Urinary tract infections (UTIs) are one of the most common bacterial infections and are an increasing burden on the health care system because of recurrence and antibiotic resistance (1, 2). The most common uropathogen is E. coli (3, 4), which is responsible for about 80 to 90% of community-acquired UTIs and 40 to 50% of nosocomially acquired UTIs (2).
KEYWORDS: Escherichia coli, flagellar motility, surface motility, uropathogen
ABSTRACT
Uropathogenic Escherichia coli (UPEC) is the causative pathogen for most uncomplicated urinary tract infections (UTIs). Motility is likely to contribute to these infections, and E. coli possesses flagellum-dependent swimming motility, flagellum-dependent surface motility (often called swarming), and the recently observed pilus-dependent surface motility. Surface motility has not been extensively studied, but for the strains that have been tested, nonpathogenic E. coli (NPEC) laboratory strains use pili, NPEC hypermotile derivatives of these laboratory strains use flagella, and UPEC strains use flagella. Using a representative of these three types of strains, we showed differences in the nutritional and pathway requirements for surface motility with respect to the glucose concentration, the glycolytic pathway utilized, acetogenesis, the tricarboxylic acid (TCA) cycle. In addition, glucose-controlled flagellum synthesis was shown for the NPEC strain but not for the hypermotile NPEC variant or the UPEC strain. The requirements for surface motility are likely to reflect major metabolic differences between strains for the pathways and regulation of energy metabolism.
IMPORTANCE Urinary tract infections (UTIs) are one of the most common bacterial infections and are an increasing burden on the health care system because of recurrence and antibiotic resistance (1, 2). The most common uropathogen is E. coli (3, 4), which is responsible for about 80 to 90% of community-acquired UTIs and 40 to 50% of nosocomially acquired UTIs (2). Virulence requires both pili and flagella, and either appendage can contribute to surface motility, although the surface motility of uropathogenic E. coli has not been examined. We found different appendage, nutrient, and pathway requirements for the surface motility of a nonpathogenic E. coli laboratory strain and a uropathogenic E. coli strain. We propose that these differences are the result of differences in the pathways and regulation of energy metabolism.
INTRODUCTION
Bacteria possess several forms of movement or motility, which includes swimming motility and chemotaxis in a liquid and translocation on a surface (5–7). Swimming motility is assessed in agar with <0.3% agar, while surface motility assays employ plates with >0.3% agar. The surface motility of Escherichia coli is called swarming, and its characteristic feature is a dependence on flagella (5, 6, 8). Swarming cells express virulence genes and show enhanced resistance to both engulfment and antibiotics (9–12). The genetic requirements for E. coli swarming were examined for mutants of the Keio collection, which contains deletions in most nonessential genes (13). Swarming motility required flagella but unexpectedly also required pili. The authors suggested that some genes for pilus synthesis were required for flagellum synthesis, which is not consistent with subsequent evidence that pilus and flagellum syntheses are mutually exclusive (14–16). Recent results show that E. coli also possesses pilus-dependent surface motility (16, 17). Our recent results show that the surface motility of common E. coli laboratory strains, including the parental strain of the Keio mutants, requires pili, but fast variants rapidly appear due to a mutation that increases the expression of the master regulator for flagellum synthesis (16). In other words, results using Keio collection mutants involved strains that expressed either pili, flagella, or both if a flagellum-synthesizing variant was generated during the motility assay. In addition to these forms of surface motility, E. coli also possesses pilus-dependent rolling motility (18, 19). The genetic and metabolic requirements for surface motility may depend on the appendage and must be reexamined in strains for which the motility appendage is unambiguously known.
Surface motility has been examined in laboratory strains of E. coli but not pathogenic strains. E. coli generally resides in the intestinal tract, but uropathogenic E. coli (UPEC), a pathotype of extraintestinal pathogenic E. coli, is generally thought to migrate to, adapt to, and colonize the urinary tract and cause urinary tract infection (UTI) (20). UPEC can infect the urinary tract, kidneys, and bloodstream, causing cystitis, pyelonephritis, and sepsis, respectively. Nutrient availability differs between the intestinal and urinary tracts (21, 22). The growth of E. coli in the intestinal tract requires multiple carbohydrates, while growth in the urinary tract is proposed to require amino acids and peptides (21, 23, 24).
The presentation of human UTIs varies from localized trigonitis to unlocalized pancystitis (25). A difference between the two types of infection may be bacterial migration. The mechanism of migration required for the establishment of a localized UTI may differ from that required for progression to pancystitis. It is generally thought that 95% of UTIs may be ascending infections, meaning that the infection begins by colonization of the periurethral area, followed by migration up the urethra into the bladder and possibly into the ureters and kidneys (26). One mechanism of migration requires flagella. The UPEC strain CFT073 required flagellin, the fliC product, to ascend to the upper urinary tract in a murine model but was not obviously needed to ascend the urethra (27). For another UPEC strain, UTI89, the loss of flagella had a subtle effect on infection by the UPEC strain UTI89 in the mouse bladder (28). When movement is considered, swimming motility is likely to be assumed. Although E. coli can migrate on a surface, surface motility has not been discussed in relation to virulence.
We compared the nutrient and pathway requirements for (i) pilus-dependent surface movement of the nonpathogenic E. coli (NPEC) strain W3110; (ii) flagellum-dependent surface movement of W3110-J1, a fast-moving W3110 variant; and (iii) the flagellum-dependent surface movement of the UPEC strain UTI89. We discuss our results in relation to nutrient and pathway requirements for CFT073 infection in a mouse model.
RESULTS
Requirement of glucose for surface motility.
