Abstract
Aims
Safety and efficacy of antithrombotic regimens in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) may differ based on clinical presentation. We sought to compare double vs. triple antithrombotic therapy (DAT vs. TAT) in AF patients with or without acute coronary syndrome (ACS) undergoing PCI.
Methods and results
A systematic review and meta-analysis was performed using PubMed to search for non-vitamin K antagonist oral anticoagulant (NOAC)-based randomized clinical trials. Data on subgroups of ACS or elective PCI were obtained by published reports or trial investigators. A total of 10 193 patients from four NOAC trials were analysed, of whom 5675 presenting with ACS (DAT = 3063 vs. TAT = 2612) and 4518 with stable coronary artery disease (SCAD; DAT = 2421 vs. TAT = 2097). The primary safety endpoint of ISTH major bleeding or clinically relevant non-major bleeding was reduced with DAT compared with TAT in both ACS (12.2% vs. 19.4%; RR 0.63, 95% CI 0.56–0.71; P < 0.0001; I2 = 0%) and SCAD (14.6% vs. 22.0%; RR 0.68, 95% CI 0.55–0.85; P = 0.0008; I2 = 66%), without interaction (P-int = 0.54). Findings were consistent for secondary bleeding endpoints, including intra-cranial haemorrhage. In both subgroups, there was no difference between DAT and TAT for all-cause death, major adverse cardiovascular events, or stroke. Myocardial infarction and stent thrombosis were numerically higher with DAT vs. TAT consistently in ACS and SCAD (P-int = 0.60 and 0.86, respectively). Findings were confirmed by multiple sensitivity analyses, including a separate analysis on dabigatran regimens and a restriction to PCI population.
Conclusions
DAT, compared with TAT, is associated with lower bleeding risks, including intra-cranial haemorrhage, and a small non-significant excess of cardiac ischaemic events in both patients with or without ACS.
Keywords: Atrial fibrillation (AF), Percutaneous coronary intervention (PCI), Double therapy (DAT), Triple therapy (TAT), Non-vitamin K antagonist oral anticoagulant (NOAC), Acute coronary syndrome (ACS)
Introduction
The optimal antithrombotic strategy for patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) remains debated.1–5 Four multicentre trials, focusing on AF patients undergoing PCI or with acute coronary syndromes (ACS), showed that double antithrombotic therapy (DAT) consisting of a non-vitamin K antagonist (VKA) oral anticoagulant (NOAC) plus a P2Y12 inhibitor (essentially clopidogrel) reduced bleeding complications without apparent increase in ischaemic risk compared with triple antithrombotic therapy (TAT), consisting of a VKA and dual antiplatelet therapy (DAPT).6–9 However, individual trials were powered for safety and not for efficacy and a recent pooled analysis of these four trials observed that the bleeding benefit was counterbalanced by a significant increase of stent thrombosis (ST) and a trend towards higher risk of myocardial infarction (MI) with DAT.10
All these trials have enrolled a variable number of AF patients with ACS or stable coronary artery disease (SCAD). The bleeding and ischaemic risks, as well as the optimal antithrombotic therapy, might differ according to the clinical presentation. We therefore investigated the safety and efficacy of DAT vs. TAT in AF patients undergoing PCI or affected by ACS according to the clinical presentation among the four NOAC-based randomized clinical trials.
Methods
The present systematic review and meta-analysis integrates the previous one by adding a stratification based on clinical presentation (ACS vs. SCAD).10 Data on clinical events in these subgroups were extracted by published reports or provided by investigators. A full description of the methodology was previously published.10 Briefly, a systematic search was performed on PubMed and led to identify four NOAC-based randomized clinical trials comparing DAT vs. TAT in AF patients with ACS or undergoing PCI, including AUGUSTUS (Open-Label, 2 × 2 Factorial, Randomized, Controlled Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients with Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention), ENTRUST-AF PCI (EdoxabaN TReatment versUS VKA in paTients with AF undergoing PCI), PIONEER-AF PCI (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention), and RE-DUAL PCI (Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With Non-valvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention). The protocol followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines and was registered on PROSPERO (CRD42019142779).
Outcome measures
The primary safety bleeding endpoint was defined as ISTH major bleeding or clinically relevant non-major bleeding (CRNMB) at longest available follow-up (between 6 and 14 months). Secondary safety outcomes included alternative bleeding definitions (trial-defined primary safety bleeding endpoint; ISTH major bleeding, ISTH CRNMB, TIMI major or minor bleeding, intra-cranial haemorrhage).
Secondary efficacy endpoints included all-cause death; trial-defined major adverse cardiovascular event (MACE), MI, stroke, and ST. Main endpoint definitions are displayed in Supplementary material online, Table S1.
Statistical analysis
Effect sizes in the overall population and ACS and SCAD subgroups were calculated with the Mantel–Haenszel random-effects estimator and expressed as risk ratios (RRs) and 95% CIs. Heterogeneity was assessed by I2 tests, with substantial heterogeneity defined as I2 >50%. Number needed to treat for benefit (NNTB) or harm (NNTH) were also calculated according to Cochrane’s recommendations: [1/ACR×(1−RR)], where ACR is the assumed control risk. Sensitivity analyses were performed to: (i) investigate the influence of individual trials on the results; (ii) test results with a fixed-effect model; (iii) investigate separately the doses of dabigatran 110 mg and 150 mg b.i.d. for the RE-DUAL PCI trial; and (iv) restrict the analysis to PCI only (due to the peculiar design of the AUGUSTUS trial, a secondary analysis on PCI population was also conducted by excluding patients presenting with ACS and managed medically).
