Abstract
COVID-19 is a respiratory infection that has been declared as a global health crisis by the WHO. It mainly affects the respiratory system. Apart from respiratory system, it also affects other organs as well including the brain. Numerous emerging reports have demonstrated that the COVID-19 has detrimental effects on neurological functions, and can lead to severe impairment of the central nervous system (CNS). The neurological manifestations linked with COVID-19 include headache, anosmia, encephalitis, epileptic seizures, Guillain-Barre syndrome, stroke and intracerebral hemorrhage alongwith multiple others complications. The CNS related complications may be severe and are linked with poor diagnosis which may worsen the condition. Therefore, there is a need to precisely understand the neurological sequelae along with upcoming clinical outcomes. Here, we present a brief review of the neurological complications and symptoms associated with COVID-19 along with brain imaging findings. Further, we have discussed about the emerging biosensing approaches which may aid in rapid, precise and mass diagnosis of COVID-19.
Keywords: COVID-19, SARS-CoV-2, Neurological complications, Biosensors, Diagnostic imaging
1. Introduction
By the end of year 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a novel pathogenic virus causing severe pulmonary infection affecting millions of people. It has killed more than three million people worldwide till April 28, 2021. In a short duration, it spread globally, evolved as a pandemic and was declared as a communal health emergency by the World Health Organization (WHO). In view of this pandemic, the infection caused by the SARS-CoV-2, the WHO termed it as Corona Virus Disease 2019 (COVID-19). COVID-19 has created global angst because of its novelty, communicability, and rapid mutation rate. Coronaviruses belong to the Torovirinae subfamily that combined with Coronavirinae falls under the Coronaviridae family of order Nidovirales [[1], [2], [3], [4], [5], [6]]. At present, a total of seven different human coronaviruses have been reported namely, 229E, HKU1, NL63, OC43, middle east respiratory syndrome (MERS)-CoV, SARS-CoV and SARS-CoV-2 (COVID-19). Among these seven different strains, 2019-nCoV, SARS-CoV and MERS-CoV evinced to be extremely pathogenic. Before SARS-CoV and MERS-CoV outburst, CoV was considered to seed milder disease. However, these two outbursts spotlighted their potential to cause serious infections and thus, they were distinguished under emerging viruses. The COVID-19 is considered as the most fatal one among all the coronaviruses [7,96].
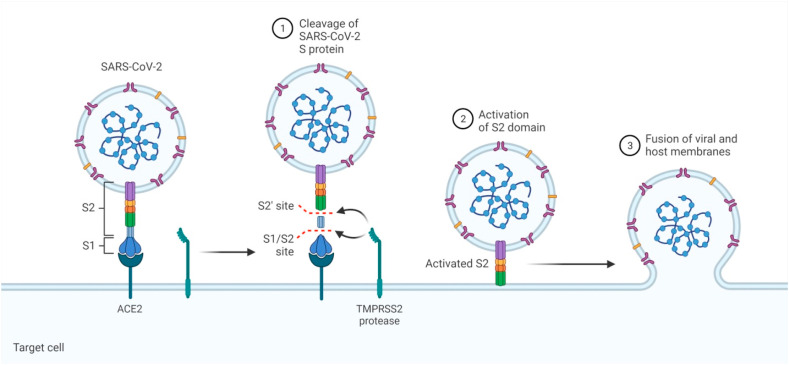