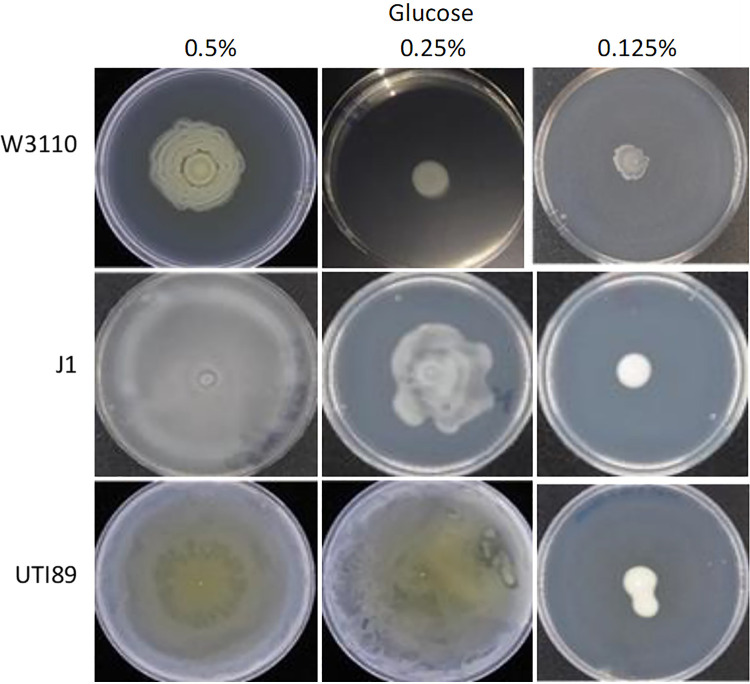
We examined the glucose requirement for surface motility for three strains: nonpathogenic W3110, which requires pili for surface movement; J1, which is a hypermotile derivative of W3110 that utilizes flagella for surface movement because of an IS5 insertion in the flhDC promoter region; and uropathogenic UTI89, which also moves with flagella (16). Electron microscopic (EM) images of these strains taken from surface motility plates confirmed their appendage requirement (Fig. 1). W3110 moved relatively slowly, rarely reached the plate’s edge during the 36-h assay, and formed a ring pattern that resembles the swarm pattern of Proteus mirabilis (29) (Fig. 2). Its movement required 0.5% glucose. J1 and UTI89 movement with 0.5% glucose covered the entire plate, with no discernible pattern. J1 did not reach the plate’s edge with 0.25% glucose, but UTI89 did. J1 and UTI89 did not move with 0.125% glucose. The glucose requirement was different for each strain, and the strains with flagellum-dependent surface motility had a lower glucose requirement.
FIG 1.
Electron microscopic images of nonpathogenic strains W3110 and J1 and uropathogenic strain UTI89. UTI89 and J1 possess flagella, which are longer than the cell length, while W3110 possess pili. The dots around UTI89 are typical for EM images of this strain and probably indicate the presence of exopolysaccharide. The bars for W3110, J1 and UTI89 are 0.5, 2, and 1 μm, respectively.
FIG 2.
Concentration requirement of glucose for surface motility.
Surface motility of mutants with defects in glucose transport and pathways of carbohydrate metabolism.
We examined surface motility in mutants with defects in glucose transport and the following pathways of central metabolism: the Embden-Meyerhof-Parnas (EMP) pathway, which is the standard glucose-degrading pathway for numerous organisms, including E. coli; gluconeogenesis; the oxidative branch of the pentose cycle; the Entner-Doudoroff (ED) pathway, which is an alternate glycolytic pathway that degrades glucose to pyruvate; and acetogenic enzymes that convert pyruvate to acetate. These pathways are important for energy generation and the biosynthesis of a variety of intermediates, such as NADPH and ribose-5-phosphate; triose phosphates for the glycerol backbone of phospholipids; 3-phosphoglycerate for serine, glycine, and cysteine synthesis; pyruvate for acetyl coenzyme A (acetyl-CoA) synthesis; and acetyl-CoA for either the tricarboxylic acid (TCA) cycle or acetate formation. Figure 3 presents a diagram of the carbohydrate pathways examined.
FIG 3.
Pathways of carbohydrate metabolism. Mutants with gene deletions that lack the indicated proteins were tested for surface motility. Enzyme abbreviations: AckA, acetate kinase; Eda, 2-keto-3-deoxygluconate 6-phosphate aldolase; Edd, phosphogluconate dehydratase; PckA, phosphoenolpyruvate carboxykinase; PfkA, phosphofructokinase isozyme A; Pta, phosphotransacetylase; PtsI, PtsH, and PtsG, components of the phosphotransferase system for glucose transport; SerB, phosphoserine phosphatase; Zwf, glucose-6-phosphate dehydrogenase. Metabolic intermediate abbreviations: acetyl-P, acetyl-phosphate; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde-3-phosphate; Glu-6-P, glucose-6-phosphate; KDGP, 2-keto-3-deoxygluconate 6-phosphate; OAA, oxaloacetate; 6-P-gluconate, 6-phosphogluconate; PEP, phosphoenolpyruvate.
The results of the mutational analysis are shown in Fig. 4 and summarized in Table 1. For W3110, surface motility required a gene for the rate-limiting enzyme of the EMP pathway, pfkA (phosphofructokinase); three genes of the major glucose transport system, ptsG, ptsH, and ptsI; the first gene of the oxidative pentose cycle, zwf (glucose-6-phosphate dehydrogenase); and a gene required for the synthesis of the 3-phosphoglycerate family of amino acids, serB (phosphoserine phosphatase). W3110 moved less well with deletions in genes of gluconeogenesis, pck (phosphoenolpyruvate carboxykinase), and the ED pathway, edd (phosphogluconate dehydratase) and eda (2-keto-3-deoxygluconate 6-phosphate aldolase). The loss of the acetogenic genes pta (phosphotransacetylase) and ackA (acetate kinase) did not impair movement.
FIG 4.
Surface motility of mutants with defects in glucose transport and pathways of carbohydrate metabolism. WT, wild type.
TABLE 1.
Summary of the surface motility of mutants with defects in various pathways
| Genotype | Pathway affected | Motilitya |
Infection model resultb | ||
|---|---|---|---|---|---|
| W3110 | J1 | UTI89 | |||
| Parental | None | ++ | ++ | ++ | + |
| ΔptsI | Glucose transport | − | ++ | + | ND |
| ΔptsH | Glucose transport | − | ++ | + | ND |
| ΔptsG | Glucose transport | − | ++ | ++ | ND |
| ΔpfkA | EMP glycolysis | − | − | ++ | + |
| Δedd | ED glycolysis | + | ++ | − | + |
| Δeda | ED glycolysis | + | ++ | + | ND |
| Δzwf | Oxidative pentose cycle | − | ++ | + | ND |
| Δpta | Acetogenesis | ++ | ++ | − | − |
| ΔackA | Acetogenesis | ++ | ++ | + | − |
| Δpck | Gluconeogenesis | + | ++ | ++ | − |
| ΔserB | Serine synthesis | − | − | − | ND |
| ΔgltA | TCA cycle | + | + | − | ND |
| ΔsdhA | TCA cycle | + | + | − | —c |
| ΔsucC | TCA cycle | + | + | − | —c |
The scoring is ++ for nearly parental movement, + for partial movement, and − for little or no movement. See Fig. 4, 13, and 14 for visualization of these qualitative results.