As previously described, the methodological quality of the randomized trials was assessed by Cochrane’s Collaboration tool for assessing risk of bias (low, unclear, or high risk of bias) and no publication bias was assessed due to the small number of studies (<10) included. Statistical significance was set at P < 0.05 (2-tailed). Data analysis was performed with Reviewer Manager (RevMan, version 5.3; Cochrane).
Results
Overall 10 193 patients (DAT = 5484 vs. TAT = 4709) from the four trials were analysed, of whom 5675 presented with ACS (DAT = 3063 vs. TAT = 2612) and 4518 with SCAD (DAT = 2421 vs. TAT = 2097).
The characteristics of the four included trials and of patients are reported in Supplementary material online, Tables S1 and S2. All trials were of high quality (Supplementary material online, Table S3).
Safety endpoints
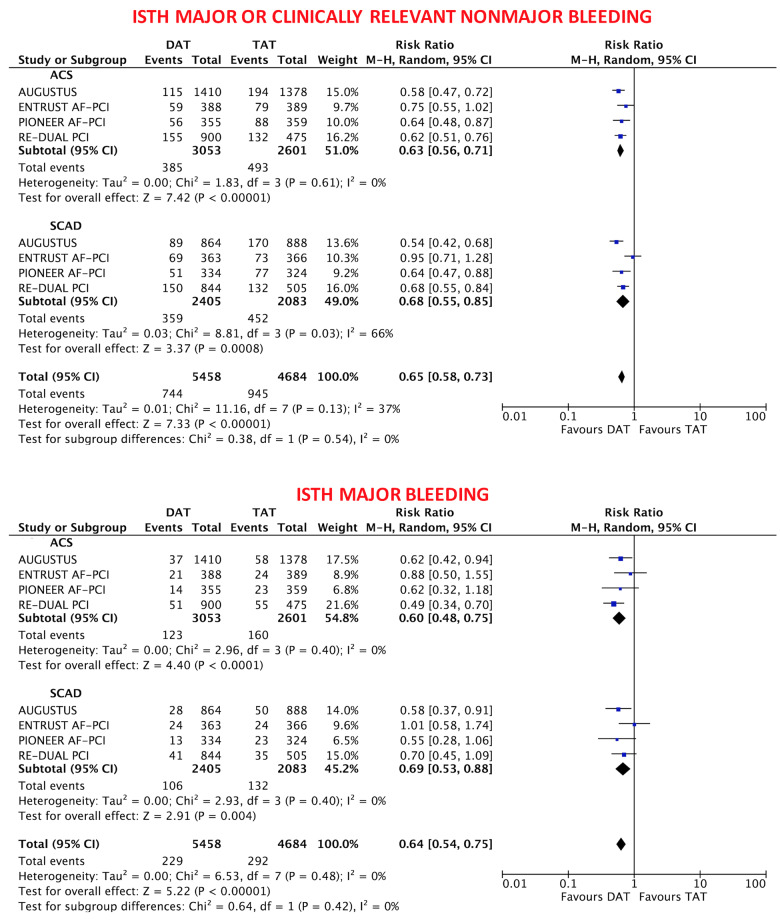
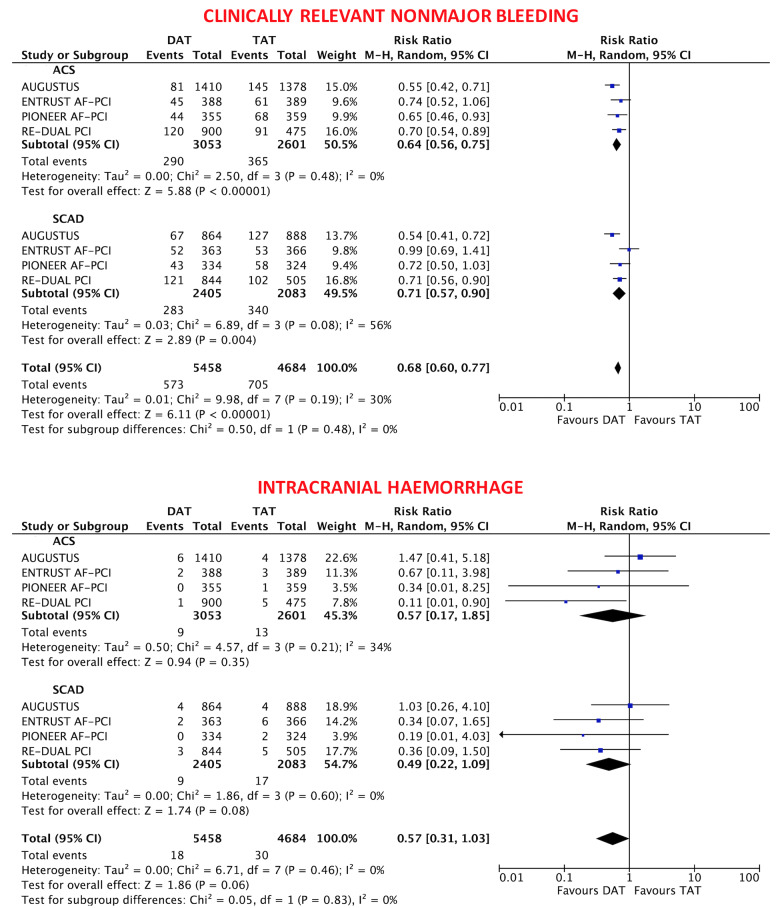
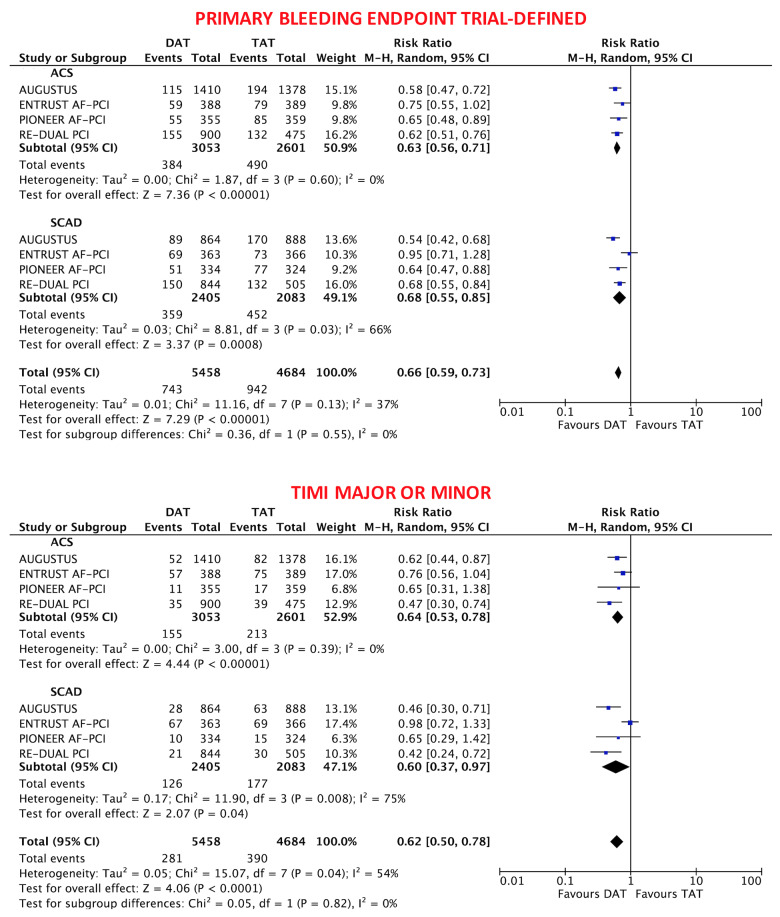
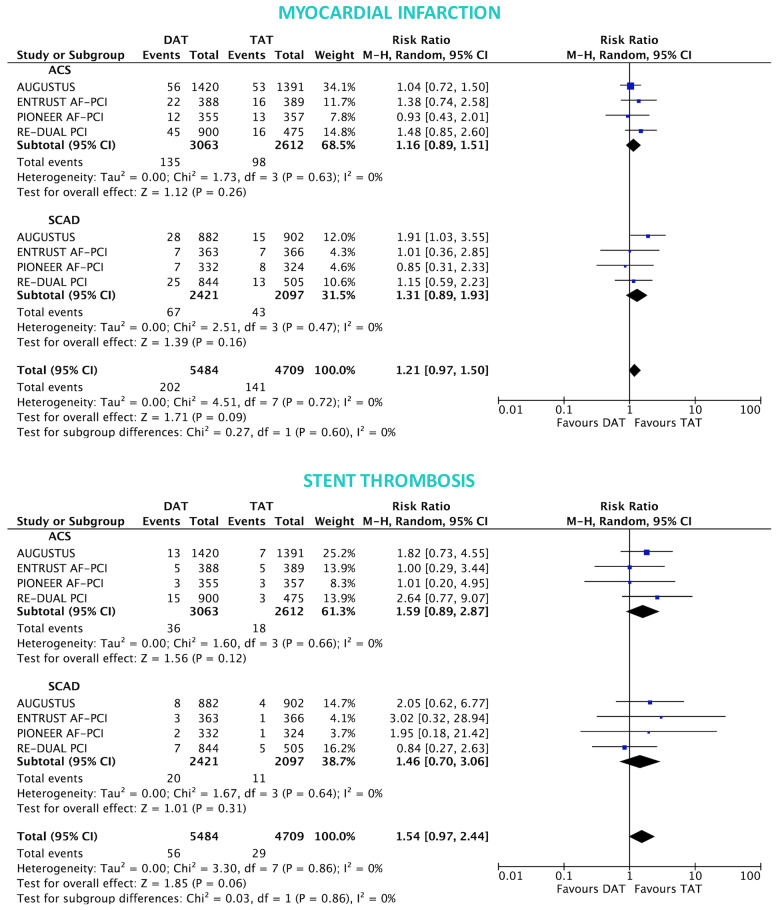
