Abstract
Background
Mild to moderate depressive symptoms are common but often remain unrecognized and treated inadequately. We hypothesized that an Internet intervention in addition to usual care is superior to care as usual alone (CAU) in the treatment of mild to moderate depressive symptoms in adults.
Methods
This trial was controlled, randomized and assessor-blinded. Participants with mild to moderate depressive symptoms (Patient Health Questionnaire, PHQ-9, score 5-14) were recruited from clinical and non-clinical settings and randomized to either CAU or a 12-week Internet intervention (Deprexis) adjunctive to usual care. Outcomes were assessed at baseline, 3 months (post-assessment) and 6 months (follow-up). The primary outcome measure was self-rated depression severity (PHQ-9). The main analysis was based on the intention-to-treat principle and used linear mixed models.
Results
A total of 1,013 participants were randomized. Changes in PHQ-9 from baseline differed significantly between groups (t<sub>825</sub> = 6.12, p < 0.001 for the main effect of group). The post-assessment between-group effect size in favour of the intervention was d = 0.39 (95% CI: 0.13-0.64). It was stable at follow-up, with d = 0.32 (95% CI: 0.06-0.69). The rate of participants experiencing at least minimally clinically important PHQ-9 change at the post-assessment was higher in the intervention group (35.6 vs. 20.2%) with a number needed to treat of 7 (95% CI: 5-10).
Conclusions
The Internet intervention examined in this trial was superior to CAU alone in reducing mild to moderate depressive symptoms. The magnitude of the effect is clinically important and has public health implications.
Keywords: Internet intervention, Cognitive behaviour therapy, Depression, Randomized controlled trial
Introduction
Depressive disorders are among the leading causes of worldwide disability [1], and mild to moderate forms of depression are particularly common [2]. Given the disappointing effectiveness beyond placebo of antidepressant medication in mild to moderate depression [3,4], Internet-based psychological interventions (hereafter: Internet interventions) could potentially augment existing treatments to improve outcomes in this population.
Internet interventions have been developed for a wide range of psychiatric conditions, including depression [5] and anxiety disorders [6]. Most Internet interventions are based on principles of cognitive behavioural therapy (CBT). They can be used in self-guided (‘stand-alone’) or clinician-guided versions. Whereas the former promise greater reach and efficiency, the latter might be advantageous in terms of effectiveness and safety. Clinician guidance can be performed even by non-expert staff, as it usually consists of brief feedback intended to motivate patients to engage with the intervention. Internet interventions can also be enhanced by administering mood monitoring and referring more severely affected patients to clinicians [7]. In one recent survey, patients expressed a preference for Internet interventions over face-to-face group therapy if the Internet intervention is augmented with weekly telephone contact; however, individual face-to-face therapy remained the majority preference in that study [8].
Recent meta-analyses have demonstrated the effectiveness of some Internet interventions for the treatment of depressive symptoms [5,9,10,11]. However, definitive, methodologically rigorous trials on safety and efficacy are still rare. Specifically, several common methodological limitations can be identified: most studies thus far have relied exclusively on self-report measures of symptom severity [5,10,11], were carried out at single sites and were led by the investigators who developed the interventions [12,13], and most have used small samples of up to 100 participants per group [5,10]. The effectiveness of Internet interventions in routine clinical settings has recently been questioned as one study failed to demonstrate the superiority of two Internet interventions with telephone guidance over usual care in participants with at least moderate depressive symptoms recruited through their general practitioners [14]. Another recent study has demonstrated the efficacy of an Internet intervention following inpatient treatment [15].
To overcome previous methodological limitations, we conducted the EVIDENT trial, a large, pragmatic, multicentre, randomized, controlled trial to test the effectiveness of an Internet intervention for depression that has shown promise in previous studies [12,13,16,17]. We compared this intervention to a care as usual (CAU) control condition in adults with mild to moderate depressive symptoms, some of whom were recruited in routine clinical settings. This was the first trial of an Internet intervention for depressive symptoms in which participants were repeatedly assessed with self- and clinician-rated measures and structured diagnostic interviews. We hypothesized that the intervention, offered adjunctively to usual care, would be superior to CAU alone in reducing depressive symptoms over the 3 months of treatment duration and up to a follow-up at 6 months after randomization.
Methods
Trial Design
This trial adheres to the guidelines of the CONSORT statement and its adaptation for Internet interventions, CONSORT-EHEALTH. The full study protocol has been published [18]. This trial was multicentre (diagnostic interviews were conducted in five sites in Germany), controlled, randomized and assessor-blinded. It was conducted in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the German Psychological Association (SM 04_2012). The trial is registered with ClinicalTrials.gov (NCT01636752). No interim analyses for effectiveness or futility were conducted. There were no changes to the study protocol after study commencement.
Participants
Participants are not referred to as patients in this study because neither a diagnosis of depression nor current treatment was an inclusion criterion. Participants were recruited via multiple settings, including inpatient and outpatient medical and psychological clinics, online forums for depression, health insurance companies and the media (e.g. newspaper and radio).
The main inclusion criterion was the presence of self-reported mild to moderate depressive symptoms, operationalized as a score between 5 and 14 on the Patient Health Questionnaire-9 (PHQ-9) [19]. Eligible participants were between 18 and 65 years of age, had Internet access and were able to communicate in German. Participants with acute suicidality or a lifetime diagnosis of bipolar disorder or schizophrenia were excluded. Participants whose symptom severity was beyond the inclusion criterion were encouraged to seek professional help and invited to participate in a separate trial that targeted individuals with severe depression symptoms [20].
All participants received written information about the aims of the study, benefits and risks of participation, and the study procedure. They were informed that they could withdraw at any time without having to disclose reasons. Informed consent was obtained online prior to the baseline assessment.
Interventions
All participants were permitted to use any form of treatment, including antidepressant medication and psychotherapy. Following a naturalistic or pragmatic design approach, CAU was not influenced by the investigators. Participants in the control condition received only CAU (hereafter referred to as the CAU group). They were offered access to the Internet intervention after the last follow-up assessment.