The results from a mouse infection model have been reviewed previously (21). ND, not determined.
These exact mutants were not tested in a mouse infection, but a deletion in the same operon had the indicated result.
J1 surface motility required pfkA but, unlike parental strain W3110, did not require the oxidative pentose cycle (zwf) or components of the major glucose transport system, ptsG, ptsH, and ptsI.
The pathway requirements for pathogenic strain UTI89 were different from those for both W3110 and J1. UTI89 movement was not affected by the loss of pfkA, ptsG, and pck and was substantially or completely impaired by the loss of ptsI, ptsH, the ED pathway genes eda and edd, zwf, the acetogenic genes ackA and pta, and serB.
Factors that affect the glucose requirement.
(i) Iron limitation. Iron can modulate the expression of genes of energy metabolism, so we examined the effect of supplemental 2,2-bipyridyl (BIP), an iron chelator, on surface motility (30). With BIP (low iron), UTI89 moved with 0.125% glucose (Fig. 5) but not without glucose (not shown). Low iron restored the movement of J1 with 0.25% glucose and allowed partial movement with 0.125% glucose. W3110 showed some movement with 0.25% glucose but no movement with 0.125% glucose (Fig. 5). Surface motility plates with BIP and no glucose did not support the growth of any of the strains (not shown). Even with low iron, flagellum-mediated motility required a lower concentration of glucose than pilus-mediated motility.
FIG 5.
Surface motility of W3110, J1, and UTI89 with 100 μM BIP and the indicated concentrations of glucose.
(ii) Glycerol, cysteine, and pyruvate. We examined whether glycerol, cysteine, and pyruvate could reduce the glucose requirement for W3110 because (i) we could not genetically test the importance of glycerol-3-phosphate (glycerol-3-P), which is required for phospholipid synthesis; (ii) a higher-than-normal level of cysteine has been shown to be a requirement for Salmonella enterica surface motility (31, 32), which is consistent with the result that a W3110 ΔserB mutant failed to move (Fig. 4); and (iii) pyruvate is a product of carbohydrate catabolism and is a major branch point of metabolism that can provide energy via the TCA cycle and generate alanine and acetate. Supplements were added to medium with 0.125% glucose and 100 μM BIP, which does not support the surface motility of W3110 (Fig. 5). A combination of 2 mM cysteine, 0.1% glycerol, and 0.1% pyruvate stimulated surface motility (Fig. 6). Cysteine omission did not prevent movement but altered the motility pattern (Fig. 6), glycerol omission severely impaired movement (Fig. 6), and pyruvate omission had no effect on movement (Fig. 6). Glycerol alone stimulated movement, but cysteine did not (Fig. 6). Glycerol replaced glucose for strains J1 and UTI89 (Fig. 7). In summary, glycerol lowered the glucose requirement and could replace glucose for the two strains with flagellum-mediated surface motility.
FIG 6.
W3110 surface motility with cysteine, pyruvate, and glycerol. The medium contained 0.125% glucose and 100 μM BIP. Cys, 2 mM cysteine; Pyr, 0.1% pyruvate; Gly, 0.1% glycerol.
FIG 7.

Surface motility with 0.5% glycerol instead of 0.5% glucose.
(iii) Supplementation with other sugars. Glucose and glycerol are not abundant in urine, but urine contains carbohydrates. The concentrations of single urinary carbohydrates are under 0.4 mM, except for glucuronate (∼2 mM in urine), but the total urinary carbohydrate content is substantial (>4 mM) (9). A mixture of glucuronate (5 mM), gluconate (1 mM), glycerol (1 mM), glucose (1 mM), mannitol (1 mM), and sorbitol (1 mM) did not support the motility of strain J1 but supported the motility of UTI89 weakly after 24 h (inner circle in Fig. 8A). The appearance of a hypermotile uropathogenic variant was apparent after 24 h, and the variant moved to the edge of the plate after 48 h (Fig. 8B). Cells from the edge of the plate were isolated and shown to move without any carbohydrate (Fig. 8C). A possible explanation for enhanced motility is an insertion in the promoter region of the flhDC operon (16, 33). PCR analysis of this region showed no insertion upstream from the flhDC structural genes (not shown). These variants were not further characterized. In summary, UTI89 migrated on a surface with a combination of carbohydrates, and a UTI89 variant migrated without a carbohydrate, which implies that UTI89 surface motility requires a carbohydrate.
FIG 8.

Surface motility of UTI89 with a carbohydrate mixture. The carbohydrate mixture contained 5 mM glucuronate, 1 mM gluconate, 1 mM glucose, 1 mM glycerol, 1 mM mannitol, and 1 mM sorbitol. (A) Motility for 24 h with the carbohydrate mixture. Notice the presence of faster-moving flares. (B) Motility for 48 h with the carbohydrate mixture. (C) Cells from the edge of the plate in panel B were incubated for 15 h in the absence of any carbohydrate.
Glucose is required for type 1 pilus-mediated surface motility.
We observed surface motility of W3110 into plates with 0.25% agar, which is used to monitor swimming motility, but only if the medium contained glucose (Fig. 9, bottom row). In the absence of glucose, W3110 swam into plates containing 0.25% agar, and such movement required flagella (FliC) (Fig. 9, top row). In the presence of glucose, W3110 moved on the surface, and this movement required pili (FimA) (Fig. 9).
FIG 9.
Motility observed in plates with 0.25% agar with and without glucose. Arrows indicate pilus-mediated surface motility, which is observed only in the presence of glucose. The bubbles for MG1655 and UTI89 with glucose resulted from CO2 generation by bacteria that swam on the agar.
