Abstract
Aims
Non-vitamin K oral anticoagulants are safe and effective for stroke prevention in patients with atrial fibrillation (AF). Data on the safety and efficacy of edoxaban in routine care are limited in Europe. We report 1-year outcomes in patients with AF treated with edoxaban in routine care.
Methods and results
ETNA-AF-Europe is a prospective, multicentre, post-authorization, observational study enrolling patients treated with edoxaban in 10 European countries, the design of which was agreed with the European Medicines Agency as part of edoxaban’s post-approval safety plan. Altogether 13 092 patients in 852 sites completed the 1-year follow-up [mean age: 73.6 ± 9.5 years; 57% male, mean follow-up: 352 ± 49 days (median: 366 days)]. Most patients had associated comorbidities (mean CHA2DS2-VASc score: 3.1 ± 1.4). Stroke or systemic embolism was reported in 103 patients (annualized event rate: 0.82%/year), and major bleeding events were reported in 132 patients (1.05%/year). Rates of intracranial haemorrhage were low [30 patients (0.24%/year)]. Death occurred in 442 patients (3.50%/year); cardiovascular (CV) death occurred in 206 patients (1.63%/year). The approved dosing of edoxaban was chosen in 83%. All-cause and CV mortality were higher in patients receiving edoxaban 30 mg vs. 60 mg, in line with the higher age and more frequent comorbidities of the 30 mg group. Major bleeding was also numerically more common in patients receiving edoxaban 30 mg vs. 60 mg.
Conclusion
The rates of stroke, systemic embolism, and major bleeding are low in this large unselected cohort of high-risk AF patients routinely treated with edoxaban.
Keywords: Non-vitamin K oral anticoagulant, Edoxaban, Real-world, Registry, Atrial fibrillation
Introduction
Atrial fibrillation (AF) is a growing epidemic, currently affecting 1.5–3% of European populations.1,2 Oral anticoagulation is key for reducing the risk of stroke in patients with AF. Non-vitamin K antagonist oral anticoagulants (NOACs) are at least as effective as vitamin K antagonists (VKAs) in controlled clinical trials, with a lower risk of intracranial haemorrhage (ICH) and overall a lower mortality than VKAs.3 Non-vitamin K oral anticoagulant use in clinical practice is increasingly becoming the standard of care. Several registries4–6 and retrospective analyses7,8 have assessed outcomes of three (apixaban, dabigatran, and rivaroxaban) of the four available NOACs, but routine clinical data on edoxaban, the fourth NOAC that entered the clinical arena, are scarce. Regulators such as the European Medicines Agency have regularly requested for data on the safety of recently approved therapies in routine clinical care as part of the post-approval safety plan of these recently approved medications.
Edoxaban, a once daily, direct factor Xa inhibitor, is approved for stroke prevention in adult patients with AF, as well as for the treatment and secondary prevention of venous thromboembolism. The recommended dose for all indications is 60 mg edoxaban once daily with a reduced dose of 30 mg once daily in patients with one or more of the following clinical factors: moderate or severe renal impairment [creatinine clearance (CrCl) 15–50 mL/min], low body weight ≤60 kg, or concomitant use of certain P-glycoprotein (P-gp) inhibitors.9 Edoxaban was shown to be effective and safe in a wide range of patient populations included in the randomized ENGAGE AF-TIMI 48 trial.10–12 However, data on its effectiveness and safety in routine clinical care is still limited in the European population.
Here, we present the 1-year outcomes of stroke, bleeding, and mortality from the Edoxaban Treatment in Routine Clinical Practice for Patients With Non Valvular Atrial Fibrillation (ETNA-AF-Europe) study conducted in unselected European patients with AF.
Methods
ETNA-AF-Europe (Clinicaltrials.gov: NCT02944019) is a multinational, multicentre, post-authorization, observational study conducted in 852 sites in 10 European countries (Austria, Belgium, Germany, Ireland, Italy, The Netherlands, Portugal, Spain, Switzerland, and UK). ETNA-AF-Europe is part of the global ETNA initiative, which is composed of separate, non-interventional prospective ETNA-AF registries in Europe, East Asia, and Japan. The design of the ETNA-AF-Europe was agreed in close collaboration with the European Medicines Agency (EMA) and has been previously published.13,14 The study was approved by the institutional review boards and independent Ethics Committees for all participating centres in compliance with the Declaration of Helsinki and Guidelines for Good Pharmacoepidemiological Practice (GPP). All participants provided written informed consent.
Unselected routine patients with AF treated with edoxaban, providing consent, were prospectively enrolled. Explicit exclusion criteria were not defined. The primary objective of ETNA-AF-Europe is to assess the routine clinical care safety of edoxaban by evaluating bleeding events, including ICH; drug-related adverse events; and cardiovascular (CV) and all-cause mortality in routine care patients with AF treated with edoxaban up to 4 years, with regard to onset (relative to treatment with edoxaban), duration, severity and outcomes of the events. Details of the inclusion criteria and secondary objectives can be found in the ETNA-AF-Europe design paper.13 Here, we present 1-year follow-up outcomes of the first 13 092 patients as captured on 31 October 2019.
Besides description of patients’ characteristics and outcomes, event rates are compared descriptively with event rates reported amongst non-Asian patients included in the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) study. In ETNA-AF-Europe, all patients were included, including a minority of Asians living in Europe. From ENGAGE AF-TIMI 48, ‘non-Asian’ self-designated patients were used to contextualize against ETNA-AF-Europe data, in view of a sub-analysis that has confirmed a preferential efficacy with edoxaban in Asian vs. non-Asian patients.15 Therefore, the side-by-side presentation of ETNA-AF-Europe and ENGAGE AF-TIMI 48 in this paper is intended to be a conservative exercise.
Statistical analysis
Baseline characteristics are summarized descriptively as frequencies (n and percentage), mean value ± standard deviation (SD) rounded to integer, or median [interquartile range (IQR)]. Based on the reported events, annualized event rates (% per year) are presented for the main safety and efficacy outcomes. Other outcomes were specified in the design paper.13 The events presented here for ETNA-AF-Europe are as reported by the site investigator.
Kaplan–Meier curves were used to present the cumulative event rates of stroke/systemic embolism (SE), major bleeding, and all-cause mortality.
A stepwise logistic regression model was used to identify the factors related to all-cause mortality and major bleeding.