Participants in the intervention group received access to the Internet intervention in addition to usual care. The intervention was a 12-week CBT-based programme (Deprexis) that has been described elsewhere in more detail [12,16]. Briefly, this programme consists of ten modules covering content that is broadly consistent with CBT (e.g. cognitive restructuring, behavioural activation, acceptance and mindfulness, problem solving), plus one summary module. The programme is interactive by engaging the user in exercises and continuously eliciting feedback in order to tailor subsequent content. It also contains audio recordings, worksheets, summary sheets and brief automatic daily messages, delivered either by SMS or e-mail. All participants who did not log in to the intervention within 2 weeks after randomization received an e-mail describing the login procedure once again.
The intervention can be used with or without guidance by a clinician [12]. In our trial, participants with an initial PHQ-9 score between 10 and 14 received the guided version because safety and efficacy considerations suggested that more intensive support would be appropriate for those with moderate rather than mild symptoms. They were actively contacted once a week by a trained e-mail supporter who provided brief feedback based on the participant's use of the programme over the previous week. Participants could respond to these messages or contact the e-mail supporter themselves. Messages were sent through a secure e-mail system embedded within the Internet intervention. Here, e-mail supporters could also check whether or not their messages were read. The primary goal of the e-mail support was to motivate participants to engage with the programme. Details on training and supervision for e-mail supporters are provided in the study protocol [18].
Outcomes
The primary outcome measure was the PHQ-9, a valid and reliable self-rating measure of depression severity whose sum score ranges from 0 to 27 [19]. A minimally clinically important individual PHQ-9 improvement was defined as a 5-point reduction [21]. The internal consistency of the PHQ-9 was good (Cronbach's alpha = 0.83). The calculation of the internal consistency was based on follow-up data as baseline data were affected by substantial restriction of range, which can distort reliability estimates [22].
Secondary outcome measures were clinician-rated severity of depression as assessed with the 24-item version of the Hamilton Depression Rating Scale (HDRS-24) [23] and the 16-item Quick Inventory of Depressive Symptomatology (QIDS-C16) [24]. Psychiatric diagnoses were established using the Mini International Neuropsychiatric Interview (MINI) [25]. Acute suicidality was assessed clinically based on a structured assessment of current suicidal ideation and past suicide attempts. The internal consistency of the QIDS-C16 and HDRS-24 based on our study data were acceptable (Cronbach's alpha = 0.75 and 0.79, respectively).
All clinician ratings were conducted via telephone by trained raters. These were mostly degree-educated psychologists but also included advanced university students majoring in psychology or medicine. They completed training conducted face to face. Before they were permitted to rate trial participants, raters had to demonstrate adequate interrater reliability on an audiotaped interview. For each rater, we also assessed interrater reliability of their fifth interview. Pearson correlations between individual raters and the trainers ranged between 0.92 and 0.96.
We also employed a measure of health-related quality of life (Short-Form Health Survey: SF-12) [26] and the Questionnaire for the Evaluation of Psychotherapeutic Progress (FEP-2) [27]. The SF-12 total scores on the two subscales (mental health and physical health) are calculated in such a way that they compare to a norm population with a mean score of 50 and a standard deviation of 10. The FEP-2 covers several domains including well-being (‘I feel at peace with myself’) and interpersonal relations (‘I have difficulties expressing negative feelings if necessary’). We also report two measures of negative effects of the intervention: any worsening of PHQ-9 and the score of the PHQ-9 suicidality item.
All self-rating measures were administered via online questionnaires at baseline, after 3 months (post-assessment) and after 6 months (follow-up assessment). Clinicians contacted participants for clinical interviews including the MINI, the HDRS-24 and the QIDS-C16 at baseline and after 3 months. For an overview of all assessments as well as reliability and validity estimates of the instruments, please refer to the study protocol [18].
Sample Size
The sample size calculation was based on the expected difference between the intervention and the control group on the main outcome variable at the post-assessment (depressive symptoms measured with the PHQ-9). Based on an estimated effect size of Cohen's d = 0.23, a power of 0.80, an alpha level of 0.05 and a dropout rate of 40%, 500 participants were needed in each condition. The effect size estimate was based on a meta-analysis [5]. Further details on the sample size calculation are described in the study protocol [18].
Randomization
Participants were randomized equally (1:1) to the two groups (intervention or control). Randomization was stratified by the PHQ-9 (PHQ-9 <10 vs. PHQ-9 ≥10). Block randomization with variable block sizes was used. The allocation schedule was created by an independent investigator with a computerized random number generator; the other investigators were blinded to this schedule. The allocation sequence was concealed from participants and researchers. Only the participants and the e-mail supporters were informed about the randomization outcome. Diagnostic interviewers were blinded to the group assignment of the participants. For individual participants, e-mail support and diagnostic interviews were conducted by separate centres to avoid accidentally unblinding interviewers.
Statistical Methods
A statistical analysis plan was written and agreed upon by the study team before the initiation of the statistical analysis. The analyses were performed with SPSS, version 22. We used linear mixed models (LMM) as they have the advantage of using all the available data of each subject. LMM analysis also offers the opportunity to choose an appropriate covariance structure reflecting the potential dependence due to repeated measurements [28]. Adjustment for baseline measure was chosen as this increases statistical power and accounts for regression to the mean [29]. No missing values were substituted for any of the statistical analyses as mixed model analyses based on all observed data are valid and unbiased methods for missing at random data [30].
The primary analysis for all the outcomes reported here was an intention-to-treat analysis, which included all randomized participants. All continuous outcomes were analysed as change from baseline, with a random intercept for the participant, time as within-group factor, study group as well as guided versus unguided treatment as fixed effect and adjustment for baseline measure. A diagonal covariance structure with unequal variances was chosen based on Akaike's Information Criterion (AIC) from a fixed set of candidate structures, namely a first-order autoregressive (AR1), or scaled identity structure, or heterogeneous versions thereof. The study hypothesis was tested on the main effect for group. For dichotomous outcomes we calculated logistic regression analyses that were adjusted for baseline values.