MG1655 is a nonpathogenic strain of E. coli that, like J1, has an IS1 element 106 bases upstream from the flhDC transcriptional start site that increases flagellum synthesis. MG1655 swam into 0.25% agar plates with or without glucose (Fig. 9). Without glucose, MG1655 ΔfliC did not swim, but with glucose, this mutant moved on the surface (Fig. 9). J1 exhibited the same properties (not shown). The same phenotype for two different strains shows that these properties are not strain dependent.
Pathogenic UTI89 swam into a plate with 0.25% agar, with or without glucose, and this movement required flagella (Fig. 9). Swimming with glucose is unexpected since glucose should prevent cyclic AMP (cAMP) synthesis, which is generally thought to be required for flagellum synthesis (16, 34, 35). However, we have shown that glucose does not prevent flagellum synthesis in several pathogenic E. coli strains, which shows that the absence of a glucose effect is not strain dependent (16). UTI89 ΔfliC, which lacks flagella, moved slightly on the surface in the presence of glucose (Fig. 9). This surface movement required pili because UTI89 ΔfliC ΔfimA failed to move (16). In summary, glucose is required for surface movement in strains lacking flagella.
We examined surface motility with 0.45% agar for W3110, MG1655, and UTI89 derivatives with a deletion of fliC, which forces these strains to move with pili. (ΔfliC ΔfimA double mutants of these strains do not move [16]). All three strains moved well with 0.5% glucose but not with 0.5% glycerol (Fig. 10A).
FIG 10.
Pilus-dependent surface motility of ΔfliC derivatives of UTI89, MG1655, and W3110. ΔfliC derivatives of UTI89 and MG1655 use pili in the absence of flagella since ΔfliC ΔfimA derivatives do not move. W3110 normally uses pili for surface motility. (A) UTI89, MG1655, and W3110 mutants lacking flagella (ΔfliC) in medium with the indicated carbohydrates. (B) Surface motility of W3110 and Δcrp and ΔcyaA derivatives in surface motility medium with 0.5% glucose. (C) Electron microscopy image of the W3110 ΔcyaA mutant showing piliated cells. The pili are in focus, but the bacterial cells are not. The bar indicates 800 nm.
The glucose-dependent movement predicts that pilus-dependent surface motility will not require cAMP because glucose inhibits cAMP synthesis (36). Furthermore, cAMP receptor protein (CRP)-cAMP represses fimB expression, which is part of the complex control of pilus synthesis (37). Such control predicts that the loss of CRP or cAMP will not affect surface motility. As expected, crp and cya mutants of W3110 still exhibited surface motility, although the pattern of motility was altered (Fig. 10B). Electron microscopy verified that the Δcya mutant had pili but not flagella (Fig. 10C).
Requirement for tryptone and the TCA cycle.
Surface motility medium contains 1% tryptone. For all types of strains, 0.75% tryptone supported movement to the same extent as 1% tryptone (Fig. 11). For J1 and UTI89, the bacteria on the 0.75% tryptone plate were not as dense as those on the 1% tryptone plate, which suggests less growth (not shown). For all strains, 0.5% tryptone supported substantially less motility, and 0.25% tryptone did not support movement (Fig. 11). The tryptone requirement does not distinguish between pilus-dependent and flagellum-dependent strains.
FIG 11.
Tryptone requirement for surface motility.
Tryptone is an enzymatic digest of casein that consists mostly of amino acids, which can function as energy sources and biosynthetic precursors. Amino acid degradation in complex mixtures is poorly characterized, but if amino acids are energy sources, then their degradation requires the TCA cycle, electron transport, and oxidative phosphorylation (38). Surface motility was examined for mutants lacking enzymes of the TCA cycle and electron transport (Fig. 12). Deletion of the following genes of W3110 had little or no effect on surface motility: nuoC, glpD, glpA, poxB, hyaA, fdhF, and menA (Fig. 13). Movement was substantially impaired, but not eliminated, for mutants with deletions of cyoA, ubiF, gltA, acnB, sucC, sucD, sdhA, and sdhB (Fig. 13). lpd, sucA, and sucB mutants could not move (Fig. 13). The latter three mutants cannot generate succinyl-CoA, which is required for meso-diaminopimelate synthesis, an essential component of peptidoglycan that cannot be synthesized from components in the medium.
FIG 12.
The TCA cycle and electron transport enzymes. Mutants with gene deletions that lack the indicated proteins were tested for surface motility. Enzyme abbreviations: AcnB, aconitate hydratase B; CyoA, cytochrome bo3 ubiquinol oxidase subunit 2; FdhF, formate dehydrogenase H; GlpA, anaerobic glycerol-3-phosphate dehydrogenase subunit A; GlpD, aerobic glycerol 3-phosphate dehydrogenase; GltA, citrate synthase; HyaA, hydrogenase 1 small subunit; Lpd, lipoamide dehydrogenase; MenA, 1,4-dihydroxy-2-naphthoate octaprenyltransferase in menaquinone synthesis; NuoC, NADH:quinone oxidoreductase subunit CD; PoxB, pyruvate oxidase; SdhA and SdhB, two components of succinate dehydrogenase; SucA and SucB, two components of α-ketoglutarate dehydrogenase; SucC and SucD, the two components of succinyl-CoA synthetase; UbiF, 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinol hydroxylase in ubiquinol biosynthesis. OAA, oxaloacetate.
FIG 13.
Surface motility of W3110 and derivatives lacking TCA cycle and electron transport chain enzymes.
For the flagellum-dependent hypermotile J1 and uropathogenic UTI89 strains, we examined mutants lacking genes for three representative enzymes of the TCA cycle: gltA, sucC, and sdhA. None of the three UTI89 mutants exhibited surface motility (Fig. 14), which suggests that flagellum-dependent movement requires the TCA cycle and oxidative phosphorylation. Such a result is not unexpected since flagellum rotation requires a proton motive force. In contrast, the J1 mutants moved, albeit poorly, and unexpectedly formed a ringed pattern like their parental strain W3110 (Fig. 14). Such a pattern is more consistent with the pilus-dependent movement of W3110, and electron microscopy showed that these mutants were piliated (Fig. 15). These results suggest that a mutational block in the TCA cycle prevents flagellum synthesis and results in pilus synthesis in strain J1.
FIG 14.

Surface motility of J1 and UTI89 mutants lacking representative TCA cycle enzymes.
FIG 15.
