showed that resistin promoted the growth, aggressiveness, and stemness of breast cancer (BC) cells through overexpression and activation of transducer and activator of transcription 3 (STAT3) [39, 40]. Interaction of TLR4 with resistin was first reported in a human epithelial kidney cell line where it mediated the pro-inflammatory effects [41]. Resistin-TLR4 interaction induced structural changes in rat hypothalamus leading to the activation of JNK and P38 pathways via adaptor protein, myeloid differentiating factor 88 (MyD88), and Toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP) [42]. Further, resistin-TLR4 interaction activated porcine alveolar macrophages via the TLR4/NF-κβ pathway [43]. It also induced STAT3 and NF-κβ signaling in BC cells leading to the acquisition of epithelial-to-mesenchymal transition (EMT) and stemness [19].
Decorin is an extracellular-matrix protein that belongs to the small leucine-rich proteoglycan (SLRP) family and is involved in cell adhesion, migration, and proliferation [44, 45]. Murine resistin is shown to interact with an isoform of decorin (ΔDAC) expressed on the adipose progenitor cells [45]. Binding of resistin to decorin induced cell proliferation and migration, leading to the expansion of white adipose tissue [45]. Receptor Tyrosine Kinase Like Orphan Receptor (ROR1) belongs to a family of tyrosine kinase receptors that are important for cell division, proliferation, angiogenesis, migration, and survival [46, 47]. The ROR1 was shown to interact with murine resistin in 3T3-L1 preadipocytes leading to the activation of extracellular signal-regulated kinases 1 and 2 (ERK 1/2). It also upregulated the suppressor of cytokine signaling 3 (SOCS3) and glucose transporter 4 to promote adipogenesis and glucose uptake [46]. However, in humans, the expression of ROR1 and decorin is rarely observed in mononuclear and macrophage cells [34].
3. Aberrant expression and regulation of resistin and its receptors in cancer
Aberrant expression of resistin and resistin receptors has been reported in multiple malignancies. More importantly, it was demonstrated that resistin levels are disparately higher in African American (AA) BCpatients, suggesting its role in racially disparate clinical outcomes [39]. An earlier study also reported resistin to be one of the differentially-expressed genes in breast tumors from AA and Caucasian American (CA) patients [48]. Elevated serum levels of resistin were also reported in stage II or III BC patients treated with chemotherapy [49]. In another study, a high level of resistin in BC tissues correlated with aggressive clinicopathological features and poor survival of patients [50]. Analysis of the TCGA database found elevated expression of resistin transcripts in the estrogen receptor (ER) negative BC subtype in AA women [51]. Higher expression of resistin is also reported in lung adenocarcinoma compared to the normal lung tissues [52]. In another study, enhanced immunostaining of resistin was observed in the marginal areas of human lung cancer (LC) tissue sections [53]. Increased serum levels of resistin are reported in endometrial cancer patients as well [54]. Elevated level of resistin has been reported among other adipocytokines and inflammatory cytokines in obese people and associated with increased risk of colorectal cancer (CRC) [55, 56]. In other studies, patients with CRC exhibited higher levels of circulating resistin as compared to the control subjects [57, 58]. Apart from the resistin, increased levels of its receptor, CAP1 was also reported in CRC and suggested to be involved in the pathogenic process via interaction with resistin [59]. Overexpression of CAP1 is also associated with disease progression in BC patients [60, 61].
Enhanced expression of CAP1 in pancreatic cancers (PC) was suggested to be involved in the aggressive behavior of (PC) cells [62]. CAP1 overexpression was also found to be associated with Hepatocellular carcinoma (HCC) metastasis and poor prognosis [63]. The upregulation of the CAP1 level was also reported in invasive esophageal squamous cell carcinoma (ESCCs) and was associated with lymph node metastasis and patient’s survival [64]. BC tumor tissue exhibited significantly higher expression of TLR4 [65]. Importantly, the expression of TLR4 was associated with BC metastasis [65]. Yang et al. showed that TLR4 expression is vital for BC cell proliferation [66]. Furthermore, knockdown of TLR4 leads to the inhibition of proliferation and survival of BC cells [66]. Increased expression of TLR4 at mRNA and protein levels was also reported in LC tissue [67]. High expression of TLR4 and MyD88 has been associated with liver metastasis, and association of both was also considered to an independent predictor of poor survival in CRC patients [68]. The aberrant expression of another receptor of resistin, decorin, was also shown to drive the progression of oral epithelial cells to carcinoma [69]. Also, the enhanced expression of ROR1 in BC was found to be associated with EMT, metastasis, and increased rates of relapse [70]. It was also shown that there is an increased expression of ROR1 following treatment with chemotherapy [71]. Importantly, higher expression of ROR1 was also associated with BC stemness [71]. Moreover, exposure of HER2+ BC cells to T-DM1 induced ROR1 expression that was associated with increased BC stem cell phenotypes [72]. Furthermore, high ROR1 levels were shown to promote leukemia cell survival and accelerate disease progression [73]. Altogether, these evidences showed that aberrant expression of resistin receptors could lead to the progression of cancer; however, extensive studies focusing on resistin and its receptor interaction should be investigated to establish a broader and more precise clinical relevance of resistin signaling in cancer.
4. Role of resistin in cancer
The development of cancer to metastatic disease is a multi-step process. There are several hallmarks of cancer, including the ability to sustain proliferation, resist apoptosis, avoid immune destruction, unlimited replicative potential, tumor-promoting inflammation, angiogenesis induction, activation of invasion and metastasis, genomic instability and deregulated cellular energetics [74–78]. Numerous lines of evidence suggest that resistin plays a significant role in the pathogenesis of different types of malignancies by impacting on these cancer hallmarks (Figure 3). Also, the role of resistin has been suggested in therapeutic resistance as well. Below we discuss these findings in detail.
Figure 3. Role of resistin in cancer.