Sensitivity Analysis
Several sensitivity analyses were conducted to determine the robustness of our results for the main outcome. In our first sensitivity analysis, the adjusted analysis, we corrected for the following baseline variables: age, sex, marital status, employment status, stratification variable (PHQ-9 <10 vs. PHQ-9 ≥10) and presence of dysthymia. These have been found to affect the effectiveness of treatment for depression [31]. Our second sensitivity analysis was a predefined per protocol analysis [18]. Here, only those participants who had used the intervention for at least two sessions over at least 1 h were compared to all participants in the CAU group.
Subgroup Analyses
We performed two subgroup analyses. In the first subgroup analysis, we tested the influence of antidepressant medication on PHQ change. In the second subgroup analysis, we tested the influence of concomitant psychiatric or psychotherapeutic treatment between baseline and post-assessment on PHQ change. The hypothesis of difference in treatment effects was tested on the group by concomitant treatment interaction. For these analyses we relied on the participants' report of their having received antidepressant medication, psychiatric care or psychotherapy.
Effect Sizes
Effect sizes are presented as Cohen's d for continuous data and numbers needed to treat (NNT) for dichotomous data. Cohen's d was calculated by subtracting the mean of the intervention group from the mean of the CAU group and dividing the result by the pooled standard deviation of the two groups. The NNT was calculated based on the success rate difference. Effect sizes were labelled as small (corresponding to d = 0.2, NNT 8.9), medium (corresponding to d = 0.5, NNT 3.6) and large (corresponding to d = 0.8, NNT 2.3). For the calculation of effect sizes, we used multiple imputation (with 50 imputations per missing value) to estimate missing scores by evaluating the relationships between observed and missing scores as well as baseline scores.
Results
Recruitment, Participant Flow
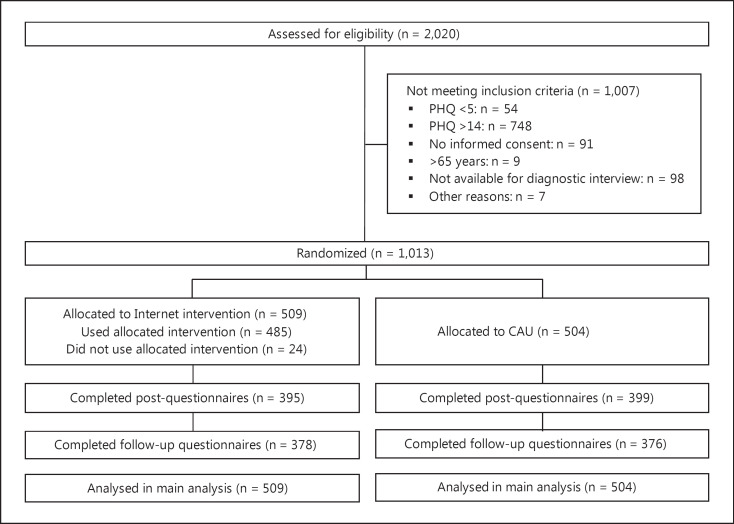
Recruitment began in August 2012 and ended in December 2013. The last 6-month follow-up assessment was performed in July 2014. For full participant flow, please refer to the flowchart (fig. 1). Briefly, 2,020 participants were assessed for eligibility, and 1,007 (49.9%) were excluded. The most common exclusion criterion was exceeding a score of 14 on the PHQ-9 (748, 37.0%). Participants excluded because of their symptom severity were given the opportunity to use the intervention in a separate study [20]. Non-completion rates for the main outcome measure were 21.6% at post-assessment (n = 219) and 24.6% at follow-up (n = 259).
Fig. 1.
Trial profile.
We computed a logistic regression analyses to explore whether any of the following variables were associated with dropout status: randomization group, age, sex, family status, educational status, baseline PHQ, baseline diagnosis of depression (clinician-assessed) or panic disorder (self-assessed). The potential predictors of dropout were chosen in keeping with meta-analytic analyses of dropout [32]. Dropout status at the post-assessment was entered as a dependent variable and the above-mentioned potential predictors of dropout were entered as independent variables. None of the variables were significantly associated with dropout status (Nagelkerke's R2 = 0.039, model χ2(17) = 25.954, p = 0.075).
Participant Characteristics
For details on the participant characteristics, please refer to table 1 and online supplementary table 1 (see www.karger.com/doi/10.1159/000445355 for all online suppl. material). Briefly, the modal participant was 45 years old, female, married, employed full-time, suffered from moderately severe self-rated depressive symptoms (PHQ-9: 12) but only mild clinician-rated depressive symptoms (HDRS-24: 17, QIDS-C16: 8), and reported having experienced two to five depressive episodes. The participants reported a markedly reduced mental health-related quality of life, but their physical health-related quality of life was comparable to a normal population. Participants in both groups reported having taken more than 20 days of disability leave in the 6 months preceding randomization. A diagnosis of a depressive disorder was not an inclusion criterion, and only 290 participants (28.6%) fulfilled the criteria for a current major depressive episode on the MINI interview. Examination of baseline characteristics revealed that randomization achieved a balance between groups on these variables. Also, both groups did not differ with respect to use of concomitant treatments either before or after randomization. Overall, 312 participants (30.8 %) reported being in outpatient psychiatric treatment and 353 participants (34.8 %) said they were in outpatient psychotherapy.
Table 1.