Coronaviruses are spherical, pleiomorphic or enveloped viruses which have a single-stranded, positive sense RNA genome size ranging from 26.2 to 31.7 kb. The RNA genome of SARS-CoV-2 is one of the biggest among all the RNA viruses. They contain six to ten open reading frames (ORFs) in their genome. The viral genome contains four different proteins encoding Viz. spike (S), nucleocapsid (N), membrane (M), and envelope (E) proteins and several non-structural proteins as well [8]. The genome of CoV is receptive for recurrent recombination evolving into new strains with revision in virulence [9]. COVID-19 is distinguished by severe clinical exhibition of the lower respiratory tract infection that includes common cold, pneumonia, rhinitis, bronchiolitis, pharyngitis, and sinusitis along with other indications such as vomiting, infrequent watery diarrhea etc. [10,97]. The epithelial cells of the respiratory tract are preyed by the coronavirus, evolving into diffused alveolar damage. It is mainly transmitted via droplets produced while sneezing, coughing and close individual-to-individual contact [11,12]. Upon entering into the host, the spike protein S1 of SARS-CoV-2 attaches to the cellular receptor angiotensin-converting enzyme 2 (ACE2). The attachment is favored by the spike receptor-binding domain (RBD) which allows direct connection with ACE2, and a S1/S2 cleavage site that is cleaved by the transmembrane protease serine 2 (TMPRSS2) as shown in Fig. 1 . This mediates the entry of the virus through surface of the plasma membrane which leads to arrival of the RNA genome of the virus inside the host cell followed by the translation of structural and non-structural proteins. The polyproteins pp1a and pp1ab are produced from the translation of ORF1a and ORF1ab, which are then cleaved to form 16 non-structural proteins. It is accompanied by grouping and enters into the lumen of the ERGIC (Endoplasmic Reticulum Golgi Intermediate Compartment) and finally via exocytosis the virions are released from the infected cell [13].
Fig. 1.
Mechanism of COVID-19 entry inside the host cell: The spike protein of SARS-CoV-2 attaches to the cellular receptor angiotensin-converting enzyme 2 (ACE2) of the host cell. The spike protein has two subunits namely the S1 subunit that regulates ACE2 attachment while other is the S2 subunit that contains the fusion peptide and transmembrane domains that allows the fusion between virus and the target cell membranes. For activation spike protein must be cleaved by the transmembrane protease serine 2 (TMPRSS2) at S1/S2 cleavage site. This mediates entry of the virus and fusion with the surface of the host plasma membrane. The figure was created with BioRender.com.
2. Neurological context of SARS-CoV-2 entry to host cells