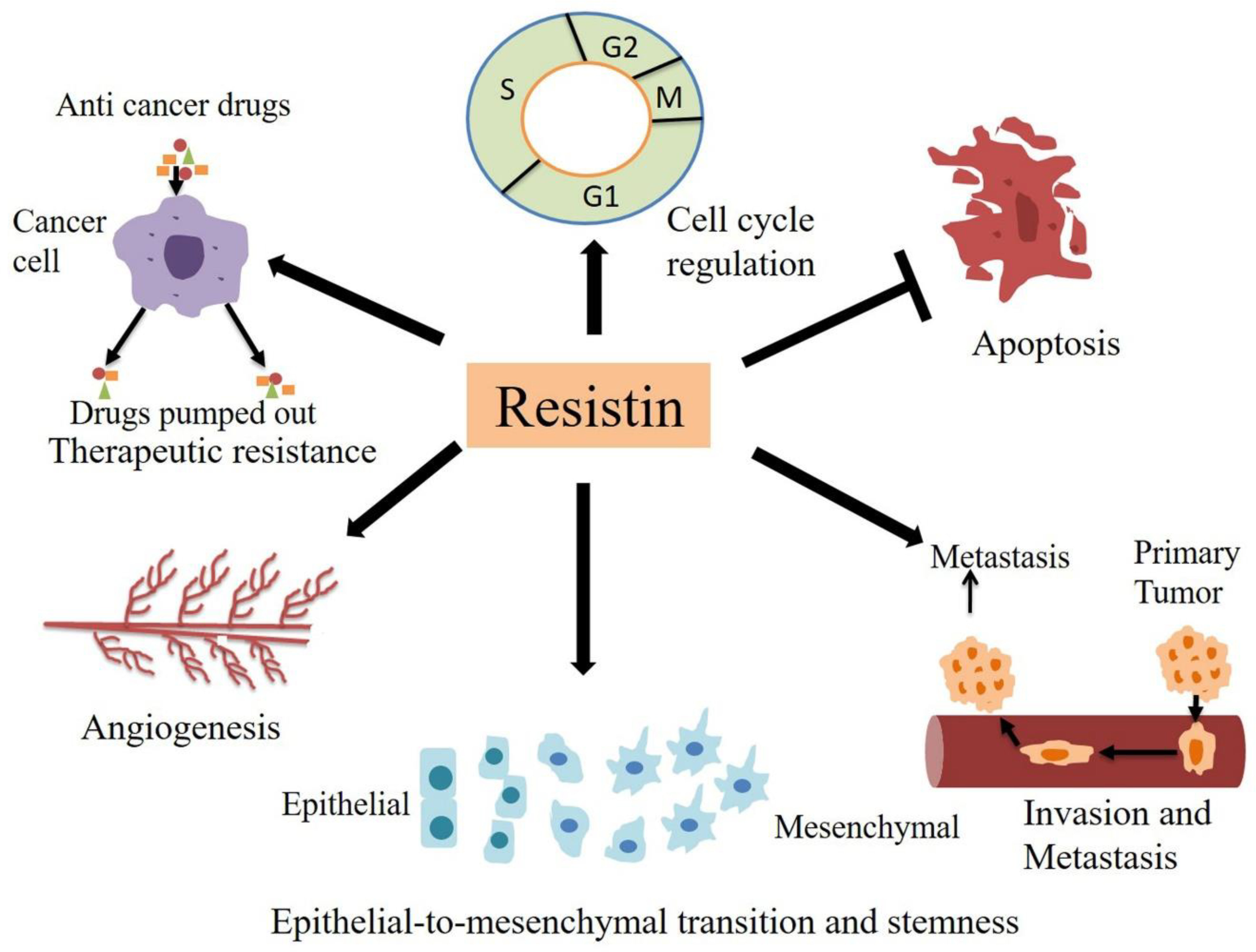
Inflammatory cytokine ‘resistin’ influence the pathobiology of cancer by inducing invasion, metastasis, epithelial to mesenchymal transition and stemness, angiogenesis, therapy resistance, and regulating cell cycle progression and apoptosis.
4.1. Cell cycle regulation and apoptosis-resistance
The cell cycle is a tightly controlled process that consists of the interphase and the mitotic phase. During interphase, cells prepare themselves for the cell division (the mitotic phase), and it is further subdivided into Gap 1(G1), Synthesis (S), and Gap 2 (G2) phases. The cellular transition from one phase to another is tightly regulated by cyclins and cyclin-dependent protein kinase (Cdks), whose expression is controlled by several growth-promoting and suppressive proteins, mostly transcription factors or their co-factors [79–87]. It appears that resistin may affect the cell cycle in a context-dependent manner. One study shows that resistin activates ERK via the activation of the TLR4-MyD88-dependent pathway. Activation of ERK promotes the upregulation of SOCS 3 that downregulates the JAK2/STAT3 pathway leading to the arrest of cells in the G1 phase. The arrest of resistin-exposed cells in the G1 phase helped them survive 5-fluorouracil treatment due to a decrease in drug uptake [88]. These findings are intriguing because many other reports show a growth-promoting action of resistin on cancer cells [39, 89, 90]. Resistin promoted prostate cancer cell proliferation through the activation of PI3K/Akt signaling [91]. Akt pathway plays a significant role in inhibiting cell cycle arrest by phosphorylating p21[92].
Apoptosis, also known as “programmed cell death,” is another growth regulatory process that maintains cellular homeostasis by getting rid of aging cells during the process of development [93]. Apoptosis is also induced when cells experience cellular or genetic damage under a disease condition to keep a check on the propagation of defective cells [94]. This regulatory process is lost or weakened in all cancer cells because of genomic or aberrant activation of various survival signaling pathways [95, 96]. In several studies, resistin has been implicated in the activation of the anti-apoptotic pathways and supports tumor cell survival. In myeloma cells, resistin upregulated NF-κB and PI3K/Akt pathways to reduce the apoptosis [97]. This study also showed that resistin enhanced the expression and drug efflux function of ATP-binding cassette (ABC) transporters via decreasing the expression and methylation levels of both DNA methyltransferases, DNMT1, DNMT3a and ABC gene promoters leading to the inhibition of chemotherapy-induced apoptosis. Overexpression of resistin in LC cells is also shown to confer apoptosis resistance as a decrease in the apoptosis-associated proteins caspase-3, and caspase-7 expression [52]. Activation of the MAPK/ERK, JAK/STAT, and Akt/PI3K signaling pathways by resistin led to apoptosis inhibition in porcine ovarian cells [98]. This study further showed that resistin decreased the expression of proapoptotic genes FADD, FAS, and CASP8 in ovarian cells. Overall, these findings suggest that resistin promotes the growth of cancer cells by impacting both cell cycle and apoptotic processes through a variety of mechanisms.