Results
Baseline characteristics
Baseline characteristics of ETNA-AF-Europe patients completing follow-up (N = 13 092) were broadly similar to the non-Asian ethnicity cohort of ENGAGE AF-TIMI 48. Mean age was slightly higher in ETNA-AF-Europe with more than half of all patients aged ≥75 years, whereas the CHA2DS2-VASc score was slightly lower and the HAS-BLED bleeding risk score was slightly higher in patients included in ETNA-AF-Europe compared with the non-Asian ENGAGE AF-TIMI 48 population (Table 1), reflecting the broader indications for edoxaban compared with the inclusion criteria of ENGAGE AF-TIMI 48.
Table 1.
Baseline demographics and clinical characteristics of patients included in ETNA-AF-Europe and ENGAGE AF-TIMI 48
| ETNA-AF-Europe |
ENGAGE AF-TIMI 48 (non-Asian cohort) |
|||||
|---|---|---|---|---|---|---|
| Total | Edoxaban 60 mg o.d. | Edoxaban 30 mg o.d. | Total | Edoxaban 60 mg o.d. | Edoxaban dose adj. to 30 mg | |
| Patients, n (%) | 13092 (100%) | 9991 (76.3%) | 3101 (23.7%) | 6056 (100%) | 4747 (78.4%) | 1309 (21.6%) |
| Male, n (%) | 7430 (56.8%) | 6061 (60.7%) | 1369 (44.1%) | 3696 (61.0%) | 3160 (66.6%) | 536 (40.9%) |
| Age (years), mean ± SD | 73.6 ± 9.46 | 71.8 ± 9.14 | 79.5 ± 7.92 | 70.9 ± 9.41 | 69.2 ± 9.17 | 77.1 ± 7.47 |
| By age sub-groups, n (%) | ||||||
| <65 years | 1994 (15.2%) | 1861 (18.6%) | 133 (4.3%) | 1544 (29.5%) | 1445 (30.4%) | 99 (7.6%) |
| 65–74 years | 4456 (34.0%) | 3896 (39.0%) | 560 (18.1%) | 2007 (33.1%) | 1731 (36.5%) | 276 (21.1%) |
| ≥75 years | 6640 (50.7%) | 4233 (42.4%) | 2407 (77.6%) | 2505 (41.4%) | 1571 (33.1%) | 934 (71.4%) |
| <85 years | 11718 (89.5%) | 9467 (94.8%) | 2251 (72.6%) | 5772 (95.3%) | 4649 (97.9%) | 1123 (85.8%) |
| ≥85 years | 1372 (10.5%) | 523 (5.2%) | 849 (27.4%) | 284 (4.7%) | 98 (2.1%) | 186 (14.2%) |
| Body weight (kg), mean ± SD | 81.0 ± 17.29 | 83.5 ± 16.75 | 72.9 ± 16.51 | 86.9 ± 19.94 | 91.2 ± 18.88 | 71.5 ± 15.61 |
| BMI (kg/m2), mean ± SD | 28.1 ± 5.11 | 28.6 ± 5.05 | 26.5 ± 5.00 | 30.3 ± 5.99 | 31.3 ± 5.87 | 26.6 ± 4.89 |
| CrCl (recalc.) (mL/min), mean ± SD | 74.3 ± 30.42 | 82.1 ± 29.19 | 50.3 ± 19.61 | 78.7 ± 32.05 | 86.6 ± 30.19 | 50.0 ± 20.05 |
| Patients fulfilling ≥1 dose adjustment criteria, n (%) | 3106 (23.7%) | 1119 (11.2%) | 1987 (64.1%) | NA | NA | NA |
| CHADS2, mean ± SD | 1.7 ± 1.07 | 1.6 ± 1.05 | 2.1 ± 1.03 | 2.9 ± 0.97 | 2.8 ± 0.94 | 3.1 ± 1.06 |
| CHA2DS2-VASc, mean ± SD | 3.1 ± 1.40 | 2.9 ± 1.37 | 3.8 ± 1.27 | 4.3 ± 1.41 | 4.2 ± 1.36 | 5.0 ± 1.39 |
| By CHA2DS2-VASc, n (%) | ||||||
| CHA2DS2-VASc score (<4), n (%) | 8184 (62.5%) | 6878 (68.8%) | 1306 (42.1%) | 1767 (29.2%) | 1601 (33.7%) | 166 (12.7%) |
| CHA2DS2-VASc score (≥4), n (%) | 4908 (37.5%) | 3113 (31.2%) | 1795 (57.9%) | 4289 (70.8%) | 3146 (66.3%) | 1143 (87.3%) |
| mod. HAS-BLED, mean ± SD | 2.5 ± 1.10 | 2.4 ± 1.08 | 2.9 ± 1.06 | 1.5 ± 0.94 | 1.4 ± 0.94 | 1.8 ± 0.89 |
| Frailtya, n (%) | ||||||
| Yes | 1392 (10.6%) | 612 (6.1%) | 780 (25.2%) | NA | NA | NA |
| No | 10820 (82.7%) | 8719 (87.3%) | 2101 (67.8%) | NA | NA | NA |
| Not known | 878 (6.7%) | 658 (6.6%) | 220 (7.1%) | NA | NA | NA |
| Previous history of CV disease, n (%) | ||||||
| Hypertension | 10088 (77.1%) | 7594 (76.0%) | 2494 (80.4%) | 5763 (95.2%) | 4546 (95.8%) | 1217 (93.0%) |
| Congestive heart failure | 777 (5.9%) | 478 (4.8%) | 299 (9.6%) | 3598 (59.4%) | 2847 (60.0%) | 751 (57.4%) |
| Myocardial infarction | 560 (4.3%) | 371 (3.7%) | 189 (6.1%) | 731 (12.1%) | 568 (12.0%) | 163 (12.5%) |
| Peripheral artery disease | 437 (3.3%) | 278 (2.8%) | 159 (5.1%) | 253 (4.2%) | 177 (3.7%) | 76 (5.8%) |
| Previous history of diabetes mellitus, n (%) | 2879 (22.0%) | 2057 (20.6%) | 822 (26.5%) | 2221 (36.7%) | 1833 (38.6%) | 388 (29.6%) |
| Previous history of COPD, n (%) | 1206 (9.2%) | 824 (8.2%) | 382 (12.3%) | 553 (9.1%) | 404 (8.5%) | 149 (11.4%) |
| Previous history of stroke and ICH, n (%) | ||||||
| Ischaemic strokeb | 778 (5.9%) | 564 (5.6%) | 214 (6.9%) | 998 (16.5%) | 772 (16.3%) | 226 (17.3%) |
| Intracranial haemorrhage | 62 (0.5%) | 45 (0.5%) | 17 (0.5%) | 7 (0.1%) | 4 (0.1%) | 3 (0.2%) |
| Previous history of bleeding, n (%) | ||||||
| Major | 129 (1.0%) | 80 (0.8%) | 49 (1.6%) | NA | NA | NA |
| Major or CRNM | 270 (2.1%) | 160 (1.6%) | 110 (3.5%) | NA | NA | NA |
| Current AF type, n (%) | ||||||
| Paroxysmal | 7039 (53.9%) | 5473 (54.9%) | 1566 (50.6%) | 1542 (25.5%) | 1194 (25.2%) | 348 (26.6%) |
| Persistent | 3159 (24.2%) | 2496 (25.0%) | 663 (21.4%) | 1372 (22.7%) | 1084 (22.8%) | 288 (22.0%) |
| Long-standing persistent and permanent | 2864 (21.9%) | 1997 (20.0%) | 867 (28.0%) | 3142 (51.9%) | 2469 (52.0%) | 673 (51.4%) |
Calculated scores for CHADS2, CHA2DS2-VASc, and HAS-BLED are reported here.