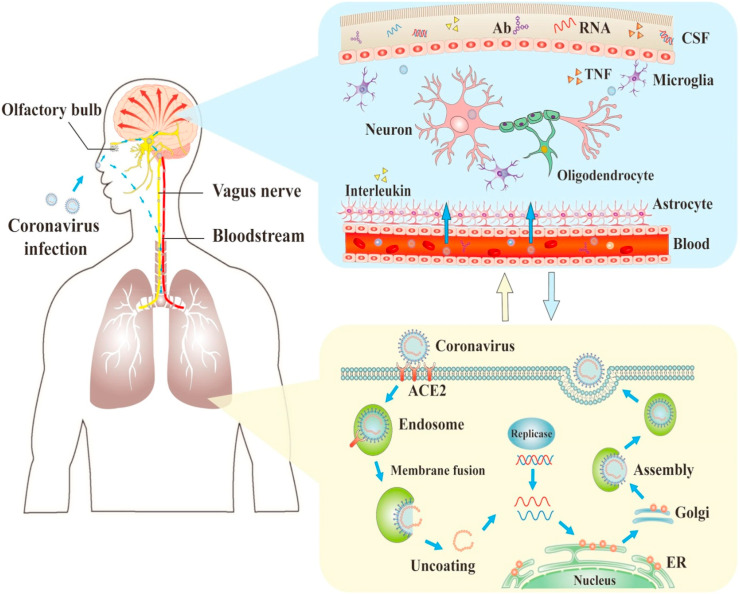
The SARS-CoV-2 is basically considered to cause infection in the respiratory tract. However, it has also been linked to neurological complications as well. Various studies have proposed the olfactory-haematogenous pathway, circulatory system, trans-neuronal machinery, lymphatic pathway as alleged pathways of coronavirus invasion into the central nervous system (CNS). Since the SARS-CoV-2 is primarily present in the respiratory tract, the olfactory nerves may be employed as a prominent route for the virus as they travel alongside axonal neurons of olfactory receptor to enter into the CNS. Once the virus reaches the nasal airways, SARS-CoV-2 can go across the basolateral surfaces of epithelial cells and enter into the blood circulation leading to infection of the CNS and other tissues as shown in Fig. 2, Fig. 3 . In circulation, SARS-CoV-2 may cause infection either in the epithelial cells of the blood-cerebrospinal fluid (CSF) barrier present in the brain ventricle or the endothelial cells of the blood-brain-barrier (BBB), or white blood cells may be employed as a vector for propagation towards the CNS. Thus, it is feasible that SARS-CoV-2 can be neuroinvasive through circulatory or respiratory haematogenous pathways [14,15]. A potential neuroinvasion route could be trans-neuronal retrograde machinery where the virus causes infection in the peripheral neurons to enter the CNS through the retrograde axonal transport. Evidence indicates the capability of SARS-CoV-2 to take over the peripheral terminals of the nerves and then through synapses achieve entry into the CNS. Apart from all these routes, the lymphatic system can also be a probable route for neuroinvasion, though exact mechanism is not known yet [16].
Fig. 2.
The process of infections and nervous system damage by COVID-19. The COVID-19 can damage the nerve via direct infection pathways, hypoxia, angiotensin converting enzyme 2 (ACE2), immune injury, and other mechanisms. Moreover, it can penetrate into the nervous system via the olfactory nerve, and also invade by neuronal pathways and blood circulation leading to neurological disorders. The figure has been reproduced with the permission from reference number [26].
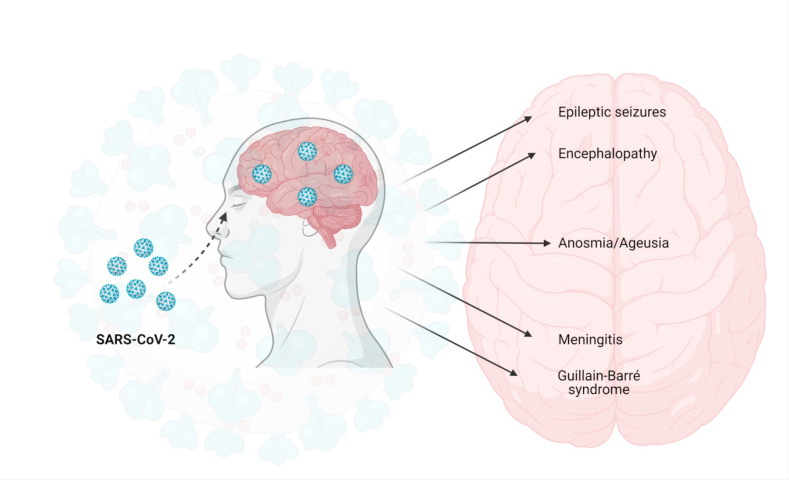
Fig. 3.
Neurological manifestations of COVID-19: The SARS-CoV-2 enters through the olfactory epithelium by utilizing the angiotensin converting enzyme 2 (ACE2) receptor. After cell entry, the virus replicates and reaches to the central nervous system (CNS) leading to various neurological manifestations such as encephalopathy, epileptic seizures, anosmia/ageusia, meningitis, Gullian-Barre syndrome etc. The figure was created with BioRender.com.
