To the Editor:
THROMBOTIC AND BLEEDING events have been implicated in the progression of coronavirus disease 2019 (COVID-19).1 , 2 This dysregulation of coagulation has been associated with poor prognoses.3 , 4 Neurologic sequelae, such as ischemic stroke and intracranial hemorrhage (ICH), have been reported in patients with COVID-19 at rates of 0.9%-to-2.3% and 0.9%, respectively.5 , 6 , 7 , 8 Limited data exist on neurologic events in patients with COVID-19 in the intensive care unit who require extracorporeal membrane oxygenation (ECMO) due to severe acute respiratory distress syndrome (ARDS).
We retrospectively reviewed adult patients with COVID-19 supported by ECMO at our tertiary care center. Inclusion criteria were (1) a positive polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and (2) cannulation for venovenous (VV) ECMO support. Patient demographics, past medical history, adverse events during hospitalization, laboratory values on day one of ECMO, ECMO variables, and outcomes were obtained through electronic medical records. Neurologic events, such as ischemic stroke, hypoxic ischemic brain injury, ICH, and cerebral microbleed (CMB), were identified based on computed tomography (CT) and magnetic resonance imaging (MRI) reports conducted anytime during ECMO support up to five days after decannulation. ICHs were defined as any hemorrhages visualized on a CT scan, and CMBs were defined as hemorrhages <5 mm, visualized on susceptibility-weighted imaging or gradient-recalled echo MRI.9 Both CMBs and ICHs were defined as hemorrhagic neurologic events. Bleeding events were categorized as in the gastrointestinal tract, at the cannulation site, and at the tracheostomy site. This study was approved by the institutional review board. All patients who require ECMO in our center receive neurocritical care consultation and standardized neuromonitoring protocol.10 All patients received a heparin bolus at the time of cannulation and heparin infusion before and during ECMO therapy, with an activated partial thromboplastin time (aPTT) goal of 50-to-65 seconds. Comparisons of demographic and clinical variables were performed using Fisher's exact test or Mann-Whitney U test as appropriate. A p value < 0.05 was considered significant.
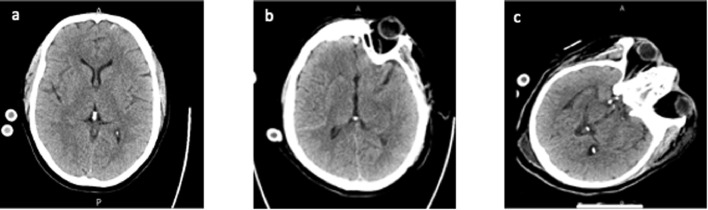
Of 16 patients (median age, 51 years [interquartile range (IQR), 38-57], male: 81%) with VV-ECMO support, four (25%) had neurologic events. The clinical characteristics and ECMO variables of the four patients on ECMO who had neurologic events are shown in Table 1 . Neurologic events included four hemorrhagic neurologic events in two patients with ICH only, one patient with CMB only, and one patient with both ICH and CMB. Among the three patients with ICH, two had subarachnoid hemorrhages and one had an intraventricular hemorrhage. There were no ischemic strokes. Excluding the patient with CMB only, which was detected on MRI four days after ECMO decannulation, the median number of days from ECMO cannulation to ICH was three days. Figure 1 shows CT without contrast imaging for the three patients who experienced ICH. All three patients with ICHs were managed by discontinuing heparin upon CT detection of a neurologic event. These patients’ laboratory values on the first day of ECMO are shown in Table 2 .
Table 1.
Clinical Characteristics and ECMO Variables
| Case | Age (y)/Sex | Past Medical History | Bleeding History | Type of Neurological Injury | SOFA Score | Apache II Score | Days on Mechanical Ventilation | Days From Admission to ECMO | Days From ECMO Cannulation to Neurological Event | Days On ECMO | Cannula Site | Cannula Size, F | P:F Ratio at Time of Cannulation | ECMO Flow (L/min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58/female | DM, CAD, HL, HTN | GI | SAH | 9 | 18 | 37 | 6 | 0 | 13 | Right IJ | 32 | 58 | 5 |
| 2 | 60/female | DM, HL, HTN | Cannulation site | CMB | 11 | 28 | 29 | 6 | 33 | 29 | Right IJ | 32 | 67 | 6.2 |
| 3 | 47/male | - | Cannulation site | SAH | 10 | 16 | 40 | 4 | 3 | 15 | Right IJ/FEM | 22/25 | 62 | 6.6 |
| 4 | 53/male | - | Cannulation site, tracheostomy | Intraventricular hemorrhage, CMB | 9 | 16 | 45 | 5 | 12 | 22 | Right IJ | 32 | 59 | 5 |
Abbreviations: AC, assist control; CAD, coronary artery disease; CMB, cerebral microbleed; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; FEM, femoral; GI, gastrointestinal; HL, hyperlipidemia; HTN, hypertension; IJ, internal jugular; PC, pressure control; P:F, pressure of arterial oxygen to fraction of inspired oxygen; SAH, subarachnoid hemorrhage; SOFA, Sequential Organ Failure Assessment.