Electron microscopy images of J1 ΔsdhA and J1 ΔsucC. The bars for ΔsdhA and ΔsucC strains are 1 and 0.6 μm, respectively.
pH of medium after motility assays.
The pathway requirements for motility are clearly different between strains. Glycolysis through PfkA is required for the motility of W3110 and J1 but not for the motility of UTI89. The loss of the TCA cycle affects all three strains, but the effect is greatest for UTI89. The motility medium is essentially unbuffered, which means that reliance on different energy-generating pathways will have different effects on medium pH: glycolysis will generate acids, and amino acid degradation via the TCA cycle will alkalinize the medium due to ammonia formation. The pHs at the movement edge for W3110, J1, and UTI89 were 4.5 to 5.0, 5.5 to 6.0, and 6.0 to 6.5, respectively (not shown). The pH indicates the relative dependence of acid-generating carbohydrate degradation versus ammonia-generating amino acid degradation for these strains: W3110 is more dependent on glycolysis, whereas UTI89 is more dependent on the TCA cycle.
DISCUSSION
Our goals were to determine the nutrient and pathway requirements for the surface motility of nonpathogenic E. coli that used pili or flagella for movement and to compare these requirements with those of a uropathogen. The strains examined were W3110, which exhibited pilus-dependent movement, and J1 and UTI89, which showed flagellum-dependent movement. J1 is a hypermotile derivative of W3110. The results are summarized in Tables 1 and 2. E. coli pilus-mediated motility has not been thoroughly characterized, but its requirements differ substantially from those for the flagellum-dependent strains, which confirms that pilus-dependent E. coli surface motility is distinct from flagellum-dependent E. coli motility.
TABLE 2.
Summary of pathway requirements for surface motility
| Function or pathway | Requirement in strain |
||
|---|---|---|---|
| W3110 | J1 | UTI89 | |
| Glucose transport | PtsG | Not PtsG | Not PtsG |
| Embden-Meyerhof-Parnas (via PfkA) | Yes | Yes | No |
| Pentose-phosphate (via Zwf) | Yes | No | Yes |
| Entner-Doudoroff | Partial | No | Yes |
| TCA cycle | Partial | Yes/partiala | Yes |
Mutants of J1 lacking TCA cycle enzymes moved partially and had switched from flagellum- to pilus-dependent movement.
A potential problem with our analysis is the possibility that P1 transduction, which was used to construct our mutants, carried a mutation in a cotransduced gene that caused the phenotype. The best way to handle this difficulty is via complementation, which also has potential problems, especially the effect of plasmid controls, which inhibited motility. To address this problem, deletions of genes for multiple enzymes of glucose transport, glucose catabolism via the oxidative pentose and Entner-Doudoroff pathways, acetogenesis, and the TCA cycle were analyzed. In all cases, when the loss of one enzyme of the pathway impaired movement, the loss of other enzymes of the same pathway also impaired movement.
Our results are generally consistent with known metabolism. Flagellum-dependent movement requires proton motive force; it is not a surprise that all TCA cycle mutants fail to move using flagella. The failure of mutants with defects in glucose metabolism to move is not surprising since all surface movements (pilus or flagellum dependent) require glucose when glucose is the sole carbon source. W3110 requires glycolysis via PfkA, which is not surprising, but UTI89 does not require PfkA. However, the latter result is consistent with the observation that UPEC strain CFT073 does not require PfkA for mouse infection (21). Because acetogenesis is required for CFT073 infection of mice (21), it is not surprising that acetogenesis is required for surface motility. The phenotypes of ptsG, eda, and pckA mutants are not consistent with previous results and are discussed below.
Energy metabolism during surface motility.
Flagellum-dependent movement of UTI89 and J1 required the TCA cycle. For UTI89, the requirement for the TCA cycle was absolute, while for J1, the requirement appeared to be partial. However, the J1 mutants with TCA cycle defects used pili for movement. Our interpretation is that J1 flagellum-dependent surface motility absolutely requires the TCA cycle but under pressure to acquire nutrients can generate variants that utilize pili for movement.
Based on several observations, flagellum-dependent motility preferentially utilized amino acids degraded via the TCA cycle over carbohydrate degradation via glycolysis. First, movement of the flagellum-dependent strains resulted in medium alkalinization, which can result only from the deamination of amino acids. Second, both flagellum-dependent strains had a lower glucose requirement than the pilus-dependent strain W3110. Finally, J1 required fewer genes of carbohydrate transport and metabolism than parental strain W3110. Despite the greater reliance on amino acids and the TCA cycle, migration by both J1 and UTI89 still required a carbohydrate.
The pilus-dependent motility of W3110 was more dependent on carbohydrate degradation and less dependent on the TCA cycle, although mutants with defects in the TCA cycle were less motile. The evidence for this conclusion is (i) the greater medium acidification for W3110 than for J1 and UTI89 and (ii) that defects in a greater number of glycolytic enzymes affected W3110 motility. These results suggest that pilus-dependent movement is more dependent on ATP from carbohydrate catabolism. Perhaps, intracellular ATP can control the conformational states of type 1 pili, which could contribute to a form of motility (39).
In summary, flagellum-dependent motility requires the TCA cycle, oxidative phosphorylation, and the proton motive force, while pilus-dependent motility has a greater reliance on ATP from glycolysis. This conclusion is consistent with observations of S. enterica swarming cells, which are morphologically and metabolically distinct from vegetative swimming cells (40). Although swarming cells require glucose, the levels of almost all enzymes of glycolysis were lower while those of several TCA cycle proteins were higher during swarming (40).
Comparison of requirements for the two strains with flagellum-dependent movement.
The common requirements for J1 and UTI89 were glucose, albeit less glucose than for the pilus-dependent strain W3110, and flagellum synthesis in the presence of glucose, which is not a property of frequently studied E. coli laboratory strains. Despite these similarities, J1 and UTI89 also differed. J1, but not UTI89, required glycolysis through PfkA. In this respect, J1 is like parental strain W3110. Conversely, UTI89, but not J1, was affected by the loss of the oxidative branch of the pentose cycle, the ED pathway, and acetogenic enzymes. Given their reduced requirement for glucose compared to W3110, carbohydrate metabolism may be important for one or more biosynthetic intermediates, and the specific glycolytic pathway used for the synthesis of the intermediate may not be important. For example, the specific pathway that generates triose-phosphates, e.g., the EMP versus the ED pathway, may not be important if triose-phosphates are made.