4.2. Cell invasion and metastasis
The gain of a motile and invasive phenotype is essential for a malignant cell to grow optimally at the primary site as well as metastasize to the distant locations via facilitating their intravasation and extravasation [99, 100]. Resistin is shown to promote the invasion and metastasis of lung adenocarcinoma cells through mechanisms involving the activation of TLR4/Src/EGFR/PI3K/NF-κβ signaling pathway [101]. Resistin also activates the ezrin, radixin, and moesin (ERM) protein to promote migration and invasion of BC cells [102]. Activation of ERM proteins occurred via calcium-mediated Src/PP2A/PKCα signaling that also enhanced the expression of vimentin, a protein associated with the gain of malignant phenotype [102]. In our earlier lab study, we found that resistin promoted the migration and invasion of BC cells by enhancing the expression and phosphorylation of STAT3 [39]. We further found that resistin promoted IL-6 expression and secretion that was involved in resistin-induced STAT3 phosphorylation. Resistin is shown to elicit the expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) via NF-κB pathway in hepatic carcinoma cells to facilitate their adhesion to endothelium and subsequent metastasis [103]. In CRC, resistin treatment increased the adhesion of cancer cells to endothelial cells via the activation of the NF-κB pathway and the expression of ICAM-1A and VCAM-1 [104]. Resistin also increased invasion in multiple liver cancer cell lines by promoting the phosphorylation of Akt and ERK signaling pathway [105]. The invasive potential of ovarian cancer (OC) cells was also increased by resistin through the inhibition of tumor suppressor miRNAs, including, let-7a, miR-200c, and miR-186 [89]. Rosendahl et al. demonstrated that adipocyte secretome promotes BC cell proliferation and motility [106]. Besides, resistin was directly associated with the differentiation of osteoclasts, which played an important role in LC bone metastasis [53]. Moreover, resistin upregulated the expression of TWIST that is known to promote the metastasis of LC cells [107, 108]. Furthermore, binding of resistin to CAP1 or TLR4 led to STAT3 activation and induced migration and invasion of (PC) cells [20]. Altogether, significant data support the role of resistin mediated signaling in tumor cell invasion and metastasis.
4.3. Epithelial-to-mesenchymal transition (EMT) and cancer stemness
Elizabeth Hay postulated the concept of EMT more than 50 years ago [109]. During EMT, epithelial cells transition into a mesenchymal phenotype through a series of biochemical and molecular changes, and this process is often associated with the gain of invasiveness and stemness properties [110, 111]. Acquisition of stem cell-like properties by cancer cells is also associated with their ability to sustain under stressed environmental conditions [112–114]. EMT and cancer stemness potential are regulated by the interplay of several signaling pathways culminating into the altered expression and activation of gene regulators, including transcription factors and miRNAs [115–119]. Activation of TGF-β, NOTCH, Hedgehog pathway, and Wnt pathways serve as a biochemical switch for EMT and gain of cancer stemness properties [119]. Their effect is mediated through a variety of transcription factors, including Zeb, Snail, and Twist, that eventually alter the expression of cadherin proteins and other proteins involved in cellular polarization [119]. We recently demonstrated that treatment of BC cells with resistin led to the activation of STAT3 as well as the overexpression of other stemness associated transcription factors Nanog and KLF4 [40]. In another study, the interaction of resistin with TLR4 was shown to mediate the activation of STAT3 and NF-κβ in BC cells leading to the induction of EMT and stemness phenotype [19]. Resistin has been reported to upregulate other transcription factor proteins involved in EMT and cancer stemness, including SNAIL, SLUG, ZEB1, and TWIST1, that alter the expression of fibronectin, vimentin, E-cadherin and claudin-1 [120]. Resistin also promotes EMT and stemness in OC cells through a miRNA-mediated mechanism [89].
4.4. Angiogenesis
Angiogenesis is the process of formation of new blood vessels from the existing ones. It is a complicated process that requires extracellular matrix degradation, increase vascular permeability, proliferation, migration, and invasion of endothelial cells, followed by the formation of new vessels [121, 122]. Angiogenesis is an essential requirement for optimal cancer growth. As the tumor grows, its demand for nutrients and oxygen increases, and thus cancer cells secrete factors to stimulate the process of angiogenesis [122, 123]. Inflammatory TME can further promote this process. Resistin was shown to induce the expression of VEGF and MMP2 in human ovarian epithelial cancer cell lines to promote angiogenesis [124]. Resistin induced the expression of VEGF-C and downregulated miR-186 in chondrosarcoma to promote lymphangiogenesis [125]. In another study, resistin promoted VEGF-A production in chondrosarcoma cells by decreasing miR-16–5p via PI3K/Akt-dependent signaling pathway to induce proliferation of endothelial progenitor cells (EPCs) [126]. Resistin is also reported to induce angiogenesis by promoting human coronary artery endothelial cells (HCAECs) proliferation, migration, and capillary-like tube formation, upregulating the expression of vascular endothelial growth factor receptors (VEGFRs) and matrix metalloproteinases (MMPs) and activating ERK1/2 and p38 pathways [127]. Also, treatment of human umbilical vein endothelial cells (HUVECs) with resistin led to the induction of VEGF production and increased endothelial cell tube formation in an in vitro model [128].
4.5. Therapeutic resistance
Therapeutic-resistance is a significant cause of cancer-associated mortality. A plethora of literature now suggests that TME plays a vital role in cancer growth and therapeutic-resistance and thus needs to be exploited [78, 129–131]. Resistin increased the expression and drug efflux function of ABC transporters in myeloma by relieving its epigenetic repression via DNA methyltransferases (DNMT1 and DNMT3a) [97]. Overexpression of ATP-binding cassette (ABC) transporters has been associated with inherent and acquired multidrug resistance in several cancers [132–134]. Resistin was also shown to upregulate the levels of fatty acid synthase (FASN), caveolin (Cav)-1, and P-glycoprotein (P-gp) in myeloma that associated with resistance to dacarbazine (DTIC) therapy [90]. Resistin also induces the expression of stemness-associated factors, KLF4, and Nanog, which have been implicated in doxorubicin resistance [40]. We also demonstrated that resistin treatment of BC cells reduced their sensitivity to doxorubicin in through STAT3 activation. Autophagy is a conserved process of self-destruction employed by cells to degrade and generate new organelles [135]. The induction of autophagy also enables cancer cells to resist drug-induced toxicity [135, 136]. Resistin was shown to induce autophagy in BC cells as a mechanism to survive during doxorubicin treatment [137]. Mechanistic studies revealed that the induction of autophagy by resistin was mediated through the activation of AMPK/mTOR/ULK1 and JNK signaling pathways. Resistin is also reported to confer cisplatin-resistance in OC cells by downregulating miRNAs let-7a, miR-200c, and miR-186 [89]. The role of resistin in the progression of several cancers is summarized in Table 1.