Fig 1.
Neuroimaging (CT without contrast) of intracranial hemorrhage in patients with COVID-19 on ECMO. (A) CT of patient one showed 5 mm linear hyperdensity within the cortex of the left frontal lobe along the anterior/inferior parafalcine region, compatible with subarachnoid hemorrhage. (B) CT of patient three showed hyperdensity in the right Sylvian fissure, compatible with subarachnoid hemorrhage. (C) CT of patient four showed subtle hyperdensity within the posterior horn of the right lateral ventricle, concerning for minimal intraventricular hemorrhage. CT, computed tomography.
Table 2.
Laboratory Findings on Day 1* of ECMO Therapy
| Case | pH | PCO2 | PO2 | Platelet Count, K/cu mm | IL-6, pg/mL | LDH, U/L | Ferritin, ng/mL | CRP, mg/dL | Medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.35 | 48 | 105 | 177 | - | 643 | 5924 | 22.5 | Pressors, hydroxychloroquine |
| 2 | 7.37 | 33 | 65 | 101 | - | 751 | 705 | 38.1 | Pressors |
| 3 | 7.37 | 38 | 62 | 114 | 231 | 920 | 1845 | 6.5 | Pressors, tocilizumab |
| 4 | 7.46 | 37 | 77 | 149 | 1359 | 476 | 1503 | 31.1 | Pressors, convalescent plasma |
Values measured on ECMO day 1 closest to time of ECMO cannulation.
Abbreviations: CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; IL-6, interleukin-6; LDH, lactate dehydrogenase.
The four patients who experienced neurologic events during ECMO support were compared with those that did not to better understand the differences between the two groups (Table 3 ). Patients with neurologic events tended to be older, albeit nonsignificantly, with a median age of 55.5 years (IQR, 50-59) versus 47 years (IQR, 36-54). Although not statistically significant, patients with neurologic events had more bleeding events while on ECMO (100% v 75%), compared with those without neurologic events. These patients also were less likely to have received remdesivir (0% v 42%), interleukin 6 (IL-6) inhibitors (25% v 75%), and steroids (0% v 33%). Ventilation and ECMO variables were similar between the groups. All 16 patients were proned, received neuromuscular blockade, and underwent mechanical ventilation. Not surprisingly, patients with neurologic events had a longer hospital stay (55.5 v 41 days). Nineteen percent (three of 16) died within 30 days, and this mortality rate was similar regardless of whether the patient experienced a neurologic event. Patients with neurologic events were significantly more likely to have lower platelet counts and higher D-dimer values versus those without. Although not statistically significant, neurologic events also were associated with lower hemoglobin, hematocrit, and PaCO2 values and higher creatinine, aspartate aminotransferase, IL-6, lactate dehydrogenase, ferritin, C-reactive protein, and aPTT values. An increased Sequential Organ Failure Assessment score on ECMO day one (median, 9.5 v 7) was significantly associated with neurologic events.
Table 3.
