Abstract
Homodimeric class I cytokine receptors are assumed to exist as preformed dimers that are activated by ligand-induced conformational changes. We quantified the dimerization of three prototypic class I cytokine receptors in the plasma membrane of living cells by single-molecule fluorescence microscopy. Spatial and spatiotemporal correlation of individual receptor subunits showed ligand-induced dimerization and revealed that the associated Janus kinase 2 (JAK2) dimerizes through its pseudokinase domain. Oncogenic receptor and hyperactive JAK2 mutants promoted ligand-independent dimerization, highlighting the formation of receptor dimers as the switch responsible for signal activation. Atomistic modeling and molecular dynamics simulations based on a detailed energetic analysis of the interactions involved in dimerization yielded a mechanistic blueprint for homodimeric class I cytokine receptor activation and its dysregulation by individual mutations.
Cytokines bind their cognate receptors to regulate hematopoiesis and immunological homeostasis. Consequently, imbalances in levels of circulating cytokines, or mutations that alter cytokine receptor function, can lead to severe pathological effects, ranging from aberrant immune responses to hematological malignancies. Therefore, a complete understanding of the dynamics and mechanisms underpinning cytokine receptor activation is critical.
The class I cytokine receptor family includes receptors for interleukins, colony-stimulating factors, and hormones. These receptors lack intrinsic kinase activity, relying instead on associated Janus kinase (JAK) proteins to initiate signal transduction. The precise activation mechanism of class I cytokine receptors, however, remains unclear (1). Originally, ligand-induced receptor dimerization was assumed to trigger signal activation (2). However this model has been replaced by more complex concepts that propose ligand-induced conformational changes of pre-dimerized receptor subunits (Fig. 1A) (1, 3, 4). Supported by extensive structural and biochemical studies, homodimeric class I cytokine receptors such as the erythropoietin (Epo) and growth hormone (GH) receptors have become a paradigm for pre-assembled receptor dimers (1, 5–7). Moreover, pre-dimerization has also been reported for numerous heterodimeric class I and class II cytokine receptors (8–12), which suggests that receptor pre-dimerization may be a generic feature of this receptor family (1, 4). However, the molecular mechanism responsible for triggering JAK activation by preformed receptor dimers has remained rather speculative. Current models cannot convincingly explain receptor dysregulation by constitutively activating, oncogenic JAK mutations. Notably, hyperactivity of JAK2 caused by somatic mutations such as Val617 → Phe (JAK2 V617F) is the most common cause of the Philadelphia chromosome–negative myeloproliferative neoplasms (Ph− MPNs) (13, 14). Gaining a mechanistic understanding of pathway activation could inspire more specific strategies to target the aberrant signaling that underlies such MPNs.
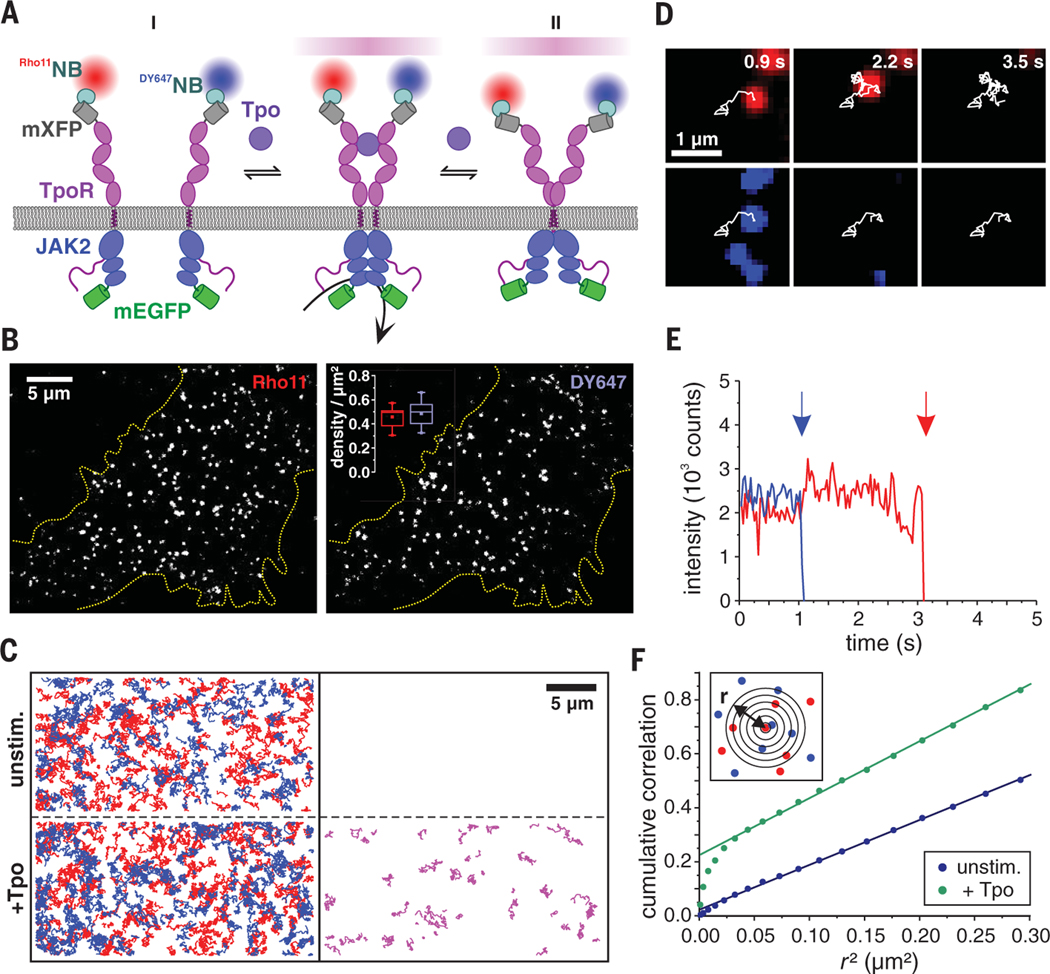
Fig. 1. Receptor monomer-dimer equilibrium quantified by dual-color single-molecule imaging.
(A) Cytokine receptor activation by ligand-induced dimerization (I) versus ligand-induced conformational change of pre-formed dimers (II) schematically depicted for TpoR. Receptor subunits fused to mXFP were labeled with anti-GFP nanobodies (NB) conjugated to Rho11 (Rho11NB) and DY647 (Dy647NB) at equal concentrations. Receptor homodimers carrying both Rho11 and DY647 are identified by co-tracking analysis (stochastically only 50% of the entire dimer population). Coexpression of JAK2 wild-type and JAK2 variants fused to mEGFP ensures unambiguous detection at the single-cell level. (B) Individual mXFP-TpoR subunits in the plasma membrane of HeLa cells after labeling with Rho11NB and Dy647NB. The densities of molecules in each channel are depicted in the inset (calculated from 15 cells). In this and later figures, box plots indicate the data distribution of the second and third quartiles (box), median (line), mean (square), and 1.5× interquartile range (whiskers). (C) TpoR tracking and co-tracking analysis shown for representative cells. Left: Trajectories (150 frames, ~4.8 s) of individual Rho11-labeled (red) and DY647-labeled (blue) TpoR subunits before (top) and after (bottom) addition of Tpo. Right: Receptor dimers identified by co-locomotion analysis. (D and E) Single-step photobleaching observed for an individual TpoR dimer (red, Rho11; blue, Dy647) in the presence of Tpo (D) and intensity-time traces with bleaching events indicated by arrows (E). (F) Spatial correlation of TpoR at single-molecule level by PICCS as schematically indicated in the inset. Representative results for a cell in the absence (blue) and presence of Tpo (green), respectively, are shown.
Directly observing the spatiotemporal organization of cytokine receptors in the plasma membrane of living cells under physiological conditions has been hindered by their very low cell surface expression levels, which are below the detection limit of conventional fluorescence microscopy (15, 16). We devised a method for quantifying the monomer-dimer equilibrium of homodimeric class I cytokine receptors at physiological densities in the plasma membrane by dual-color single-molecule fluorescence imaging in combination with posttranslational cell surface labeling. We found that thrombopoietin receptor (TpoR), Epo receptor (EpoR), and GH receptor (GHR) are monomeric and randomly distributed in the plasma membrane in the resting state, yet efficiently dimerize upon ligand binding. By quantifying the monomer-dimer equilibrium for various receptor and JAK variants, we obtained a comprehensive energetic landscape of ligand- and oncogene-induced receptor dimerization, revealing finely tuned, additive interactions involving JAK and receptor domains. On the basis of these quantitative insights in conjunction with atomistic molecular dynamics (MD) simulations, we suggest a mechanism of homodimeric class I cytokine receptor activation by ligand-induced dimerization, which can explain their dysregulation by individual mutations.