Table 1:
Role of resistin in different cancers
| Type of cancer | Effect | Mechanism of action | References |
|---|---|---|---|
| Breast | Invasion and Migration | Calcium-dependent c-Src and PKCα-ezrin pathway | [102] |
| Metastasis | Induction of EMT and stemness | [120] | |
| Doxorubicin-resistance | AMPK/mTOR/ULK1 and JNK signaling pathways | [137] | |
| Chemoresistance and stemness | STAT 3 pathway | [40] | |
| Progression | Induction of EMT and stemness by TLR4/NF-κB/S TAT3 signaling pathway | [19] | |
| Colorectal | CRC stage progression | Higher levels of resistin | [138] |
| G1 arrest of cells | ERK/ SOCS3/ JAK2/STAT3 pathway | [88] | |
| Inhibition to apoptosis by 5-fluorouracil | G1 arrest of cells | [88] | |
| Endothelial adhesion | NF-κβ pathway and expression of ICAM-1A and VCAM-1 | [104] | |
| Myeloma | Multidrug resistance | NF-κB and PI3K/Akt pathways and ABC transporter | [97] |
| Ovarian | Angiogenesis | PI3K/Akt-Sp1 pathway and VEGF expression | [124] |
| Invasion and Cisplatin-resistance | Induction of EMT and stemness by tumor suppressor miRNAs, let-7a, miR-200c, and miR-186 | [89] | |
| Gastric | SDF-1 expression | p38 MAPK and NF-κβ pathway | [139] |
| Prostate | Cell proliferation | PI3K/Akt signaling pathways | [91] |
| Liver | Adhesion to human umbilical vein endothelial cells (HUVECs) | NF-κB-regulated ICAM-1 and VCAM-1 expression | [103] |
| Pancreatic | Proliferation, migration, invasion and gemcitabine resistance | STAT3 signaling pathway | [20] |
| Lung | Development and metastasis | Expression of Wolf-Hirschhorn syndrome candidate 1 (WHSC1) histone methyltransferase and TWIST | [53] |
| Invasion and metastasis | TLR4/Src/EGFR/PI3K/NF-κβ pathway | [101] |
5. Conclusion and future perspectives
Resistin has emerged as an important inflammatory cytokine over the past two decades. A growing body of evidence also supports an association of resistin in cancer pathogenesis and therapeutic outcomes. Resistin not only enhances the growth and aggressiveness of cancer cells but is also involved in diminishing the therapeutic efficacy of several anti-cancer drugs. Higher circulating levels of resistin positively correlate with increased tumor stage, size, and lymph node metastasis in various cancer subtypes. Therefore, it would be interesting to see if resistin could be useful as a potential clinical biomarker in cancer. Since enhanced levels of resistin can be detected under various inflammatory conditions, including obesity, it may not alone be used as a specific diagnostic marker. However, it could still be useful in predicting the risk of cancer development, especially in people who are already genetically predisposed. Further, considering the role of resistin in cancer aggressiveness and therapy-resistance, its utility as a prognostic biomarker and therapeutic response predictor could be explored. Therapeutic targeting of resistin would also be challenging due to its interaction with multiple known receptor proteins and diverse impact on downstream signaling pathways. It is crucial that we develop a precise mechanistic understanding of resistin action in cancer cells and identify the protein that mediates or modify its effect on cancer phenotypes. It is also important that we characterize all the proteins that bind to resistin and can positively or negatively influence its downstream oncogenic signaling. More and more studies are needed to study the collective clinical significance of resistin and its known receptors in cancer. Overall, resistin and its interactors hold great significance in the clinical management of cancer. Inhibiting the effects of resistin on cancer cells, either by neutralizing antibody, antagonism of resistin receptor or by antisense oligonucleotides or RNA interference, could be useful therapeutic strategies against cancer. Targeting mechanisms regulating resistin and its receptors could also be useful in cancer prevention and therapy. Thus, biological understanding of resistin action and careful strategizing for the development of therapeutics targeting resistin and its receptor axis has enormous implications in the effective management of cancer.
Highlights.
Inflammation is intricately linked with cancer either as a cause or a consequence.
Resistin is a pro-inflammatory cytokine whose serum levels are elevated in cancer patients.
Aberrant expression of resistin receptors is also reported in cancer and associated with disease aggressiveness.
Resistin signaling plays multiple roles in cancer development and could be exploited for cancer prevention and therapy.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health/National Cancer Institute [CA204801, CA231925 (to S Singh) and CA185490, CA224306 (to AP Singh)] and the University of South Alabama Mitchell Cancer Institute.