The primary safety bleeding endpoint of ISTH major bleeding or CRNMB was significantly reduced with DAT compared with TAT in both ACS (12.2% vs. 19.4%; RR 0.63, 95% CI 0.56–0.71; P < 0.0001; I2 = 0%) and SCAD (14.6% vs. 22.0%; RR 0.68, 95% CI 0.55–0.85; P = 0.0008; I2 = 66%) without interaction (interaction P = 0.54; Figure 1). In both ACS and SCAD subgroups, this benefit was consistently driven by reductions of both major (ACS: 3.9% vs. 6.4%; RR 0.60, 95% CI 0.48–0.75; P < 0.0001; I2 = 0%; SCAD: 4.4% vs. 6.4%; RR 0.69, 95% CI 0.53–0.88; P = 0.004; I2 = 0%; interaction P = 0.42) and CRNMB (ACS: 9.1% vs. 14.3%; RR 0.64, 95% CI 0.56–0.75; P < 0.0001; I2 = 0%; SCAD: 11.5% vs. 16.6%; RR 0.71, 95% CI 0.57–0.90; P = 0.004; I2 = 56%; interaction P = 0.48; Figures 1 and 2). The results remained consistent when alternative bleeding definitions were adopted (Figure 3). DAT was associated with a borderline 43% reduction of intra-cranial haemorrhage (P = 0.06, Figure 2) compared with TAT, with consistent effects among ACS (0.31% vs. 0.53%; RR 0.57, 95% CI 0.17–1.85; P = 0.35; I2 = 34%) and SCAD patients (0.38% vs. 0.83%; RR 0.49, 95% CI 0.22–1.09; P = 0.08; I2 = 0%; interaction P = 0.83; Figure 2).
