Abstract
Objectives
Prior research has reported an increased risk of fatality for patients with cancer, but most studies investigated the risk by comparing cancer to non-cancer patients among COVID-19 infections, where cancer might have contributed to the increased risk. This study is to understand COVID-19’s imposed HR of fatality while controlling for covariates, such as age, sex, metastasis status and cancer type.
Methods
We conducted survival analyses of 4606 cancer patients with COVID-19 test results from 16 March to 11 October 2020 in UK Biobank and estimated the overall HR of fatality with and without COVID-19 infection. We also examined the HRs of 13 specific cancer types with at least 100 patients using a stratified analysis.
Results
COVID-19 resulted in an overall HR of 7.76 (95% CI 5.78 to 10.40, p<10−10) by following 4606 patients with cancer for 21 days after the tests. The HR varied among cancer type, with over a 10-fold increase in fatality rate (false discovery rate ≤0.02) for melanoma, haematological malignancies, uterine cancer and kidney cancer. Although COVID-19 imposed a higher risk for localised versus distant metastasis cancers, those of distant metastases yielded higher overall fatality rates due to their multiplicative effects.
Discussion
The results confirmed prior reports for the increased risk of fatality for patients with COVID-19 plus hematological malignancies and demonstrated similar findings of COVID-19 on melanoma, uterine, and kidney cancers.
Conclusion
The results highlight the heightened risk that COVID-19 imposes on localised and haematological cancer patients and the necessity to vaccinate uninfected patients with cancer promptly, particularly for the cancer types most influenced by COVID-19. Results also suggest the importance of timely care for patients with localised cancer, whether they are infected by COVID-19 or not.
Keywords: COVID-19, medical informatics, BMJ health informatics
Introduction
In localised cancers or haematological malignancies, timely cancer diagnosis and treatment is critical for increasing a patient’s survivability. Otherwise, localised cancers may progress into distant (distant organ systems) metastasis,1 2 while distant metastases may become uncontrollable, both of which result in more fatalities.3–5 However, with COVID-19 evidently impacting cancer care, diagnosis and treatment delays are inevitable due to the unavailability of medical resources, potential exposure risks of COVID-19 in medical facilities and complications of treatment (eg, chemotherapies worsening the fatality rate)6 7 attributed to the weaker immune systems of patients with cancer. Therefore, attention to the timeliness of therapy for patients with cancer is encouraged to minimise the risk of fatality.3 Still, the extent of risk that delays in cancer therapies add for persons with COVID-19 is not known8 9 and is likely to vary depending on cancer type,10 stage, grade and treatment.9 11 Therefore, estimating the risk COVID-19 imposes on each type of cancer is critical. Although prior research has been conducted, most studied the HR or OR by comparing patients with cancer to non-cancer patients among patients with COVID-19, which did not reflect the impact of COVID-19 on specific cancer types, could be confounded by the therapy types12–14 and is limited by the small sample size available for specific cancer types. Exceptions included Passamonti et al’s15 study, which reported 536 haematological cancer patients with COVID-19 infection at 66 hospitals in Italy. Those patients with severe or critical COVID-19 infections had an HR of 4.08 for mortality when taking mild severity as reference in Italy.15 However, the association between severity and fatality is evident, and the study was not designed to study the added risk of fatality from COVID-19 infection for the haematological malignancies. Here, we report a study comparing fatality rates among persons with a wide range of cancer diagnoses with and without COVID-19 while controlling for age, sex and type of cancer. Age and sex are essential biological variables underlying the fatality of COVID-1916–19 and thus should be controlled for. We further studied the added COVID-19 risk to fatality for specific cancer types to aid oncologists in making optimal treatment decisions from various risk factors, several of which may be contradictory, such as delay of care and infection risk.