3. Neurological manifestations of COVID-19
There is strong evidence that SARS-CoV-2 infection in brain can cause several neurological disorders and alterations ranging from nonspecific to moderate to severe conditions. Systemic neurological and nonspecific symptoms include headache, dizziness, fatigue and myalgia that range from 30 to 45% in COVID-19 patients. Moderate symptoms include anosmia or hyposmia, disturbance in taste, smell and visual dysfunctioning. Paralysis, epilepsy and consciousness disorders have also been reported. Severe symptoms include cerebrovascular events like intracerebral hemorrhage, cerebral venous thrombosis and ischemic stroke. While long term consequence of infection can lead to demyelinating and neurodegenerative disorders. Therefore, the understanding of neuroinvasive mechanisms and related interactions is crucial to understand the potential pathological outcomes and to design new pharmacological interventions and diagnostic strategies for the management of COVID-19 [17,18]. Neurological complications of COVID-19 appear to be both diverse and infrequent in behavior. The neurological manifestations may be the result of metabolic abnormalities activated by the infection, autoimmune response against virus or consequence of direct effect of the virus on central or peripheral nervous system [19]. In following subsections, we provide brief summary of some of the important neurological manifestations linked with COVID-19.
3.1. Anosmia & ageusia
Loss of smell (anosmia) and loss of taste (ageusia) are typical signs of COVID-19. Disabilities in olfactory and gustatory functions of COVID-19 patients are possibly due to the virus mediated damage to the epithelial cells of the oral and nasal mucosa. A large number of ACE2 receptors are expressed in nasopharynx, oral mucosa, and olfactory epithelial cells. When the virus attaches to the ACE2 receptor in oral and nasal mucosa, it inhibits the activity of sensory receptor cells that are responsible for gustation and olfaction [19,20]. It is reported that the SARS-CoV-2 penetrates along the olfactory bulb and eventually results in fast, transneuronal outspread of the virus to attached areas of the brain as well (Fig. 2). Hence, the invasion and multiplication of SARS-CoV-2 at early stages of the infection results in damage to the olfactory nerves and therefore, explains the mechanism behind anosmia [21]. While in the case of ageusia as well, ACE2 receptors are present in large number on the epithelial cells of the oral mucosa. When SARS-CoV-2 binds to the receptor on oral epithelial cells, it results in the dysfunction of oral cavity causing ageusia in the initial stages of the viral infection. A study of 68 COVID-19 patients displayed that abnormal taste and smell are resolutely associated with COVID-19 [22]. In a European study of 417 patients, loss of smell was reported in 86% of the COVID-19 patients while oral dysfunctioning was seen in 82% of the patients. However, a remarkable variation is seen in COVID-19 patients of Asia and Europe in terms of taste and smell perception. A study in China revealed that there were only 5% of the COVID-19 patients suffering from taste and smell impairments. This is in contrast to the European study of COVID-19 patients where the frequency of olfactory and gustatory dysfunction was found to be 33.9–88.0%. This difference may be due to the ACE2 polymorphism or due to the difference in allele frequency of ACE2 receptor that results in variation in outcomes and symptoms of COVID-19. In a nutshell, anosmia and ageusia are the most usual neurological symptoms of COVID-19 and the subjects showing these symptoms should be screened for COVID-19 especially during this pandemic [23,24].
3.2. Encephalopathy
Brain encephalopathy is a pathobiological activity which progresses from hours to days resulting in altered personality, cognition, consciousness (alongwith clinical presentations of coma or delirium) and behavioral changes. In a retrospective case study of 214 COVID-19 patients in China, 25% (53) of the patients had CNS associated symptoms like dizziness in 17% (16) patients, impaired consciousness in 7% (16) patients and headache in 13% (28). In a French study of critical patients with COVID-19 infection, 84% had neurological symptoms that included encephalopathy and corticospinal associated symptoms [23]. Three more case studies of COVID-19 encephalopathy have been reported, one each from Iran, Iraq and the USA spanning within an age group from 30 to 75 years. In all the three cases cough and fever were the initial symptoms accompanied by onset of asthenia and confusion. Magnetic resonance imaging (MRI) and computerized tomography (CT) scanning of brain were found to be unremarkable. Though, in Italy and USA electroencephalogram (EEG) was employed for the confirmation of encephalopathy [25].