Clinical characteristics at baseline and treatment utilization between baseline and post-assessment
| Intervention (n = 509; 50.1%) | CAU (n = 504; 49.9%) | |
|---|---|---|
| Diagnosis | ||
| Any depressive disorder (MINI) | 285 (56.0) | 266 (52.8) |
| Current MDE (MINI) | 154 (30.3) | 136 (27.0) |
| Dysthymia (MINI) | 180 (35.4) | 196 (38.8) |
| Panic disorder (WSQ) | 162 (31.9) | 159 (31.5) |
| Social phobia (WSQ) | 245 (48.2) | 229 (45.3) |
| Alcohol abuse (WSQ) | 28 (5.5) | 24 (4.8) |
| Severity | ||
| PHQ-9 | 10.23±2.41 | 10.34±2.40 |
| HDRS-24 | 16.81 ±7.30 | 16.74±7.63 |
| QIDS-C16 | 8.84±4.02 | 8.72 ±4.04 |
| SF-12 mental health | 31.24±7.71 | 31.44±7.62 |
| SF-12 physical health | 47.72 ±9.36 | 47.41 ±9.51 |
| FEP-2 total mean | 2.91 ±0.45 | 2.95 ±0.46 |
| PHQ-9 ≥10 | 317 (62.4) | 317 (62.8) |
| History (self-report) | ||
| ≤ 1 depressive episodes | 96 (18.9) | 94 (18.6) |
| 2–5 depressive episodes | 205 (40.4) | 225 (44.6) |
| 6–10 depressive episodes | 110 (21.7) | 92 (18.2) |
| >10 depressive episodes | 97 (19.1) | 94 (18.7) |
| Treatment in the past 6 months | ||
| Psychotherapy | 184 (36.2) | 169 (33.5) |
| Outpatient psychiatric treatment | 153 (30.1) | 159 (31.5) |
| Inpatient psychiatric treatment | 41 (8.1) | 36 (7.1) |
| Treatment between baseline and post-assessment | ||
| Psychotherapy | 127 (32.3) | 140 (35.1) |
| Outpatient psychiatric treatment | 106 (27.0) | 108 (27.1) |
| Inpatient psychiatric treatment | 5 (1.3) | 9 (2.3) |
| Antidepressant medication | 193 (48.9) | 204 (51.3) |
| Disability leave, days | ||
| 6 months prior to baseline | 20.14±43.76 | 25.28 ±50.06 |
Data are presented as n (%) or means ± SD, as appropriate. WSQ = Web Screening Questionnaire. The observed between-group differences in treatment utilization between baseline and post-assessment were not statistically significant (psychotherapy, χ2 = 1.04, p = 0.307; outpatient psychiatric treatment, χ2 = 0.06, p = 0.814; inpatient psychiatric treatment, χ2 = 1.20, p = 0.273; antidepressant medication, χ2 = 0.455, p = 0.500).
Intervention Usage
A total of 509 participants were randomized to the intervention group. The mean number of sessions with a duration of at least 10 min was 8.32 (SD = 4.71), and the mean total usage time was 429.70 min (about 7 h; SD = 294.0). In the intervention group, 308 participants had a PHQ-9 score greater than 9 and therefore received e-mail support; 268 of these (87.02%) read at least one of the messages they received. Participants received 10.5 messages on average (range 2-23); 39.93% of participants (n = 123) sent at least one message, and 19.16% (n = 59) sent three or more messages. The number of participants who used the Internet intervention at least once was 485 (95.3%), and 473 (92.9%) used the intervention for at least two sessions over a total duration of at least 60 min and thus met our per protocol definition.
Main Outcome
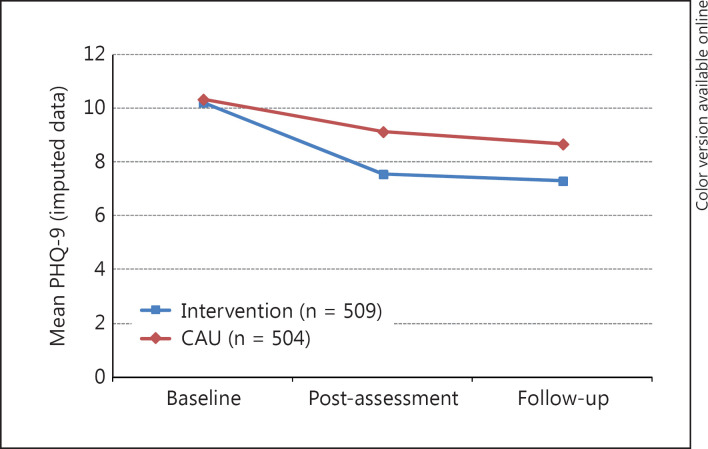
Based on LMM analysis of the intention-to-treat sample, the intervention had a significant effect on the main outcome measure, the change of PHQ-9. Whereas scores decreased in both groups, changes in PHQ-9 differed significantly (t825 = 6.12, p < 0.001) between groups (fig. 2). In the intervention group, PHQ-9 scores decreased by 1.57 (95% CI: 1.07-2.07) points more than in the CAU group, on average.
Fig. 2.
PHQ-9 scores at baseline, post-assessment and follow-up (imputed data). LMM analysis showed that changes in PHQ-9 differed significantly between groups (t825 = 6.12, p < 0.001).
As shown in table 2, between-group effect sizes for the PHQ were in the small to medium range (post-assessment: d = 0.39, 95% CI: 0.13-0.64; follow-up: d = 0.32, 95% CI: 0.06-0.59). The within-group effect sizes between baseline and post-assessment were large (d = 0.81, 95% CI: 0.61-1.01) for the intervention group and small to medium (d = 0.34, 95% CI: 0.11-0.56) for the control group. Between baseline and follow-up, within-group effect sizes were d = 0.86 (95% CI: 0.65-1.07) and d = 0.47 (95% CI: 0.25-0.68), respectively.
Table 2.
Descriptive statistics and effect sizes of main and secondary outcomes (imputed data)
| Post-assessment | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| intervention (n = 509) | CAU (n = 504) | Cohen's d | 95% CI | intervention (n = 509) | CAU (n = 504) | Cohen's d | 95% CI | |
| PHQ-9 | 7.54±4.04 | 9.15±4.30 | 0.39 | 0.13 to 0.64 | 7.31 ±4.18 | 8.69±4.41 | 0.32 | 0.06 to 0.59 |
| HDRS-24 | 12.82±7.49 | 14.25 ±7.96 | 0.19 | −0.29 to 0.66 | n.a. | |||
| QIDS-C16 | 6.73 ±4.44 | 7.22 ±4.38 | 0.11 | −0.16 to 0.38 | n.a. | |||
| SF-12 mental health | 37.26± 10.06 | 35.19±8.87 | 0.22 | −0.37 to 0.80 | 38.14± 10.60 | 36.71 ±10.49 | 0.14 | −0.51 to 0.79 |
| SF-12 physical health | 47.74±9.58 | 47.21 ±9.45 | 0.06 | −0.53 to 0.64 | 47.88 ±9.69 | 47.22 ±9.48 | 0.07 | −0.52 to 0.66 |
| FEP-2 | 2.56±0.61 | 2.74±0.62 | 0.30 | 0.26 to 0.33 | 2.51 ±0.63 | 2.71 ±0.67 | 0.31 | 0.27 to 0.35 |
| Minimally clinically | ||||||||
| important PHQ-9 | ||||||||
| improvement | 181 (35.6) | 102 (20.2) | NNT = 7 | 5 to 10 | 191 (37.5) | 128 (25.4) | NNT = 8 | 6 to 16 |
Data are presented as n (%) or means ± SD, as appropriate. n.a. = Not applicable.