Random distribution and diffusion of TpoR in the plasma membrane
For time-lapse dual-color single-molecule imaging at the plasma membrane of live HeLa cells by total internal reflection fluorescence microscopy (TIRFM), receptor subunits were stochastically labeled with photostable fluorescent dyes by using equal concentrations of anti-GFP (green fluorescent protein) nanobodies conjugated to either Rho11 (Rho11NB) or DY647 (DY647NB) (Fig. 1A). To this end, EpoR, TpoR, and GHR were N-terminally fused to monomeric enhanced GFP (mEGFP) that had been rendered nonfluorescent by the mutation Tyr66 → Phe (mXFP). Because the endogenous JAK2 levels in HeLa cells are negligibly low, JAK2 fused to mEGFP (JAK2-mEGFP) was coexpressed with the receptor subunit to verify functional integrity at the single-cell level. Efficient association of JAK2-mEGFP with the receptor (fig. S1), as well as uncompromised activity of mXFP-tagged receptor and JAK2mEGFP (figs. S2 and S3A), were confirmed. Representative results of dual-color single-molecule imaging experiments obtained for mXFP-TpoR expressed in HeLa cells are summarized in Fig. 1, B to F. After labeling with Rho11NB and DY647NB, individual TpoR subunits randomly diffusing in the plasma membrane could be discerned (Fig. 1B and movie S1). The presence of individual receptor subunits was confirmed by single-step photobleaching at elevated laser power (movie S2 and fig. S4A).Long–time scale single-molecule localization microscopy of TpoR confirmed largely homogeneous spatiotemporal distribution across the plasma membrane (fig. S4B). Despite coexpression of JAK2, a low cell surface receptor density was obtained, with typically 0.4 to 0.5 molecules/μm2 in each spectral channel and a Rho11/Dy647 ratio close to unity (Fig. 1B). Taking into account an effective labeling degree of ~70% achieved by both Rho11NB and DY647NB (fig. S4C), the total cell surface concentration of receptor subunits was ~1 to 1.5 molecules/μm2. Very similar cell surface densities of endogenous TpoR were found in UT-7/Tpo cells, a megakaryocytic cell line commonly used for functional studies on TpoR (17), as quantified by flow cytometry experiments with fluorescence-labeled Tpo (fig. S4D). These observations confirm the physiological relevance of low cell surface expression in our model system and suggest that TpoR cell surface densities are under tight control.
Ligand-induced TpoR dimerization revealed by single-molecule co-tracking
Receptor dimerization was quantified by colocalization and co-tracking of individual receptor subunits detected in both spectral channels. We recognized that, statistically, only half of the dimerized receptors would be labeled with two different colors (18). This method was calibrated on the basis of negative and positive control experiments with a model transmembrane protein that was dimerized via a monoclonal antibody (movie S3 and fig. S5A). For TpoR coexpressed with JAK2, very few (on average less than one per cell) co-trajectories of Rho11NB- and DY647NB-labeled receptors could be detected in the absence of ligand (Fig. 1C and movie S4). By contrast, strong TpoR dimerization was observed upon addition of Tpo, yielding a large number of single-molecule co-trajectories (Fig. 1C and movie S4). The stoichiometry of TpoR homodimers was confirmed by single- and dual-color photobleaching experiments (Fig. 1, D and E, and fig. S5B). The significant increase in Rho11 fluorescence upon photobleaching of DY647 indicated FRET within co-locomoting TpoR dimers, confirming molecular proximity within ligand-induced TpoR dimers (Fig. 1E and fig. S5C). TpoR dimerization levels increased up to a concentration of 10 nM Tpo and then decreased at elevated concentrations, yielding a bell-shaped dose-dimerization curve that essentially matched the bell-shaped dose-response curve of STAT5 phosphorylation (fig. S3, A and B). This concentration-dependent self-inhibition can be explained by a gradual shift from a 1:2 to a 1:1 Tpo:TpoR stoichiometry. Ligand-induced receptor dimerization was accompanied by a small yet significant decrease of ~25% in the overall diffusion constant (fig. S5D and table S1) that can be ascribed to the increased friction within the membrane for receptor dimers relative to monomers (19, 20). Furthermore, the fraction of immobile receptors increased after addition of ligand (table S1), which is in line with receptor endocytosis upon activation (21, 22). Very similar diffusion properties in the absence and presence of Tpo were obtained for TpoR labeled via the 21–amino acid E3 tag (23) (fig. S5D and table S1), confirming minimal bias of receptor diffusion and interaction by the mXFP tag. Significantly lower levels of ligand-induced dimerization were observed in the absence of JAK2 (Fig. 2A). The diffusion constants of TpoR monomers and dimers were ~15% lower when JAK2 was coexpressed (fig. S5E and table S1).
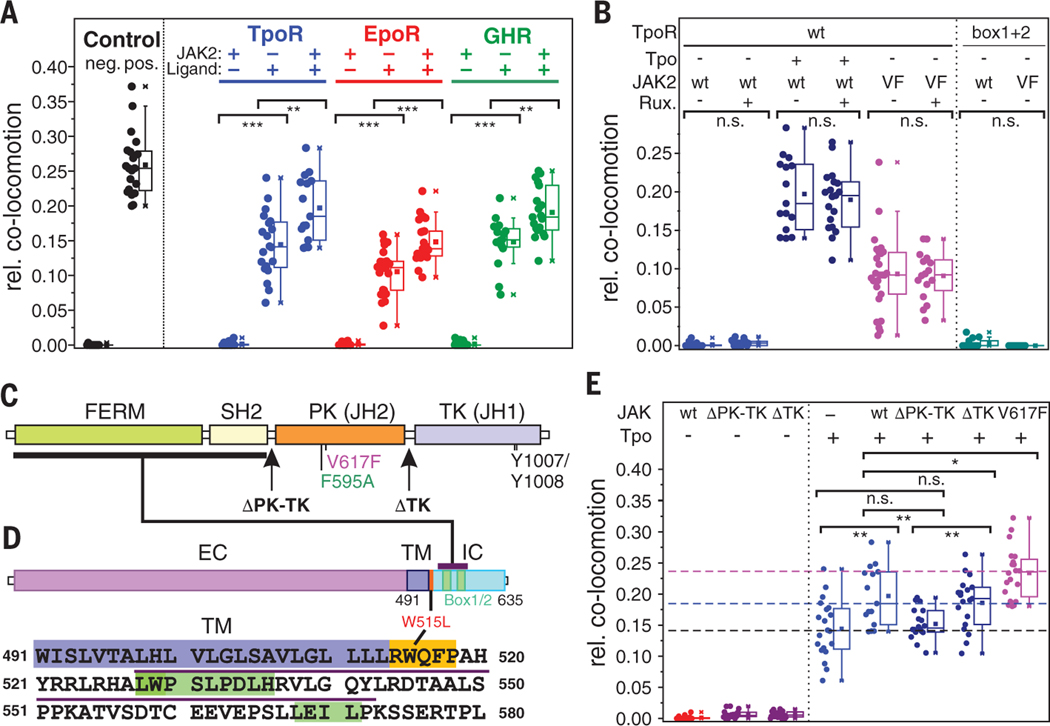
Fig. 2. JAK2 regulates ligand-induced dimerization of TpoR, EpoR, and GHR.
(A) Relative number of co-trajectories observed for positive and negative control proteins as well as for unstimulated TpoR, EpoR, and GHR and after stimulation with the respective ligand with and without coexpression of JAK2. (B) Comparison of dimerization levels in the absence and presence of the JAK2 inhibitor ruxolitinib (left) and dimerization levels of TpoR Box1+2 mutant (right) coexpressed with JAK2 wild-type (wt) or V617F (VF). (C) Primary structure of JAK2 comprising FERM-SH2 (FS), pseudokinase (PK), and tyrosine kinase (TK) domains. Positions of the C-terminal truncations ΔTK and ΔPK-TK as well as key residues and mutations are high-lighted. (D) Primary structure of TpoR including extracellular (EC), transmembrane (TM), and intracellular (IC) domains. The primary sequence of the TM domain (blue) followed by a functionally critical amphipathic motif (orange) and the intracellular domain (ICD) including the Box motifs (green) is shown below. The putative JAK2 binding sequence is indicated by a purple overline. Amino acid abbreviations: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; Y, Tyr. (E) Ligand-induced dimerization of TpoR coexpressed with different JAK2 variants as identified in (C). Dashed lines mark the mean dimerization levels in the absence (black) and presence of JAK2 wt (blue) or JAK2 V617F (magenta), respectively. In (A), (B), and (E), each data point represents the analysis from one cell with a minimum of 10 cells measured for each condition. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001; n.s., not significant.