3.3. Infectious toxic encephalopathy
Infectious toxic encephalopathy, which is also called as acute toxic encephalitis, is a kind of brain reversible dysfunction syndrome that happens due to factors like hypoxia, systemic toxaemia and metabolic disorders during the course of acute infection. The major pathological alterations include edema which upon CSF analysis showed no evidence of inflammation. Patients with COVID-19 infection exhibit diverse and complex range of symptoms. Initially mild symptoms may arise such as dysphoria, headache and delirium [26,27]. In severe cases, patients may encounter loss of consciousness, disorientation, coma and paralysis. COVID-19 patients often experience severe viremia and hypoxia that can further lead to toxic encephalopathy [26]. Furthermore, 40% of the COVID-19 patients suffer with brain dysfunction symptoms including edema in the brain tissues in severe cases. Collectively, these studies indicate that COVID-19 may lead to mild to severe infectious toxic encephalopathy [28].
The communication between the nervous system and the immune system has an ancient evolutionary origin as it has been believed that every physiological action including immune reactions are under the control of brain. It is evident that immune system and neuronal system cross-talk and both are employed in immune response [98,99]. This signaling is mediated by various molecules such as opioid peptides like enkephalin and endorphins. These neuropeptides were originally found in the CNS, and were later identified in immune cells and possess immunomodulatory properties [29]. Endorphins and enkephalins can have impact on various immunological activities like antibody synthesis, lymphocyte proliferation, and natural killer cell mediated cytotoxicity. They are physiological regulators of the immunological response as well as act as humoral mediators between the immune system and the CNS [30]. They can act as immunomodulators and enhancers of the biological response [31]. It has been reported that opioid peptides have role in immune modulation. Enkephalin can vitalize the release of pro-inflammatory cytokines like interleukin (IL)-6 [32]. Moreover, it influences the intracellular signal transduction in T-cells and is involved in humoral immunity resulting in antibody production. Kowalski et al., reported that enkephalin promotes proliferation of B and T cell and can enhance the population of T helper and T cytotoxic cells [33]. The presence of these opioid peptides mRNAs has been found in different cells like the expression of enkephalin was identified in monocytes and T lymphocytes, in fetal thymocytes alongwith B and T lymphocytes. It has been previously reported that they also exert anti-inflammatory action as in the inflamed tissue, the delivery of endorphins via lymphocyte trafficking system would increase the analgesic and anti-inflammatory effect [29]. Gilmore et al., reported that endorphins plays protective role against neurotropic murine coronavirus, MHV-JHM (JHMV). The virus can cause paralytic-demyelinating disease and encephalitis in susceptible strains of rats and mice. A single dose of beta-endorphin decreased the occurrence of viral-stimulated paralytic-demyelinating disease by 40–50% followed by remarkable decrease in viral replication in the brain [34]. Hence, it can be concluded from the discussion that endorphins and enkephalins could be beneficial in boosting immunity in COVID-19 as they possess immunomodulatory properties. However, further extensive studies need to be done for better understanding.
3.4. Seizures
Seizures may arise as an outcome of acute systemic sickness, adverse effect of medication or as a primary neurological pathology in COVID-19 patients and can result in a broad range of symptoms from subtle twitching, convulsive activity to lethargy. In severe cases, untreated seizures can rapidly accelerate towards the convulsive status epilepticus and more commonly non-convulsive status epilepticus that are related with high morbidity and mortality. Seizures are generally uncommon neurological symptom in COVID-19 patients [35,36]. Mao and coworkers revealed that out of 214 critical COVID-19 patients, only one patient was recorded with acute symptomatic seizures. While in some of the case studies triggering of new onset seizures by COVID-19 infection was reported [37]. Numerous studies have reported seizures in children having COVID-19 infection. Consistent seizures with paroxysmal episodes were described in 2 infants with virus on nasopharyngeal swab but no respiratory symptoms [38]. In a case study of 168 hospitalized children with COVID-19 infection, seizures were reported in 3% of the children in which one had preexisting febrile seizures and the other three had previous epilepsy. Vollono and coworkers reported incidence of non-convulsive status epilepticus in an elderly patient after COVID-19 infection [39].