As only the moderately depressed subgroup received e-mail support, we compared the results for this with the mildly depressed subgroup (online suppl. table 4). Between-group effects at the post-assessment were slightly higher in the moderately depressed subgroup: d = 0.44 (95% CI: 0.18-0.70) versus d = 0.31 (95% CI: 0.09-0.54) but less so at follow-up: d = 0.35 (95% CI: 0.08-0.61) versus d = 0.30 (95% CI: −0.05 to 0.55).
Secondary Outcomes
Both the mixed model analysis for continuous outcomes and the logistic regression analysis for dichotomous outcomes demonstrated statistically significant (p < 0.01) effects for clinician-rated depression severity (HDRS-24, QIDS-C16), mental health-related quality of life (SF-12) and overall progress (FEP-2), but not physical health-related quality of life (SF-12) and diagnosis of depressive episode. For the exact test statistics, please refer to online supplementary table 2. Means, ratios and effect sizes are presented in table 2. Effect sizes were small to medium for the statistically significant secondary outcomes based on self-ratings (SF-12 MH, FEP 2) and lower for the statistically significant secondary outcomes based on clinician ratings (HDRS-24 and QIDS-C16). Effect sizes for the clinician ratings were small to medium when calculating them based on the observed data instead of the imputed data (online suppl. table 3). The effects on the self-ratings were stable at follow-up.
Negative Effects
The two predefined negative effects, worsening of PHQ and suicidality, were more pronounced in the control group that in the intervention group. Observed means and observed ratios of negative effects are presented in table 3.
Table 3.
Negative effects of the intervention (imputed data)
| Post-assessment | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| intervention (n = 509) | CAU (n = 504) | Cohen's A | 95% CI | intervention (n = 509) | CAU (n = 504) | Cohen's A | 95% CI | |
| PHQ-9 suicidality item | 1.20±0.47 | 1.27±0.53 | 0.14 | 0.10 to 0.17 | 1.16±0.42 | 1.27±0.54 | 0.23 | 0.19 to 0.26 |
| PHQ-9 worsening | 107 (21.0) | 165 (32.7) | NNT = 9 | 6 to 16 | 101 (19.8) | 184 (28.4) | NNT = 6 | 5 to 9 |
Data are presented as n (%) or means ± SD, as appropriate.
Sensitivity Analyses
We performed several sensitivity analyses to determine the robustness of our results for the main outcome. The first sensitivity analysis, the adjusted analysis, showed that adjustment for the predetermined baseline variables did not change the results substantially (t769 = 5.73, p < 0.001). In the intervention group, the PHQ-9 score decreased by 1.52 points (95% CI: 1.00-2.04) more than in the CAU group. The second sensitivity analysis was a per protocol analysis. We found that the treatment effect on PHQ-9 change was robust to the per protocol analysis: changes in PHQ-9 differed significantly (t799 = 6.347, p < 0.001) between groups. In the intervention group, the PHQ-9 score decreased by 1.65 points (95% CI: 1.14-2.16) more than in the CAU group.
Subgroup Analyses
Antidepressant Medication. We observed a significant group by medication interaction (t820 = −2.01, p = 0.045). Participants receiving medication had a smaller difference in decrease in PHQ score between the intervention and CAU groups (-1.05, 95% CI: −2.08 to −0.23). Between-group effects were lower among those receiving medication at the post-assessment: d = 0.27 (95% CI: −0.16 to 0.71) versus d = 0.54 (95% CI: 0.15-0.93) and at follow-up: d = 0.14 (95% CI: −0.30 to 0.57) versus d = 0.54 (95% CI: 0.13-0.96; online suppl. table 5; online suppl. fig. 1).
Concomitant Psychiatric or Psychotherapeutic Treatment. We observed a significant group by concomitant treatment interaction (t820 = −2.19, p = 0.029). Participants receiving concomitant treatment had a smaller difference in decrease in PHQ score between the intervention and CAU group (-1.12, 95% CI: −2.13 to −0.12). Between-group effects were lower among those receiving concomitant treatment at the post assessment [d = 0.24 (-0.19 to 0.66) vs. d = 0.48 (0.17 to 0.80)] and at follow-up [d = 0.12 (-0.32 to 0.56) vs. d = 0.46 (0.13 to 0.78); online suppl. table 6].
Discussion
To our knowledge, this is the largest randomized controlled trial to date of a psychological Internet intervention for adult depressive symptoms. It is one of the first that demonstrates the effectiveness of such an intervention not only on self-rated but also on clinician-rated measures. In participants with mild to moderate depressive symptoms, we found statistically significant effects of the intervention (Deprexis) on these measures and on measures of mental health-related quality of life and general well-being as well as interpersonal functioning. These effects were stable at the follow-up assessment conducted 6 months after randomization. The size of the effect for the main outcome measure, the PHQ-9, was in the small to medium range (d = 0.39).
The effect was somewhat larger in those participants who were not currently in psychotherapeutic or psychiatric treatment (d = 0.48) and for participants who were not on antidepressant medication (d = 0.54). In a separate study with a sample of more severely depressed adults we found the opposite effect: here, the benefit of this intervention was particularly strong with medication [20]. These findings confirm the notion that long-term antidepressant medication can also be disadvantageous in a population such as ours that reports mild to moderate depressive symptoms and multiple depressive episodes [33]. It has been shown that psychological treatment might even be more effective if antidepressants are slowly discontinued [34]. Our findings may also suggest, however, that the Internet intervention we studied is most effective among those participants with mild to moderate depressive symptoms who do not already receive treatment through other means.
