1. Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It primarily causes respiratory symptoms and often presents with flu-like symptoms such as fever, cough, tiredness, and headache. Severe COVID-19 is associated with cytokine storm, an acute systemic hyperinflammatory syndrome defined by elevated circulating cytokine levels and secondary organ dysfunction, including the brain. Herein, we aimed to analyse clinical manifestations of COVID-19-related encephalopathy (CORE) and other cytokine storm-associated encephalopathy (CySE) disorders, with a focus on akinetic-mutism, in a bio-behavioral perspective, suggesting that they may be interpreted as maladaptive sickness behavioral responses caused by cytokine storm.
2. Clinical and investigative findings of CORE and other CySE disorders
CORE is a disorder characterized by a predominant electro-clinical frontal lobe dysfunction, whose underlying mechanisms have been partially unravelled (Pilotto et al., 2021; Muccioli et al., 2020; Pensato et al., 2021; Toniolo et al., 2021). Even though a direct pathogenic role of SARS-CoV-2 was initially proposed, coronavirus RNA is almost invariably undetectable in cerebrospinal fluid (CSF). In addition, CSF analysis, neuroimaging, neuropathological findings, and the clinical response to immunotherapies, argue for an immune-mediated pathogenesis driven by cytokine-mediated neuroinflammation, rather than an infectious agent targeting the brain (Pilotto et al., 2021; Muccioli et al., 2020; Pensato et al., 2021; Toniolo et al., 2021). Several studies have shown a direct correlation between cytokine storm intensity and severity of neurological manifestations in CORE. Accordingly, common clinical manifestations of CORE, such as delirium, dysexecutive syndrome, and pyramidal signs, are observed in up to 85% of COVID-19 patients in intensive care units (Helms et al., 2020). Delirium has also been reported in up to 28% of elderly patients with COVID-19 presenting to the emergency department, especially those with prior cognitive impairment (Kennedy et al., 2020), yet it may also occur in younger adults with no known comorbidities. Notably, encephalopathy has been reported as a recurrent neurological complication of other cytokine storm disorders, including sepsis and cytokine release syndrome related to chimeric antigen receptor (CAR) T-cells therapy, a novel treatment for refractory malignancies (Pensato et al., 2021; Lee et al., 2019). We recently reviewed these encephalopathies, revealing a remarkable overlap of clinical and investigative features across conditions, suggesting that cytokine storm-associated encephalopathy (CySE) may represent a clinical spectrum of disorders sharing cytokine-mediated neuroinflammation as the overarching underlying mechanism (Pensato et al., 2021).
Delirium and a frontal lobe dysfunction characterized by expressive aphasia, behavioral abnormalities, dysexecutive syndrome and akinetic mutism have emerged as recurrent presentations in patients with CORE (Muccioli et al., 2020; Pensato et al., 2021; Toniolo et al., 2021). Akinetic mutism is an uncommon neurological manifestation secondary to frontal-subcortical dysfunction, usually related to a brain lesion (Arnts et al., 2020). Perhaps surprisingly, it has been reported in the absence of underlying structural brain abnormalities in up to 13% of admitted COVID-19 patients (Nersesjan et al., 2021), and is also reported in neurotoxicity related to CAR T-cells therapy, where it represents a distinctive feature (Muccioli et al., 2020; Pensato et al., 2021; Lee et al., 2019). Akinetic mutism is characterized by a decrease of goal-directed behavioral and emotional responses, leading to near absence of spontaneous movement (akinetic) and speech (mutism), yet with relatively preserved consciousness (Arnts et al., 2020). A predominant frontal involvement in these encephalopathies is supported by functional investigations such as electroencephalography (EEG), fluorodeoxyglucose (FDG)-positron emission tomography (PET) and perfusion magnetic resonance imaging (MRI) (Pensato et al., 2021; Toniolo et al., 2021). The biological underpinnings of these distinctive clinical manifestations still remain a conundrum.