Methods
We conducted a retrospective survival study using UK Biobank (UKB)20 under the UKB COVID-19 policy.21 Started in 2006, UKB is a government funded biobank with longitudinal COVID-19 test results, death registries, cancer registries and inpatient records for approximately 500 000 patients.22 COVID-19 tests started on 16 March 2020 for symptomatic patients, during which testing capacity was limited and results were provided by Public Health England.23 There were roughly 67 000 living cancer subjects at the beginning of COVID-19 testing. Inclusion criteria for the study were: (1) subjects of British ancestry with a history of hospitalisation in UKB (updated to the end of September 2020) and (2) subjects conducted a COVID-19 test no later than 11 October 2020, for which we could obtain a 21-day follow-up in the death registry from National Health Service (NHS) Digital and NHS Central Register, UK. The inclusion criteria resulted in 6528 cancer patients. We then excluded 893 patients with cancer whose cancer diagnoses were 10 or more years ago, without another primary cancer or recurrence in the record since they are unlikely in remission and more closely resemble non-cancer patients. We further excluded five cancer patients with inconsistent self-report of sex and 1024 ‘non-melanoma skin cancer patients’ reported with truncated International Classification of Diseases, Tenth Revision (ICD-10) codes in the UKB, which conflates non-lethal basal cell carcinomas with lethal forms of non-melanoma skin cancers. The final cancer cohort comprised 4606 patients, where 288 (6.3%) were positive for COVID-19 (table 1). We also built a randomised non-cancer cohort of 4606 patients for comparative studies, which matched the COVID-19 status, sex, age (per 5-year bin) and specific laboratory testing facility of the corresponding patients with cancer. We exclude seven non-cancer patients with COVID-19 tests conducted after death during the sampling.
Table 1.
Demographic and clinical characteristics of 4606 COVID-19 tested cancer subjects in the UK Biobank
| COVID-19 positive associated | COVID-19 negative associated | |||||
| Fatalities | (%) | Survivors | Fatalities | (%) | Survivors | |
| Race | ||||||
| British ancestry | 64 | (22.2) | 224 | 153 | (3.5) | 4165 |
| Age (years) | ||||||
| 50–59 | 2 | (7.1) | 26 | 8 | (2.9) | 271 |
| 60–69 | 6 | (9.7) | 56 | 26 | (3.0) | 850 |
| 70–79 | 42 | (26.4) | 117 | 94 | (3.5) | 2562 |
| 80–84 | 14 | (35.9) | 25 | 25 | (4.9) | 482 |
| Sex | ||||||
| Male | 40 | (23.8) | 128 | 90 | (3.8) | 2285 |
| Female | 24 | (20.0) | 96 | 63 | (3.2) | 1880 |
| History of cancer (years) | ||||||
| >10 | 18 | (27.7) | 47 | 35 | (3.4) | 1008 |
| 5<years ≤10 | 15 | (18.5) | 66 | 24 | (2.3) | 1005 |
| 1<years ≤5 | 3 | (13.0) | 20 | 8 | (4.3) | 178 |
| ≤1 | 28 | (23.5) | 91 | 86 | (4.2) | 1974 |
We analysed the case fatality rate (CFR) of patients with cancer and their cancer types using a multivariate Cox proportional hazard model.24 COVID-19 status was obtained from the patient’s first test result (negative controls remained so throughout). Control covariates included ethnicity, age, sex, and cancer status. Age was treated as a continuous variable, and cancer status was categorised as localised versus distant metastatic based on UKB ICD-10 codes from inpatient records where distant metastasis status was characterised by ICD-10 codes of C78 (metastatic to respiratory and digestive organs), C79 (metastatic to other and unspecified sites) and C80 (metastasis without specific sites or multiple sites), whereas localised metastatic status were patients without any of the three codes, possibly including C77 (spread to lymph nodes) as well. R’s survival package25 was used to diagnose the model and plot Kaplan-Meier curves.
Results
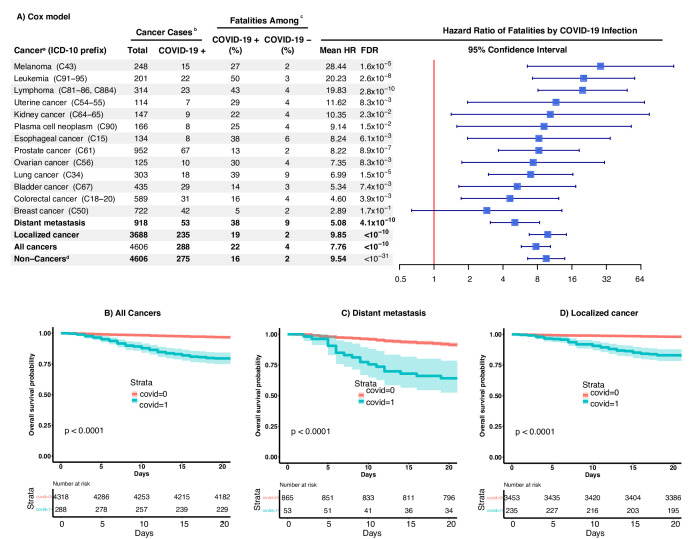
Quartiles of time from COVID-19 diagnosis to death for patients with cancer (64 total) are 5, 10 and 14 days, respectively. The CFR within 21 days of diagnosis for COVID-19 positive patients with cancer was sixfold higher than that of COVID-19 negative ones (22.2% (64 out of 288) versus 3.5% (153 out of 4318)) (table 1). Distant metastasis patients demonstrated a higher fatality rate, where CFR within 21 days of a positive COVID-19 diagnosis was nearly double that of patients with localised cancer (37.7% versus 18.7%) (figure 1). No matter the spread of cancer, the CFR of COVID-19 within 21 days (22.2%) was higher than that of non-cancer patients when positive (15.6%), from the cohort using matched covariates such as test result, sex, age and testing venue (a proxy of the hospitalisation system). A multivariate Cox model was built to study