Glucose transport during surface motility.
Glucose transport in laboratory strains of E. coli requires PtsI (enzyme I), PtsH (the Hpr protein), Crr (the glucose-specific enzyme IIA component), and PtsG (the glucose-specific enzyme IIBC component). W3110 motility required PtsH, PtsI, and PtsG. J1 motility did not require any of these components but still required a carbohydrate. A minor non-phosphotransferase system (PTS) glucose uptake system (e.g., GalP [41]) may be sufficient for J1’s reduced carbohydrate requirement. UTI89 did not require PtsG but at least partially required PtsH and PtsI. An additional glucose transport mechanism could explain both the nonessentiality of PtsG in UTI89 and flagellum synthesis in the presence of glucose. Glucose transport via PtsG and Crr controls cAMP synthesis: an alternate glucose transporter that does not inhibit cAMP synthesis would account for flagella in the presence of glucose. Glucose-independent flagellum synthesis has also been observed for the UPEC strains PNK-004 and PNK-006 (16). PtsG-independent glucose transport and flagellum synthesis in the presence of glucose may be adaptations to the urinary tract environment.
Comparing metabolic requirements for surface motility and UTIs.
The pathway requirement for UTIs has been extensively studied with the uropathogen CFT073 in a competitive-fitness mouse model (21, 42). The EMP pathway was dispensable for bladder infection but was required for kidney infection. On the other hand, CFT073 mutants lacking TCA cycle, acetogenesis, and gluconeogenesis enzymes were less fit in a murine model (21, 42). Like CFT073 infection, UTI89 surface motility required the TCA cycle and acetogenesis and did not require PfkA. However, a major difference is that CFT073 infection required gluconeogenesis (Pck), but UTI surface motility did not.
Genetic analysis has suggested that CFT073 infection required amino acid catabolism via the TCA cycle but not carbohydrate catabolism via PfkA (21). The lack of a role for carbohydrates in UPEC metabolism during UTI is not established. First, because surface motility required a carbohydrate but did not require PfkA, the absence of a phenotype for a mutant lacking PfkA is not sufficient to prove that carbohydrates are not required for growth. Second, genes for lactose and sorbitol catabolism are induced in intracellular bacterial communities during UTIs, and their loss reduced UTI89 virulence (43). Third, the loss of the Vpe carbohydrate permease, which transports an unknown carbohydrate, impaired the virulence of the uropathogen AL511 (44). We propose that carbohydrate metabolism for both UTI89 and CFT073 occurs via PfkA-independent pathways. For example, the loss of ED pathway enzymes results in defective UTI89 motility, which implies that the ED pathway contributes to glucose metabolism.
The metabolic requirements for UTI89 motility, which can be assessed in a defined and controlled environment, can provide insight into the metabolism of uropathogens, including CFT073. The requirements for the TCA cycle and acetogenesis and the lack of a requirement for PfkA for both UTI89 motility and CFT073 infectivity may suggest a UPEC-specific metabolism that is an adaptation to the urinary tract environment. However, UTI89 motility, but not CFT073 infectivity, requires the ED pathway, and CFT073 infectivity, but not UTI89 motility, requires gluconeogenesis. These differences may be a function of either varied requirements for surface motility and infection or strain-specific differences. Strain-specific differences should not be considered surprising since each UPEC strain must adapt to a different urinary tract environment. UPEC strains are becoming increasingly antibiotic resistant. The identification of UPEC-specific enzymes or processes that do not show strain-to-strain variations, possibly a UPEC-specific glucose transporter, could be crucial for the identification of targets for the development of antibacterial therapies.
MATERIALS AND METHODS
Bacterial strains.
E. coli strain W3110, hypermotile strain J1 (derived from W3110), NPEC strain MG1655, and uropathogenic strain UTI89 were used as parental strains. Table 3 lists the derivatives of these strains used in the study. The mutant alleles came from the Keio strain collection and contained deletions in which a kanamycin resistance gene replaced the gene of interest (45). The deletion/insertion was transferred by P1 transduction into the various parental strains (46). UTI89 was the uropathogen studied because of the ease of transduction. Other pathogenic strains can be transduced with P1, but the transduction procedure is more complex.
TABLE 3.
List of bacterial strains
| Parental strain | Mutant(s) constructed | Parental source |
|---|---|---|
| W3110 | ΔackA, ΔacnB, Δcrp, ΔcyaA, ΔcyoA, Δeda, Δedd, ΔfdhF, ΔfimA, ΔfliC, ΔfliC ΔfimA, ΔglpA, ΔglpD, ΔgltA, ΔhyaA, Δlpd, ΔmenA, ΔnuoC, Δpck, ΔpfkA, ΔpoxB, Δpta, ΔptsG, ΔptsH, ΔptsI, ΔsdhA, ΔsdhB, ΔserB, ΔsucA, ΔsucB, ΔsucC, ΔsucD, ΔubiF, Δzwf | Laboratory strain |
| J1 | ΔackA, Δeda, Δedd, ΔgltA, Δpck, ΔpfkA, Δpta, ΔptsG, ΔptsH, ΔptsI, ΔserB, ΔsdhA, ΔsucC, Δzwf | Laboratory strain (derived from W3110) |
| UTI89 | ΔackA, Δeda, Δedd, ΔfliC, ΔgltA, Δpck, ΔpfkA, Δpta, ΔptsG, ΔptsH, ΔptsI, ΔserB, ΔsdhA, ΔsucC, Δzwf | Laboratory strain |
| MG1655 | ΔfliC | Coli Genetic Stock Center (Yale University) |
The kanamycin resistance gene was left in place, which implies the possibility of polarity. The conclusions of this work do not depend on whether gene expression is polar past the insertion. However, for several reasons, the potential effects of polarity are likely to be minor. First, genes downstream from the insertion can be expressed from the resistance gene, although expression may not be the same as that in the intact operon. Second, the insertion should not have downstream effects on the following monocistronic genes: acnB, crp, cyaA, fdhF, fliC, glpD, gltA, menA, pck, pfkA, ptsG, ubiF, and zwf. Third, insertions in eda, lpd, pta, ptsI, sdhB, and sucD are in the last gene of an operon and should not have a polar effect. Fourth, insertions in cyoA, fimA, glpA, hyaA, and nuoC were intended to eliminate a multiprotein complex: polarity is irrelevant. Finally, the insertion of the following genes may affect downstream genes but will affect only genes coding for proteins of the same pathway: ackA of the ackA-pta operon and acetogenesis, edd of the edd-eda operon and the ED pathway, ptsH of the ptsHI operon and carbohydrate transport, and the suc and sdh genes of their respective operons of the TCA cycle.