Abbreviations:
- CAP1
adenylyl cyclase-associated protein 1
- DMT
DNA methyltransferases
- ER
estrogen receptor
- ERK
extracellular-signal-regulated kinase
- EMT
epithelial-to-mesenchymal transition
- ERM
ezrin, radixin, and moesin
- FIZZ3
found in Inflammatory Zone 3
- ICAM-1
intercellular adhesion molecule-1
- JNK
c-Jun N-terminal kinase
- MyD88
myeloid differentiating factor 88
- NF-κβ
nuclear factor κβ
- PKA
protein kinase A
- ROR1
receptor tyrosine kinase-like orphan receptor 1
- SLRP
small leucine-rich proteoglycan
- SOCS3
suppressor of cytokine signaling 3
- STAT3
transducer, and activator of transcription 3
- TIRAP
toll/interleukin-1 receptor domain-containing adaptor protein
- TLR4
toll-like receptor 4
- TME
tumor microenvironment
- VCAM-1
vascular cell adhesion molecule
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Heron M, Anderson RN, Changes in the Leading Cause of Death: Recent Patterns in Heart Disease and Cancer Mortality, NCHS Data Brief (254) (2016) 1–8. [PubMed] [Google Scholar]
- [2].G.B.D. Mortality, C. Causes of Death, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015, Lancet 388(10053) (2016) 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J Clin 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [4].Lu H, Ouyang W, Huang C, Inflammation, a key event in cancer development, Mol Cancer Res 4(4) (2006) 221–33. [DOI] [PubMed] [Google Scholar]
- [5].Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB, Inflammation and cancer, Ann Afr Med 18(3) (2019) 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grivennikov SI, Greten FR, Karin M, Immunity, inflammation, and cancer, Cell 140(6) (2010) 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coussens LM, Werb Z, Inflammation and cancer, Nature 420(6917) (2002) 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Philip M, Rowley DA, Schreiber H, Inflammation as a tumor promoter in cancer induction, Semin Cancer Biol 14(6) (2004) 433–9. [DOI] [PubMed] [Google Scholar]
- [9].Multhoff G, Molls M, Radons J, Chronic inflammation in cancer development, Front Immunol 2 (2011) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hussain SP, Harris CC, Inflammation and cancer: an ancient link with novel potentials, Int J Cancer 121(11) (2007) 2373–80. [DOI] [PubMed] [Google Scholar]
- [11].Stone TW, McPherson M, Gail Darlington L, Obesity and Cancer: Existing and New Hypotheses for a Causal Connection, EBioMedicine 30 (2018) 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basen-Engquist K, Chang M, Obesity and cancer risk: recent review and evidence, Curr Oncol Rep 13(1) (2011) 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lamb A, Chen LF, Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer, J Cell Biochem 114(3) (2013) 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C, Role of tumor microenvironment in tumorigenesis, J Cancer 8(5) (2017) 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gatti-Mays ME, Balko JM, Gameiro SR, Bear HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky K, Abdul Sater H, Sparano JA, Cescon D, Page DB, McArthur H, Adams S, Mittendorf EA, If we build it they will come: targeting the immune response to breast cancer, NPJ Breast Cancer 5 (2019) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deshmukh SK, Srivastava SK, Tyagi N, Ahmad A, Singh AP, Ghadhban AAL, Dyess DL, Carter JE, Dugger K, Singh S, Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: a closer look at the surroundings, Carcinogenesis 38(8) (2017) 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jamaluddin MS, Weakley SM, Yao Q, Chen C, Resistin: functional roles and therapeutic considerations for cardiovascular disease, Br J Pharmacol 165(3) (2012) 622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dalamaga M, Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives, Biomark Med 8(1) (2014) 107–18. [DOI] [PubMed] [Google Scholar]
- [19].Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, Tsai CH, Chiu WC, Chu-Sung Hu S, Lu CW, Yang YF, Chiu CC, Ou-Yang F, Wang YM, Hou MF, Yuan SS, Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties, Oncogene 37(5) (2018) 589–600. [DOI] [PubMed] [Google Scholar]
- [20].Zhang M, Yan L, Wang GJ, Jin R, Resistin effects on pancreatic cancer progression and chemoresistance are mediated through its receptors CAP1 and TLR4, J Cell Physiol 234(6) (2019) 9457–9466. [DOI] [PubMed] [Google Scholar]
- [21].Deshmukh SK, Srivastava SK, Poosarla T, Dyess DL, Holliday NP, Singh AP, Singh S, Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy, Ann Transl Med 7(20) (2019) 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV, Serum resistin (FIZZ3) protein is increased in obese humans, J Clin Endocrinol Metab 88(11) (2003) 5452–5. [DOI] [PubMed] [Google Scholar]
- [23].Schaffler A, Buchler C, Muller-Ladner U, Herfarth H, Ehling A, Paul G, Scholmerich J, Zietz B, Identification of variables influencing resistin serum levels in patients with type 1 and type 2 diabetes mellitus, Horm Metab Res 36(10) (2004) 702–7. [DOI] [PubMed] [Google Scholar]
- [24].Gong WJ, Zheng W, Xiao L, Tan LM, Song J, Li XP, Xiao D, Cui JJ, Li X, Zhou HH, Yin JY, Liu ZQ, Circulating resistin levels and obesity-related cancer risk: A meta-analysis, Oncotarget 7(36) (2016) 57694–57704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sood A, Shore SA, Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies, J Allergy (Cairo) 2013 (2013) 785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA, The hormone resistin links obesity to diabetes, Nature 409(6818) (2001) 307–12. [DOI] [PubMed] [Google Scholar]
- [27].Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, Rothenberg ME, Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung, Am J Physiol Lung Cell Mol Physiol 293(2) (2007) L305–13. [DOI] [PubMed] [Google Scholar]
- [28].Schwartz DR, Lazar MA, Human resistin: found in translation from mouse to man, Trends Endocrinol Metab 22(7) (2011) 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M, Resistin release by human adipose tissue explants in primary culture, Biochem Biophys Res Commun 300(3) (2003) 674–8. [DOI] [PubMed] [Google Scholar]
- [30].Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA, Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators, Biochem Biophys Res Commun 300(2) (2003) 472–6. [DOI] [PubMed] [Google Scholar]
- [31].Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW, Comparative studies of resistin expression and phylogenomics in human and mouse, Biochem Biophys Res Commun 310(3) (2003) 927–35. [DOI] [PubMed] [Google Scholar]
- [32].Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ, The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications, Gene 305(1) (2003) 27–34. [DOI] [PubMed] [Google Scholar]
- [33].Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L, Disulfide-dependent multimeric assembly of resistin family hormones, Science 304(5674) (2004) 1154–8. [DOI] [PubMed] [Google Scholar]