individual risk factors and showed an HR of 7.76 (95% CI 5.78 to 10.40, p<10−10) for COVID-19 positive patients with cancer compared with COVID-19 negative patients with cancer after controlling for ethnicity, sex, age and metastatic versus localised cancer confounders (figure 1). The model suggested increased fatality rates by COVID-19 infections particularly for patients with melanoma, lymphoma, leukaemia, uterine or kidney cancer. The HRs of COVID-19 were 10-fold higher for these five cancer diagnoses using stratified analyses with matched cancer types (figure 1). As expected, distant metastasis was a risk factor for fatality, with an HR of 3.92 (95% CI 2.99 to 5.13, p<10−10) compared with localised cancers. Age remained an important factor, with an HR of 1.04 per year (95% CI 1.02 to 1.07; p=3.1×10−4), implying an over 10-fold higher fatality risk for the 60-year difference that may exist between the seniors and youths. Sex was not deemed significant. No factor was found to significantly deviate from the model assumption (p>0.25). Logistic regression led to similar but slightly inflated results because the logistic model does not require constant risk over time, whereas the Cox model does.
Figure 1.
(A) HRs of COVID-19 associated death among patients with cancer and types of cancers. (a) Results were from stratified analyses using the patients stratifying conditions listed in the column named ‘cancer’. All analyses used Cox model with covariates of sex, age, COVID-19 infection status, and cancer status (localised or distant metastasis) except for stratified analyses of localised cancer, distant metastasis, and non-cancers. Cancer subtypes were analysed when they were composed of 100 or more total cases. (b) Four patients with cancer with inconsistent self-reporting and gene sex were excluded from the study. (c) Fatality event was assessed for the 21 days following the first COVID-19 positive testing or the first COVID-19 negative testing (negative controls remained so throughout) and was available for all cancer subjects under study. (d) Sixty non-cancer patients did not match the testing facility with a patient with cancer due to lacking subjects of matching all factors (eg, age). (B-D) Kaplan-Meier curves for COVID-19 positive vs. negative cancer patients: (B) all cancer patients, (C) patients with distant metastasis, and (D) patients with only localized cancer.
Discussion
Our study demonstrated that COVID-19 adds 10-fold more risk to 21-day fatality rates for patients with melanoma, lymphoma, leukaemia, uterine and kidney cancer with a positive COVID-19 infection versus no COVID-19 infection. Our results for lymphoma and leukaemia patients confirm reports from Italy, while the findings of increased 21-day mortality rates in COVID-19 infections among melanoma, uterine and kidney cancer patients have not been reported previously. The findings do support prior kidney injury reports among patients with COVID-19 as well.26 Fortunately, our study suggests that COVID-19 does not impose a larger risk to distant metastasised cancers as compared with localised cancers (eg, lymphoma and leukaemia) in general. However, the overall fatality rate in distant metastases was still about twice that of localised cancers due to the multiplicative effect of HRs in the model. It should be noted that fatality rates were dependent on cancer type, and COVID-19 did impose larger risk to distant metastasis of some types of cancer, such as melanoma (HR 49.37, 95% CI 3.70 658.85), prostate cancer (HR 22.11, 95% CI 6.15 to 79.54) and ovarian cancer (HR 13.04, 95% CI 2.61 to 65.14), based on a stratified analysis using only metastasis patients (online supplemental figure 1). In all cases, higher rates of fatality among patients with COVID-19 of older age were consistent with literature.16 17 27 Our results focused on fatality are complementary to those few studies focused on oncological procedures unimpacted by COVID-19. Indeed, investigations of the National Cancer Data Base on prostate radiotherapy28 and breast cancer surgeries29 report unchanged overall survival during COVID-19.
bmjhci-2021-100341supp001.pdf (84.8KB, pdf)
The strengths of this study include the large UKB cancer cohort size for patients with COVID-19 and its reliable death registry. Limitations include the unavailability of complete cancer stage and grade, plus a relatively small sample size for some specific cancer types. Furthermore, the study was unable to include other pre-existing conditions that may have been associated with the fatality, and the conclusions may be limited to symptomatic patients and hospitalised patients due to the inclusion criteria.
