Abstract
Background
DiGeorge syndrome, also known as ‘CATCH 22’, is the most common deletion in humans and is one of the velocardiofacial syndromes. It is characterized by a specific facial phenotype, and structural and functional abnormalities in the cardiac and endocrine systems. One form of endocrine system dysfunction is hypocalcaemia, which causes arrhythmic events and can result in a transient loss of consciousness. We present a case highlighting the late diagnosis of DiGeorge syndrome in a patient with recurrent episodes of syncope due to suspected arrhythmic events secondary to hypocalcaemia.
Case summary
A 44-year-old woman was referred for further investigation of recurrent syncope episodes and documented transient QT-prolongation with hypocalcaemia. Previous detailed cardiological examination, including invasive procedures such as coronary angiography and cardiac magnetic resonance tomography, was unremarkable. Slight characteristic facial dysmorphia and transient hypocalcaemia were strongly suggestive of DiGeorge syndrome. The diagnosis was confirmed by genetic testing. Calcium substitution was initiated and no recurrent episodes of syncope or arrhythmic events were reported during 12 months of follow-up.
Discussion
Clinical presentation and time of manifestation of the DiGeorge syndrome varies widely depending on the mutation expression extent. An atypical disease course may delay the diagnosis and appropriate management of affected patients. In this case, confirmation of the diagnosis allowed the initiation of appropriate treatment, reducing the risk for further events. Given that syncope and arrhythmia can be the first and only manifestation of late-onset DiGeorge syndrome, specialists in adult cardiology need to be aware of this presentation.
Keywords: DiGeorge syndrome, Syncope, Long QT, 22q11.2, Hypocalcaemia, Arrhythmia, Case report
Learning points
DiGeorge syndrome being the most common deletion mutation in humans is not infrequently diagnosed in adulthood.
Syncope resulting from arrhythmias due to electrolyte disturbances can be part of a congenital endocrine disorder.
In case of recurrent loss of consciousness accompanied by hypocalcaemia in combination with characteristic facial dysmorphia one should consider the diagnosis of the DiGeorge syndrome.
Introduction
Syncope is defined as transient loss of consciousness (TLOC) due to cerebral hypoperfusion, characterized by a rapid onset, short duration, and spontaneous complete recovery.1 There are many different causes for a loss of consciousness, but only a few are classified as a syncope. These are reflex syncope, orthostatic hypotension, and cardiac syncope. Epileptic seizure is defined as a non-syncopal form of TLOC. Both cardiac arrhythmias and seizures may result from hypocalcaemia, which might be part of a congenital disorder called DiGeorge syndrome.
DiGeorge syndrome occurs as a result of the most common genetic deletion in humans (in chromosome 22q11.2). This results in abnormal pharyngeal arch development with resulting defective development of the parathyroid glands, thymus, and the conotruncal region of the heart, and cleft palate.2 Affected individuals often present with characteristic facial features, which may include small, low-set ears, short width of eye openings, hooded eyes, an enlarged nose tip (bulbous), and a flattened groove in the upper lip (Figure 1).3
Figure 1.
Typical facial features in DiGeorge syndrome: malar flatness, fullness of eyelids (hooded eyelids), hypertelorism, broad nasal bridge and broad/round nasal tip, narrow nares, small mouth and round/broad and slightly low set ears (reproduced with permission from Oskarsdottir et al.3 of John Wiley & Sons).
The clinical spectrum of DiGeorge syndrome is broad and depends on the phenotypic expression of the gene mutation. Approximately 180 clinical scenarios have been described.3 Diagnosis after age 10 years is defined as late-onset DiGeorge syndrome. The true incidence of such cases has not yet been estimated. In one study where 70/228 patients were late-diagnosed, the most common first presentation in hospital was hypocalcaemic seizure.4 Evaluation of survival probability in 309 adults with DiGeorge syndrome demonstrated that severe congenital heart disease (CHD) was a significant predictor for mortality (survival probability to age 45 years was 72% in those with CHD vs. 95% in those who had no major CHD; P < 0.0001).5 The cardiovascular system is involved in 82% of patients with DiGeorge sydnrome,6 with symptomatic heart failure in 75%.7
We report a middle-aged female patient with recurrent episodes of syncope due to low calcium level as the result of primary hypoparathyroidism in the context of previously undiagnosed DiGeorge syndrome.