Dissociation of ligand-induced TpoR dimers was observed very rarely within the experimental time frame. A reliable determination of complex lifetimes was therefore not possible because of a lack of co-tracking fidelity as well as photobleaching. These observations suggest that the stability of ligand-induced TpoR dimers is high relative to the imaging time resolution. To exclude the possibility that TpoR pre-dimerization was missed by co-tracking analysis because of very short dimer lifetimes in the absence of ligand, we quantified the spatial organization of receptor subunits in the plasma membrane by particle image cross-correlation spectroscopy (PICCS) (24) of individual TpoR subunits detected in both channels. Spatial cross-correlation above the background was observed only after adding the ligand (Fig. 1F, fig. S6, C and D, and table S2), yielding dimerization levels similar to those determined by co-locomotion analysis. Quantification of the relative number of co-trajectories yielded ligand-induced dimerization levels that were 75% of those of a positive control based on a model transmembrane protein that was dimerized by a high-affinity monoclonal antibody (Fig. 2A and movie S3). Antibody-induced dimerization of the model transmembrane protein reduced its diffusion constant in a way similar to that observed for ligand stimulation of TpoR (table S1).
Comparable spatiotemporal dynamics of TpoR, EpoR, and GHR dimerization
For EpoR and GHR, very similar properties with respect to diffusion and interaction in the plasma membrane were observed: The receptor cell surface expression was low, as previously found for EpoR in erythroid cell types (15) and in binding experiments with labeled GH bound to endogenous GHR in GH-responsive (25) HuH7 cells (figs. S7 and S8). Uncorrelated receptor diffusion was observed in the absence of ligand, and efficient dimerization occurred in the presence of ligand with a bell-shaped concentration dependence (Fig. 2A, movies S5 and S6, and figs. S3B, S7, A and B, and S8, A and B). Similar levels of ligand-induced dimerization (50% and 60% for EpoR and GHR, respectively) were observed, which depended on the presence of JAK2, as well as similar changes in the diffusion properties (Fig. 2A, figs. S7C and S8C, and table S1). However, assays with GHR using cells cultured in the presence of fetal calf serum were strongly biased by traces of bovine GH (fig. S8D). For this reason, dimerization was quantified after serum starving and scavenging of residual bovine GH by adding purified soluble GHR ectodomain. Under these conditions, ligand-induced dimerization was unambiguously confirmed for GHR. By contrast, antagonistic Epo (Ser126 → Glu) and GH (Gly146 → Arg) mutants (26, 27) did not dimerize their receptors in the plasma membrane (figs. S7D and S8E).
JAK2 PK domains contribute to receptor dimerization
The increased levels of ligand-induced dimerization that were obtained for all three receptors in the presence of JAK2 (Fig. 2A) suggest that the associated JAKs energetically contribute to receptor dimerization. To exclude the possibility that dimerization was enhanced by downstream signal activation, we compared TpoR dimerization in the absence and presence of the JAK2 inhibitor ruxolitinib, which remained unchanged (Fig. 2B). These observations suggested stabilizing interactions between the associated JAKs, which have been implicated in regulating the activation of receptor tyrosine kinases (28). JAKs comprise four domains: The intimately connected N-terminal FERM and SH2-like (FS) domains bind the receptor through its membrane proximal box 1 and box 2motifs (Fig. 2, C and D), whereas the C-terminal tyrosine kinase (TK) domain is regulated by the adjacent pseudokinase (PK) domain, which lacks tyrosine kinase activity. To identify the role played by the TK and PK domains in contributing to the additional binding energy, we quantified TpoR dimerization in the presence of JAK2 variants in which the TK domain (JAK2 ΔTK) or both TK and PK domains (JAK2 ΔPK-TK) were truncated (Fig. 2C). These experiments established that stabilization of ligand-induced dimers was maintained in the absence of the TK domain but was abrogated for JAK2 ΔPK-TK (Fig. 2E).
The oncogenic JAK2 V617F mutation induces ligand-independent receptor dimerization
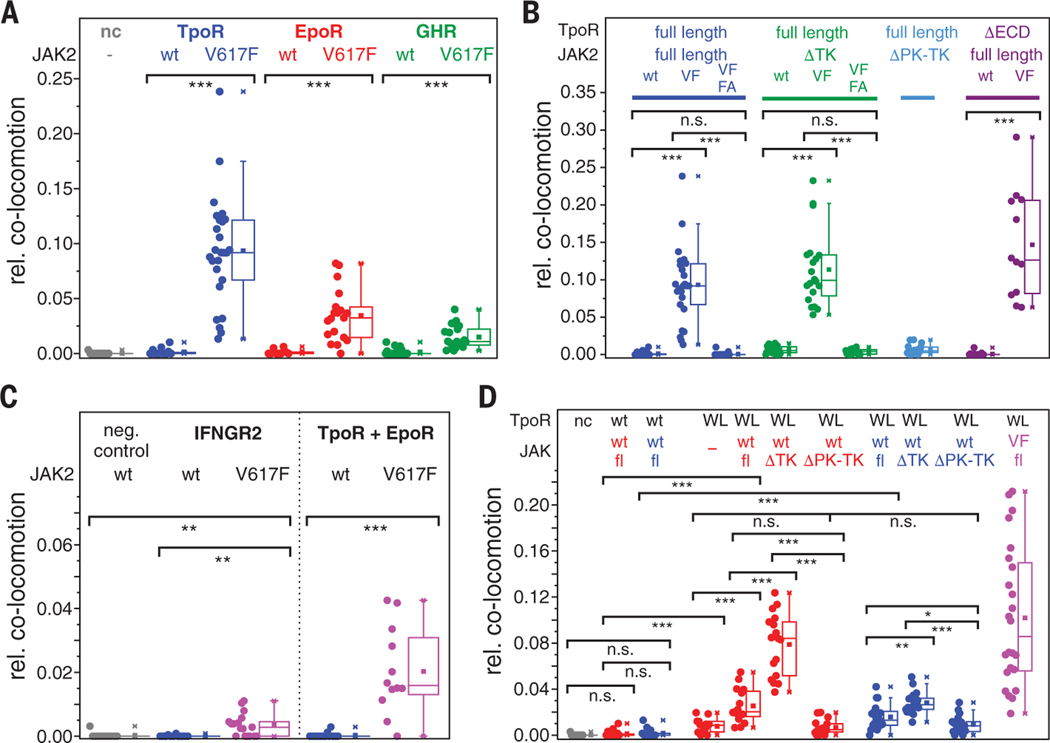
These data suggest that intermolecular JAK2 interactions involving the PK domains may be important for JAK activation. The JAK2 PK domain is the primary site of somatic mutations in Ph– MPNs (14, 29). For example, JAK2 V617F is hyperactive (fig. S2C), and the consequent factor-independent proliferation relies on the coexpression of a homodimeric class I cytokine receptor, usually either EpoR or TpoR, at the cell surface (30, 31). However, the mechanisms underlying JAK2 V617F activation remain elusive. Strikingly, coexpression of JAK2 V617F yielded substantial ligand-independent dimerization of TpoR, EpoR, and GHR (Fig. 3A and movies S4 to S6). Relative to ligand-induced dimerization, ligand-independent dimerization in the presence of JAK2 V617F reached ~50% of the maximum level for TpoR, ~25% for EpoR, and ~10% for GHR, respectively. Single-molecule photobleaching confirmed that JAK2 V617F induced formation of TpoR dimers, and single-molecule FRET (smFRET) confirmed the molecular proximity of both subunits (fig. S9A). Ligand-independent JAK2 V617F–induced receptor dimers showed diffusion properties that were very similar to those of the corresponding ligand-induced receptor dimers in the presence of wild-type JAK2 (table S1 and figs. S5E, S7E, and S8G). Somewhat higher FRET efficiencies were observed for ligand-independent dimerization of TpoR in the presence of V617F relative to TpoR dimers formed in the presence of Tpo (fig. S9A), suggesting differences in the structural organization of the receptor ectodomains in these dimers. Ligand-independent dimerization of TpoR in the presence of JAK2 V617F did not rely on JAK2 TK activity, as confirmed by treatment with ruxolitinib (Fig. 2B). Moreover, no dimerization of TpoR by JAK2 V617F was found for a box 1/box 2mutant (TpoR Box 1+2) that is defective in JAK recruitment to the receptor (Fig. 2B).
Fig. 3. Oncogenic JAK2 and TpoR mutants drive ligand-independent receptor dimerization.