AF, atrial fibrillation; BMI, body mass index; CrCl, creatinine clearance; COPD, chronic obstructive pulmonary disease; CRNM, clinically relevant non-major; CV, cardiovascular; ICH, intracranial haemorrhage; NA, not available; o.d., once daily; SD, standard deviation.
There was no specific definition for frailty; it was left to the discretion of the physician to categorise a patient as frail.
The ENGAGE AF-TIMI 48 numbers refer to ischaemic/embolic stroke.
In ETNA-AF-Europe, the mean body weight was 81 ± 17 kg and body mass index (BMI) was 28 ± 5 kg/m2. The mean (± SD) and median follow-up duration was 352 ± 49 and 366 days. AF at first diagnosis was symptomatic in 54% and asymptomatic in 34% of the patients. The remaining 12% were reported as unknown by the investigator. The most common type of reported symptoms among the symptomatic patients at baseline were palpitations (69%), followed by dyspnoea (60%), and fatigue (58%). The median time between first diagnosis of AF and enrolment was 4.6 months (IQR: 0.4–29.9). Frequent comorbidities included hypertension (77%), diabetes (22%), valvular heart disease (18%), congestive heart failure (6%), and history of myocardial infarction (4%).
Over three-quarters of patients (76%) were prescribed the full dose of edoxaban (edoxaban 60 mg). Compared with those receiving edoxaban 60 mg, patients receiving edoxaban 30 mg were older and had a lower body weight and CrCl. Furthermore, patients receiving edoxaban 30 mg had more comorbidities and a greater proportion of these patients had a prior history of stroke and bleeding (Table 1). The overall adherence to the summary of product characteristics (SmPC) dosing recommendations was observed in 83% (10 859 of 13 092) patients. Non-adherence to the SmPC was observed in 11% of patients receiving edoxaban 60 mg vs. 36% of those receiving the 30 mg dose. Overall, 1191 of 13 092 patients (9.1%) permanently discontinued from edoxaban treatment, and 11 901 of 13 092 patients (90.9%) were still receiving edoxaban at the end of 1-year follow-up.
Patients who were prescribed the reduced dose of edoxaban (30 mg/day) despite the SmPC dosing recommendation for receiving edoxaban 60 mg had a higher mean age, mean CHA2DS2-VASc and modified HAS-BLED scores, a lower mean CrCl, and similar mean body weight and BMI (Table 2) compared with those receiving the recommended full dose. Patients who were prescribed edoxaban 60 mg despite the SmPC dosing recommendation for receiving the reduced dose had a lower mean age, lower mean CHA2DS2-VASc and modified HAS-BLED scores, a higher mean CrCl, and similar mean body weight and BMI vs. those receiving the recommended reduced 30 mg dose (Table 2).
Table 2.
Baseline characteristics of patients included in ETNA-AF-Europe receiving edoxaban 60 mg and 30 mg doses in line and not in line with SmPC recommendations
| Patients with the SmPC recommendation for 60 mg (N = 9986) |
Patients with the SmPC recommendation for 30 mg (N = 3106) |
|||
|---|---|---|---|---|
| 60 mg recommended dose | 30 mg non-recommended dose | 30 mg recommended dose | 60 mg non-recommended dose | |
| Patients, N (%) | 8872 (67.8%) | 1114 (8.5%) | 1987 (15.2%) | 1119 (8.5%) |
| Male, n (%) | 5697 (64.2%) | 656 (58.9%) | 713 (35.9%) | 364 (32.5%) |
| Age (years), mean | 71.1 | 76.4 | 81.3 | 77.3 |
| (calc.) CHA2DS2-VASc, mean | 2.8 | 3.4 | 4.0 | 3.6 |
| (calc.) mod. HAS-BLED, mean | 2.6 | 3.2 | 3.1 | 3.1 |
| CrCl (recalculated) (mL/min), mean | 86.4 | 69.6 | 41.6 | 51.9 |
| Body weight (kg), mean | 85.7 | 84.8 | 66.7 | 66.3 |
| BMI (kg/m2), mean | 29.1 | 29.4 | 25.0 | 24.6 |
Mortality, stroke, and major bleeding
During the 1-year follow-up period, the overall rates of major bleeding, stroke, and systemic embolism as well as all-cause mortality were low and linearly increasing over time (Figure 1). Specifically, 442 patients died (3.5%/year), of whom 206 patients (1.6%/year) died of CV causes (Table 3).
Figure 1.