Timeline
| Date | Event | Therapy |
|---|---|---|
| Childhood | Diagnosis of epilepsy | No special therapy was indicated |
| December 2014 |
First syncope. Cranial computed tomography with neither cerebral ischaemia nor intracranial bleeding. Coronary angiography and cardiac magnetic resonance imaging have ruled out a structural heart disease. Electrocardiogram (ECG) showed a prolonged QTc interval of 520 ms in the context of hypocalcaemia (total calcium of 1.4 mmol/L). ECG showed a prolonged QTc-interval under hypocalcaemia. Further laboratory testing has revealed primary hypoparathyroidism. |
Initiation of calcium and vitamin D substitution therapy |
| April 2019 |
Second syncope. The patient admits not taking calcium. |
|
| Present admission (May 2019) |
|
|
| October 2019 |
|
|
Case presentation
A 44-year-old woman was admitted to hospital for further evaluation of recurrent episodes of syncope with a long QT-interval associated with hypocalcaemia. The patient had experienced epileptic seizures as a child, but no treatment had been required. No further epileptic events were reported after puberty. Otherwise, past medical and surgical history was unremarkable. The patient denied taking recreational drugs or consuming alcohol. There was no history of inherited diseases among relatives.
The patient experienced her first syncope episode in 2014 while she was doing housework; this occurred without any prodrome or signs of vasovagal reaction (i.e. fainting, nausea, sweating, blurred vision, tingling of lips or fingertips). The fall caused by the syncope led to a fracture of the atlas arch and she was admitted to the hospital. On admission, vital parameters were oxygen saturation 98%, blood pressure 124/65 mmHg, heart rate 80/min; there was no cyanosis, oedema, or bibasilar rales in the lungs. She was awake and oriented, with no focal neural deficits. Cranial computed tomography (CCT) ruled out cerebral pathologies. The electrocardiogram (ECG) on admission showed a prolonged QT interval with corrected QT (QTc) of 520 ms, associated with a hypocalcaemia (calcium level 1.4 mmol/L) (Figure 2). Echocardiography showed apical hypokinesia and coronary angiography revealed normal coronary arteries. Cardiac magnetic resonance tomography ruled out structural heart disease and there were no signs of myocarditis. Serial ECG showed a normal QTc when serum calcium levels were within the normal range. No significant arrhythmias were documented on 7-day-Holter monitoring.
Figure 2.
Electrocardiogram with prolonged QTc, 2014 (A); recording speed 50 mm/s (B). Corrected QT interval (QTc) was estimated using Bazett formula.
Hypocalcaemia was interpreted as part of a newly diagnosed primary hypoparathyroidism, and substitution therapy with vitamin D (1000 U/day) and calcium (500 mg twice daily) was started. However, the patient admitted to not having taken medication shortly before a second syncope event that occurred suddenly at home in 2019, which resulted in fracture to the jaw and hand. Physical and emotional stress at that time were denied. Physical examination including neurological assessment was unremarkable. CCT revealed neither intracranial bleeding nor an ischaemic event. Levetiracetam 500 mg twice a day was prescribed by neurologist due to an increased epileptic activity on electroencephalography. In the absence of other possible causes, this event was assumed to also be related to hypocalcaemia.
ECG on admission to our hospital (Figure 3) showed no significant conduction disorders and no repolarization abnormalities. Telemonitoring was unremarkable except for a single 3-min episode of a self-terminated supraventricular tachycardia with sudden onset and a heart rate of 140 beats/min without any haemodynamic compromise. Laboratory tests showed hypocalcaemia (1.76 mmol/L) due to reduced parathormone production; all other electrolytes were within normal ranges; haemoglobin was 12 mg/dL. High-sensitivity troponin was not elevated. Although it had not previously been documented, we noted mild facial dysmorphia (i.e. prominent nose with large tip and hypoplastic nares, small dysmorphic ears). The combination of transient hypocalcaemia with subtle facial characteristics were thought to be indicative of one of the velocardiofacial syndromes, namely DiGeorge syndrome. Subsequent genetic test showed a microdeletion in 22q11.2, confirming the diagnosis (Figure 4).
Figure 3.
Electrocardiogram on current admission.