Media and growth conditions.
For growth on solid medium, strains were streaked on LB agar plates (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl, 15 g/liter Difco agar) and incubated at 37°C for 15 h. For liquid cultures, bacteria were grown in LB broth with 25 μg/ml kanamycin (when appropriate) at 37°C with aeration (250 rpm) for 12 h.
Motility assays.
(i) Surface motility. Bacterial strains were streaked on an LB agar plate. After growth overnight, a single colony was inoculated in 1 ml of glucose-tryptone medium (0.5% glucose, 1% tryptone, and 0.25% NaCl) and incubated at 37°C for 6 h with aeration. Surface motility plates contained glucose-tryptone medium with 0.45% Eiken agar and were dried at room temperature for 4 to 5 h after pouring. Changes to the glucose-tryptone medium are indicated in Results. The motility plates did not contain antibiotics. One microliter from a 6-h-grown culture was inoculated at the center of the surface motility plate. Plates were placed in a humid incubator set at 33°C for nonpathogenic strains or at 37°C for UPEC strains, and surface motility was documented at 36 h. Assays for the nonpathogenic strains were conducted at 33°C to ensure reproducibility: assays at 37°C were highly variable for NPEC strains because cells started moving at different times. Assays at 37°C for W3110 frequently result in the generation of hypermotile variants. All assays were performed at least three times. Surface motility was extremely sensitive to conditions. The motility of the parental controls depended on the batch of the plates; for example, compare the results for parental strain W3110 in Fig. 2 and 4. The motility of W3110 stopped if plates were removed from the incubator and examined for several minutes.
(ii) Swimming motility. Bacterial strains were streaked on LB, and a single colony was inoculated into 1 ml of glucose-tryptone medium and grown for 6 h. Swim plates (1% tryptone, 0.25% NaCl, 0.25% Eiken agar) were stab inoculated at the center with 1 μl from the 6-h-grown culture and incubated at 33°C for 16 h in a humid incubator. All assays were performed in triplicate.
Electron microscopy.
Cells from surface motility plates were collected from the edge of movement and fixed with 2.5% glutaraldehyde. Bacteria were absorbed onto Formvar carbon-coated copper grids for 1 min. Grids were washed with distilled water and stained with 1% phosphotungstic acid for 30 s. A total of 500 to 1,000 cells were observed before choosing what to record. Samples were viewed on a JEOL 1200 EX transmission electron microscope at UT Southwestern Medical Center.
Images were taken of multiple areas on multiple windows of the grids. The images were chosen to represent the population seen. A minimum of 10 images containing more than one cell were taken. The images selected were representative of the entire population of bacteria of each strain. Cells were resuspended in 100 μl of glutaraldehyde to ensure that there was no observer bias. Cells had been removed from the plate using multiple methods (e.g., removal with inoculation loops or wooden dowels) to ensure that there was no bias with selecting cells using glutaraldehyde resuspension. No differences were seen.
On rare occasions, we saw a flagellated cell in a population of piliated cells, but this was exceedingly rare in that only 1 cell out of the 500 to 1,000 observed would present that way.
PCR amplification of the flhDC promoter region.
The flhDC promoter region was PCR amplified using FlhDp forward and reverse primers as described previously (47). The PCR product was then subjected to gel electrophoresis in a 0.8% agarose gel at 130 V for 30 min.
pH of medium after motility.
pH paper was placed directly on the plate. The pH determinations were performed at least 10 times.
ACKNOWLEDGMENTS
This work was supported in part by a UT Dallas Collaborative Biomedical Research Award grant program. The electron microscopy was performed at UT Southwestern, which is supported by NIH grant 1S10OD021685-01A1.
REFERENCES
- 1.Stamm WE, Norrby SR. 2001. Urinary tract infections: disease panorama and challenges. J Infect Dis 183(Suppl 1):S1–S4. 10.1086/318850. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 3.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284. 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz WA. 2011. Uropathogenic bacteria leave a mark. Lab Invest 91:816–818. 10.1038/labinvest.2011.51. [DOI] [PubMed] [Google Scholar]
- 5.Kearns DB. 2010. A field guide to bacterial swarming motility. Nat Rev Microbiol 8:634–644. 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrichsen J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol Rev 36:478–503. 10.1128/BR.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partridge JD, Harshey RM. 2013. Swarming: flexible roaming plans. J Bacteriol 195:909–918. 10.1128/JB.02063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harshey RM, Matsuyama T. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc Natl Acad Sci U S A 91:8631–8635. 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison C, Lai HC, Hughes C. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol 6:1583–1591. 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 10.Ammendola A, Geisenberger O, Andersen JB, Givskov M, Schleifer KH, Eberl L. 1998. Serratia liquefaciens swarm cells exhibit enhanced resistance to predation by Tetrahymena sp. FEMS Microbiol Lett 164:69–75. 10.1111/j.1574-6968.1998.tb13069.x. [DOI] [PubMed] [Google Scholar]
- 11.Lai S, Tremblay J, Deziel E. 2009. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ Microbiol 11:126–136. 10.1111/j.1462-2920.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Killam T, Sood V, Surette MG. 2003. Swarm-cell differentiation in Salmonella enterica serovar Typhimurium results in elevated resistance to multiple antibiotics. J Bacteriol 185:3111–3117. 10.1128/jb.185.10.3111-3117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K. 2007. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J Bacteriol 189:950–957. 10.1128/JB.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane MC, Simms AN, Mobley HL. 2007. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J Bacteriol 189:5523–5533. 10.1128/JB.00434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini S, Slauch JM, Aldridge PD, Rao CV. 2010. Role of cross talk in regulating the dynamic expression of the flagellar Salmonella pathogenicity island 1 and type 1 fimbrial genes. J Bacteriol 192:5767–5777. 10.1128/JB.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambagaspitiye S, Sudarshan S, Hogins J, McDill P, De Nisco NJ, Zimmern PE, Reitzer L. 2019. Fimbriae and flagella mediated surface motility and the effect of glucose on nonpathogenic and uropathogenic Escherichia coli. bioRxiv 10.1101/840991. [DOI]