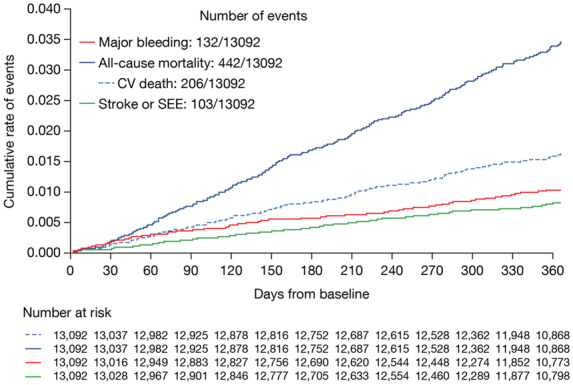
Kaplan–Meier curve showing cumulative event rates for major bleeding, stroke/systemic embolism, and all-cause mortality in patients at 1-year follow-up.
Table 3.
Safety and efficacy outcomes in all patients included in ETNA-AF-Europe and ENGAGE AF-TIMI 48
| Annualized event rates, n (%/year) (95% CI) | ETNA-AF-Europe |
ENGAGE AF-TIMI 48 (non-Asian cohort) |
||||
|---|---|---|---|---|---|---|
| Total | Edoxaban 60 mg o.d. | Edoxaban 30 mg o.d. | Total | Edoxaban 60 mg o.d. | Edoxaban dose adj. to 30 mg | |
| Patients, N (%) | 13092 (100%) | 9991 (76.3%) | 3101 (23.7%) | 6056 (100%) | 4747 (78.4%) | 1309 (21.6%) |
| Primary outcomes | ||||||
| Major bleeding | 132 (1.05) (0.88–1.25) | 85 (0.88) (0.70–1.09) | 47 (1.61) (1.18–2.14) | 468 (2.95) | 358 (2.84) | 110 (3.37) |
| Major or CRNM bleeding | 293 (2.35) (2.09–2.63) | 200 (2.08) (1.81–2.39) | 93 (3.22) (2.60–3.94) | 1437 (10.15) | 1121 (9.99) | 316 (10.77) |
| Major GI bleeding | 51 (0.40) (0.30–0.53) | 25 (0.26) (0.17–0.38) | 26 (0.89) (0.58–1.30) | 254 (1.58) | 200 (1.56) | 54 (1.62) |
| ICHa | 30 (0.24) (0.16–0.34) | 23 (0.24) (0.15–0.36) | 7 (0.24) (0.10–0.49) | 66 (0.40) | 48 (0.37) | 18 (0.53) |
| Cardiovascular mortality (sensitivity analysis)b | 206 (1.63) (1.42–1.87) | 108 (1.11) (0.91–1.35) | 98 (3.34) (2.71–4.07) | 456 (2.74) | 287 (2.17) | 169 (4.89) |
| All-cause mortality | 442 (3.50) (3.18–3.84) | 231 (2.38) (2.09–2.71) | 211 (7.19) (6.25–8.23) | 677 (4.06) | 437 (3.31) | 240 (6.95) |
| Secondary outcomes | ||||||
| Any stroke or systemic embolism | 103 (0.82) (0.67–0.99) | 74 (0.77) (0.60–0.96) | 29 (0.99) (0.66–1.42) | 245 (1.51) | 167 (1.29) | 78 (2.34) |
| Ischaemic stroke | 70 (0.56) (0.43–0.70) | 50 (0.52) (0.38–0.68) | 20 (0.68) (0.42–1.05) | 202 (1.24) | 138 (1.07) | 64 (1.91) |
| Haemorrhagic stroke | 14 (0.11) (0.06–0.19) | 12 (0.12) (0.06–0.22) | 2 (0.07) (0.01–0.25) | 34 (0.21) | 24 (0.18) | 10 (0.30) |
| Myocardial infarction | 66 (0.52) (0.41–0.67) | 44 (0.45) (0.33–0.61) | 22 (0.75) (0.47–1.14) | 120 (0.73) | 81 (0.62) | 39 (1.17) |
ETNA-AF-Europe and ENGAGE AF-TIMI 48 data are not intended for comparison.
CRNM, clinically relevant non-major bleeding; GI, gastrointestinal; ICH, intracranial haemorrhage.
Intracranial haemorrhage included epidural, subdural, subarachnoid, intracerebral, or unknown haemorrhage.
Cardiovascular mortality sensitivity is a conservative exercise because also patients with an unknown or unconfirmed cause of death are calculated as CV death.
Major bleeding events occurred in 132 patients (1.05%/year), in whom 51 (0.4%/year) were gastro-intestinal bleeds and 30 (0.2%/year) were intracranial bleeds. Stroke or systemic embolism occurred in 103 patients (0.8%/year) (Table 3). All-cause mortality, CV mortality, any rates of stroke/SEE, and major bleeding events were lower in ETNA-AF-Europe vs. the non-Asian ethnicity cohort of ENGAGE AF-TIMI 48 (Table 3, Supplementary material online, Figures S1 and S2).
Figure 2.
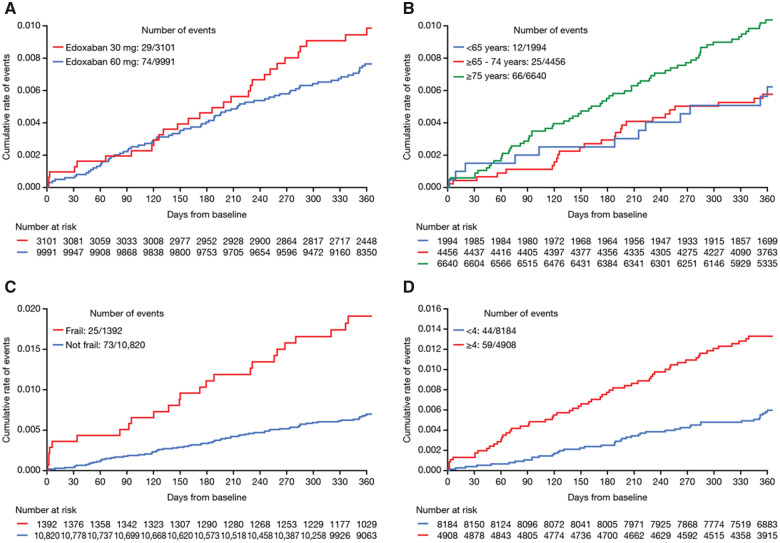
Kaplan–Meier curves showing cumulative event rates for stroke/systemic embolism in patients at 1-year follow-up classified by: (A) edoxaban dose, (B) age groups, (C) frailty status, and (D) CHA2DS2-VASc groups.