- [34].Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, Kim HS, Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes, Cell Metab 19(3) (2014) 484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Matviw H, Yu G, Young D, Identification of a human cDNA encoding a protein that is structurally and functionally related to the yeast adenylyl cyclase-associated CAP proteins, Mol Cell Biol 12(11) (1992) 5033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Freeman NL, Lila T, Mintzer KA, Chen Z, Pahk AJ, Ren R, Drubin DG, Field J, A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization, Mol Cell Biol 16(2) (1996) 548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T, SWISS-MODEL: homology modelling of protein structures and complexes, Nucleic Acids Res 46(W1) (2018) W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Avtanski D, Chen K, Poretsky L, Resistin and adenylyl cyclase-associated protein 1 (CAP1) regulate the expression of genes related to insulin resistance in BNL CL.2 mouse liver cells, Data Brief 25 (2019) 104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, Dyess DL, Dal Zotto V, Carter JE, Singh S, Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation, Oncotarget 6(13) (2015) 11231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Deshmukh SK, Srivastava SK, Zubair H, Bhardwaj A, Tyagi N, Al-Ghadhban A, Singh AP, Dyess DL, Carter JE, Singh S, Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes, Cancer Lett 396 (2017) 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI, Resistin competes with lipopolysaccharide for binding to toll-like receptor 4, J Cell Mol Med 14(6B) (2010) 1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, Taouis M, Central resistin overexposure induces insulin resistance through Toll-like receptor 4, Diabetes 62(1) (2013) 102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li B, Fang J, Zuo Z, Yin S, He T, Yang M, Deng J, Shen L, Ma X, Yu S, Wang Y, Ren Z, Cui H, Activation of the porcine alveolar macrophages via toll-like receptor 4/NF-kappaB mediated pathway provides a mechanism of resistin leading to inflammation, Cytokine 110 (2018) 357–366. [DOI] [PubMed] [Google Scholar]
- [44].Jarvinen TA, Prince S, Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition, Biomed Res Int 2015 (2015) 654765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG, An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells, Cell Stem Cell 9(1) (2011) 74–86. [DOI] [PubMed] [Google Scholar]
- [46].Sanchez-Solana B, Laborda J, Baladron V, Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor, Mol Endocrinol 26(1) (2012) 110–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Green JL, Kuntz SG, Sternberg PW, Ror receptor tyrosine kinases: orphans no more, Trends Cell Biol 18(11) (2008) 536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stewart PA, Luks J, Roycik MD, Sang QX, Zhang J, Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer, PLoS One 8(12) (2013) e82460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Coskun T, Kosova F, Ari Z, Sakarya A, Kaya Y, Effect of oncological treatment on serum adipocytokine levels in patients with stage II-III breast cancer, Mol Clin Oncol 4(5) (2016) 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS, Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification, Gynecol Oncol 125(3) (2012) 742–50. [DOI] [PubMed] [Google Scholar]
- [51].Vallega KA, Liu N, Myers JS, Yu K, Sang QX, Elevated Resistin Gene Expression in African American Estrogen and Progesterone Receptor Negative Breast Cancer, PLoS One 11(6) (2016) e0157741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhao CC, Chen J, Niu RF, Liu Y, Zhang CG, Increased resistin suggests poor prognosis and promotes development of lung adenocarcinoma, Oncol Rep 40(6) (2018) 3392–3404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [53].Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, Chen YW, Yang CJ, Hung JY, Huang MS, Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway, Carcinogenesis 34(11) (2013) 2600–9. [DOI] [PubMed] [Google Scholar]
- [54].Hlavna M, Kohut L, Lipkova J, Bienertova-Vasku J, Dostalova Z, Chovanec J, Vasku A, Relationship of resistin levels with endometrial cancer risk, Neoplasma 58(2) (2011) 124–8. [DOI] [PubMed] [Google Scholar]
- [55].Guffey CR, Fan D, Singh UP, Murphy EA, Linking obesity to colorectal cancer: recent insights into plausible biological mechanisms, Curr Opin Clin Nutr Metab Care 16(5) (2013) 595–600. [DOI] [PubMed] [Google Scholar]
- [56].Vazzana N, Riondino S, Toto V, Guadagni F, Roselli M, Davi G, Ferroni P, Obesity-driven inflammation and colorectal cancer, Curr Med Chem 19(34) (2012) 5837–53. [DOI] [PubMed] [Google Scholar]
- [57].Danese E, Montagnana M, Minicozzi AM, Bonafini S, Ruzzenente O, Gelati M, De Manzoni G, Lippi G, Guidi GC, The role of resistin in colorectal cancer, Clin Chim Acta 413(7–8) (2012) 760–4. [DOI] [PubMed] [Google Scholar]
- [58].Joshi RK, Lee SA, Obesity related adipokines and colorectal cancer: a review and meta-analysis, Asian Pac J Cancer Prev 15(1) (2014) 397–405. [DOI] [PubMed] [Google Scholar]
- [59].Mihajlovic M, Ninic A, Sopic M, Miljkovic M, Stefanovic A, Vekic J, Spasojevic-Kalimanovska V, Zeljkovic D, Trifunovic B, Stjepanovic Z, Zeljkovic A, Association among resistin, adenylate cyclase-associated protein 1 and high-density lipoprotein cholesterol in patients with colorectal cancer: a multi-marker approach, as a hallmark of innovative predictive, preventive, and personalized medicine, EPMA J 10(3) (2019) 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu X, Yao N, Qian J, Huang H, High expression and prognostic role of CAP1 and CtBP2 in breast carcinoma: associated with E-cadherin and cell proliferation, Med Oncol 31(3) (2014) 878. [DOI] [PubMed] [Google Scholar]
- [61].Yu XF, Ni QC, Chen JP, Xu JF, Jiang Y, Yang SY, Ma J, Gu XL, Wang H, Wang YY, Knocking down the expression of adenylate cyclase-associated protein 1 inhibits the proliferation and migration of breast cancer cells, Exp Mol Pathol 96(2) (2014) 188–94. [DOI] [PubMed] [Google Scholar]
- [62].Yamazaki K, Takamura M, Masugi Y, Mori T, Du W, Hibi T, Hiraoka N, Ohta T, Ohki M, Hirohashi S, Sakamoto M, Adenylate cyclase-associated protein 1 overexpressed in pancreatic cancers is involved in cancer cell motility, Lab Invest 89(4) (2009) 425–32. [DOI] [PubMed] [Google Scholar]
- [63].Liu Y, Cui X, Hu B, Lu C, Huang X, Cai J, He S, Lv L, Cong X, Liu G, Zhang Y, Ni R, Upregulated expression of CAP1 is associated with tumor migration and metastasis in hepatocellular carcinoma, Pathol Res Pract 210(3) (2014) 169–75. [DOI] [PubMed] [Google Scholar]
- [64].Li M, Yang X, Shi H, Ren H, Chen X, Zhang S, Zhu J, Zhang J, Downregulated expression of the cyclase-associated protein 1 (CAP1) reduces migration in esophageal squamous cell carcinoma, Jpn J Clin Oncol 43(9) (2013) 856–64. [DOI] [PubMed] [Google Scholar]