Figure 1.
Main bleeding endpoints in double antithrombotic therapy vs. triple antithrombotic therapy according to clinical presentation. Random-effects risk ratios and 95% confidence intervals for main bleeding endpoints. DAT, double antithrombotic therapy; ISTH, International Society on Thrombosis and Haemostasis; M–H, Mantel–Haenszel; TAT, triple antithrombotic therapy.
Figure 2.
Clinically relevant non-major bleeding and intra-cranial haemorrhage in double antithrombotic therapy vs. triple antithrombotic therapy according to clinical presentation. Random-effects risk ratios and 95% confidence intervals clinically relevant non-major bleeding and intra-cranial haemorrhage.
Figure 3.
Alternative bleeding definitions in double antithrombotic therapy vs. triple antithrombotic therapy according to clinical presentation. Random-effects risk ratios and 95% confidence intervals for primary bleeding endpoint trial-defined and thrombolysis in myocardial infarction major or minor bleeding.
Efficacy endpoints
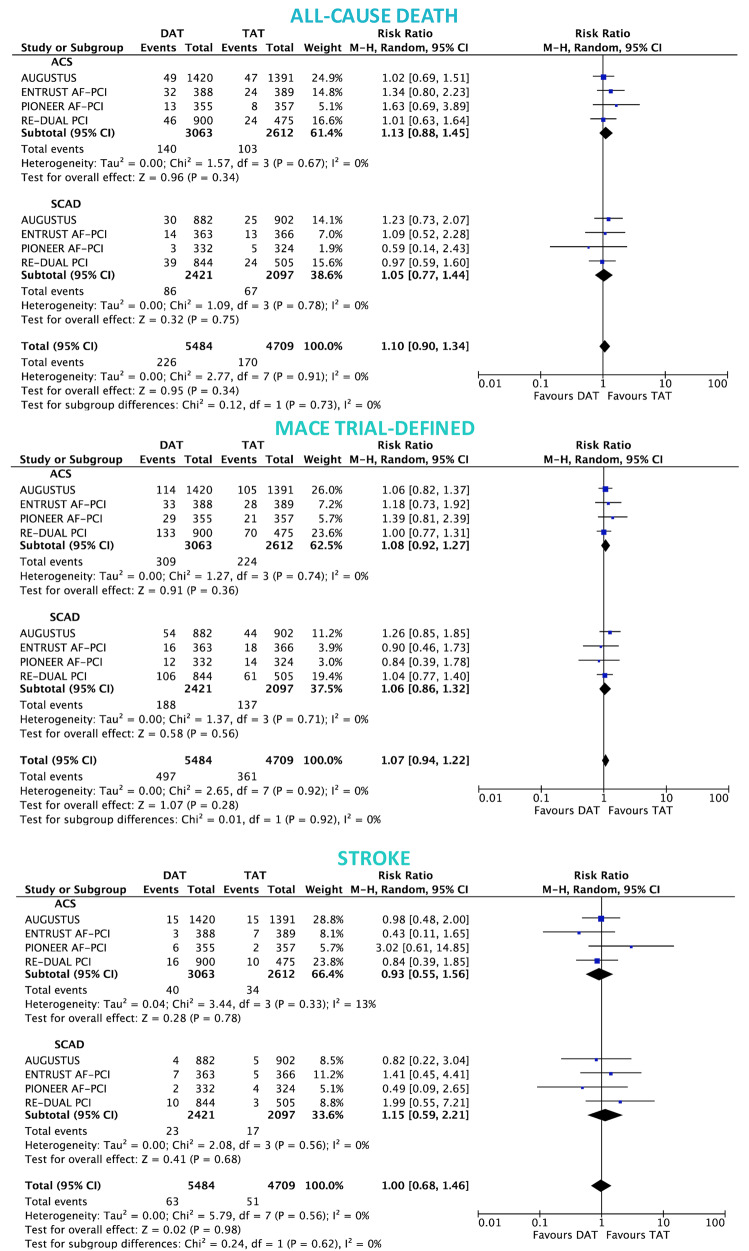
In both ACS and SCAD subgroups, there was no significant difference between DAT and TAT for all-cause death (ACS: 4.5% vs. 4.0%; RR 1.13, 95% CI 0.88–1.45; P = 0.34; I2 = 0%; SCAD: 3.5% vs. 3.3%; RR 1.05, 95% CI 0.77–1.44; P = 0.75; I2 = 0%; interaction P = 0.73; Figure 4), MACE (ACS: 9.6% vs. 8.9%; RR 1.08, 95% CI 0.92–1.27; P = 0.36; I2 = 0%; SCAD: 7.3% vs. 6.9%; RR 1.06, 95% CI 0.86–1.32; P = 0.56; I2 = 0%; interaction P = 0.92; Figure 4) and stroke (ACS: 1.3% vs. 1.3%; RR 0.93, 95% CI 0.55–1.56; P = 0.78; I2 = 13%; SCAD: 0.9% vs. 0.8%; RR 1.15, 95% CI 0.59–2.21; P = 0.68; I2 = 0%; interaction P = 0.62; Figure 4). The rates of MI and ST were slightly but not significantly higher with DAT in both ACS: (4.4% vs. 3.7%; RR 1.16, 95% CI 0.89–1.51; P = 0.26; I2 = 0% and 1.1% vs. 0.7%; RR 1.59, 95% CI 0.89–2.87; P = 0.12; I2 = 0%; respectively) and SCAD groups (2.8% vs. 2.1%; RR 1.31, 95% CI 0.89–1.93; P = 0.16; I2 = 0%; interaction P = 0.60 and 0.8% vs. 0.6%; RR 1.46, 95% CI 0.70–3.06; P = 0.31; I2 = 0%; respectively; interaction P = 0.86; Figure 5).