The intervention effect we observed can be regarded as clinically significant, particularly for a low-threshold intervention that has the potential to have an impact at a population level. Whereas only about 1 in 5 participants in the control condition experienced a clinically important improvement in depressive symptoms on the PHQ-9, more than 1 in 3 improved substantially in the intervention group. This corresponds to an NNT of 7, indicating that 7 participants would have to be treated for 1 more participant to experience clinically relevant symptomatic improvement. The corresponding relative risk of 0.57 (95% CI: 0.46-0.72) is well below the threshold for clinical significance (relative risk of 0.80 or less) defined by the National Institute of Clinical Excellence (NICE) [35].
The effect size we observed for the main outcome measure is remarkably high given the fact that this trial targeted a population with mild to moderate rather than more severe depressive symptoms. Meta-analyses show that initial severity moderates the treatment effect not only in studies of antidepressant efficacy [3] but also in studies of Internet interventions for depression, with larger effects typically occurring among more severely depressed subjects [9]. Some [36,37,38] but not all studies [17,39,40] in participants with subthreshold depression found lower effect sizes (d = 0.17-0.27) than the effect we could demonstrate.
The effects observed in our study compare favourably not only with results from previous studies of Internet interventions for depression but also with studies of other depression treatments [41]. The effect size we observed is slightly higher than the effect observed in one previous study [13] of the same intervention (d = 0.36) but somewhat lower than the effects (d = 0.57-0.66) found for this intervention in studies that did not focus on mild to moderate depressive symptoms [12,16,20] and in two recent studies [42,43] in depressed patients with epilepsy (d = 0.46) and multiple sclerosis (d = 0.53). All effect sizes reported for this intervention (Deprexis) compare favourably with those of other unguided interventions (d = 0.05-0.78) [10]. The effect size we observed in those receiving e-mail support in our study was lower than that observed in other studies of this intervention (d = 1.14) [12] and some but not all of the other guided interventions summarized in a meta-analysis (d = 0.29-1.20) [10].
Several strengths and limitations should be noted. A first major strength concerns the large sample size, which yields more precise treatment effect estimates compared to smaller studies [44]. A second major strength is that we performed clinical assessments of diagnostic status and symptom severity in all participants. This allowed us to confirm that the intervention effects are not limited to self-report measures but are also evident when measured by structured, validated clinician rating scales. Diagnostic status has been assessed clinically in some [12,20] but not all [13,16] previous studies of the intervention we examined. None of the studies in three meta-analyses on the effects of Internet interventions for depression employed clinical assessments of symptom severity over time [5,10,11].
A third strength concerns the fact that we recruited participants from a broad array of settings, including those receiving routine care, which enhances the study's external validity. A fourth strength is that a population with clinically relevant symptoms and functional impairment was studied. Although the study population experienced only mild to moderate depressive symptoms, they reported substantial and long-lasting impairment. For example, about a third of the participants met the criteria for dysthymic disorder. Participants also reported a mean of 20 days of disability leave in the 6 months prior to randomization. Compared to other studies of mild to moderate depressive symptoms, the within-group effect size in our control group was remarkably small, suggesting that these participants did not just improve on their own, even though about a third of them reported being in psychotherapy and a third reported receiving outpatient psychiatric treatment. One [12] but not other previous studies [13,16,20] of the same intervention excluded patients who were currently in psychotherapy or who were not on a stable medication. A comparison to a pure waitlist control group, then, might have yielded even larger intervention effects.
Further strengths concern our attention to adverse effects and adequate intervention dosage. It should be noted that we also looked at negative effects of the Internet intervention, although we ought to have employed more precise measures of negative effects [20,45]. Finally, most participants met our predefined criterion of per protocol use of the intervention. Almost 93% used the intervention for at least two sessions and at least 1 h. This contrasts with lower treatment adherence in previous trials [16,20] and may be attributed to the fact that all participants had contact with clinicians during the telephone interview preceding the randomization.
There are also limitations to consider when interpreting the study's results. The first limitation concerns the non-completion rate at the post-assessment (21.6%) as well as the follow-up assessment (24.6%). Non-completion rates were higher in one [16] but not other [12,13,20] previous trials of this intervention. One further limitation is that while participants in the CAU group received any treatment they actively sought, they did not receive an active control condition as part of the study. Therefore, participants could not be blinded to treatment allocation, and we cannot infer the extent to which the observed effects are specific to the particular intervention we studied. Another limitation is that our design does not permit us to disentangle whether the small effect differences between the mildly versus moderately depressed subgroups can be attributed to symptom severity differences, as such, or to the fact that only the moderately depressed subgroup received e-mail support. Future research is required to better understand how, when and for whom support can enhance the effects of Internet interventions, but our results demonstrate that this intervention can produce meaningful effects even when none or minimal support is provided. Also, we did not systematically assess whether participants in the CAU group used an Internet intervention outside the study.
Finally, there are limitations to the external validity of our trial. Participants in our study were largely self-selected and may have been more motivated than others to use this intervention. Patients seeking treatment via the Internet and patients seeking traditional psychotherapy have been found to have higher levels of education than the general population [46]. Results may not generalize to all persons with mild to moderate depressive symptoms, then. However, they may generalize to those who are motivated and able to use an Internet intervention, and to those with more severe forms of depression, as we reported in a separate trial [20].
On the basis of the robust evidence yielded by this and previous smaller trials, we conclude that evidence-based Internet interventions should be added to the repertoire of currently available treatments for depressive symptoms. Implementing effective Internet interventions in routine care could substantially alter the way mild to moderate depressive symptoms are treated in general medicine, where interventions must be not only effective but also easily accessible. Whether this intervention is as effective as medication or psychotherapy could be investigated in head-to-head trials. A particularly important next step, though, concerns the broader dissemination of this and other evidence-based psychological Internet interventions.
Disclosure Statement
J.P.K. has received payments for presentations, workshops and books on psychotherapy for chronic depression and on psychiatric emergencies. B.M. is employed as research director at GAIA AG, the company that developed, owns and operates the Internet intervention investigated in this trial. All the other authors report no relationships with commercial interests.