(A) Ligand-independent dimerization of TpoR, EpoR, and GHR by JAK2 V617F. Co-locomotion was quantified for TpoR, EpoR, and GHR coexpressed with either JAK2 or JAK2 V617F, compared to a negative control (nc). (B) Ligand-independent dimerization by JAK2 V617F is driven by the pseudokinase domain. Co-locomotion was analyzed for the indicated receptor and JAK2 variants. For ΔECD-TpoR, dimerization by JAK2 V617F was quantified by single-molecule FRET (see movie S7). (C) Homo- and heterodimerization of cytokine receptors by JAK2 V617F. Left: Homodimerization of IFNGR2. Right: Heterodimerization of EpoR and TpoR, which were orthogonally labeled via mXFP and SNAPf-tag, respectively (see movie S8). (D) Ligand-independent dimerization of TpoR W515L (WL) in the absence and presence of different JAK2 (red) and TYK2 (blue) variants and JAK2 V617F (VF, magenta). In (A) to (D), each data point represents the analysis from one cell with a minimum of 10 cells measured for each condition. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Additional molecular determinants contribute to JAK2 V617F–mediated dimerization
These results support our hypothesis that JAK2 V617F short-circuits ligand-induced receptor dimerization and highlight the critical role of the PK domain. We therefore explored in more detail the molecular determinants of JAK2 V617F–induced dimerization of TpoR. Combination of V617F with the mutation Phe595 → Ala (F595A), which effectively abolishes constitutive activation by V617F (32), largely neutralized ligand-independent dimerization of TpoR by JAK2 V617F (Fig. 3B). In the absence of the tyrosine kinase domain (DTK), JAK2 V617F dimerized TpoR in a ligand independent manner and remained sensitive to the combination with F595A (Fig. 3B). Strikingly, dimerization and activation of TpoR by JAK2 V617F occurred independently of the receptor ectodomain (Fig. 3B, movie S7, and fig. S9, B and C). Taken together, these results suggest that the V617F mutation enhances interactions between the PK domains. Further enhanced dimerization levels of TpoR were observed in the presence of Tpo and JAK2 V617F (Fig. 2E). The substantial differences observed between JAK2 V617F–induced dimerization of EpoR, TpoR, and GHR, however, suggested that the receptor itself also contributes to dimerization. We therefore explored whether JAK2 V617F was capable of driving homodimerization of the interferon-γ receptor subunit IFNGR2. Significant dimerization, although much lower than with EpoR, TpoR, and GHR, was observed for IFNGR2 (Fig. 3C), confirming the critical role of the receptor in JAK2 V617F–mediated dimerization. JAK2 V617F was even capable of driving substantial heterodimerization between mXFP-TpoR and SNAPf-EpoR (Fig. 3C and movie more stable dimerization was observed upon S8); this result served to corroborate that ligand-independent dimerization was largely driven by JAK2 V617F.
Membrane-proximal amphipathic segment is involved in receptor dimerization
To shed light on potential interactions directly mediated by the receptor, we focused on the juxtamembrane (JM) amphipathic motif of TpoR (Fig. 2D), which is critical in regulating TpoR activation (33). Most prominently, the mutation Trp515 → Leu (W515L) is an oncogenic mutation that constitutively activates TpoR signaling (34). Structural studies on the transmembrane (TM) and JM domains of TpoR by solid-state nuclear magnetic resonance have suggested that W515L enhances interaction of the TM domains (35). Similar weak interactions between the TM domains have been reported for EpoR (36) and GHR (6, 7). Strikingly, we observed substantial dimerization of TpoR W515L in the absence of ligand (Fig. 3D). Although ligand-independent dimerization of TpoR W515L in the absence of JAK2 was weak and transient, stronger and more stable dimerization was observed upon coexpression of JAK2 (Fig. 3D and movie S9), confirming cooperative interactions mediated by TpoR TM-JM and JAK2 PK domains. Like-wise, a significant (but lesser) increase in ligand-independent dimerization was observed for TpoRW515Lwhen coexpressing TYK2 instead of JAK2. TYK2 complements the activity of TpoR in the absence of JAK2, although with lower potency (37). Whereas deletion of the TK domains further increased ligand-independent TpoR W515L dimerization (Fig. 3D), truncation of the PK domain decreased dimerization to the level in the absence of JAK2/TYK2 (Fig. 3D), confirming productive PK-PK interactions in W515L-induced dimers. Ligand-independent dimerization was enhanced upon coexpression of TpoR W515Lwith JAK2 V617F (Fig. 3D), highlighting the additive nature of these interactions, in line with their additivity in ligand-independent signaling activities (38).
Additive interactions control the assembly of the signaling complex
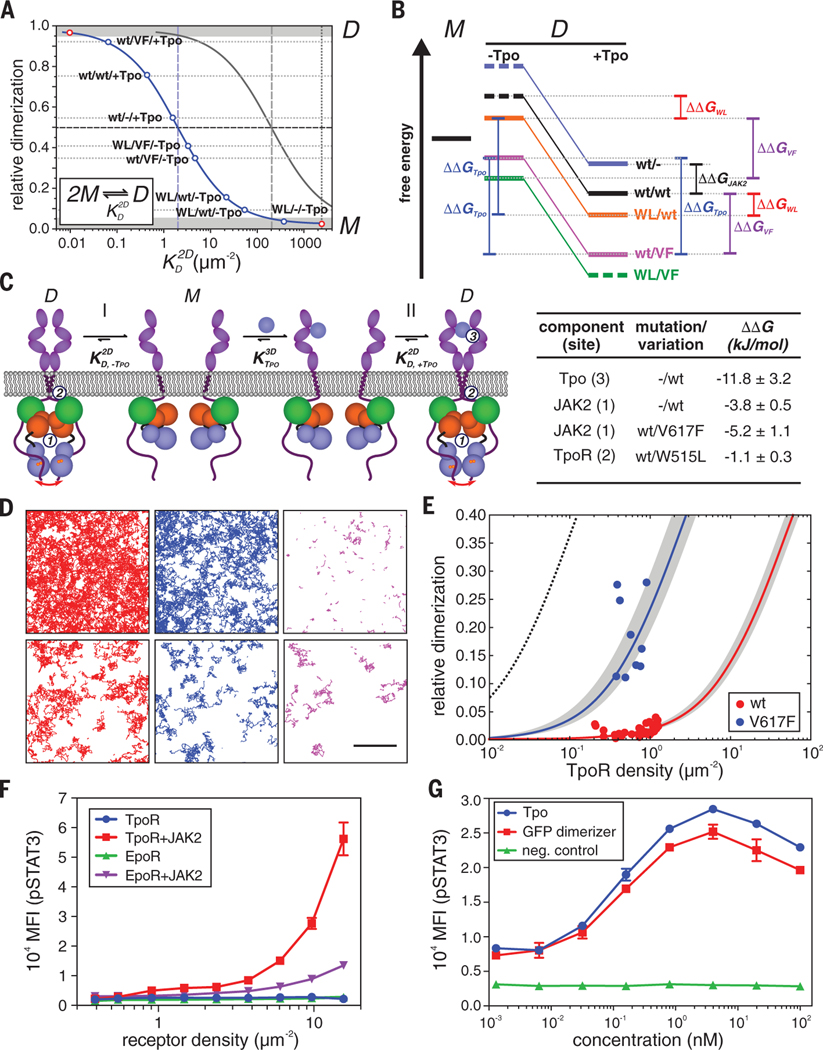
To quantify the different energetic contributions involved in the assembly of the TpoR signaling complex, we converted dimerization levels into two-dimensional equilibrium dissociation constants according to the law of mass action for a monomer-dimer equilibrium (Fig. 4A and table S3). These were used to estimate the energetic contributions, ΔΔG, of the different interaction sites within the receptor, as schematically outlined in Fig. 4B. The ΔΔG values were determined under the assumption that ligand-mediated dimerization, as well as the interactions mediated by the JAK2 PK domains and by the TM/JM domains, additively contribute to the total binding energy (Fig. 4C). Exploiting the availability of the constitutively dimerizing mutants TpoR W515L and JAK2 V617F, we obtained a comprehensive energetic picture for the different interactions (Fig. 4B). These moderate binding energies highlight the cooperativity of finetuned subtle interactions regulating receptor dimerization. Our results indicate a low intrinsic dimerization affinity of the TpoR/JAK2 subunits, which is attributed to interactions between the JAKPK domains and the TM/JM region of the receptor (sites 1 and 2, respectively, in Fig. 4C). At physiological receptor densities at the plasma membrane, the total binding energy of these interactions is not sufficient to yield significant dimerization in the absence of ligand (Fig. 4C). Thus, the additional binding energy provided by ligand-mediated receptor cross-linking effectively shifts the equilibrium toward receptor dimers (Fig. 4C). Likewise, a small increase in the binding energy of the intrinsic interactions upon single point mutations in the constitutive interacting sites can shift the equilibrium toward receptor dimers, as observed for TpoR W515L and JAK2 V617F.