- [65].Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, del Casar JM, Lamelas ML, Gonzalez-Quintana JM, Vizoso FJ, Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis, BMC Cancer 10 (2010) 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang H, Zhou H, Feng P, Zhou X, Wen H, Xie X, Shen H, Zhu X, Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion, J Exp Clin Cancer Res 29 (2010) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang YB, He FL, Fang M, Hua TF, Hu BD, Zhang ZH, Cao Q, Liu RY, Increased expression of Toll-like receptors 4 and 9 in human lung cancer, Mol Biol Rep 36(6) (2009) 1475–81. [DOI] [PubMed] [Google Scholar]
- [68].Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, Kudo E, Shimada M, Sano T, High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer, Br J Cancer 102(5) (2010) 908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Banerjee AG, Bhattacharyya I, Lydiatt WM, Vishwanatha JK, Aberrant expression and localization of decorin in human oral dysplasia and squamous cell carcinoma, Cancer Res 63(22) (2003) 7769–76. [PubMed] [Google Scholar]
- [70].Cui B, Zhang S, Chen L, Yu J, Widhopf GF 2nd, Fecteau JF, Rassenti LZ, Kipps TJ, Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis, Cancer Res 73(12) (2013) 3649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ, Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody, Proc Natl Acad Sci U S A 116(4) (2019) 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Islam SS, Uddin M, Noman ASM, Akter H, Dity NJ, Basiruzzman M, Uddin F, Ahsan J, Annoor S, Alaiya AA, Al-Alwan M, Yeger H, Farhat WA, Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1, EBioMedicine 43 (2019) 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cui B, Ghia EM, Chen L, Rassenti LZ, DeBoever C, Widhopf GF 2nd, Yu J, Neuberg DS, Wierda WG, Rai KR, Kay NE, Brown JR, Jones JA, Gribben JG, Frazer KA, Kipps TJ, High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia, Blood 128(25) (2016) 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [75].Hanahan D, Weinberg RA, The hallmarks of cancer, Cell 100(1) (2000) 57–70. [DOI] [PubMed] [Google Scholar]
- [76].Ward PS, Thompson CB, Metabolic reprogramming: a cancer hallmark even warburg did not anticipate, Cancer Cell 21(3) (2012) 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Menyhart O, Harami-Papp H, Sukumar S, Schafer R, Magnani L, de Barrios O, Gyorffy B, Guidelines for the selection of functional assays to evaluate the hallmarks of cancer, Biochim Biophys Acta 1866(2) (2016) 300–319. [DOI] [PubMed] [Google Scholar]
- [78].Khan MA, Zubair H, Anand S, Srivastava SK, Singh S, Singh AP, Dysregulation of metabolic enzymes in tumor and stromal cells: Role in oncogenesis and therapeutic opportunities, Cancer Lett 473 (2020) 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].John PC, Mews M, Moore R, Cyclin/Cdk complexes: their involvement in cell cycle progression and mitotic division, Protoplasma 216(3–4) (2001) 119–42. [DOI] [PubMed] [Google Scholar]
- [80].Nurse P, Cyclin dependent kinases and cell cycle control (nobel lecture), Chembiochem 3(7) (2002) 596–603. [DOI] [PubMed] [Google Scholar]
- [81].Hochegger H, Takeda S, Hunt T, Cyclin-dependent kinases and cell-cycle transitions: does one fit all?, Nat Rev Mol Cell Biol 9(11) (2008) 910–6. [DOI] [PubMed] [Google Scholar]
- [82].Gerard C, Goldbeter A, Temporal self-organization of the cyclin/Cdk network driving the mammalian cell cycle, Proc Natl Acad Sci U S A 106(51) (2009) 21643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G, Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis, EMBO J 12(1) (1993) 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Harbour JW, Dean DC, The Rb/E2F pathway: expanding roles and emerging paradigms, Genes Dev 14(19) (2000) 2393–409. [DOI] [PubMed] [Google Scholar]
- [85].Balmanno K, Cook SJ, Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells, Oncogene 18(20) (1999) 3085–97. [DOI] [PubMed] [Google Scholar]
- [86].Shaulian E, Karin M, AP-1 in cell proliferation and survival, Oncogene 20(19) (2001) 2390–400. [DOI] [PubMed] [Google Scholar]
- [87].Bartusel T, Schubert S, Klempnauer KH, Regulation of the cyclin D1 and cyclin A1 promoters by B-Myb is mediated by Sp1 binding sites, Gene 351 (2005) 171–80. [DOI] [PubMed] [Google Scholar]
- [88].Singh S, Chouhan S, Mohammad N, Bhat MK, Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3, FEBS Lett 591(10) (2017) 1371–1382. [DOI] [PubMed] [Google Scholar]
- [89].Qiu L, Zhang GF, Yu L, Wang HY, Jia XJ, Wang TJ, Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition, Sci Rep 8(1) (2018) 12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Malvi P, Chaube B, Singh SV, Mohammad N, Vijayakumar MV, Singh S, Chouhan S, Bhat MK, Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state, Cancer Metab 6 (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, Kim MK, Kim W, Myung SC, Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation, BJU Int 108(2 Pt 2) (2011) E77–83. [DOI] [PubMed] [Google Scholar]
- [92].Lawlor MA, Alessi DR, PKB/Akt: a key mediator of cell proliferation, survival and insulin responses?, J Cell Sci 114(Pt 16) (2001) 2903–10. [DOI] [PubMed] [Google Scholar]
- [93].Elmore S, Apoptosis: a review of programmed cell death, Toxicol Pathol 35(4) (2007) 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Norbury CJ, Hickson ID, Cellular responses to DNA damage, Annu Rev Pharmacol Toxicol 41 (2001) 367–401. [DOI] [PubMed] [Google Scholar]
- [95].Bauer JH, Helfand SL, New tricks of an old molecule: lifespan regulation by p53, Aging Cell 5(5) (2006) 437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wong RS, Apoptosis in cancer: from pathogenesis to treatment, J Exp Clin Cancer Res 30 (2011) 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Pang J, Shi Q, Liu Z, He J, Liu H, Lin P, Cui J, Yang J, Resistin induces multidrug resistance in myeloma by inhibiting cell death and upregulating ABC transporter expression, Haematologica 102(7) (2017) 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rak A, Drwal E, Wrobel A, Gregoraszczuk EL, Resistin is a survival factor for porcine ovarian follicular cells, Reproduction 150(4) (2015) 343–55. [DOI] [PubMed] [Google Scholar]
- [99].van Zijl F, Krupitza G, Mikulits W, Initial steps of metastasis: cell invasion and endothelial transmigration, Mutat Res 728(1–2) (2011) 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM, Cancer Invasion: Patterns and Mechanisms, Acta Naturae 7(2) (2015) 17–28. [PMC free article] [PubMed] [Google Scholar]
- [101].Gong WJ, Liu JY, Yin JY, Cui JJ, Xiao D, Zhuo W, Luo C, Liu RJ, Li X, Zhang W, Zhou HH, Liu ZQ, Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-kappaB pathway, Cancer Sci 109(8) (2018) 2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, Kim HS, Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation, Sci Rep 6 (2016) 18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yang CC, Chang SF, Chao JK, Lai YL, Chang WE, Hsu WH, Kuo WH, Activation of AMP-activated protein kinase attenuates hepatocellular carcinoma cell adhesion stimulated by adipokine resistin, BMC Cancer 14 (2014) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Huang WS, Yang JT, Lu CC, Chang SF, Chen CN, Su YP, Lee KC, Fulvic Acid Attenuates Resistin-Induced Adhesion of HCT-116 Colorectal Cancer Cells to Endothelial Cells, Int J Mol Sci 16(12) (2015) 29370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Miethe C, Zamora M, Torres L, Raign KG, Groll CJ, Price RS, Characterizing the differential physiological effects of adipocytokines visfatin and resistin in liver cancer cells, Horm Mol Biol Clin Investig 38(2) (2019). [DOI] [PubMed] [Google Scholar]