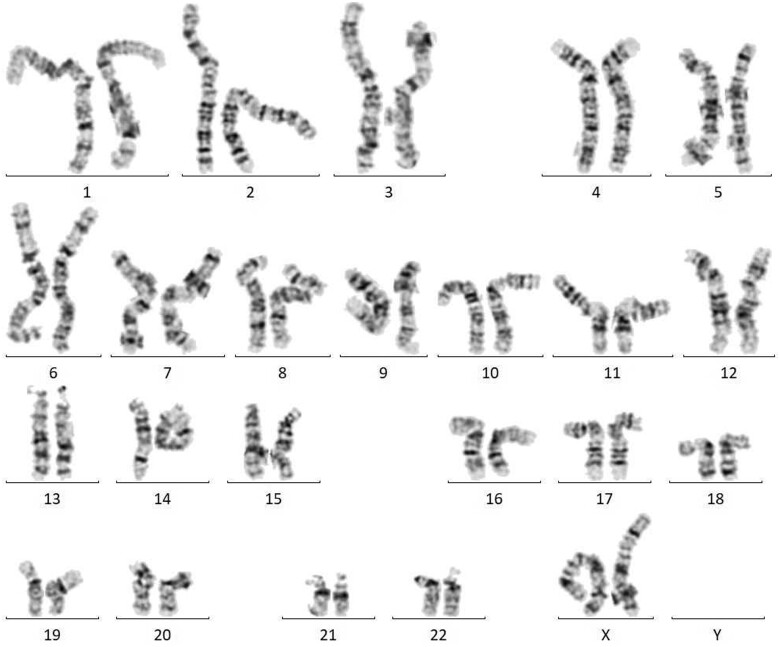
Figure 4.
Karyotype: 46, XX, del (22) (q11.2q11.2). Small part of chromosome 22 is missing due to microdeletion resulting in shortened chromosome arm.
There are two possible mechanisms of hypocalcaemia inducing a TLOC: arrhythmogenic syncope or seizure. Because our patient showed prolonged QTc immediately after the first episode, we assumed that she had experienced an arrhythmogenic syncope, and that this was caused by a hypocalcaemia-induced long QT syndrome. Therefore, it was decided to implant an event recorder for the detection of arrhythmic events. Substitution therapy with calcium carbonate 500 mg twice daily and vitamin D 1000 U/day was initiated. The calcium level was monitored every 3 months and dosages adjusted by an endocrinologist as required. The patient was also examined by neurologist. Although we made a strong recommendation for psychiatric evaluation, the patient declined. No further organ systems were affected. No recent syncope was reported during 12 months of follow-up and there were no arrhythmic events stored by the event-recorder.
Discussion
Syncope is a common symptom, affecting 40% of people once in their life.8 The underlying aetiology is diverse, and includes cardiac and non-cardiac causes. Adverse outcomes are associated not only with high-risk syncope due to underlying disease but also with formally non-high-risk syncope in high-risk settings. Therefore, it is important not only to stratify patient risk but also to initiate appropriate management to prevent further events.
DiGeorge syndrome is widely known as ‘CATCH 22’ syndrome, a mnemonic which summarizes the spectrum of organ systems involved (Table 1). The estimated incidence of DiGeorge syndrome is approximately 1 in 4000–6000 live births.9 The underlying disorder is microdeletion in the 22q11.2 region of the 22nd chromosome, but the pathophysiology is not fully understood. Most cases (90–95%) are the result of a de novo mutation. The affected region is one of the most complex areas in genome, and includes such genes as Tbx1 and DGCR8 which are responsible for coding proteins regulating the normal development of the cardiovascular and neural systems.10 Animal data have recorded similar abnormalities due to mutations in these genes as seen in patients with DiGeorge syndrome.11 Clinical manifestations depend on the extent of gene variation and range from mild to severe congenital heart disease (CHD), cellular immunodeficiency, and hypoparathyroidism. Each of these abnormalities is highly indicative of DiGeorge syndrome and should be further investigated. In cases that present with non-specific symptoms, DiGeorge syndrome might be diagnosed late, resulting in a delay in appropriate treatment. Among the most common clinical signs of the DiGeorge syndrome in adults are endocrine disorders including hypothyroidism and hypoparathyroidism, thrombocytopenia with epistaxis, recurrent infections due to low T-cell count, and psychotic and mood disorders.12 Patients diagnosed late usually do not have severe cardiac anomalies, nor do they develop congestive heart failure. However, arrhythmic events are observed and are associated with electrolyte disturbances, especially hypocalcaemia due to insufficient parathyroid gland activity.
Table 1.
DiGeorge syndrome, also known under the name ‘CATCH 22’
| ‘C’ | Cardiac defects |
| ‘A’ | Abnormal faces |
| ‘T’ | Thymic hypoplasia |
| ‘C’ | Cleft palate |
| ‘H’ | Hypocalcaemia |
| 22 | Chromosome 22 |
This mnemonic helps to memorize the most common manifestations of the disease and originates from the name of Joseph Heller’s novel ‘Catch 22’, 1953.