- 17.Kurihara S, Suzuki H, Oshida M, Benno Y. 2011. A novel putrescine importer required for type 1 pili-driven surface motility induced by extracellular putrescine in Escherichia coli K-12. J Biol Chem 286:10185–10192. 10.1074/jbc.M110.176032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson BN, Ding AM, Nilsson LM, Kusuma K, Tchesnokova V, Vogel V, Sokurenko EV, Thomas WE. 2007. Weak rolling adhesion enhances bacterial surface colonization. J Bacteriol 189:1794–1802. 10.1128/JB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas WE, Nilsson LM, Forero M, Sokurenko EV, Vogel V. 2004. Shear-dependent ‘stick-and-roll’ adhesion of type 1 fimbriated Escherichia coli. Mol Microbiol 53:1545–1557. 10.1111/j.1365-2958.2004.04226.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiles TJ, Kulesus RR, Mulvey MA. 2008. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol 85:11–19. 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reitzer L, Zimmern P. 2020. Rapid growth and metabolism of uropathogenic Escherichia coli in relation to urine composition. Clin Microbiol Rev 33:e00101-19. 10.1128/CMR.00101-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savageau MA. 1983. Escherichia coli habitats, cell types, and molecular mechanisms of gene control. Am Nat 122:732–744. 10.1086/284168. [DOI] [Google Scholar]
- 23.Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, Conway T. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci U S A 101:7427–7432. 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, Leatham MP, Lins JJ, Allen RL, Laux DC, Cohen PS, Conway T. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun 76:1143–1152. 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi Z, MacLennan G, Childs SJ, Zimmern PE. 2019. Is trigonitis a neglected, imprecise, misunderstood, or forgotten diagnosis? Low Urin Tract Symptoms 11:182–188. 10.1111/luts.12264. [DOI] [PubMed] [Google Scholar]
- 26.Bacheller CD, Bernstein JM. 1997. Urinary tract infections. Med Clin North Am 81:719–730. 10.1016/s0025-7125(05)70542-3. [DOI] [PubMed] [Google Scholar]
- 27.Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A 104:16669–16674. 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright KJ, Seed PC, Hultgren SJ. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun 73:7657–7668. 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauprich O, Matsushita M, Weijer CJ, Siegert F, Esipov SE, Shapiro JA. 1996. Periodic phenomena in Proteus mirabilis swarm colony development. J Bacteriol 178:6525–6538. 10.1128/jb.178.22.6525-6538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo SW, Kim D, Latif H, O’Brien EJ, Szubin R, Palsson BO. 2014. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat Commun 5:4910. 10.1038/ncomms5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turnbull AL, Surette MG. 2008. l-Cysteine is required for induced antibiotic resistance in actively swarming Salmonella enterica serovar Typhimurium. Microbiology (Reading) 154:3410–3419. 10.1099/mic.0.2008/020347-0. [DOI] [PubMed] [Google Scholar]
- 32.Oguri T, Schneider B, Reitzer L. 2012. Cysteine catabolism and cysteine desulfhydrase (CdsH/STM0458) in Salmonella enterica serovar Typhimurium. J Bacteriol 194:4366–4376. 10.1128/JB.00729-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker CS, Pruss BM, Matsumura P. 2004. Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J Bacteriol 186:7529–7537. 10.1128/JB.186.22.7529-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokota T, Gots JS. 1970. Requirement of adenosine 3′,5′-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol 103:513–516. 10.1128/JB.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp PD, Ong WK, Paley S, Billington R, Caspi R, Fulcher C, Kothari A, Krummenacker M, Latendresse M, Midford PE, Subhraveti P, Gama-Castro S, Muniz-Rascado L, Bonavides-Martinez C, Santos-Zavaleta A, Mackie A, Collado-Vides J, Keseler IM, Paulsen I. 12 November 2018. The EcoCyc database. EcoSal Plus 2018. 10.1128/ecosalplus.ESP-0006-2018. [DOI] [PMC free article] [PubMed]
- 36.Pastan I, Perlman R. 1970. Cyclic adenosine monophosphate in bacteria. Science 169:339–344. 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- 37.Schwan WR. 2011. Regulation of fim genes in uropathogenic Escherichia coli. World J Clin Infect Dis 1:17–25. 10.5495/wjcid.v1.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reitzer L. 25 July 2005. Catabolism of amino acids and related compounds. EcoSal Plus 2005. 10.1128/ecosalplus.3.4.7. [DOI] [PubMed]
- 39.Hospenthal MK, Waksman G. 2019. The remarkable biomechanical properties of the type 1 chaperone-usher pilus: a structural and molecular perspective. Microbiol Spectr 7:PSIB-0010-2018. 10.1128/microbiolspec.PSIB-0010-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim W, Surette MG. 2004. Metabolic differentiation in actively swarming Salmonella. Mol Microbiol 54:702–714. 10.1111/j.1365-2958.2004.04295.x. [DOI] [PubMed] [Google Scholar]
- 41.Henderson PJ, Giddens RA, Jones-Mortimer MC. 1977. Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem J 162:309–320. 10.1042/bj1620309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alteri CJ, Smith SN, Mobley HL. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog 5:e1000448. 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conover MS, Hadjifrangiskou M, Palermo JJ, Hibbing ME, Dodson KW, Hultgren SJ. 2016. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 7:e00104-16. 10.1128/mBio.00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Jehanne V, Pichon C, du Merle L, Poupel O, Cayet N, Bouchier C, Le Bouguenec C. 2012. Role of the Vpe carbohydrate permease in Escherichia coli urovirulence and fitness in vivo. Infect Immun 80:2655–2666. 10.1128/IAI.00457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 47.Fahrner KA, Berg HC. 2015. Mutations that stimulate flhDC expression in Escherichia coli K-12. J Bacteriol 197:3087–3096. 10.1128/JB.00455-15. [DOI] [PMC free article] [PubMed] [Google Scholar]