Fig. 4. Energy landscape of TpoR dimerization and its mechanistic interpretation.
(A) Determination of 2D equilibrium dissociation constants from the dimerization levels observed under different conditions. Each dot corresponds to a dimerization experiment where the label denotes TpoR [wt or W515L (WL)]/JAK2 [wt or V617F (VF)]/ligand (+/–Tpo). The 2D law of mass action is depicted for a monomer-dimer (M-D) equilibrium at a total receptor surface concentration of 2 μm–2 (blue) and 200 μm–2 (gray). Dimerization levels that cannot be unambiguously quantified by co-tracking are indicated by gray zones. (B) Semi-quantitative energy diagram of the M-D equilibrium in the absence (–Tpo) and presence (+Tpo) of ligand as derived from (A). Experiments involving different TpoR/JAK2 combinations are depicted in different colors; energy levels for determining different values of ΔΔG are indicated. Energetic contributions ΔΔG obtained for different combinations of components and mutations are listed in the table below. (C) Proposed mechanism of homodimeric cytokine receptor activation deduced from live-cell dimerization assays: In the absence of ligand (I), the basal level of dimerization caused by interactions mediated via the JAK2 PK domains (1) and TM/JM domains (2) is negligible because substantially exceeds the receptor surface concentration in the plasma membrane. Ligand binding provides the additional binding energy (3) required to shift the equilibrium toward the dimeric state. Oncogenic mutations enhancing interactions 1 or 2 shift the equilibrium toward the dimeric state in a ligand-independent manner (II). (D to F) Intrinsic dimerization and activation of TpoR and EpoR. (D) Representative smFRET experiments with TpoR coexpressed with JAK2-mEGFP wt (top) and V617F (bottom) showing single-molecule trajectories of the donor (red) as well as the acceptor upon direct excitation (blue) and via smFRET (magenta) detected within 150 frames (5 s). Total receptor densities were 1.2/μm2 for JAK2 wt and 0.4/μm2 for JAK2 V617F. Scale bar, 5 μm. (E) Relative ligand-independent dimerization levels as a function of receptor density for full-length TpoR in the presence of JAK2 wt and V617F and fit by the law of mass action for a monomer-dimer equilibrium (solid lines). Confidence intervals of the fit are indicated as gray zones. The dimerization curve in the presence of Tpo calculated from the corresponding is shown for comparison (black dotted line). (F) Ligand-independent activation of STAT3 phosphorylation upon overexpression of TpoR and EpoR, respectively, together with JAK2 wt in HeLa cells. pSTAT3 and receptor cell surface densities were quantified by phospho-flow analysis. As a negative control, coexpression of JAK2 was omitted. (G) Activation of mXFP-TpoR by dimerization with an NB-based cross-linker that binds the mXFP-tag. For comparison, activation by Tpo in the presence and absence of TpoR (neg. control) is shown. In (F) and (G), error bars denote SEM.
Weak intrinsic receptor dimerization correlates with constitutive activation
These analyses predict a low intrinsic dimerization affinity for homodimeric class I cytokine receptors. To experimentally quantify the of ligand-independent receptor dimerization, we used single-molecule FRET (smFRET) for direct detection of dimer formation via sensitized fluorescence. Thus, ligand-independent interaction of the receptors could be reliably detected and quantified even at relatively high receptor densities, which were not compatible with robust co-tracking analysis. Under these conditions, ligand-independent TpoR dimers could be observed in the presence of wild-type JAK2, whereas much higher levels were observed in the presence of JAK2 V617F even at much lower receptor densities (Fig. 4D, fig. S9D, and movie S10). Substantially shorter smFRET trajectories were found for TpoR dimers in the presence of wild-type JAK2 compared to JAK2 V617F, suggesting rather transient dimerization under wild-type conditions. These observations support our hypothesis that the V617F mutation promotes receptor dimerization by stabilizing inter-JAK2 interactions. Relative dimerization increased in a receptor density–dependent manner (Fig. 4E). By fitting the law of mass action, a two-dimensional of 110 (±60)/μm2 was determined for TpoR in the presence of wild-type JAK2 compared to 5 (±2)/μm2 for JAK2 V617F, in good agreement with the values determined from co-locomotion analysis (table S3). Similar results were observed with variants of EpoR and TpoR lacking the extracellular domains (EpoR-ΔECD and TpoR-ΔECD, respectively) (fig. S9, E and F, and table S3). These observations are in line with the activation of EpoR via synthetic cross-linkers (19, 39). Likewise, efficient activation of TpoR was achieved by dimerization through binding of a flexible cross-linker based on the NB against the mXFP-tag (Fig. 4G).
Dimerizing and nondimerizing oncogenic JAK2 mutations
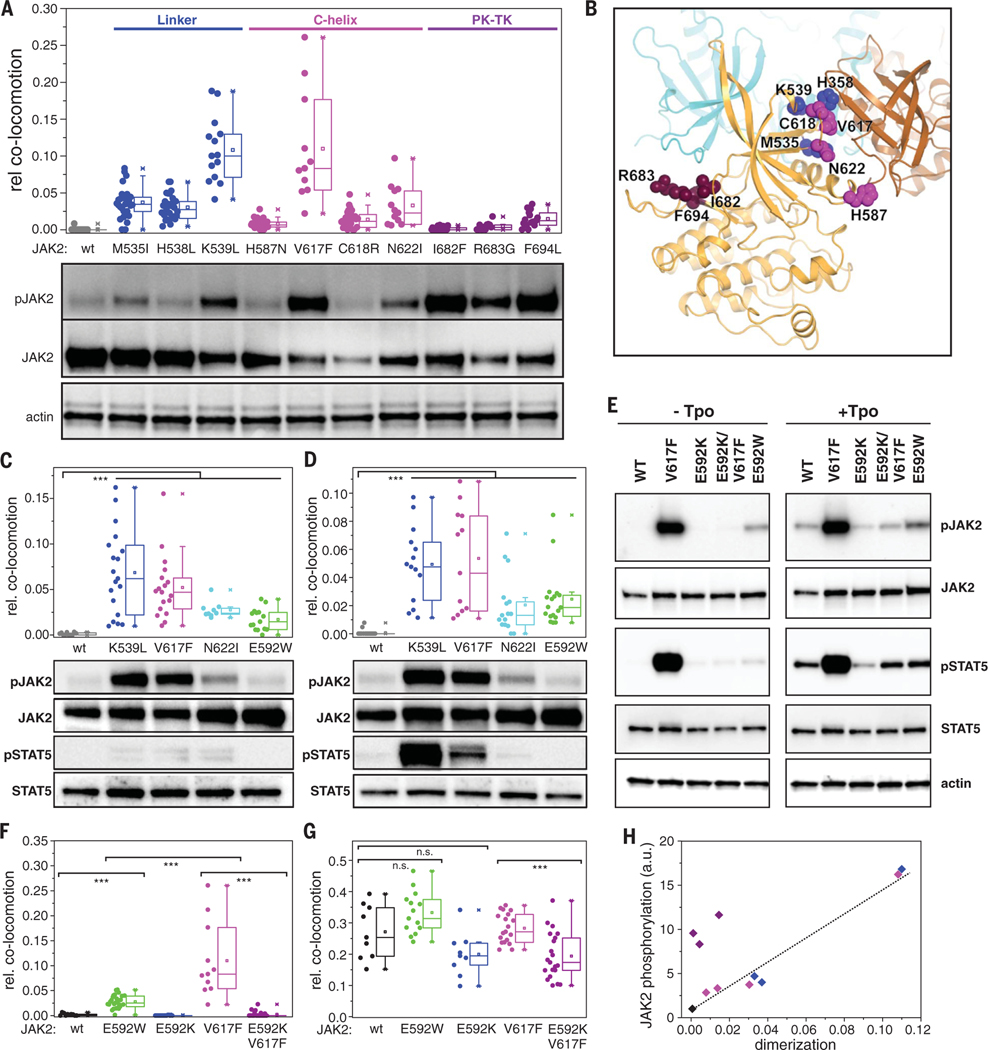
To further investigate the mechanism of signal activation, we compared various oncogenic mutations in the JAK2 PK domain (Fig. 5, A to D) (40). Three groups of mutations were chosen covering the FS-PK linker region (M535I, H538L, and K539L; group I), residues in the proximity of the αC helix (H587N, C618R, N622I; group II), and a hotspot at the autoinhibitory PK-TK interface (41, 42) (I682F, R683G, F694L; group III) (Fig. 5, A and B). Whereas ligand-independent activation was confirmed for all these JAK2 mutants, consistent TpoR dimerization was observed only for mutations within the first two groups (Fig. 5, A and B, and fig. S10). In contrast, JAK2 mutations at the PK-TK interface (group III) yielded very low dimerization levels relative to their potent constitutive activation. This disconnect suggests that gain-of-function mutations in the PK cause JAK2 hyperactivity via distinct mechanisms: (i) loss of PK-mediated autoinhibition, facilitating trans-autophosphorylation of the TK domains in the context of receptor-JAK2 monomers (group III), and (ii) stabilization of receptor-JAK2 dimers (groups I and II). Similar dimerization and activation patterns were observed for EpoR and GHR upon expression of selected mutants from groups I and II (Fig. 5, C and D), supporting the common mechanistic basis for mutational hyperactivation of different receptors.