Patients who were older (ranged between <65 and ≥75 years), frail patients (as defined by the investigator), and patients with higher CHA2DS2-VASc scores (≥4) were at higher risk of events (Figures 2–4).
Dosing effects
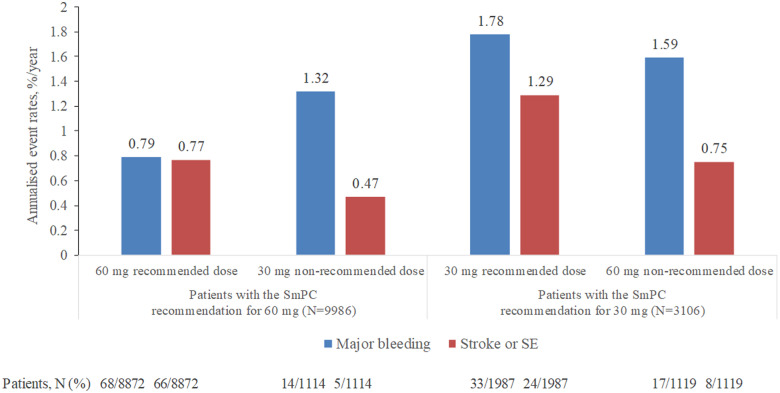
All-cause mortality, CV mortality, and rates of major bleeding were higher in patients receiving edoxaban 30 mg dose vs. those receiving edoxaban 60 mg (Table 3). Patients who were prescribed the reduced dose of edoxaban 30 mg despite the SmPC dosing recommendation for receiving edoxaban 60 mg had higher rates of all-cause death, CV death, and major bleeding in comparison with those receiving the recommended edoxaban 60 mg. However, rates of stroke/SEE and ischaemic stroke were not higher in those receiving the non-recommended 30 mg vs. the recommended 60 mg dose (Figure 5, Supplementary material online, Table S1). Patients who were prescribed edoxaban 60 mg despite the SmPC dosing recommendation for receiving the reduced dose had lower annualized event rates of all-cause death, CV death, major bleeding, any stroke/SEE, and ischaemic stroke in comparison with those receiving the recommended edoxaban 30 mg (Figure 5, Supplementary material online, Table S1).
Figure 5.
Major bleeding and stroke/SEE in ETNA-AF-Europe patients dosed in line and not in line with SmPC recommendations. SE, systemic embolism; SmPC, summary of product characteristics.
Using the stepwise logistic regression model, the factors related to all-cause mortality and major bleeding were identified (Supplementary material online, TablesS2 andS3). The main factors included older age (defined as ≥85 years and ≥75 years), history of chronic obstructive lung disease, congestive heart failure, CHA2DS2-VASc (≥4), female sex, and edoxaban dose 30 mg o.d. A higher frequency of these variables was observed in patients receiving edoxaban 30 mg o.d., a finding that relates with an increased risk of events in patients receiving 30 mg vs. 60 mg dose.
Discussion
To our knowledge, this report provides the first outcome data on patients treated with edoxaban in routine care, enrolled into ETNA-AF-Europe, the largest prospective, non-interventional study investigating a single NOAC to date. The results illustrate low rates of bleeding and ischaemic events, thereby complement data from the ENGAGE AF-TIMI 48, the landmark randomized trial that demonstrated superior safety and non-inferior efficacy of edoxaban vs. warfarin. The strengths of the ETNA-AF-Europe study include a large sample size, a prospective study design, an international setting, and the lack of explicit exclusion criteria, validating the reliability and generalizability of data.
Various registries and retrospective analyses have looked into how the outcomes of randomized NOAC trials may translate into routine practice in terms of favourable safety and effectiveness of NOACs. Low rates of stroke and major bleeding were observed in patients receiving rivaroxaban in routine clinical practice (XANTUS registry).16 In the 1-year follow-up of EURObservational Research Programme on AF (EORP-AF) Long-Term General Registry, the cumulative event-free survival from the main outcomes was the highest amongst patients treated only with NOACs vs. other antithrombotic agents.17 Data from retrospective analyses have also shown lower risks of stroke/SE and lower-to-comparable bleeding events with NOACs vs. VKAs.18,19 Findings noted in ETNA-AF-Europe now complement the outcomes reported in other real-world registries.
Lower rates of major bleeding and stroke/SEE were observed in ETNA-AF-Europe compared with the non-Asian ethnicity cohort of ENGAGE AF-TIMI 48, while mortality and CV deaths were comparable between the two data sets. The ENGAGE AF-TIMI 48 inclusion criteria allowed the enrolment of patients with a CHADS2 score of at least 2, whereas the population in ETNA-AF-Europe was unselected with respect to CHADS2 score at baseline. This translated into a lower CHA2DS2-VASc score in the ETNA-AF-Europe vs. ENGAGE AF-TIMI 48 (3.1 vs. 4.3) which might have contributed towards different event rates but may represent more generalized prescribing of edoxaban since the randomized trial and marketing authorization.
There was a good overall adherence of 83% (10 859 out of 13 092 patients) to the SmPC dosing recommendations, suggesting that the dose reduction criteria of edoxaban are generally followed in routine clinical care. Notably, the permanent discontinuation rate was relatively low (9.1%) compared with other registries (e.g. discontinuation rate of 20.1% at 1 year was noted in the XANTUS registry).16
A comparison of outcomes in patients receiving the two doses generated interesting observations. More deaths and numerically higher bleeding events were reported in patients receiving the reduced edoxaban 30 mg/day regimen. This can potentially be explained by the fact that the criteria for dose reduction (renal impairment, lower body weight, and concomitant P-gp inhibitor therapy) are markers for patients at high risk of thromboembolic and bleeding events. In line with this hypothesis, patients receiving edoxaban 30 mg were older (80 vs. 72 years) and had almost one point higher CHA2DS2-VASc score (3.8 vs. 2.9) than those receiving edoxaban 60 mg. Findings observed in ETNA-AF-Europe were in line with those reported in the non-Asian cohort of ENGAGE AF-TIMI 48, with higher rates of bleeding, stroke, and mortality noted in the edoxaban 30 mg vs. 60 mg dose group (Table 2, Figures 3–5). Of note, there was no clear signal for a higher stroke rate.