Comparison Between Patients on ECMO With and Without Neurologic Complications
| All patients (N = 16) | No Neurologic Event (n = 12) | Neurologic Event (n = 4) | p Value* | |
|---|---|---|---|---|
| Demographic/past medical history | ||||
| Age, y, median (IQR) | 51 (38-57) | 47 (36-54) | 55.5 (50-59) | 0.129 |
| Sex, male, n (%) | 13 (81) | 11 (92) | 2 (50) | 0.136 |
| Race, n (%) | 0.118 | |||
| African American | 5 (31) | 2 (17) | 3 (75) | |
| Hispanic | 10 (63) | 9 (75) | 1 (25) | |
| BMI, kg/m2, median (IQR) | 31.9 (29.4-35.5) | 32.4 (30.5-35.5) | 29.4 (29.1-34.8) | 0.363 |
| Smoker, n (%) | 1 (6) | 0 (0) | 1 (25) | 0.250 |
| Hypertension, n (%) | 7 (44) | 5 (42) | 2 (50) | 1.000 |
| Diabetes mellitus, n (%) | 6 (38) | 4 (33) | 2 (50) | 0.604 |
| Hyperlipidemia, n (%) | 4 (25) | 2 (17) | 2 (50) | 0.245 |
| Coronary artery disease, n (%) | 1 (6) | 0 (0) | 1 (25) | 0.250 |
| Medications | ||||
| Neuromuscular blockade, n (%) | 16 (100) | 12 (100) | 4 (100) | 1.000 |
| Inhaled nitric oxide, n (%) | 14 (88) | 10 (83) | 4 (100) | 1.000 |
| Vasopressors, n (%) | 12 (75) | 8 (67) | 4 (100) | 0.516 |
| Hydroxychloroquine, n (%) | 2 (13) | 1 (8) | 1 (25) | 0.450 |
| Remdesivir, n (%) | 5 (31) | 5 (42) | 0 (0) | 0.245 |
| IL6 inhibitor, n (%) | 10 (63) | 9 (75) | 1 (25) | 0.118 |
| Steroids, n (%) | 4 (25) | 4 (33) | 0 (0) | 0.516 |
| Convalescent plasma, n (%) | 5 (31) | 4 (33) | 1 (25) | 1.000 |
| Ventilation | ||||
| Proned, n (%) | 16 (100) | 12 (100) | 4 (100) | 1.000 |
| Airway pressure release ventilation, n (%) | 9 (56) | 7 (58) | 2 (50) | 1.000 |
| Mechanical ventilation, n (%) | 16 (100) | 12 (100) | 4 (100) | 1.000 |
| P:F ratio at time of cannulation, median (IQR) | 60.5 (53.5-64) | 60.5 (51-64) | 60.5 (58.5-64.5) | 0.627 |
| Pre-ECMO ventilation, median (IQR) | 6.5 (4.5-7) | 7 (4.5-8) | 5.5 (4.5-6.5) | 0.387 |
| Pre-tracheostomy ventilation, median (IQR) | 14 (13-20) | 14 (12-20) | 15.5 (14-19) | 0.509 |
| Mechanical ventilation support time, d, median (IQR) | 40 (25-51.5) | 41 (20.5-60.5) | 38.5 (33-42.5) | 0.808 |
| ECMO Variables | ||||
| Support time, day, median (IQR) | 27 (15-33) | 24 (13-41) | 29 (25-30) | 0.626 |
| Cannulation site, RIJ, n (%) | 12 (75) | 9 (75) | 3 (75) | 1.000 |
| Speed, rpm, median (IQR) | 3855 (3508-3943) | 3883 (3450-3993) | 3830 (3658-3873) | 0.716 |
| Flow, L/min, median (IQR) | 5 (4.7-5.9) | 5 (4.4-5.7) | 5.6 (5-6.4) | 0.180 |
| Sweep, L/min, median (IQR) | 5.3 (4-7.5) | 5.3 (4.3-7) | 5.5 (3.5-7.5) | 0.712 |
| AC, n (%) | 14 (88) | 11 (92) | 3 (75) | 0.450 |
| Adverse events and complications, n (%) | ||||
| Acute kidney injury | 7 (44) | 5 (42) | 2 (50) | 1.000 |
| Bleeding event | 13 (81) | 9 (75) | 4 (100) | 0.529 |
| Thrombotic event | 6 (38) | 4 (33) | 2 (50) | 0.604 |
| Pneumonia | 12 (75) | 9 (75) | 3 (75) | 1.000 |
| Sepsis | 4 (25) | 3 (25) | 1 (25) | 1.000 |
| Right ventricular dysfunction | 5 (31) | 4 (33) | 1 (25) | 1.000 |
| Outcomes | ||||
| Hospitalization time, d, median (IQR) | 51 (36-66) | 41 (24-77) | 55.5 (43.5-69) | 0.695 |
| Tracheostomy on ECMO, n (%) | 14 (88) | 10 (83) | 4 (100) | 0.550 |
| Death within 30 d of hospital admission, n (%) | 3 (19) | 2 (17) | 1 (25) | 0.673 |
| Laboratory values, median (IQR)† | ||||