3.5. Inflammation & cytokine storm
COVID-19 infection can escort to severe inflammation together with cytokine storm that results into thrombosis or COVID-19 associated coagulopathy (CAC). COVID-19 can attach to the toll like receptors (TLR) that results in the secretion and release of IL-1. Receptor activation triggered a series of action starting with the synthesis of pro IL-1 that results in inflammation activation. In COVID-19 infection or in any other viral infection, type I interferon is released that can lead to inflammation and suppression of the immune system [40]. A remarkable difference in the concentration of IL-6 among COVID-19 non-effectors and effectors has been remarked where the concentration of IL-6 is 1.7 times greater in non-survivors in comparison to that of survivors. Additionally, the neuro-inflammation is characterized by cytokine storm mainly due to IL-6. This results in a rush of cytokine storm that includes IL-2, IL-7, monocyte chemoattractant protein 1 (MCP1), interferon-γ, macrophage inflammatory protein 1-α, inducible protein 10 and tumor necrosis factor-α (TNF-α) leading to hyper-inflammation. This cascade of systemic inflammation can lead to severe encephalopathy that may even cause stroke. Hence, blocking IL-6 and inflammation is the main focus for current clinical trials that have employed IL-6 receptor blocking agent like tocilizumab where increasing levels of IL-6 is firmly linked with the requirement of mechanical ventilation [41]. While in extreme situations, remarkably augmented levels of pro-inflammatory cytokines, elevated levels of neutrophils, and lymphopenia have been observed. Cytokine storm is linked with increased vascular hyper-permeability, multisystem dysfunction and coagulopathies. Around 5% of the patients with COVID-19 infection develop multiple organ dysfunctions, acute respiratory distress syndrome (ARDS) and septic shock. Cytokine storm is possibly the reason for multiple pathological complications of COVID-19 infection [42,43].
3.6. Smoking
Smoking has been presumed to be undoubtedly linked with adverse prognosis of disease, as numerous evidences have spotlighted the adverse effects of tobacco on lungs and its usual association with a number of respiratory diseases including COVID-19. Moreover, smoking is injurious to the immune system and its receptivity to infections makes smokers more susceptible to infections. Numerous studies have displayed that the mortality rate of smokers due to COVID-19 is twice more likely than the non-smokers. Further, smokers were also noted to be more vulnerable to respiratory diseases with severe symptoms as compared to that of non-smokers. Additionally, the probability of COVID-19 patients to meet neurological manifestations can be accelerated by smoking and alcoholism. Smoking can increase the chances of COVID-19 patients to show neurological symptoms due to the functional interaction among the nicotinic receptor and human ACE2. A functional link is developed due to co-expression of nicotinic acetylcholine receptor and ACE2. Hence, while smoking due to stimulation of acetylcholine receptor by nicotine, the expression of ACE2 is augmented and thus, more prone to infection [[44], [45], [46]]. Several studies have been done to analyze the linkage between COVID-19 and smoking. Zhou et al., conducted a study on 191 COVID-19 patients, where 137 survived and 54 deaths were reported. Among the dead patients, 19% were smokers in comparison to that of 4% amid those survived [47]. Similarly, Zhang and coworkers studied clinical symptoms of 140 individuals having COVID-19 infection. The study revealed that amid severe patients, 6.9% were previous smokers, 3.4% were present smokers in comparison to that of non-severe patients where 3.7% were previous smokers and no case of current smokers [48]. A wide population study of 1099 COVID-19 was presented by Guan et al., in China. Out of 1099 patients, 173 had serious symptoms and 926 had casual symptoms. Among the severe patients, 5.2% were previous smokers while 16.9% were present smokers, in comparison to non-severe patients where 1.3% were previous smokers and 11.8% were present smokers. Moreover, the patients that either required critical care, mechanical ventilation or died in which 7.6% were previous smokers and 25.5% were present smokers [49]. It is worth mentioning that behavior of smoking is associated with inhalation and repeated hand to mouth act that is firmly advised to be avoided for reducing COVID-19 contamination.