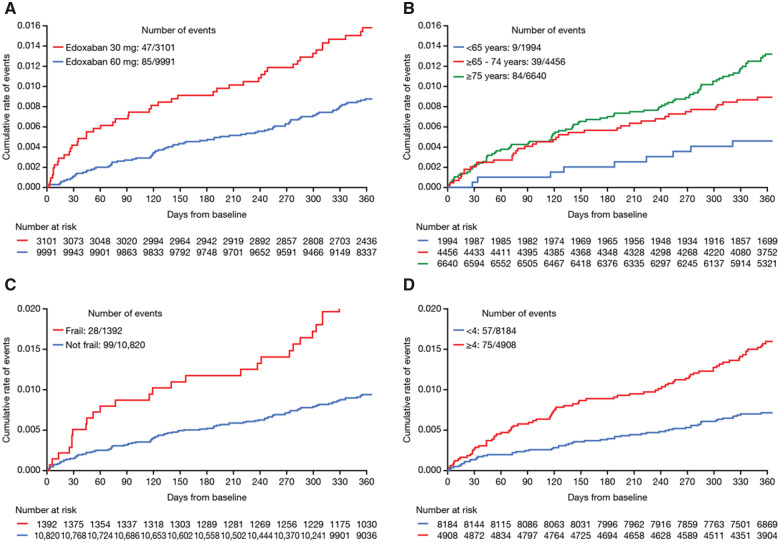
Figure 3.
Kaplan–Meier curves showing cumulative event rates for major bleeding events in patients at 1-year follow-up classified by: (A) edoxaban dose, (B) age groups, (C) frailty status, and (D) CHA2DS2-VASc groups. Major bleeding was defined as a clinically overt bleeding event (i.e. bleeding that is visualized by examination or radiologic imaging) that meets at least one of the following: (A) Fatal bleeding. (B) Symptomatic bleeding in a critical area or organ such as, retroperitoneal, intracranial, intraocular, intraspinal, intra-articular, pericardial, and intramuscular with compartment syndrome. (C) A clinically overt bleeding event that causes a fall in haemoglobin level of 2.0 g/dL (>1.24 mMol/L) or more; or a fall of haematocrit of 6.0% or more, adjusted for transfusion.
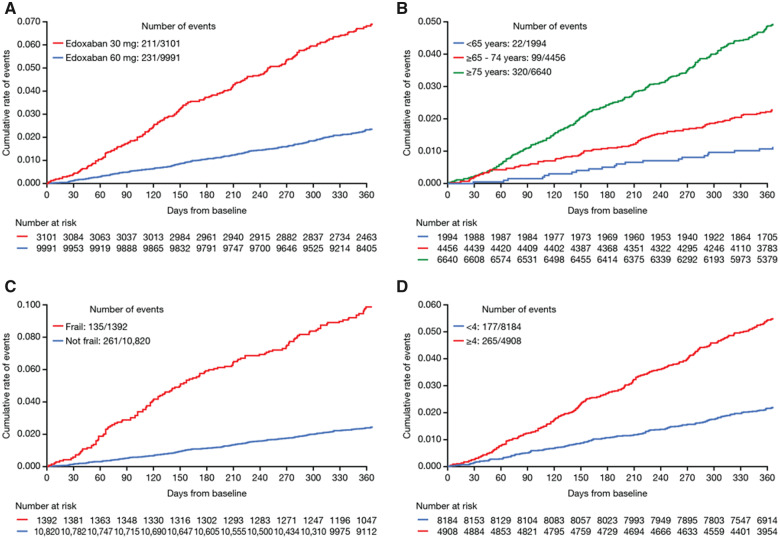
Figure 4.
Kaplan–Meier curves showing cumulative event rates for all-cause mortality in patients at 1-year follow-up classified by: (A) edoxaban dose, (B) age groups, (C) frailty status, and (D) CHA2DS2-VASc groups.
A further exploratory analysis of patients receiving non-recommended doses yielded noteworthy observations. Overall, patients treated with the non-recommended doses of edoxaban 30 mg and 60 mg daily had an intermediate risk profile, as observed both in the baseline characteristics and in the study outcomes. These findings infer that physicians prescribed doses not in line with the SmPC recommendation in patients that had ‘midway’ baseline characteristics between the higher and lower dose recommendations, and based on their clinical judgement, clinicians selected a higher or lower dose as they deemed appropriate or that awareness of dose reduction criteria was not scrutinized in detail or that prescribing occurred in settings outside of the formal anticoagulation clinic settings. This may explain the higher rate of annualized events of all-cause death, CV death, and major bleeding in patients receiving the non-recommended 30 mg vs. those receiving the recommended 60 mg dose. Similar patterns have been observed in patients treated with other NOACs, with under-dosing observed to be associated with higher risk for adverse events.20,21 Importantly, the current data set is not powered to assess small differences in stroke rates, but there was no signal for a higher stroke rate in patients receiving the non-recommended low dose of edoxaban. However, there are too few events in patients taking the non-recommended doses to determine a clinically relevant, substantial difference compared with those taking the recommended doses. A longer follow-up of ETNA-AF-Europe may provide more information on the effects of non-approved dosing.13
Advancing age and accumulation of comorbidities, including vascular disease, heart failure, diabetes, chronic kidney disease, as well as frailty are believed to increase thromboembolic and bleeding risks in patients with AF.22 In line with existing data for elderly patients,22 the ETNA-AF-Europe registry reported higher rates of clinical outcomes in elderly patients. Noticeably, the real-world penetration of anticoagulation in elderly and frail patients remains low. It is despite this population being at a higher risk for ischaemic events by virtue of their age and burden of associated comorbidities,23 and may be driven by a fear of bleeding associated with the use of oral anticoagulation.24 Furthermore, VKAs remain commonly used.25 Compared with VKAs, NOACs have been associated with lower risks of thromboembolic events, major bleeding, and all-cause death in octogenarian and frail patients.6,8,26,27 The ETNA-AF-Europe dataset demonstrates a low and constant rate of ICH in elderly and younger patients with AF, replicating findings observed in patients receiving other anticoagulants16 and the constant ICH rate across edoxaban plasma concentrations in ENGAGE AF-TIMI 48 in non-Asian patients.28 Increased rates of other bad outcomes were observed in frail vs. non-frail patients, as simply reported by investigators, included in the ETNA-AF-Europe registry. The data accumulated so far including ETNA-AF-Europe reinforce the ability of NOACs, and particularly edoxaban, to improve outcomes and quality of life in elderly and frail patients.