Fig. 5. Dimerization interface of JAK2 PK domains.
(A) Ligand-independent dimerization of TpoR (top) and associated JAK2 phosphorylation (bottom) in the presence of oncogenic mutations within the JAK2 PK domain. Residues are grouped and colored according to their location within the PK structure: FS-PK linker (blue); αC helix (magenta), and PK-TK interface (purple). (B) Putative intermolecular PK-PK interface derived from the MD simulations, with one PK domain colored orange and the other brown. The positions of the residues mutated in (A) are mapped onto the orange PK domain. Superimposed in cyan is the TK domain in its autoinhibitory configuration (intramolecular) relative to the orange PK domain; the TK domain would clash with the second (brown) PK domain. (C and D) Ligand-independent dimerization of EpoR (C) and GHR (D) (top) and associated JAK2 phosphorylation (bottom) for selected constitutively active JAK2 mutants. (E to G) Altering dimerization and activation by perturbation of the putative PK-PK interface via mutagenesis of Glu592. (E) Activity of different JAK2 mutants in HeLa cells stably expressing mXFP-TpoR. Phosphorylation of JAK2 and STAT5 in the absence of ligand (left) and after stimulation with Tpo (right) was probed by Western blot. [(F) and (G)] Dimerization of TpoR associated with different JAK2 mutants in the absence (F) and presence (G) of Tpo. (H) Correlation of receptor dimerization with activation for constitutively active JAK2 mutants [same color coding as in (A)]. Error bars are omitted for clarity. In (A), (C), (D), (F), and (G), each data point represents the analysis from one cell with a minimum of nine cells measured for each condition. ***P ≤ 0.001.
Putative PK-PK interface mediates receptor dimerization and activation
Interestingly, groups I and II both localize to a putative PK-PK interface (43, 44), which has been proposed based on the JAK1 PK crystal structure (45). This interaction is mediated by the N lobe of the PK domain, predominantly the αC-helix, and the linker connecting the FS and PK domains (43, 45, 46). We focused on residue Glu592, which is located at the center of the interface (Fig. 5B). In the context of TpoR, the mutation Glu592→Ala did not alter basal (non–cytokine-mediated) JAK2 activation (33); however, activity increased upon introduction of a large hydrophobic residue [Glu592 →Trp (E592W); Fig. 5E and fig. S11A], and this correlated with ligand-independent dimerization of TpoR (Fig. 5F). In contrast, switching charge at this position [Glu592 → Lys (E592K)] led to slightly reduced levels of activation and dimerization in the presence of Tpo (Fig. 5, E and G) and substantially suppressed the hyperactivity of V617F (E592K/V617F) by reducing dimerization (Fig. 5, E and F, and fig. S11A).
TpoR density–dependent activation assays with JAK2 Glu592 mutants showed that E592W shifted the onset of constitutive activation to lower receptor densities relative to the wild type, whereas no constitutive activation was detectable for E592K (fig. S11B). These results highlight the changes in the intrinsic dimerization affinities resulting from mutation of the PK-PK interface. JAK2 E592W also constitutively dimerized and activated EpoR and GHR (Fig. 5, C and D). Very similar patterns of dimerization and activation were found for the combination of JAK2 Glu592 mutants with EpoR using pSTAT5 as a functional readout (fig. S11, C and D), highlighting the generic relevance of this JAK2 interaction site for homodimeric cytokine receptors. Overall, constitutive receptor dimerization and activation showed a striking correlation for all mutants located in close proximity to the PKPK interface (Fig. 5H), thereby confirming the relevance of the PK-PK interface for JAK2 activation and dysregulation.
Atomistic model of a transmembrane signaling complex
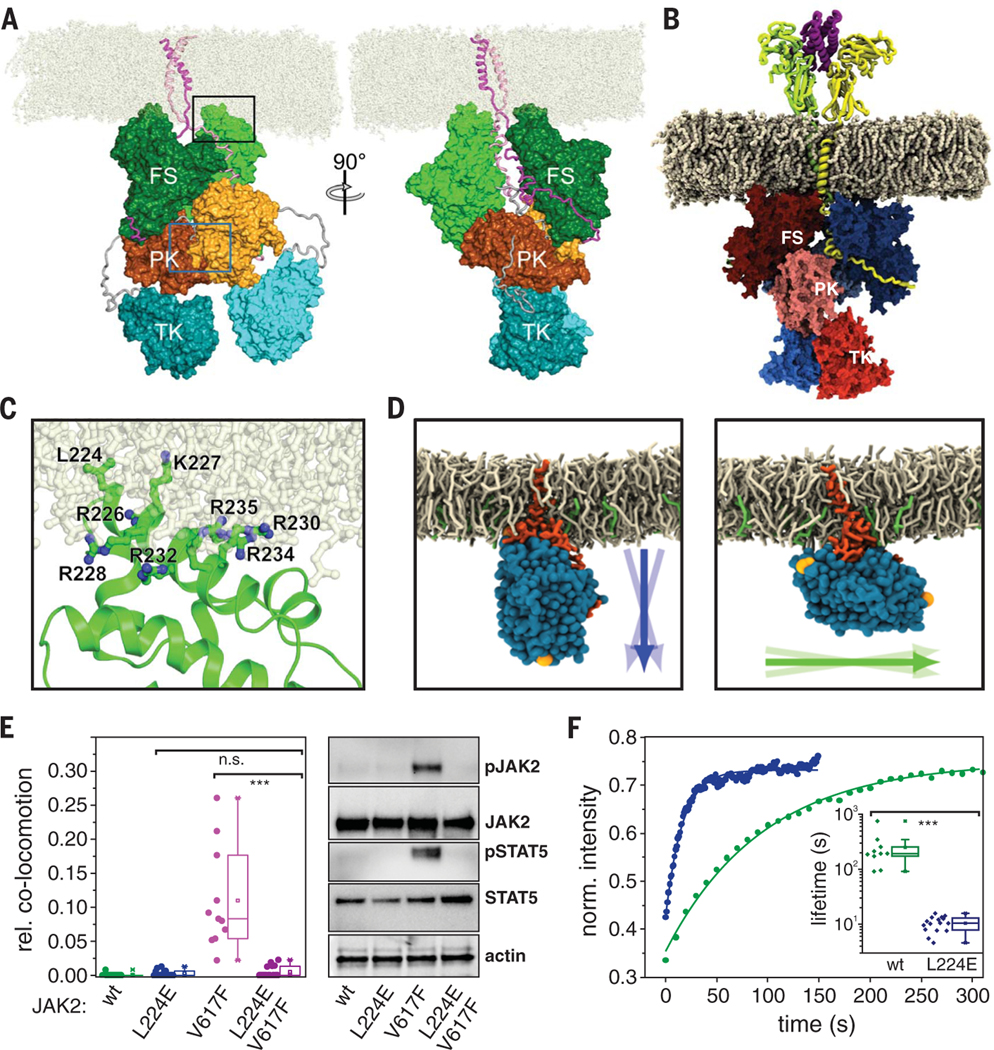
On the basis of existing structural data and our experimental validation of a JAK2 PK-PK interface, we generated atomistic models of TpoR and EpoR dimers within membranes. Ligand-independent dimerization was modeled for JAK2 in complex with TpoR-ΔECD (Fig. 3B; Fig. 4, E and F; and fig. S9, B and C). We performed multiple independent 1-μs all-atom MD simulations for this system integrated into a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane (simulation system S1AA; table S4) [see (18) for model system construction]. For comparison, we generated a corresponding model of JAK2 bound to EpoR including the ectodomains dimerized by Epo (simulation systems 4AA and 5AA; table S4) [see (18) for an independent construction of this model system]. These systems represent very high receptor densities (~5000 receptors/μm2), and there-fore we expect the equilibrium to be fully shifted toward the dimer even in the absence of the ligand. Despite starting with different complex geometries (18), these MD simulations converged into similar structural organization of JAK2 homodimers (Fig. 6, A and B, movies S11 and S12, and fig. S12, A to C). In both cases, JAK2 adopts a stable extended orientation perpendicular to the membrane normal, with a slightly more tilted orientation in the EpoR complex (fig. S12B). The FERM domains were found to strongly interact with the inner leaflet of the lipid bilayer through the hydrophobic residue Lys224 and several positively charged residues from helix a3 in the F2 subdomain (Fig. 6C and fig. S13, A to C), which may account for the decreased receptor diffusion constants in the presence of JAK2 (figs. S5E, S7E, and S8G). These JAK2-lipid interactions stabilize the orientation of the complex with respect to the membrane. In contrast to the crystal structure of the JAK2 FS domains (used here as the starting geometry), which suggested that EpoR dimerization is mediated by FS domains (47), we observed no stable contacts between the FS domains during our simulations. The contacts observed in the crystal structure predominantly dissociated during the simulations of the EpoR-JAK2 complex (fig. S13, D to F), and the residues involved in these contacts were found anchored into the membrane (fig. S13G). Structural organization of JAK2 at the membrane was largely independent of the lipid composition, as confirmed by atomistic MD simulations with lipid mixtures comprising cholesterol and phosphatidylinositides in addition to POPC (simulation system S3AA; table S4 and figs. S12, A to C, and S13, B and C). The tight coupling of the FS domains with the membrane enforces an appropriate orientation for optimal intermolecular PK-PK interaction of JAKs within the receptor dimers (Fig. 6A, left) involving several residues that we experimentally found to be implicated in dimerization (Fig. 5B). The TK domainswere the most mobile parts of the otherwise stable complex (Fig. 6, A and B, fig. S12, and movies S11 and S12). The simulation results support the schematic model shown in Fig. 4C, in which receptor dimerization shifts the cis PK-TK autoinhibitory interaction to a trans PK-PK interaction and thus liberates the TK domains.