3. Maladaptive sickness behavior in CORE and other CySE disorders
Sickness behavior is an innate, evolutionarily conserved, coordinated behavioral, autonomic and endocrine response shared by mammals confronted with stressors such as predators or infections (Cunningham and Maclullich, 2013). Distinctive motor and autonomic patterns are engendered by different brain structures, including prefrontal cortex, limbic system, hypothalamus, thalamus and midbrain nuclei. As a result, the individuals affected display motor quiescence, apathy, anhedonia, anorexia, lethargy, fever and sympathoinhibition, resulting in a general disengagement from the environment. This stereotypical adaptive response translates into individual, and social advantages. Indeed, fever boosts immune responses, and motor and autonomic inhibition preserves metabolic energy. Additionally, immobility and lack of interest for the external environment limits the exposure to potential threats and the risk of infecting relatives. Concomitantly, it may increase the individual's chance of survival by showing a desperate need for kins assistance.
Elevated cytokine levels, particularly IL-1, IL-6 and TNF-alfa, are considered the primum movens of the sickness behavioral response (Cunningham and Maclullich, 2013). Indeed, these key molecular players of the immune system act as potent neuromodulators, signaling systemic inflammation to different brain structures, especially the hypothalamus (Cunningham and Maclullich, 2013). Cytokines exert this function by communicating directly with circumventricular organs or through afferent vagus nerve fibers, although they can also be produced by the central nervous system activated microglia. After that, the hypothalamus orchestrates adaptive biological reactions to restore homeostasis engendering sickness behavior (Cunningham and Maclullich, 2013). Conversely, external or interoceptive stressors, including systemic inflammation, may also result in a coordinated hypothalamic response consisting of behavioral rage and sympathetic hyperactivation, referred to as “fight-or-flight”.
These coordinated responses reflect immune activation and may become maladaptive and dangerous under particular conditions, similarly to other evolutionary conserved responses meant to restore homeostasis. Sepsis-associated encephalopathy and delirium have been proposed as a framework model of this maladaptive response.
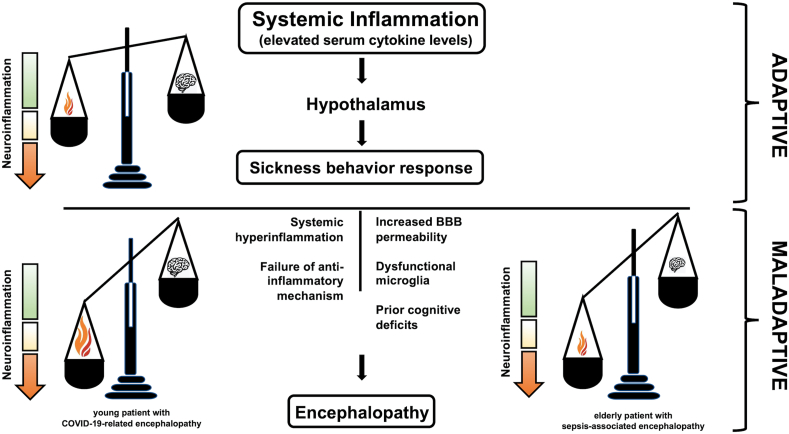
Studies in the elderly and individuals with prior cognitive impairment (who are the most at risk of developing sepsis-associated encephalopathy, as well as CORE) have shed light into potential mechanisms leading to maladaptive sickness behavior. These include increased blood-brain barrier permeability and dysfunctional microglia, which may act as an amplifier for neuroinflammation (Cunningham and Maclullich, 2013). As cytokine storm-associated encephalopathy has been observed recurrently also in young, cognitively healthy individuals, the maladaptive sickness behavior may be interpreted as an imbalance between systemic inflammation and neuropsychological responses. Therefore, both an exaggerated systemic inflammation in a healthy brain (e.g., COVID-19-associated cytokine storm), or a susceptible brain confronted with a relatively mild inflammatory stimulus (e.g., urinary tract infection in the elderly) may be sufficient to result in a disproportionate cytokine-mediated neuroinflammation, leading to encephalopathy (Fig. 1). Further supporting this hypothesis, many overlapping features are shared among mild manifestations of CORE and sickness behavior, such as apathy, anhedonia, decreased responses to external stimuli and lethargy. Therefore, manifestations of severe encephalopathy, such as akinetic mutism or hypoactive delirium, may represent the extreme end of this psychoneuroimmunological spectrum. The reasons why a subgroup of patients confronted with similar inflammatory stress develop hyperactive delirium remain more controversial, even though it may represent the complementary maladaptive “flight-or-fight” response. This speculative, yet comprehensive, behavioral view may explain the distinctive neurological manifestations observed in CORE and other cytokine storm-associated encephalopathies, as already proposed for sepsis-associated encephalopathy.