Acknowledgements
This study was funded by the German Federal Ministry of Health (II A 5-2512 FSB 052). The funding body had no role in the design of the study, data collection, analysis, or interpretation of the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors wish to thank GAIA AG (Hamburg, Germany), which gave technical support and made the Internet intervention (Deprexis) available at no cost to the participants in the trial. The full EVIDENT study team consists of the following: Sandra Nolte, Matthias Rose (local principal investigator), Anna Paulitschek, Leonie Gmöhling, and Leonie Schickedanz (Berlin); Thomas Berger (Bern); Viola Gräfe and Wolfgang Greiner (local principal investigator; Bielefeld); Mirja Behrens, Cecile Hoermann, Anna J. Katharina Jahns, Thies Lüdtke, Björn Meyer, Steffen Moritz (local principal investigator), Johanna Schröder, Amit Gulati, and Eik Vettorazzi (Hamburg); Carla Gamon, Fritz Hohagen (principal investigator), Martin Kolbe, Philipp Klein (local principal investigator), Antje Roniger, and Christina Späth (Lübeck); Alice Arndt, Liv Glindemann, Wolfgang Lutz (local principal investigator), David Rosenbaum, and Kathinka Wolter (Trier), and Flora Bach, Elisabeth Beck, Kristina Fuhr, Martin Hautzinger (local principal investigator), Katharina Krisch, and Melanie Wahl (Tübingen).
S.M. and F.H. contributed equally to this work.
verified
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Busch MA, Maske UE, Ryl L, Schlack R, Hapke U. [Prevalence of depressive symptoms and diagnosed depression among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:733–739. doi: 10.1007/s00103-013-1688-3. [DOI] [PubMed] [Google Scholar]
- 3.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Andersson G, Paxling B, Roch-Norlund P, Ostman G, Norgren A, Almlov J, Georen L, Breitholtz E, Dahlin M, Cuijpers P, Carlbring P, Silverberg F. Internet-based psychodynamic versus cognitive behavioral guided self-help for generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81:344–355. doi: 10.1159/000339371. [DOI] [PubMed] [Google Scholar]
- 7.Kok G, Burger H, Riper H, Cuijpers P, Dekker J, van MH, Smit F, Beck A, Bockting CL. The three-month effect of mobile internet-based cognitive therapy on the course of depressive symptoms in remitted recurrently depressed patients: results of a randomized controlled trial. Psychother Psychosom. 2015;84:90–99. doi: 10.1159/000369469. [DOI] [PubMed] [Google Scholar]
- 8.Berle D, Starcevic V, Milicevic D, Hannan A, Dale E, Brakoulias V, Viswasam K. Do patients prefer face-to-face or internet-based therapy? Psychother Psychosom. 2015;84:61–62. doi: 10.1159/000367944. [DOI] [PubMed] [Google Scholar]
- 9.Bower P, Kontopantelis E, Sutton A, Kendrick T, Richards DA, Gilbody S, Knowles S, Cuijpers P, Andersson G, Christensen H, Meyer B, Huibers M, Smit F, van SA, Warmerdam L, Barkham M, Bilich L, Lovell K, Liu ET. Influence of initial severity of depression on effectiveness of low intensity interventions: meta-analysis of individual patient data. BMJ. 2013;346:f540. doi: 10.1136/bmj.f540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson R, Andersson G. Internet-based psychological treatments for depression. Expert Rev Neurother. 2012;12:861–869. doi: 10.1586/ern.12.63. [DOI] [PubMed] [Google Scholar]
- 11.Cuijpers P, Donker T, Johansson R, Mohr DC, van SA, Andersson G. Self-guided psychological treatment for depressive symptoms: a meta-analysis. PLoS One. 2011;6:e21274. doi: 10.1371/journal.pone.0021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger T, Hammerli K, Gubser N, Andersson G, Caspar F. Internet-based treatment of depression: a randomized controlled trial comparing guided with unguided self-help. Cogn Behav Ther. 2011;40:251–266. doi: 10.1080/16506073.2011.616531. [DOI] [PubMed] [Google Scholar]
- 13.Moritz S, Schilling L, Hauschildt M, Schröder J, Treszl A. A randomized controlled trial of internet-based therapy in depression. Behav Res Ther. 2012;50:513–521. doi: 10.1016/j.brat.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, Araya R, Barkham M, Bower P, Cooper C, Gask L, Kessler D, Lester H, Lovell K, Parry G, Richards DA, Andersen P, Brabyn S, Knowles S, Shepherd C, Tallon D, White D. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ. 2015;351:h5627. doi: 10.1136/bmj.h5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordy H, Wolf M, Aulich K, Burgy M, Hegerl U, Husing J, Puschner B, Rummel-Kluge C, Vedder H, Backenstrass M. Internet-delivered disease management for recurrent depression: a multicenter randomized controlled trial. Psychother Psychosom. 2016;85:91–98. doi: 10.1159/000441951. [DOI] [PubMed] [Google Scholar]
- 16.Meyer B, Berger T, Caspar F, Beevers CG, Andersson G, Weiss M. Effectiveness of a novel integrative online treatment for depression (Deprexis): randomized controlled trial. J Med Internet Res. 2009;11:e15–e15. doi: 10.2196/jmir.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoifodt RS, Lillevoll KR, Griffiths KM, Wilsgaard T, Eisemann M, Waterloo K, Kolstrup N. The clinical effectiveness of web-based cognitive behavioral therapy with face-to-face therapist support for depressed primary care patients: randomized controlled trial. J Med Internet Res. 2013;15:e153. doi: 10.2196/jmir.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JP, Berger T, Schröder J, Späth C, Meyer B, Lutz W, Hautzinger M, Rose M, Gräfe V, Hohagen F, Andersson G, Vettorrazi E, Moritz S. The EVIDENT-trial: protocol and rationale of a multicenter randomized controlled trial testing the effectiveness of an online-based psychological intervention. BMC Psychiatry. 2013;13:239. doi: 10.1186/1471-244X-13-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer B, Bierbrodt J, Schröder J, Berger T, Beevers CG, Weiss M, Jacob G, Späth C, Andersson G, Lutz W, Hautzinger M, Löwe B, Rose M, Hohagen F, Caspar F, Greiner W, Moritz S, Klein JP. Effects of an Internet intervention (Deprexis) on severe depression symptoms: randomized controlled trial. Internet Interventions. 2015:48–59. [Google Scholar]