| WBC, K/cu mm | 13.2 (9.0-17.8) | 13.2 (9.5-17) | 13.7 (9.0-19.3) | 0.904 |
| Hemoglobin, g/dL | 11.2 (9.2-11.9) | 11.4 (9.3-12.6) | 9.0 (7.6-10.7) | 0.129 |
| Hematocrit, % | 33.8 (29.8-38.9) | 35.5 (31.3-39.3) | 28.9 (24.9-34.2) | 0.069 |
| Platelets, K/cu mm | 281 (174-332) | 297 (239-341) | 132 (108-163) | 0.005 |
| Creatinine, mg/dL | 1.0 (0.8-1.6) | 1.0 (0.7-1.5) | 1.5 (1.2-2.9) | 0.160 |
| Lactate, mmol/L | 2.2 (1.4-3) | 1.8 (1.3-2.9) | 3.2 (2-4.2) | 0.129 |
| AST, U/L | 60 (45-78) | 54 (39-73) | 81 (67-104) | 0.052 |
| ALT, U/L | 43 (33-57) | 41 (33-61) | 44 (35-50) | 1.000 |
| Bilirubin (total), mg/dL | 0.6 (0.4-1.3) | 0.5 (0.4-1.4) | 0.9 (0.5-1.3) | 0.583 |
| IL-6, pg/mL | 290 (167-710) | 290 (167-697) | 795 (231-1,359) | 0.346 |
| LDH, U/L | 558 (453-668) | 515 (428-616) | 697 (560-836) | 0.115 |
| Ferritin, ng/mL | 1099 (785-1,906) | 996 (608-1,906) | 1674 (1,104-3,885) | 0.396 |
| CRP, mg/dL | 17.2 (8.2-35.3) | 14.3 (7-35.3) | 26.8 (14.5-34.6) | 0.467 |
| D-dimer, mg/L | 18 (4-30) | 7 (3-20) | 30 (27-30) | 0.031 |
| Fibrinogen, mg/dL | 469 (322-717) | 551 (396-675) | 496 (165-768) | 1.000 |
| INR | 1.1 (1-1.2) | 1.1 (1-1.2) | 1.25 (1.1-1.4) | 0.342 |
| PT, s | 11.7 (11-13) | 11.7 (10.8-12.6) | 13.1 (11.2-14.9) | 0.280 |
| aPTT, s | 48.9 (37.7-57.5) | 42.8 (36.6-57.5) | 51.1 (46.7-77.1) | 0.240 |
| pH | 7.39 (7.37-7.44) | 7.39 (7.37-7.44) | 7.37 (7.36-7.42) | 0.463 |
| PaCO2, mmHg | 47 (38-50) | 48 (45-52) | 38 (35-43) | 0.060 |
| PaO2, mmHg | 70 (67-77) | 70 (68-76) | 71 (64-91) | 0.952 |
| ΔPaCO2, mmHg | 36 (30-43) | 39 (30-43) | 33 (27-48) | 0.557 |
| ΔPaO2, mmHg | 7 (−18 to 13) | 8 (−9 to 13) | −5 (−30 to 9) | 0.228 |
| Severity of illness, median (IQR) | ||||
| RESP score | 1 (0-3) | 1.5 (0.8-3) | 0.5 (−0.5 to 1.3) | 0.243 |
| SOFA score | 8 (6.5-9.5) | 7 (6-8.5) | 9.5 (9-10.5) | 0.027 |
| Apache II score | 16 (11-20) | 13 (10-20) | 17 (16-23) | 0.179 |
Abbreviations: AC, assist-control; ALT, alanine transferase; aPTT, activated partial thromboplastin time; AST, aspartate transferase; BMI, body mass index; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; IL-6, interleukin 6; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; P:F, pressure of arterial oxygen to fraction of inspired oxygen; PT, prothrombin time; RESP, Respiratory ECMO Survival Predication; RIJ, right internal jugular; SOFA, Sequential Organ Failure Assessment; WBC, white blood cells.
Mann-Whitney U and Fisher's exact tests, as appropriate.
Values measured on ECMO day 1 closest to time of ECMO cannulation.
Our analysis provided several important findings. First, no patient had an ischemic stroke, and ICHs were common, with a rate of 25%. We hypothesized that the prevalence of neurologic events in patients with COVID-19 ARDS with ECMO support would be similar to other patients with ARDS with ECMO support. Several prior studies have reported that patients treated with VV-ECMO for non–COVID-19-related respiratory failure are at higher risk for ICH than ischemic stroke.11 , 12 The Extracorporeal Life Support Organization registry study reported that the rates of infarct and ICH in 983 patients with COVID-19 on ECMO were 0.7% and 6% ICHs, respectively.13 Our data showed an ICH rate much greater than that of non–COVID-19 patients on ECMO.