Fig. 6. Structural organization of homodimeric cytokine receptor signaling complexes in the membrane.
(A) Snapshot (t = 1 μs) from all-atom MD simulations of JAK2 bound to TpoR (TM and IC domains) forming a homodimeric complex (system S1AA; movie S11). JAK2 is colored green (FS), orange (PK), and cyan (TK). Protomer 1 is in dark colors with domains labeled; protomer 2 is in light colors and unlabeled. The FS-PK and PK-TK linkers are colored gray. TpoR is colored magenta (bound to JAK2 protomer 1) and pink (bound to JAK2 protomer 2). POPC lipid molecules are colored off-white. The PK-PK interface region highlighted by the green rectangle is shown in Fig. 5B. (B) Snapshot (t = 1 μs) from an all-atom MD simulation of a homodimeric complex of JAK2 bound to EpoR (residues Pro31 to Ser335, system S4AA) in the presence of Epo (movie S12). (C) Membrane binding of the F2 subdomain of FS stabilizes the orientation of JAK2 relative to the membrane [enlarged view of the region indicated by the black rectangle in (A)]. The side chains of Lys224 and the seven Lys and Arg residues in α3 that change orientation and flexibility upon interaction with the membrane are highlighted. (D to F) Functional role of Lys224 in TpoR dimerization and activation. (D) Representative orientation of JAK2 FS wt (left) and L224E (right) observed in MD simulations (systems S14CG and S16CG, respectively). Arrows indicate the orientation of the FS domain and its variation during the simulations. (E) Ligand-independent dimerization of TpoR (left) as well as JAK2 and STAT5 phosphorylation (right) observed for JAK2 wt and V617F upon combination with L224E. (F) Stability of JAK2 FS wt and L224E binding to TpoR probed by live-cell micropatterning (fig. S14C) in combination with FRAP. Representative FRAP curves are shown for JAK2 wt (green) and L224E (blue); the inset, using the same colors, shows a statistical analysis of dissociation rate constants. Each data point represents the analysis from one cell with a minimum of 10 cells measured for each condition. ***P ≤ 0.001.
Membrane anchoring of the FERM domain regulates dimerization affinity
MD simulations predicted an important role for FERM domain anchoring into the membrane via Lys224, a conserved hydrophobic residue within the JAK family (Leu in JAK1 and TYK2, Val in JAK3), which is surface-exposed in all FERM domain structures. To functionally test this prediction, we introduced a negative charge in this position [Lys224→Glu (L224E)]. MD simulations confirmed a pronounced reorientation of JAK2 L224E relative to the wild type (simulation systems S14CG and S16CG; Fig. 6D, table S4, and fig. S14, A and B). Strikingly, introduction of L224E led to markedly reduced ligand-independent TpoR, EpoR, and GHR dimerization and activation by JAK2 V617F (Fig. 6E and fig. S14, C and D); this finding supports the key role of Lys224 in orienting JAK2 at the membrane to allow productive PK-PK interactions. Cell micropatterning experiments revealed that the L224E mutation diminished without completely abrogating JAK2 binding to the receptors (fig. S14E). The binding stability of the FS interaction with TpoR at the plasma membrane was reduced by a factor of ~20, as quantified by FRAP experiments (Fig. 6F). Ligand-induced receptor dimerization and activation were still observed for JAK2 L224E, although with reduced potency relative to wild-type JAK2 (fig. S14, F and G), which was also observed for the mutation E592K that directly compromised the PK-PK interface. Likewise, JAK2 L224E abrogated ligand-independent TpoR activation at elevated receptor densities (fig. S14H). JAK2 L224E also strongly reduced ligand-stimulated activation of EpoR and GHR (fig. S14G), which supports the key role of membrane anchoring across the homodimeric class I cytokine receptor family. Taken together, these results confirm that the L224E mutation does not compromise the structural integrity of JAK2; rather, it destabilizes the intermolecular interaction between JAK2 monomers by altering the orientation of the receptor complex, as predicted by our MD simulations.
Oncogenic mutations alter and stabilize the receptor dimerization interface
The PK-PK interaction in the signaling complex remained highly stable during the dimer simulations (systems S1AA to S5AA), corroborating the relevance of this interaction for the structural organization of the signaling complex. This stability arises from a combination of hydrophobic and polar interactions at the interface (tables S5 and S6). Interactions of certain key residues, such as Glu592 and Phe595, were found to be very stable, as exemplified by persistent contacts with their counterparts in the other monomer (table S5). These results are consistent with a previous suggestion that the JAK2 V617F mutation promotes activation by forming an intramolecular pi-stacking network between Phe617, Phe594, and Phe595 (46, 48). This hypothesis was tested by atomistic MD simulations for the isolated V617F mutant PK-PK dimer (systems S6AA and S7AA; table S4). Comparison of the interacting residue pairs in these two cases (table S6) highlights that some of the interactions at the interface are strikingly reorganized. Many of these interaction pairs involve a residue from the N-terminal linker (residues 526 to 539) connecting the PK domain to the FS domain. This linker has been shown to play a role in JAK2 activation (49). The reorganization of the interface leads to an increase in the number of intermolecular contacts for JAK2 V617F relative to the wild type in systems S6AA and S7AA (table S6). Free energy calculations conducted on JAK2 wildtype (simulation S6AA) and V617F (simulation S7AA) systems using the MM-PBSA (Molecular Mechanics Poisson-Boltzmann Surface Area) scheme (50) displayed consistently lower binding free energy values for the mutated PK dimer relative to the wild type (ΔΔGwt-VF =–32.7 ± 16.2 kJ/mol). Such free energy differences indicate stabilization of the V617F dimer over the wild type, as also observed in our experiments (see Fig. 4).
To assess the role of the TpoR TM/JM segment, we simulated the receptor TM/JM helices (system S8AA and S9AA; table S4). They were found to align in a rather tilted orientation (helix tilt angle 37° ± 2°), with W491 and W515 partitioning into the water/membrane interface, as is typical for Trp residues. The W515L mutation increased the helix tilt to 41° ± 1°, likely imposing constraints on the TM domain that favor dimerization. Such involvement of the amphipathic JM segment in regulating TM interactions via tilting is qualitatively supported by spectroscopic studies on reconstituted TM-JM peptides (35). Coarse-grained simulations (systems S10CG to S13CG; table S4) corroborate this result, showing a highly tilted X-shape to be the most stable TM dimer structure (figs. S15 and S16) (18).