- [106].Rosendahl AH, Bergqvist M, Lettiero B, Kimbung S, Borgquist S, Adipocytes and Obesity-Related Conditions Jointly Promote Breast Cancer Cell Growth and Motility: Associations With CAP1 for Prognosis, Front Endocrinol (Lausanne) 9 (2018) 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA, Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis, Cell 117(7) (2004) 927–39. [DOI] [PubMed] [Google Scholar]
- [108].Fu J, Qin L, He T, Qin J, Hong J, Wong J, Liao L, Xu J, The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis, Cell Res 21(2) (2011) 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hay E, Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In Epithelial–Mesenchymal Interactions, Fleischmajer R and Billingham RE (eds), Baltimore, MD, USA: Williams & Wilkins Co; (1968) 31–55. [Google Scholar]
- [110].Lamouille S, Xu J, Derynck R, Molecular mechanisms of epithelial-mesenchymal transition, Nat Rev Mol Cell Biol 15(3) (2014) 178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang Y, Weinberg RA, Epithelial-to-mesenchymal transition in cancer: complexity and opportunities, Front Med 12(4) (2018) 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Prasad S, Ramachandran S, Gupta N, Kaushik I, Srivastava SK, Cancer cells stemness: A doorstep to targeted therapy, Biochim Biophys Acta Mol Basis Dis 1866(4) (2020) 165424. [DOI] [PubMed] [Google Scholar]
- [113].Aponte PM, Caicedo A, Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment, Stem Cells Int 2017 (2017) 5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kaseb HO, Fohrer-Ting H, Lewis DW, Lagasse E, Gollin SM, Identification, expansion and characterization of cancer cells with stem cell properties from head and neck squamous cell carcinomas, Exp Cell Res 348(1) (2016) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zaravinos A, The Regulatory Role of MicroRNAs in EMT and Cancer, J Oncol 2015 (2015) 865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang J, Ma L, MicroRNA control of epithelial-mesenchymal transition and metastasis, Cancer Metastasis Rev 31(3–4) (2012) 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hu Y, Tang H, MicroRNAs regulate the epithelial to mesenchymal transition (EMT) in cancer progression, Microrna 3(2) (2014) 108–17. [DOI] [PubMed] [Google Scholar]
- [118].Hao J, Zhang Y, Deng M, Ye R, Zhao S, Wang Y, Li J, Zhao Z, MicroRNA control of epithelial-mesenchymal transition in cancer stem cells, Int J Cancer 135(5) (2014) 1019–27. [DOI] [PubMed] [Google Scholar]
- [119].Garg M, Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer, World J Stem Cells 5(4) (2013) 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, Lavi A, Poretsky L, Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms, Cytokine 120 (2019) 155–164. [DOI] [PubMed] [Google Scholar]
- [121].Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D, Extracellular matrix modulates angiogenesis in physiological and pathological conditions, Biomed Res Int 2014 (2014) 756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Nishida N, Yano H, Nishida T, Kamura T, Kojiro M, Angiogenesis in cancer, Vasc Health Risk Manag 2(3) (2006) 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Folkman J, Tumor angiogenesis: therapeutic implications, N Engl J Med 285(21) (1971) 1182–6. [DOI] [PubMed] [Google Scholar]
- [124].Pang L, Zhang Y, Yu Y, Zhang S, Resistin promotes the expression of vascular endothelial growth factor in ovary carcinoma cells, Int J Mol Sci 14(5) (2013) 9751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC, Chen WC, Wang SW, Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells, Biochem Pharmacol 154 (2018) 234–242. [DOI] [PubMed] [Google Scholar]
- [126].Chen SS, Tang CH, Chie MJ, Tsai CH, Fong YC, Lu YC, Chen WC, Lai CT, Wei CY, Tai HC, Chou WY, Wang SW, Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16–5p in human chondrosarcoma cells, Cell Death Dis 10(1) (2019) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C, Adipokine resistin promotes in vitro angiogenesis of human endothelial cells, Cardiovasc Res 70(1) (2006) 146–57. [DOI] [PubMed] [Google Scholar]
- [128].Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D’Asta M, Caforio L, Caruso A, Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes, J Endocrinol 189(3) (2006) 691–9. [DOI] [PubMed] [Google Scholar]
- [129].Son B, Lee S, Youn H, Kim E, Kim W, Youn B, The role of tumor microenvironment in therapeutic resistance, Oncotarget 8(3) (2017) 3933–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Correia AL, Bissell MJ, The tumor microenvironment is a dominant force in multidrug resistance, Drug Resist Updat 15(1–2) (2012) 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ma S, Pradeep S, Hu W, Zhang D, Coleman R, Sood A, The role of tumor microenvironment in resistance to anti-angiogenic therapy, F1000Res 7 (2018) 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer 2(1) (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [133].Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T, Katsumata N, Kang YK, Nishio K, Fujiwara Y, Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients, Breast Cancer Res Treat 99(1) (2006) 9–17. [DOI] [PubMed] [Google Scholar]
- [134].Mohelnikova-Duchonova B, Brynychova V, Oliverius M, Honsova E, Kala Z, Muckova K, Soucek P, Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues, Pancreas 42(4) (2013) 707–16. [DOI] [PubMed] [Google Scholar]
- [135].Kohli L, Roth KA, Autophagy: cerebral home cooking, Am J Pathol 176(3) (2010) 1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS, Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer, PLoS One 7(7) (2012) e42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Liu Z, Shi A, Song D, Han B, Zhang Z, Ma L, Liu D, Fan Z, Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction, Am J Cancer Res 7(3) (2017) 574–583. [PMC free article] [PubMed] [Google Scholar]
- [138].Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S, Kato K, Hamaguchi T, Shimada Y, Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer, Cancer Sci 101(5) (2010) 1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hsieh YY, Shen CH, Huang WS, Chin CC, Kuo YH, Hsieh MC, Yu HR, Chang TS, Lin TH, Chiu YW, Chen CN, Kuo HC, Tung SY, Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/NFkappaB signaling pathway in gastric cancer cells, J Biomed Sci 21 (2014) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]