Figure 4.
Death, major adverse cardiovascular events, and stroke in double antithrombotic therapy vs. triple antithrombotic therapy according to clinical presentation. Random-effects risk ratios and 95% confidence intervals for all-cause death, major adverse cardiovascular events, and stroke.
Figure 5.
Myocardial infarction and stent thrombosis in double antithrombotic therapy vs. triple antithrombotic therapy according to clinical presentation. Random-effects risk ratios and 95% confidence intervals for myocardial infarction and stent thrombosis. Note: stent thrombosis definition was definite ST for the RE-DUAL PCI, ENTRUST-AF PCI and PIONEER-AF PCI trials, and definite or probable ST for AUGUSTUS.
Additional analyses
Bleeding endpoints remained consistent across clinical presentation subgroups when dabigatran 110 or 150 mg were analysed separately (Supplementary material online, Figures S1–S6). Ischaemic endpoints showed consistent results when dabigatran 110 or 150 mg were analysed separately (Supplementary material online, Figures S7–S11), although DAT with dabigatran 110 mg seemed to be associated with somewhat greater MI and ST risks in ACS but not SCAD patients (Supplementary material online, Figures S10 and S11).
Results remained consistent when AUGUSTUS patients with ACS not undergoing PCI were excluded (Supplementary material online, Figures S12–S16), although the benefit of DAT in the ACS subgroup in terms of intra-cranial haemorrhage became greater (Supplementary material online, Figure S13) as the risk of MI, while the risk of ST slightly reduced (Supplementary material online, Figure S16).
When removing one study at a time, consistent results between ACS and SCAD subgroups were confirmed for the primary bleeding endpoint (Supplementary material online, Table S4). Results remained consistent when a fixed-effects model was adopted (Supplementary material online, Table S5).
The NNTB and NNTH were calculated for safety and efficacy endpoints in both ACS and SCAD (Supplementary material online, Table S6). We also calculated NNTB and NNTH for multiple risk strata for both ISTH major bleeding and MI and analysed the net benefit (NNTB<NNTH) or harm (NNTB>NNTH) in ACS and SCAD subgroups, observing that DAT had greater benefit than harm in ACS patients but not for SCAD patients in whom the bleeding benefit did not seem to exceed the higher MI rates (Figure 6; Supplementary material online, Tables S7 and S8).
Figure 6.
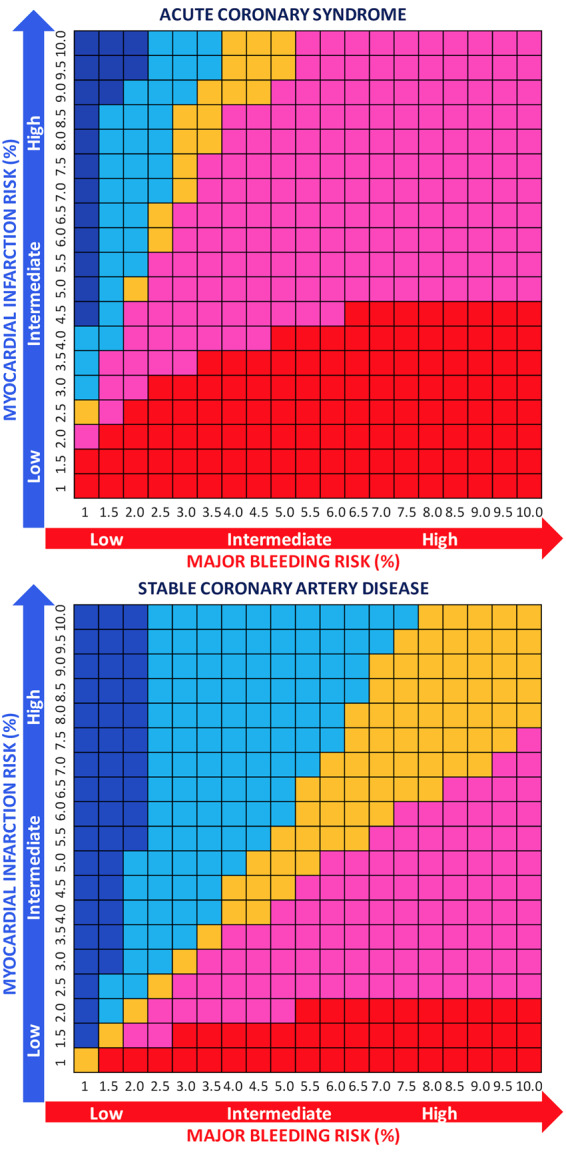
Number needed to treat for benefit or harm for double antithrombotic therapy vs. triple antithrombotic therapy according to risk of major bleeding and myocardial infarction in acute coronary syndrome and stable coronary artery disease subgroups. At each risk (%) ranging from 1% to 10% for both major bleeding and MI, the difference between NNTB and NNTH was calculated. Red indicates that the net benefit of double antithrombotic therapy vs. triple antithrombotic therapy is in favour of bleeding (NNTB < NNTH, thus reduction of bleeding is higher than the risk of MI) and its intensity refers to greater (dark red) or lower (light red/pink) benefit (with the cut-off range selected arbitrarily to be −100–100), while blue indicates a net ischaemic harm (NNTB > NNTH, thus the reduction of bleeding is lower than the risk of MI) and its intensity refers to greater (dark blue) or lower (light blue) harm (with the cut-off range selected arbitrarily to be −100–100). Orange indicates a neutral effect (NNTB = NNTH; we arbitrarily selected a range from −10 to 10 to consider the effect as neutral).