Discussion
Our live-cell single-molecule imaging experiments establish that the prototypic homodimeric class I cytokine receptors EpoR, TpoR, and GHR are monomeric in the basal state and are dimerized by their ligand. We therefore propose a molecular mechanism with ligand-induced dimerization as the fundamental switch initiating activation of these receptors, as originally proposed by Wells and co-workers (2). This mechanism contradicts the current view of pre-dimerized, inactive receptors that are activated by a ligand-induced conformational change (4). Although we confirmed weak intrinsic receptor dimerization affinities that involve multiple interaction interfaces, we also found that receptor pre-dimerization is negligible at physiological expression levels yet accounts for a basal signaling activity. Thus, our quantitative studies did not provide any evidence for inactive receptor dimers but rather revealed a strict correlation of receptor dimerization and activation. The weak intrinsic dimerization affinities, however, explain the observation of pre-dimerized receptors by techniques such as protein fragment complementation or cysteine cross-linking (7, 51, 52), which irreversibly form cross-links between weakly interacting subunits and thus shift the equilibrium toward receptor dimers. By contrast, the single-molecule assays used in this study allow direct visualization and quantification of the monomer-dimer equilibrium at physiological receptor expression levels in living cells. Moreover, TIRF imaging in combination with extracellular posttranslational labeling ensures selective detection of receptor dimerization at the plasma membrane. The large fraction of the receptor that resides in endosomal vesicles may bias other methods of interaction analysis because of increased local concentrations during endocytic trafficking. Despite overexpression, we observed low cell surface densities for these cytokine receptors, indicating that receptor cell surface concentrations are highly regulated so as to minimize ligand-independent dimerization and activation.
In addition to identifying ligand-induced dimerization as the key step of receptor activation, we have established the importance of the interaction between JAK PK domains within receptor dimers at the plasma membrane. So far, the critical regulatory function of the PK domain has been appreciated at the level of intramolecular inhibition of TK activity (41, 42), and the numerous constitutively JAK-activating mutations have been interpreted accordingly. Here, we have shown that a substantial proportion of constitutively activating PK mutations, including JAK2 V617F, act by altering and strengthening the intermolecular interactions involving the PK-PK dimerization interface. These mutations drive cytoplasmic stabilization of receptor-JAK dimers, bypassing stabilization of dimers via extracellular cytokine binding. Our insights suggest that the design of agents that interfere with dimerization by direct or allosteric targeting of the PK-PK interface could improve therapeutic intervention for MPNs and potentially other hematological malignancies and immunological disorders. Equally, our work demonstrates that although the extracellular domain is not required for oncogenic signaling, antagonism of receptor dimerization at the extracellular interface could be exploited to destabilize the active dimer, using a strategy similar to that used for the modulation of EpoR signaling by engineered dimerizers (19, 39).
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Hikade, H. Kenneweg, W. Kohl, L. Roberts, and the University of York Technology Facility for technical support; J. van der Heyden (Ghent University) for providing rabbit GHR cDNA; D. Richter and P. Selenschik for providing evaluation tools; and the Center for Scientific Computing (CSC)–IT Center for Science (Espoo, Finland) for computing resources.
Funding:
Supported by Deutsche Forschungsgemeinschaft grants SFB 944 P8/Z, PI 405/14-1, and PI 405/15-1 (J.P. and R.K.); long-term EMBO fellowship ALTF 454–2017 (S.W.); NIH grant 5R01AI101256 (S.R.H.); the Academy of Finland Center of Excellence program, the Sigrid Jusélius Foundation, the European Research Council (CROWDED-PRO-LIPIDS), and the Helsinki Institute of Life Science (HiLIFE) Fellow project (I.V.); the Academy of Finland, the Sigrid Jusélius Foundation, and the University of Helsinki (V.S.); NIH grant R01-AI51321, the Ludwig and Mathers Foundations, and HHMI (K.C.G.); CRUK grant A24593 (I.S.H.); and Horizon 2020 Framework program grant 714680, the Wellcome Trust, and Royal Society Sir Henry Dale Fellowship 202323/Z/16/z (I.M.).
Footnotes
Competing interests:
The authors declare no competing interests.
Data and materials availability:
Results from single-molecule imaging and phospho-flow cytometry experiments are available at DOI:10.5281/zenodo.3588316. The single-molecule evaluation software used for tracking and co-tracking analysis has been archived via GitHub (DOI:10.5281/zenodo.3588413). Final structures of the simulated systems, as well as all simulation files and protein structures needed to carry out the simulations are available at DOI:10.5281/zenodo.3555291. Materials are available from the authors upon request.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Atanasova M, Whitty A, Crit. Rev. Biochem. Mol. Biol 47, 502–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham BC et al. , Science 254, 821–825 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Stroud RM, Wells JA, Sci. STKE 2004, re7 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Waters MJ, Brooks AJ, Biochem. J 466, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ, Proc. Natl. Acad. Sci. U.S.A 99, 9858–9863 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RJ et al. , Nat. Struct. Mol. Biol 12, 814–821 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Brooks AJ et al. , Science 344, 1249783 (2014). [Google Scholar]
- 8.Tenhumberg S et al. , Biochem. Biophys. Res. Commun 346, 649–657 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Zaks-Zilberman M, Harrington AE, Ishino T, Chaiken IM, J. Biol. Chem 283, 13398–13406 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause CD et al. , Cell Res. 16, 55–69 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Krause CD et al. , Mol. Cell. Proteomics 1, 805–815 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Krause CD et al. , Biochem. Biophys. Res. Commun 340, 377–385 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Levine RL et al. , Cancer Cell 7, 387–397 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Vainchenker W, Constantinescu SN, Oncogene 32, 2601–2613 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T, Blood 77, 2583–2590 (1991). [PubMed] [Google Scholar]
- 16.McKinstry WJ et al. , Blood 89, 65–71 (1997). [PubMed] [Google Scholar]
- 17.Komatsu N et al. , Blood 87, 4552–4560 (1996). [PubMed] [Google Scholar]
- 18.See supplementary materials.
- 19.Moraga I et al. , Cell 160, 1196–1208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung I et al. , Nature 464, 783–787 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock IS, Chen MM, King JR, Kaushansky K, Blood 112, 2222–2231 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulut GB, Sulahian R, Yao H, Huang LJS, Blood 122, 3964–3972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano Y et al. , ACS Chem. Biol 3, 341–345 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Semrau S, Holtzer L, González-Gaitán M, Schmidt T, Biophys. J 100, 1810–1818 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK, J. Clin. Endocrinol. Metab 85, 4712–4720 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Fuh G et al. , Science 256, 1677–1680 (1992). [DOI] [PubMed] [Google Scholar]
- 27.Elliott S, Lorenzini T, Chang D, Barzilay J, Delorme E, Blood 89, 493–502 (1997). [PubMed] [Google Scholar]
- 28.Endres NF et al. , Cell 152, 543–556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvennoinen O, Hubbard SR, Blood 125, 3388–3392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X et al. , Proc. Natl. Acad. Sci. U.S.A 102, 18962–18967 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Huang LJ, Lodish HF, J. Biol. Chem 283, 5258–5266 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN, PLOS ONE 5, e11157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staerk J et al. , Blood 107, 1864–1871 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pikman Y et al. , PLOS Med. 3, e270 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defour JP et al. , Proc. Natl. Acad. Sci. U.S.A 110, 2540–2545 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constantinescu SN et al. , Proc. Natl. Acad. Sci. U.S.A 98, 4379–4384 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royer Y, Staerk J, Costuleanu M, Courtoy PJ, Constantinescu SN, J. Biol. Chem 280, 27251–27261 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Pecquet C et al. , Blood 115, 1037–1048 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Mohan K et al. , Science 364, eaav7532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O, Cytokine 118, 48–63 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Shan Y et al. , Nat. Struct. Mol. Biol 21, 579–584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lupardus PJ et al. , Proc. Natl. Acad. Sci. U.S.A 111, 8025–8030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubbard SR, Front. Endocrinol 8, 361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammarén HM et al. , J. Allergy Clin. Immunol 143, 1549–1559.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toms AV et al., Nat. Struct. Mol. Biol 20, 1221–1223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandaranayake RM et al. , Nat. Struct. Mol. Biol 19, 754–759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrao RD, Wallweber HJ, Lupardus PJ, eLife 7, e38089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnanasambandan K, Magis A, Sayeski PP, Biochemistry 49, 9972–9984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L et al. , J. Biol. Chem 284, 26988–26998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumari R, Kumar R, Open Source Drug Discovery Consortium,A. Lynn, J. Chem. Inf. Model 54, 1951–1962 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Remy I, Wilson IA, Michnick SW, Science 283, 990–993 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Matthews EE et al. , FASEB J. 25, 2234–2244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.