3.7. Guillain-Barré Syndrome
Guillain-Barré Syndrome (GBS) has been reported as a notable parainfectious neurological sequelae of COVID-19 [50]. It arises when the immune system injures and attacks its own nerves that are present in the exterior region of spinal cord or brain and injures myelin sheath [51]. The syndrome is associated with a state of acute flaccid paralysis that generally presents progressive symmetric sickness [52]. Generally, GBS is triggered by campylobacteriosis and viral infections like Epstein-Barr virus (EBV), human immunodeficiency virus (HIV) and Zika virus [53]. As it is evident that COVID-19 infection has an effect over the nervous system and the occurrence of GBS with SARS-CoV-2 is not surprising as infection by other viruses have also been shown in the etiology of GBS [54]. The linkage of COVID-19 with GBS is a temporal association that shows a post-infective immune-mediated process. The reason behind this association is an autoimmune reaction in which antibodies are produced against the surface glycoprotein that shares molecular similarity with peripheral nerve components resulting in neurological complications. There may be immune response associated injury in GBS patients having COVID-19 because after infection the concentration of inflammatory cytokines like IL-6, activities of macrophages, T-lymphocytes and endothelial cells are increased resulting in complement activation, coagulation, vascular leakage and eventually organ damage [55].
Previous studies have shown involvement of other coronaviruses with neurological disease such as in a retrospective study of MERS-CoV, a patient suspected with GBS was found to be overlapping with Bickerstaff's encephalitis [50,56]. GBS associated with COVID-19 patients showed normal MRI data of the CNS but an increase in post-contrast has been reported in lumbar and cervical nerve roots [56]. It generally develops from several days to weeks of infection [57]. Caress et al., reviewed 37 cases of COVID-19 associated GBS, where brain imaging results of 28% patients showed cranial nerve abnormalities. Moreover, spinal imaging displayed abnormal features in 40% patients such as myelopathy, root enhancement and leptomeningeal enhancement [50]. One more study was conducted by Abdullahi et al., on 16 patients where COVID-19 was validated with RT-PCR while GBS was diagnosed with clinical testing such as muscle strength testing. They conducted CSF analysis and neurophysiological examination, each of them revealed abnormalities consistent with GBS diagnosis criteria [55]. Virani et al., also reported a case having GBS linked with COVID-19 showing complaints of weakness and numbness in lower extremities for 2 days. Further, the progression of weakness to high fever and diarrhea was diagnosed [58]. A team of doctors in Italy performed a study on five patients and observed that the interval from the onset of COVID-19 symptoms and the arousal of initial symptoms of GBS ranges from 5 to 10 days. They reported that patients develop lower-limb weakness paresthesia, facial diplegia accompanied by flaccid tetraparesis and ataxia, as first symptom of GBS linked with COVID-19. Though no patient in the study showed dysautonomic features. MRI showed increased signal changes of the caudal nerve roots, facial nerve; CSF analysis revealed a decreased white-cell count and anti-ganglioside antibodies; electrophysiological studies data showed low amplitude of compound muscle action potential, progressive motor distal latencies and fibrillation potentials were present during electromyography [57]. Treatment of this association includes exchange of plasma, intravenous immunoglobulins, supportive care and regular monitoring. Therefore, patients showing GBS should be tested for COVID-19 for early evaluation and treatment.