- 21.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Stauffer JM, Mendoza JL. The proper sequence for correcting correlation coefficients for range restriction and unreliability. Psychometrika. 2001;66:63–68. [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;9((suppl 20)):22–33. [PubMed] [Google Scholar]
- 26.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Lutz W, Schürch E, Stulz N, Böhnke , JR, Schöttke H, Rogner J, Wiedl KH. Entwicklung und psychometrische Kennwerte des Fragebogens zur Evaluation von Psychotherapieverläufen (FEP) Diagnostica. 2009;55:106–116. [Google Scholar]
- 28.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 29.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell ML, Fiero M, Horton NJ, Hsu CH. Handling missing data in RCTs; a review of the top medical journals. BMC Med Res Methodol. 2014;14:118. doi: 10.1186/1471-2288-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van HL, Schoevers RA, Dekker J. Predicting the outcome of antidepressants and psychotherapy for depression: a qualitative, systematic review. Harv Rev Psychiatry. 2008;16:225–234. doi: 10.1080/10673220802277938. [DOI] [PubMed] [Google Scholar]
- 32.Karyotaki E, Kleiboer A, Smit F, Turner DT, Pastor AM, Andersson G, Berger T, Botella C, Breton JM, Carlbring P, Christensen H, de GE, Griffiths K, Donker T, Farrer L, Huibers MJ, Lenndin J, Mackinnon A, Meyer B, Moritz S, Riper H, Spek V, Vernmark K, Cuijpers P. Predictors of treatment dropout in self-guided web-based interventions for depression: an ‘individual patient data’ meta-analysis. Psychol Med. 2015;45:2717–2726. doi: 10.1017/S0033291715000665. [DOI] [PubMed] [Google Scholar]
- 33.Fava GA. Rational Use of Antidepressant Drugs. Psychother Psychosom. 2014;83:197–204. doi: 10.1159/000362803. [DOI] [PubMed] [Google Scholar]
- 34.Guidi J, Fava GA, Fava M, Papakostas GI. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med. 2011;41:321–331. doi: 10.1017/S0033291710000826. [DOI] [PubMed] [Google Scholar]
- 35.National Institute., for Clinical Excellence Depression: Management of depression in primary and secondary care. London, The British Psychological Society and The Royal College of Psychiatrists. 2004 [Google Scholar]
- 36.Spek V, Nyklicek I, Smits N, Cuijpers P, Riper H, Keyzer J, Pop V. Internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years old: a randomized controlled clinical trial. Psychol Med. 2007;37:1797–1806. doi: 10.1017/S0033291707000542. [DOI] [PubMed] [Google Scholar]
- 37.Morgan AJ, Jorm AF, Mackinnon AJ. Email-based promotion of self-help for subthreshold depression: Mood Memos randomised controlled trial. Br J Psychiatry. 2012;200:412–418. doi: 10.1192/bjp.bp.111.101394. [DOI] [PubMed] [Google Scholar]
- 38.Hallgren M, Kraepelien M, Ojehagen A, Lindefors N, Zeebari Z, Kaldo V, Forsell Y. Physical exercise and Internet-based cognitive behavioural therapy in the treatment of depression: randomised controlled trial. Br J Psychiatry. 2015;207:227–234. doi: 10.1192/bjp.bp.114.160101. [DOI] [PubMed] [Google Scholar]
- 39.Lintvedt OK, Griffiths KM, Sorensen K, Ostvik AR, Wang CE, Eisemann M, Waterloo K. Evaluating the effectiveness and efficacy of unguided internet-based self-help intervention for the prevention of depression: a randomized controlled trial. Clin Psychol Psychother. 2013;20:10–27. doi: 10.1002/cpp.770. [DOI] [PubMed] [Google Scholar]
- 40.Buntrock C, Ebert D, Lehr D, Riper H, Smit F, Cuijpers P, Berking M. Effectiveness of a web-based cognitive behavioural intervention for subthreshold depression: pragmatic randomised controlled trial. Psychother Psychosom. 2015;84:348–358. doi: 10.1159/000438673. [DOI] [PubMed] [Google Scholar]
- 41.Cuijpers P, van SA, Warmerdam L, Smits N. Characteristics of effective psychological treatments of depression: a metaregression analysis. Psychother Res. 2008;18:225–236. doi: 10.1080/10503300701442027. [DOI] [PubMed] [Google Scholar]
- 42.Schröder J, Brückner K, Fischer A, Lindenau M, Köther U, Vettorazzi E, Moritz S. Efficacy of a psychological online intervention for depression in people with epilepsy: A randomized controlled trial. Epilepsia. 2014;55:2069–2076. doi: 10.1111/epi.12833. [DOI] [PubMed] [Google Scholar]
- 43.Fischer A, Schröder J, Vettorazzi E, Wolf OT, Pöttgen J, Lau S, Heesen C, Moritz S, Gold SM. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. The Lancet Psychiatry. 2015;2:217–223. doi: 10.1016/S2215-0366(14)00049-2. [DOI] [PubMed] [Google Scholar]
- 44.Warlow C. Advanced issues in the design and conduct of randomized clinical trials: the bigger the better? Stat Med. 2002;21:2797–2805. doi: 10.1002/sim.1283. [DOI] [PubMed] [Google Scholar]
- 45.Rozental A, Andersson G, Boettcher J, Ebert DD, Cuijpers P, Knaevelsrud C, Ljótsson B, Kaldo V, Titov N, Carlbring P. Consensus statement on defining and measuring negative effects of internet interventions. Internet Interventions. 2014;1:12–19. [Google Scholar]
- 46.Titov N, Andrews G, Kemp A, Robinson E. Characteristics of adults with anxiety or depression treated at an internet clinic: comparison with a national survey and an outpatient clinic. PLoS One. 2010;5:e10885. doi: 10.1371/journal.pone.0010885. [DOI] [PMC free article] [PubMed] [Google Scholar]