In addition, severity of illness and coagulopathy may be important risk factors in ECMO-associated ICH. Although the pathophysiology of ICH in patients with COVID-19 likely is multifactorial, one hypothesis is that the cytokine-induced endothelial damage and breakdown of the blood brain barrier in patients with COVID-19 may increase risk of ICH.14 Additionally, sepsis-induced coagulopathy may result in consumptive coagulopathy, exacerbating bleeding risk. Tang et al4 reported high D-dimer and fibrin degradation product levels in patients with COVID-19 with severe pneumonia, suggestive of disseminated intravascular coagulation with enhanced fibrinolysis. Lersy et al15 found that CMBs in patients with COVID-19 were associated significantly with high D-dimer levels. These findings were consistent with our study, showing significantly higher D-dimer levels and Sequential Organ Failure Assessment scores in patients with neurologic events versus those without (Table 3).
Lastly, lower platelet counts and higher aPTT values on ECMO day one were associated with neurologic events, indicating anticoagulation is an important factor in ECMO-associated ICH in patients with COVID-19. Lower hemoglobin values in these patients may indicate worsening coagulopathy, predisposing patients to bleeding events. Furthermore, ICHs may be related to an acquired von Willebrand syndrome due to the high sheer stress of the ECMO circuit.16 , 17
Caution should be taken in interpreting these findings, however, because this study had a small sample size, and our center has a rigorous standardized neuromonitoring protocol that may increase the sensitivity of the detection of neurologic events.10 Diagnosis of neurologic event was only made upon imaging findings, and routine CT was not recommended without neurologic symptoms due to limited resources and a desire to avoid unnecessary exposures during the pandemic. Thus, only 12 of the 16 patients received a CT scan during ECMO, causing a selection bias. Although the absence of ischemic stroke is interesting, this diagnosis is more difficult to make than ICH due to poor sensitivity of CT scans for early ischemia. In the absence of comparative analysis with a control group, we cannot provide definitive evidence of COVID-19 infection conferring independent risk of neurologic events during ECMO support. However, our study still provided valuable information suggesting an increased ICH risk in patients with COVID-19 on ECMO. Future research is warranted to corroborate our findings and describe the risk factors for this critically ill population. Given the devastating outcome of neurologic events in patients with COVID-19 on ECMO, the utility of routine neuroimaging and reevaluation of anticoagulation strategy should be further explored.
Acknowledgments
Conflict of Interest
None.
References
- 1.Görlinger K, Dirkmann D, Gandhi A, et al. COVID-19–associated coagulopathy and inflammatory response: What do we know already and what are the knowledge gaps? Anesth Analg. 2020;131:1324–1333. doi: 10.1213/ANE.0000000000005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzeffi MA, Chow JH, Tanaka K. COVID-19 associated hypercoagulability: Manifestations, mechanisms, and management. Shock. 2021;55:465–471. doi: 10.1097/SHK.0000000000001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin X, Duan Y, Bao T, et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothstein A, Oldridge O, Schwennesen H, et al. Acute cerebrovascular events in hospitalized covid-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamakawa M, Kuno T, Mikami T, et al. Clinical characteristics of stroke with COVID-19: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannapadi NV, White B, Woo Choi C, et al. Clinically silent brain injury and perioperative neurological events in patients with left ventricular assist device: A brain autopsy study [e-pub ahead of print] ASAIO J. 2021 doi: 10.1097/MAT.0000000000001317. Accessed May 2, 2021. [DOI] [PubMed] [Google Scholar]
- 10.Cho S-M, Ziai W, Mayasi Y, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020;66:388–393. doi: 10.1097/MAT.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 11.Luyt C-E, Bréchot N, Demondion P, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42:897–907. doi: 10.1007/s00134-016-4318-3. [DOI] [PubMed] [Google Scholar]
- 12.Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33:42–50. doi: 10.1177/0267659118766435. [DOI] [PubMed] [Google Scholar]
- 13.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lersy F, Willaume T, Brisset J-C, et al. Critical illness-associated cerebral microbleeds for patients with severe COVID-19: Etiologic hypotheses [e-pub ahead of print] J Neurol. 2021 doi: 10.1007/s00415-020-10313-8. Accessed May 2, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalbhenn J, Schlagenhauf A, Rosenfelder S, et al. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant. 2018;37:985–991. doi: 10.1016/j.healun.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Uriel N, Pak S-W, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]