Discussion
In the present meta-analysis of the four NOAC-based multicentre randomized clinical trials, we investigated the safety and efficacy profile of DAT vs. TAT in 10 193 AF patients undergoing PCI according to clinical presentation (ACS or SCAD).
Main findings are summarized as follows (Figure 7):
Figure 7.
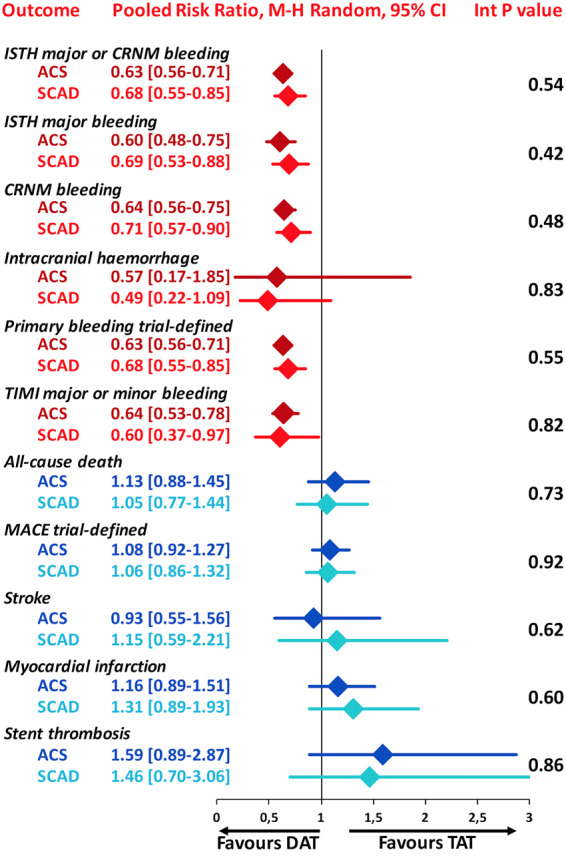
The summary of safety and efficacy endpoints in double antithrombotic therapy vs. triple antithrombotic therapy demonstrating there is a consistent effect across acute coronary syndrome and stable coronary artery disease subgroups. Pooled random-effects risk ratios with 95% confidence intervals and interaction P values for safety and efficacy endpoints. CRNM, clinically relevant non-major; DAT, double antithrombotic therapy; ISTH, International Society on Thrombosis and Haemostasis; MACE, major adverse cardiovascular events; TAT, triple antithrombotic therapy; TIMI, thrombolysis in myocardial infarction.
There was no difference in the treatment effects with respect to primary and secondary bleeding endpoints between ACS and SCAD patients treated with DAT or TAT, confirming a consistent reduction of bleeding with DAT in patients with or without ACS.
There was no difference in treatment effects with respect to any cardiac or cerebrovascular ischaemic outcome between ACS and SCAD patients treated with DAT vs. TAT, suggesting that the small numerically higher rates of non-fatal cardiovascular ischaemic events with DAT may occur irrespective of the clinical presentation.
Aspirin has represented for decades the cornerstone for antiplatelet therapy in patients with cardiovascular diseases, however, in the last few years an alternative approach has emerged, the so called ‘aspirin-free strategy’, evaluating the safety and efficacy of a single-antiplatelet therapy with a P2Y12 inhibitor alone.5,11 The clinical setting of patients with AF undergoing PCI, who need both OAC and antiplatelet agents, has been among the first setting in which aspirin was withdrawn from DAPT while clopidogrel monotherapy was continued. After the WOEST trial, four NOAC-based trials of AF patients undergoing PCI or with ACS have been conducted and tested this approach. These studies demonstrated that DAT was consistently associated with lower bleeding risk compared with TAT in the absence of a significant trade-off in terms of main ischaemic endpoint.6–9 However, all of them were mostly underpowered for identifying differences of more rare, yet of major clinical relevance, bleeding events such as intra-cranial haemorrhages, or ischaemic endpoints, including MI or ST. A meta-analysis of these four trials detected a significant reduction of intra-cranial haemorrhage with DAT when compared with TAT, and showed that the bleeding benefit associated with DAT came with a trade-off of cardiac, but not cerebrovascular, ischaemic events.10,12
Risks and benefits of antithrombotic regimens differ in patients with or without ACS. ACS patients suffer from higher risk of ischaemic events compared with SCAD patients and benefit more from prolonged DAPT duration, suggesting that a more potent and/or prolonged DAPT is beneficial among ACS patients.13 Thus, clinical presentation is an important driver for the decision-making on type and duration of DAPT.4 In patients with AF undergoing PCI, who require oral anticoagulation for the prevention of thrombo-embolic complications, the balance of benefits and risks of different antithrombotic regimens is more complex and the supporting evidence more limited. In a sub-analysis of the PIONEER-AF PCI, Kerneis et al.,14 observed consistent findings in several subgroups including those who required urgent revascularization, although a specific sub-analysis for ACS vs. SCAD was not performed. In the sub-analysis from the RE-DUAL trial, Oldgren et al.,15 found that the benefits of both dabigatran 110 and 150 mg DAT compared with warfarin TAT in reducing bleeding risks were consistent across subgroups of patients with or without ACS, as were the results on ischaemic endpoints. Windecker et al.,16 recently reported AUGUSTUS trial results stratified by clinical presentation (ACS managed medically, ACS undergoing PCI, elective PCI) demonstrating that the superior safety and similar efficacy of DAT was consistent across subgroups. Also, Vranckx et al.,17 recently reported that the edoxaban-based regimen provided consistent safety and similar efficacy irrespective of clinical presentation. However, these individual sub-analyses have limited power to identify whether clinical presentation is a treatment modifier for the effects of DAT vs. TAT.