A trend towards a greater rate of major bleeding events occurred within the first month of initiating the study in high-risk patients, including those receiving edoxaban 30 mg, the elderly and frail population, and those with CHA2DS2-VASc score ≥4; while stroke/SEE and all-cause mortality were more evenly distributed over the 1 year of follow-up. This is most likely related to unmasking of bleeding predisposition in frail patients that had just initiated the treatment regimen. Similar observations were made with warfarin, showing the highest risk of haemorrhage at the initiation of anticoagulant therapy.29
Limitations
We acknowledge limitations with this routine clinical care study. ENGAGE AF-TIMI 48, a controlled phase III trial, was better equipped to ensure medication adherence, while routine care (ETNA-AF-Europe) usually relies on patients to take medications dispensed without further checks by a pharmacy. Another difference is the reduced ability to chase missing information in routine care vs. a randomized trial. Although follow-up rates in ETNA-AF-Europe were good; however, the numbers were lower than those available for ENGAGE AF-TIMI 48 at 1 year. In ETNA-AF-Europe, direct comparison of casual treatment effects was not possible as there was no randomized selection of therapy and only patients treated with edoxaban were here eligible. This data set captures events in patients treated with edoxaban early after its approval, potentially selecting patients and health professionals who are ‘early adopters’. The observational nature of therapy with an already approved anticoagulant may decrease the alertness to report non-serious events leading to incomplete capturing of events. Owing to the observational design of the study, systematic information on laboratory and other investigations is not available as would be in a trial setting. Furthermore, the open-label nature of the study may have introduced bias due to knowledge about treatment.
Conclusions
Edoxaban therapy in routine practice in ETNA-AF-Europe has reported low ischaemic and haemorrhagic events, including ICH in the first year after initiation in an unselected cohort of patients with AF, despite half of all patients being aged ≥75 years. The adherence to recommended trial dosing was high (83%). Low event rates were found across ages and comorbidities, including elderly and very elderly patients, frail patients and in patients with increased stroke risk. These data illustrate the effectiveness and safety of edoxaban in routine care in Europe captured in ETNA-AF-Europe, the first NOAC post-authorization safety study approved by the European Medicines Agency and mandated to have minimal disruption to routine care.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Supplementary Material
Acknowledgements
This study was supported by Daiichi Sankyo Europe GmbH, Munich, Germany. Editorial support was provided by Shelley Narula from inScience Communications, Springer Healthcare Ltd, UK, and was funded by Daiichi Sankyo Europe GmbH, Munich, Germany in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Declaration of Helsinki
The study complies with the Declaration of Helsinki, that the study was approved by the institutional review boards and independent ethics committees for all participating centres. All participants provided written informed consent.
Funding
This study was funded by Daiichi Sankyo Europe GmbH, Munich, Germany.
Conflict of interest: J.R.d.G. reports personal fees from Daiichi Sankyo during the conduct of the study; grants from Abbott, Atricure, Boston Scientific and Medtronic; personal fees from Atricure, Bayer, Daiichi Sankyo, Johnson & Johnson, Medtronic, Novartis, and Servier; and other from RhythmCARE outside the submitted work. T.W.W. has received fees, honoraria and research funding from Astra-Zeneca, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Medtronic, Menarini Pharma, Novartis, and Sanofi-Aventis. P.K. has received speaker’s and committee membership from Daiichi-Sankyo. He is the Lead Investigator of the HRB Stroke Clinical Trials Network Ireland, which has received grant funding from the Irish government, Irish Heart Foundation, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Amgen, and A Menarini. P.M. is an ETNA-AF investigator and has received lecture and research fees from Daiichi-Sankyo, Bayer, Boehringer Ingelheim, and Pfizer/BMS. J.C.D. has received honoraria for lectures from Bayer, Boehringer Ingelheim, and Bristol-Myers Squibb. J.C.D. has also received research grants from Boston Scientific, Sorin Group, Biotronik, and Abbott. C.d.A. has received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Atricure, Cardiotek, Biosense Webster and research grants on behalf of the centre from Biotronik, Medtronic, St Jude Medical Abbot, Livanova, Boston Scientific Biosense Webster. E.L.-d.-S. reports personal fees from Daiichi Sankyo; grants and personal fees from Servier, ZOLL Medical and Becton Dickinson; grants from NeuroproteXeon and MedImmune LLC, during the conduct of the study.
J.W. reports personal fees and non-financial support from Biotronik, Boehringer Ingelheim, and Daiichi-Sankyo; personal fees from Akzea, Bayer Vital, MSD, Berlin-Chemie and Siemens Healthineers, outside the submitted work. J.S. reports personal fees from Amgen, Astra Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Medscape, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll; grants and personal fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi-Sankyo, and Medtronic; other from CorXL, outside the submitted work. P.L. acts as a consultant for AbbVie, Actelion, Amgen, Astellas, Bayer, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Gilead, GSK, Hospira, Impeto Médical, Janssen, MSD, Mundipharma, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi and Sanofi Pasteur MSD. A.B. is founder of Amore Health Ltd, receives honorarium from Daiichi Sankyo, Pfizer, BMS, Bayer, Novartis, Roche, Napp, Boehringer Ingelheim for lecturing and scientific advice outside the submitted work. W.Z., P.L., M.C.M., and P.-E.R. are employees of Daiichi Sankyo Europe GmbH, Munich, Germany. R.D.C. reports grants, personal fees and non-financial support from Daiichi Sankyo, during the conduct of the study; personal fees from Boehringer Ingelheim, Bayer, BMS/Pfizer, Novartis, Sanofi and Roche, outside the submitted work.
P.K. reports non-financial support and other from Daiichi Sankyo Europe, during the conduct of the study; research support from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Heart Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies, outside the submitted work. In addition, P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783).