Our findings reinforce the clinical importance of timely treatment of COVID-19 among older cancer patients with localised cancers.1 Timely care is especially important for those with haematological malignancies, melanoma, uterine or kidney cancer due to the notable additional risk of fatality from COVID-19 infection plus the added risk of metastasis due to the delay of therapies, which leads to an even higher likelihood of fatality because of the multiplicative effects of risk. The findings also support specific guidelines30 emphasising the importance of timely care for COVID-19 infected patients with cancer and strongly support a change in COVID-19 vaccine strategy with haematological malignancies in particular because the benefit of vaccination far outweighs its risk of side effects.31 32
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number 28979.
Footnotes
Twitter: @haiquanlab, @LussierY
HL and EB contributed equally.
Contributors: All authors contributed to the conception, analysis, and interpretation of the results. YL, CB, HL, and EC conceived the study. HL led the analysis. CB and YL directed the oncology discussion. EB undertook the data processing and wrote the first draft. XZ and LA performed the model diagnosis. WL and CK designed the figures. All authors revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Dirie J, Mahesan T, Hart E, et al. Delivering safe and timely cancer care during COVID-19: lessons and successes from the transition period. BJU Int 2021. 10.1111/bju.15343. [Epub ahead of print: 21 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones D, Neal RD, Duffy SRG, et al. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol 2020;21:748–50. 10.1016/S1470-2045(20)30242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34. 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol 2020;21:1035–44. 10.1016/S1470-2045(20)30392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:904–13. 10.1016/S1470-2045(20)30310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee LY, Cazier JB, Starkey T. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. The Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol 2020;38:3538–46. 10.1200/JCO.20.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Chen L, Chen L. COVID-19 in persons with haematological cancers. Leukemia 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncology 2020;6:1108. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol 2020;6:557–9. 10.1200/GO.20.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020;7:e737–45. 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natale F, Ghio D, Tarchi D. COVID-19 cases and case fatality rate by age. European Commission 2020;52:154–64. [Google Scholar]
- 17.Ghisolfi S, Almås I, Sandefur JC, et al. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob Health 2020;5:e003094. 10.1136/bmjgh-2020-003094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehingia N, Raj A. Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob Health 2021;9:e14–15. 10.1016/S2214-109X(20)30464-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo Clinic Proceedings . Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Elsevier, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, et al. Uk Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanji MY, Aung N, Chahal CAA, et al. COVID-19 and the UK Biobank-Opportunities and challenges for research and collaboration with other large population studies. Front Cardiovasc Med 2020;7:156. 10.3389/fcvm.2020.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins R. What makes UK Biobank special? Lancet 2012;379:1173–4. 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 23.Chadeau-Hyam M, Bodinier B, Elliott J, et al. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK Biobank data. Int J Epidemiol 2020;49:1454–67. 10.1093/ije/dyaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therneau TM, Grambsch PM. The COX model. modeling survival data: extending the COX model. Springer, 2000: 39–77. [Google Scholar]
- 25.Therneau TM, Lumley T. Package ‘survival’. R Top Doc 2015;128:28–33. [Google Scholar]
- 26.Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr 2020;14:1133–42. 10.1016/j.dsx.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann C, Wolf E. Older age groups and country-specific case fatality rates of COVID-19 in Europe, USA and Canada. Infection 2021;49:111–6. 10.1007/s15010-020-01538-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dee EC, Mahal BA, Arega MA, et al. Relative timing of radiotherapy and androgen deprivation for prostate cancer and implications for treatment during the COVID-19 pandemic. JAMA Oncol 2020;6:1630–2. 10.1001/jamaoncol.2020.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minami CA, Kantor O, Weiss A, et al. Association between time to operation and pathologic stage in ductal carcinoma in situ and early-stage hormone receptor-positive breast cancer. J Am Coll Surg 2020;231:434–47. 10.1016/j.jamcollsurg.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol 2020;21:629–30. 10.1016/S1470-2045(20)30217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribas A, Sengupta R, Locke T, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2021;11:233–6. 10.1158/2159-8290.CD-20-1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai A, Gainor JF, Hegde A. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nature Reviews Clinical Oncology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjhci-2021-100341supp001.pdf (84.8KB, pdf)