Patients with ACS are at higher risks of ST and recurrent MI after PCI. Therefore, despite the absence of supporting evidence, multiple international guidelines or position papers have suggested caution in selecting a DAT instead of a TAT regimen early after intervention in this patient population.3,4,18–20 While there is large consensus that the trade-off between predicted ischaemic and bleeding risks should guide the early vs. late adoption of a DAT regimen in AF patients undergoing PCI, some guidelines and position statements endorsed ACS presentation per se among the drivers for a TAT instead of a DAT regimen.3,4,18–20
Although several meta-analyses have been conducted on patients with AF undergoing PCI,10,21,22 this is the first one to specifically address the subgroups of ACS and SCAD and does not support this position for two main observations. First, ACS patients also suffer from heightened major bleeding risk and they derived, in this pooled analysis, slightly greater absolute risk benefit with DAT instead of TAT, resulting in a slightly lower number needed to treat for benefit compared with SCAD patients. Secondly, the absolute risk difference as well as the relative risk increase for MI or ST with DAT compared with TAT was not higher in ACS compared with SCAD patients. These observations were unexpected and might reflect the synergistic role of NOACs, when administered at full doses, with a P2Y12 inhibitor monotherapy for the prevention of coronary ischaemic events. Interestingly, the only signal that DAT was associated with higher MI (RR: 1.87; 95% CI 1.04–3.36) and ST (RR: 3.73; 95%CI 1.06–13.15) risks compared with TAT was observed in patients treated with dabigatran 110 mg, but not dabigatran 150 mg.
Hence, ACS or SCAD presentation per se does not justify the default adoption of a given post-PCI antithrombotic regimen in patients taking NOAC at FDA approved stroke prevention regimens, rather concurs, together with other established ischaemic, and bleeding risk factors, to the decision-making on the optimal secondary prevention antithrombotic regimens.
Study limitations
This is a study-level meta-analysis without access to individual patient data, which carries well-known inherent limitations. Due to missing information on ACS or SCAD presentation, the present analysis excluded 41 (0.4%) among the 10 234 originally included patients across the four selected trials, which explains the apparently inconsistent findings on ST in this compared with a prior meta-analysis.10 Randomization was stratified according to clinical presentation only in the ENTRUST-AF PCI and AUGUSTUS trials. Finally, our results mainly apply to a clopidogrel-based therapy (>90% of patients received this P2Y12 inhibitor), therefore, whether the use of strategies to identify poor-responders (such as genotype or platelet function tests or risk score application)23,24 or the use of alternative P2Y12 inhibitors, such as ticagrelor or prasugrel, might reduce thrombotic risks while preserving the bleeding benefit remains to be investigated.
Conclusions
DAT is associated with a reduction in bleeding complications, including major and intra-cranial haemorrhages compared with TAT, irrespective of clinical presentation and is associated with a small increase of non-fatal cardiovascular ischaemic events in both ACS and SCAD patients.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Funding
The present study did not receive any funding.
Conflict of interest: G.G. reports consultant/speaker fees from Daiichi Sankyo, outside the submitted work. C.P.C. reports research grants from Amgen, Boehringer-Ingelheim (BI), Bristol-Myers Squibb (BMS), Daiichi Sankyo, Janssen, Merck, and Pfizer; and consulting fees from Aegerion, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, BI, BMS, Corvidia, Eli Lilly, HLS Therapeutics, Innovent, Janssen, Kowa, Merck, Pfizer, Rhoshan, and Sanofi. C.M.G. receives research funds from Janssen and Johnson & Johnson. He receives consulting funds from Janssen, Johnson & Johnson, and Bayer. A.G. discloses honoraria and speaker fees from Astra Zeneca, Bayer Health Care, Berlin-Chemie, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Medtronic, Novartis, and Omeicos. Research has been supported by Josef-Freitag Stiftung, and Deutsche Herzstiftung e. V. outside the submitted work. R.D.L. reports grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG and grants from Amgen Inc., GlaxoSmithKline, Medtronic PLC, and Sanofi-aventis. S.W. reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed, outside the submitted work. P.V. discloses personal fees from Daiichi Sankyo, AstraZeneca, Bayer Health Care, and Terumo outside the submitted work. M.V. reports grants and personal fees from Abbott, Terumo, and Astrazeneca and personal fees from Chiesi, Bayer, Daiichi Sankyo, Amgen, Alvimedica, grants from Medicure, Biosensors, and Idorsia, outside the submitted work. J.O. reports consultant/advisory boards (including study steering committees and data safety monitoring boards) and lecture fees to his institution from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daichii-Sankyo, Pfizer, Portola, Roche Diagnostics, and Sanofi.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
References
- 1. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP.. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective-2018 update. Circulation 2018;138:527–536. [DOI] [PubMed] [Google Scholar]
- 2. Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, Ten Berg J, Gersh BJ, Bhatt DL, Angiolillo DJ.. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:83–99. [DOI] [PubMed] [Google Scholar]