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 2. Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM.. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 4. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma CS, Zint K, Elsaesser A, Bartels DB, Lip GY.. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol 2017;69:777–785. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Lee YS, Kim TH, Cha MJ, Lee JM, Park J, Park JK, Kang KW, Shim J, Uhm JS, Park HW, Choi EK, Kim JB, Kim C, Kim J, Joung B.. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: a COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF). Korean J Intern Med 2020;35:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monelli M, Molteni M, Cassetti G, Bagnara L, De Grazia V, Zingale L, Zilli F, Bussotti M, Totaro P, De Maria B, Dalla Vecchia LA.. Non-vitamin K oral anticoagulant use in the elderly: a prospective real-world study—data from the REGIstry of patients on Non-vitamin K oral Anticoagulants (REGINA). Vasc Health Risk Manag 2019;15:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li G, Lip GYH, Holbrook A, Chang Y, Larsen TB, Sun X, Tang J, Mbuagbaw L, Witt DM, Crowther M, Thabane L, Levine M.. Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies. Eur J Epidemiol 2019;34:173–190. [DOI] [PubMed] [Google Scholar]
- 8. Deitelzweig S, Luo X, Gupta K, Trocio J, Mardekian J, Curtice T, Lingohr-Smith M, Menges B, Lin J.. Comparison of effectiveness and safety of treatment with apixaban vs. other oral anticoagulants among elderly nonvalvular atrial fibrillation patients. Curr Med Res Opin 2017;33:1745–1754. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Lixiana: Summary of product characteristics 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/lixiana#product-information-section (6 July 2020).
- 10. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG, Nordio F, Murphy SA, Kimura T, Jin J, Lanz H, Mercuri M, Braunwald E, Antman EM.. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2016;5:e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E.. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation 2016;134:24–36. [DOI] [PubMed] [Google Scholar]
- 12. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, Aylward P, White H, Zamorano JL, Antman EM, Ruff CT.. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol 2016;68:1169–1178. [DOI] [PubMed] [Google Scholar]
- 13. De Caterina R, Kelly P, Monteiro P, Deharo JC, de Asmundis C, Lopez-de-Sa E, Weiss TW, Waltenberger J, Steffel J, de Groot JR, Levy P, Bakhai A, Zierhut W, Laeis P, Reimitz PE, Kirchhof P.. Design and rationale of the Edoxaban Treatment in routiNe clinical prActice for patients with Atrial Fibrillation in Europe (ETNA-AF-Europe) study. J Cardiovasc Med (Hagerstown) 2019;20:97–104. [DOI] [PubMed] [Google Scholar]
- 14. De Caterina R, Kelly P, Monteiro P, Deharo JC, de Asmundis C, Lopez-de-Sa E, Weiss TW, Waltenberger J, Steffel J, de Groot JR, Levy P, Bakhai A, Zierhut W, Laeis P, Kerschnitzki M, Reimitz PE, Kirchhof P; on behalf of the ETNA-AF-Europe investigators. Characteristics of patients initiated on edoxaban in Europe: baseline data from edoxaban treatment in routine clinical practice for patients with atrial fibrillation (AF) in Europe (ETNA-AF-Europe). BMC Cardiovasc Disord 2019;19:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao TF, Chen SA, Ruff CT, Hamershock RA, Mercuri MF, Antman EM, Braunwald E, Giugliano RP.. Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J 2019;40:1518–1527. [DOI] [PubMed] [Google Scholar]
- 16. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, Potpara T, Dan GA, Kalarus Z, Tavazzi L, Maggioni AP, Lip GYH; EORP-AF Long-Term General Registry Investigators. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: the EORP-AF General Long-Term Registry. Europace 2019;21:1013–1022. [DOI] [PubMed] [Google Scholar]
- 18. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, Pan X, Baser O, Deitelzweig S.. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke 2018;49:2933–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Denas G, Gennaro N, Ferroni E, Fedeli U, Saugo M, Zoppellaro G, Padayattil Jose S, Costa G, Corti MC, Andretta M, Pengo V.. Effectiveness and safety of oral anticoagulation with non-vitamin K antagonists compared to well-managed vitamin K antagonists in naive patients with non-valvular atrial fibrillation: propensity score matched cohort study. Int J Cardiol 2017;249:198–203. [DOI] [PubMed] [Google Scholar]
- 20. Amarenco P, Haas S, Hess S, Kirchhof P, Lambelet M, Bach M, Turpie AGG, Camm AJ.. Outcomes associated with non-recommended dosing of rivaroxaban: results from the XANTUS study. Eur Heart J Cardiovasc Pharmacother 2019;5:70–79. [DOI] [PubMed] [Google Scholar]
- 21. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Singer DE, Peterson ED, Piccini JP; ORBIT-AF Investigators and Patients. Off-label dosing of non-vitamin k antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 2016;68:2597–2604. [DOI] [PubMed] [Google Scholar]
- 22. Sabbag A, Yao X, Siontis KC, Noseworthy PA.. Anticoagulation for stroke prevention in older adults with atrial fibrillation and comorbidity: current evidence and treatment challenges. Korean Circ J 2018;48:873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madhavan M, Holmes DN, Piccini JP, Ansell JE, Fonarow GC, Hylek EM, Kowey PR, Mahaffey KW, Thomas L, Peterson ED, Chan P, Allen LA, Gersh BJ.. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J 2019;211:77–89. [DOI] [PubMed] [Google Scholar]
- 24. Hanon O, Vidal JS, Le Heuzey JY, Kirchhof P, De Caterina R, Schmitt J, Laeis P, Mannucci PM, Marcucci M.. Oral anticoagulant use in octogenarian European patients with atrial fibrillation: a subanalysis of PREFER in AF. Int J Cardiol 2017;232:98–104. [DOI] [PubMed] [Google Scholar]
- 25. Pugh D, Pugh J, Mead GE.. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011;40:675–683. [DOI] [PubMed] [Google Scholar]
- 26. Kim HM, Choi EK, Park CS, Cha MJ, Lee SY, Kwon JM, Oh S.. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in octogenarian patients with non-valvular atrial fibrillation. PLoS One 2019;14:e0211766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hohmann C, Hohnloser SH, Jacob J, Walker J, Baldus S, Pfister R.. Non-vitamin K oral anticoagulants in comparison to phenprocoumon in geriatric and non-geriatric patients with non-valvular atrial fibrillation. Thromb Haemost 2019;119:971–980. [DOI] [PubMed] [Google Scholar]
- 28. Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, Deenadayalu N, Jarolim P, Betcher J, Shi M, Brown K, Patel I, Mercuri M, Antman EM.. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015;385:2288–2295. [DOI] [PubMed] [Google Scholar]
- 29. Garcia DA, Lopes RD, Hylek EM.. New-onset atrial fibrillation and warfarin initiation: high risk periods and implications for new antithrombotic drugs. Thromb Haemost 2010;104:1099–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.