After confirmation of the diagnosis, the next step is to screen patients for other known disease manifestations (psychiatric evaluation, cardiovascular and endocrine disorders, immunological status, etc). In case of mental retardation, test results need to be communicated in a way that patients can understand. Adults with DiGeorge syndrome are prone to develop psychiatric disorders, especially schizophrenia (approximately 20-fold increased risk compared with non-affected subjects).12 The high incidence of endocrine disorders should prompt a regular evaluation by an endocrinologist. Given that patients with corrected heart defects might develop recurrences and need re-operation, close cardiologic follow-up is also recommended.
Reproductive fitness of patients with DiGeorge syndrome usually remains intact.13 Although there is a 50% risk of disease inheritance, contraception is not automatically indicated. Patients should be provided with detailed information on possible complications during pregnancy and delivery, and should be closely monitored to detect abnormalities in foetal development. The disease poses a risk both for mother and child. A multidisciplinary approach should be initiated in such cases. Considering the increasing knowledge about the syndrome, an annual genetic consultation is extremely important for these patients.
Conclusion
Late-diagnosed DiGeorge syndrome is still relatively rare in adult medicine, so there is a high probability of missing the diagnosis in patients with mild clinical manifestations. Furthermore, patients will present in non-paediatric settings where physicians are not familiar with this disorder. In our patient, we suspected the congenital disorder due to the presence of mild facial dysmorphia and provided an interdisciplinary approach with involvement of electrophysiologists, a paediatric cardiologist, and genetic counsellor. Being informed about the variety of possible clinical scenarios will help physicians to suspect and further investigate for this disorder, facilitating the initiation of appropriate management for these patients.
Lead author biography

Khuraman Isgandarova studied medicine at the Azerbaijan Medical University and at the Charité University, Berlin, Germany. At present, she is a 5th-year resident in training at the Herz- und Diabeteszentrum NRW, University Hospital of the Ruhr University Bochum in Bad Oeynhausen, Germany. Her main interests are electrophysiology including genetic mechanisms of arrhythmias.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
English language editing assistance was provided by Nicola Ryan, independent medical writer, funded by Department of Cardiology, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
References
- 1. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A. et al. ; ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 2. Kraus C, Vanicek T, Weidenauer A, Khanaqa T, Stamenkovic M, Lanzenberger R. et al. DiGeorge syndrome: relevance of psychiatric symptoms in undiagnosed adult patients. Wien Klin Wochenschr 2018;130:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skarsdttir S, Holmberg E, Fasth A, Strmland K.. Facial features in children with the 22q11 deletion syndrome. Acta Paediatr 2008;97:1113–1117. [DOI] [PubMed] [Google Scholar]
- 4. Yoo DY, Kim HJ, Cho KH, Kwon EB, Yoo EG.. Delayed diagnosis of 22q11 deletion syndrome due to late onset hypocalcemia in a 11-year-old girl with imperforated anus. Ann Pediatr Endocrinol Metab 2017;22:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van L, Heung T, Graffi J, Ng E, Malecki S, Van Mil S. et al. All-cause mortality and survival in adults with 22q11.2 deletion syndrome. Genet Med 2019;21:2328–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Digilio M, Marino B, Capolino R, Dallapiccola B.. Clinical manifestations of deletion 22q11.2 syndrome (DiGeorge/Velo-Cardio-Facial syndrome). Images Paediatr Cardiol 2005;7:23–34. [PMC free article] [PubMed] [Google Scholar]
- 7. Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H. et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet 1997;34:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fomin AB, Pastorino AC, Kim CA, Pereira CA, Carneiro-Sampaio M, Abe-Jacob CM, DiGeorge syndrome: a not so rare disease. Clinics (Sao Paulo, Brazil )2010;65:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solbiati M, Casazza G, Dipaola F, Rusconi AM, Cernuschi G, Barbic F. et al. Syncope recurrence and mortality: a systematic review. Europace 2015;17:300–308. [DOI] [PubMed] [Google Scholar]
- 10. Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA. et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics 2003;112:101–107. [DOI] [PubMed] [Google Scholar]
- 11. Sellier C, Hwang VJ, Dandekar R, Durbin-Johnson B, Charlet-Berguerand N, Ander BP. et al. Decreased DGCR8 expression and miRNA dysregulation in individuals with 22q11.2 deletion syndrome. PLoS One 2014;9:e103884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD. et al. Clinical features of 78 adults with 22q11 deletion syndrome. Am J Med Genet A 2005;138A:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fung W, Butcher N, Costain G, Andrade D, Boot E, Chow E. et al. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet Med 2015;17:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.