- 3. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 4. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann F-J, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 5. Gargiulo G, Windecker S, Vranckx P, Gibson CM, Mehran R, Valgimigli M.. A critical appraisal of aspirin in secondary prevention: is less more? Circulation 2016;134:1881–1906. [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH.. Committee R-DPS and investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 7. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA.. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 8. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH and Investigators A. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 9. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A.. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335–1343. [DOI] [PubMed] [Google Scholar]
- 10. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M.. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–3767. [DOI] [PubMed] [Google Scholar]
- 11. Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, Gibson CM, Steg PG, Lopes RD, Gwon HC, Storey RF, Franchi F, Bhatt DL, Serruys PW, Angiolillo DJ.. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 2018;15:480–496. [DOI] [PubMed] [Google Scholar]
- 12. Gargiulo G, Goette A, Vranckx P, Valgimigli M.. Higher risk of stent thrombosis with double therapy with direct oral anticoagulants: cherry picking the populations of interest does not help. Eur Heart J 2020;41:1701–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa F, Vranckx P, Leonardi S, Moscarella E, Ando G, Calabro P, Oreto G, Zijlstra F, Valgimigli M.. Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J 2015;36:1242–1251. [DOI] [PubMed] [Google Scholar]
- 14. Kerneis M, Gibson CM, Chi G, Mehran R, AlKhalfan F, Talib U, Pahlavani S, Mir M, Bode C, Halperin JL, Nafee T, Peterson ED, Verheugt FWA, Wildgoose P, van Eickels M, Lip GYH, Fox KAA, Cohen M.. Effect of procedure and coronary lesion characteristics on clinical outcomes among atrial fibrillation patients undergoing percutaneous coronary intervention: insights from the PIONEER AF-PCI trial. JACC Cardiovasc Interv 2018;11:626–634. [DOI] [PubMed] [Google Scholar]
- 15. Oldgren J, Steg PG, Hohnloser SH, Lip GYH, Kimura T, Nordaby M, Brueckmann M, Kleine E, Ten Berg JM, Bhatt DL, Cannon CP.. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur Heart J 2019;40:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Windecker S, Lopes RD, Massaro T, Jones-Burton C, Granger CB, Aronson R, Heizer G, Goodman SG, Darius H, Jones WS, Aschermann M, Brieger D, Cura F, Engstrom T, Fridrich V, Halvorsen S, Huber K, Kang HJ, Leiva-Pons JL, Lewis BS, Malaga G, Meneveau N, Merkely B, Milicic D, Morais J, Potpara TS, Raev D, Sabate M, de Waha-Thiele S, Welsh RC, Xavier D, Mehran R, Alexander JH and Investigators A. Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome treated medically or with percutaneous coronary intervention or undergoing elective percutaneous coronary intervention: insights from the AUGUSTUS trial. Circulation 2019;140:1921–1932. [DOI] [PubMed] [Google Scholar]
- 17. Vranckx P, Valgimigli M, Eckardt L, Lewalter T, Unikas R, Marin F, Schiele F, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Tijssen J., Goette A.. Edoxaban in atrial fibrillation patients with percutaneous coronary intervention by acute or chronic coronary syndrome presentation: a pre-specified analysis of the ENTRUST-AF PCI trial. Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goette A. Antithrombotic therapy after coronary artery stenting in atrial fibrillation: dual therapy encompassing NOAC plus P2Y12 inhibitor is ready for prime time! Ann Transl Med 2019;7:S270–S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lip GYH, Collet J-P, Haude M, Byrne R, Chung EH, Fauchier L, Halvorsen S, Lau D, Lopez-Cabanillas N, Lettino M, Marin F, Obel I, Rubboli A, Storey RF, Valgimigli M, Huber K, Potpara T, Blomström Lundqvist C, Crijns H, Steffel J, Heidbüchel H, Stankovic G, Airaksinen J, Ten Berg JM, Capodanno D, James S, Bueno H, Morais J, Sibbing D, Rocca B, Hsieh M-H, Akoum N, Lockwood DJ, Gomez Flores JR, Jardine R; ESC Scientific Document Group. 2018 joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS). Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019;21:192–193. [DOI] [PubMed] [Google Scholar]
- 20. Rubboli A, Valgimigli M, Capodanno D, Lip GYH.. Choices in antithrombotic management for patients with atrial fibrillation undergoing percutaneous coronary intervention. Questions (and answers) in chronological sequence. Eur Heart J Cardiovasc Pharmacother 2021;7:68–73. [DOI] [PubMed] [Google Scholar]
- 21. Capodanno D, Di Maio M, Greco A, Bhatt DL, Gibson CM, Goette A, Lopes RD, Mehran R, Vranckx P, Angiolillo DJ.. Safety and efficacy of double antithrombotic therapy with non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, Granger CB, Verheugt FWA, Li J, Ten Berg JM, Sarafoff N, Vranckx P, Goette A, Gibson CM, Alexander JH.. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol 2020;5:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, Ten Berg JM, Sibbing D, Price MJ.. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC Cardiovasc Interv 2020;13:606–617. [DOI] [PubMed] [Google Scholar]
- 24. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, Gurbel P, Jeong YH, Mehran R, Moliterno DJ, Neumann FJ, Pereira NL, Price MJ, Sabatine MS, So DYF, Stone GW, Storey RF, Tantry U, Trenk D, Valgimigli M, Waksman R, Angiolillo DJ.. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.