Fig. 1.
Schematic representation of adaptive and maladaptive sickness behavioral responses. Elevated cytokine levels, resulting from various potential triggers, including SARS-COV-2 virus, act as neuromodulators informing the hypothalamus about an inflammatory stressor. The hypothalamus orchestrates adaptive biological responses to restore homeostasis, engendering sickness behavior. Therefore, both an exaggerated systemic inflammation in a healthy brain (e.g., COVID-19-associated cytokine storm) or a susceptible brain confronted with a relatively mild inflammatory stimulus (e.g., urinary tract infection in the elderly) may be sufficient to result in a disproportionate cytokine-mediated neuroinflammation, leading to encephalopathy.
Akinetic mutism in the absence of structural brain lesions, especially if transitory, represents a specific clinical finding of these conditions, possibly reflecting a cytokine-mediated maladaptive sickness behavior.
Although the mass vaccination strategies adopted worldwide will hopefully limit severe COVID-19 cases, acknowledging the pathophysiological mechanisms underpinning akinetic mutism and other neurological manifestations observed in this condition remains of primary importance. Indeed, various immunotherapies investigated in CORE, including steroids, intravenous immunoglobulin and anti-cytokines monoclonal antibodies may have a beneficial role also in other cytokine storm-associated encephalopathies.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Arnts H., van Erp W.S., Lavrijsen J.C.M., van Gaal S., Groenewegen H.J., van den Munckhof P. On the pathophysiology and treatment of akinetic mutism. Neurosci. Biobehav. Rev. 2020;112:270–278. doi: 10.1016/j.neubiorev.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Cunningham C., Maclullich A.M. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav. Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Helfand B.K.I., Gou R.Y. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Santomasso B.D., Locke F.L. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. Covid-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020;88(4):860–861. doi: 10.1002/ana.25855. Epub 2020 Aug 14. PMID: 32715524. [DOI] [PubMed] [Google Scholar]
- Nersesjan V., Amiri M., Lebech A.M., Roed C., Mens H., Russell L., Fonsmark L., Berntsen M., Sigurdsson S.T., Carlsen J., Langkilde A.R., Martens P., Lund E.L., Hansen K., Jespersen B., Folke M.N., Meden P., Hejl A.M., Wamberg C., Benros M.E., Kondziella D. Central and peripheral nervous system complications of COVID-19: a prospective tertiary center cohort with 3-month follow-up. J. Neurol. 2021:1–19. doi: 10.1007/s00415-020-10380-x. Epub ahead of print. PMID: 33438076; PMCID: PMC7803470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensato U., Muccioli L., Cani I., Janigro D., Zinzani P.L., Guarino M., Cortelli P., Bisulli F. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol. 2021;8(4):968–979. doi: 10.1002/acn3.51348. Epub 2021 Mar 29. PMID: 33780166; PMCID: PMC8045903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Masciocchi S., Volonghi I., De Giuli V., Caprioli F., Mariotto S., Ferrari S., Bozzetti S., Imarisio A., Risi B., Premi E., Benussi A., Focà E., Castelli F., Zanusso G., Monaco S., Stefanelli P., Gasparotti R., Zekeridou A., McKeon A., Ashton N.J., Blennov K., Zetterberg H., Padovani A. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciaa1933. ciaa1933. Epub ahead of print. PMID: 33395482; PMCID: PMC7799260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo S., Di Lorenzo F., Scarioni M., Frederiksen K.S., Nobili F. Is the frontal lobe the primary target of SARS-CoV-2? J Alzheimers Dis. 2021;81(1):75–81. doi: 10.3233/JAD-210008. PMID: 33720900. [DOI] [PubMed] [Google Scholar]