4. Neurological imaging
4.1. Radiological findings
The reports of neurological complications are growing as the COVID-19 pandemic progresses. These lesions can be due to direct attack of the virus on the nervous system, post-infectious or para-infectious immune-associated disease, and systemic effects causing neurological manifestations [59,60]. The findings and outcomes of brain radioimaging in COVID-19 patients associated with neurological manifestations offer a valuable opportunity to understand the pathogenesis and helps in the early diagnosis. Similarity with these reported radiological patterns will help neurologists and radiologists to understand the progressiveness of the virus. Various brain imaging patterns of COVID-19 patients having neurological complications have been reported. Katal et al., reported a case of meningitis linked with COVID-19 where the patient had fever, fatigue, sore throat and headache. After few days the patient experienced seizures and lost consciousness. Brain imaging was done that displayed hippocampus and signal abnormality in the right mesial temporal lobe hyperintense in fluid-attenuated inversion recovery (FLAIR) images; diffusion-weighted imaging (DWI) predicted periventricular diffusion restriction with the temporal horn of the right lateral ventricle while contrast-enhanced imaging displayed no specific abnormal intracranial elevation. All these imaging results revealed encephalitis and right lateral ventriculitis [61]. Similarly, a new study was conducted on 73 patients which showed neurological manifestations, 41.1% displayed normal brain MRI reports. While 58.9% patients showed abnormalities in neuroimaging such as perfusion abnormalities (47.7%), acute ischemic infarcts (23.3%), multiple microhemorrhages (11.3%), multifocal white matter lesions (5.5%), restricted diffusion foci of the corpus callosum (CLOCC, 4.1%), hypoxia-induced lesions (4.1%), basal ganglia lesions (5.5%), meningeal enhancement (4.8%), neuritis (2.7%) and posterior reversible encephalopathy syndrome (PRES) (2.7%), central pontine myelinolysis (4.1%), cerebral venous thrombosis (1.4%). Studies have also mentioned about the imaging findings of basal ganglia abnormalities and multifocal white matter lesions [62]. Furthermore, one more study was conducted by Kermer et al., on 37 patients where white matter deformities were explained in three patterns i.e., white matter microhemorrhages (24%), multifocal white matter hyperintense lesions on FLAIR (30%) and signal abnormalities in the medial temporal lobe (43%). It was concluded that abnormalities in medial temporal lobe signal were similar to as that seen in autoimmune or viral encephalitis while micro-hemorrhages displayed multiple patterns. The study also revealed that the prognosis would worsen in these patients due to the presence of hemorrhage [63].
4.2. Positron emission tomography (PET) of brain
Nuclear medicine operations are not regularly utilized in COVID-19 patients [64]. Though, this is believed that fluorodeoxyglucose-positron emission tomography (FDG-PET-CT) based molecular imaging is capable to give relevant data on the pathophysiological condition of the disease [65]. In COVID-19 patients with neurological manifestations, fewer cases have explained metabolic abnormalities. Delorme et al., performed a case series where FDG-PET-CT imaging was done in 4 COVID-19 patients showing encephalitis symptoms [66]. In all the patients PET data reflected a definite pattern of metabolic abnormalities including hypermetabolism in the cerebellar vermis and hypometabolism in the orbitofrontal or prefrontal cortices. None of these patients showed specific abnormalities related to CSF on MRI reports. The study concluded that the mechanism of infection is either immune-mediated or parainfectious cytokine storm rather than neuroinvasion directly [61]. Grimaldi et al., performed brain FDG-PET and observed cerebellum hypermetabolism alongwith diffused cortical hypometabolism in COVID-19 patients [67]. Likewise, one more study was done in which anosmia of COVID-19 was examined by utilizing FDG-PET-CT and revealed hypometabolism of the left orbitofrontal cortex in association with anosmia [68]. These studies along with MRI suggested decreased neuronal activities with functional alterations in COVID-19 patients having neurological symptoms.
5. Biosensors for COVID-19
A significant number of virus or viral particle detection methods are available but they are associated with certain limitations which make their practical utilization difficult [69]. The major limitations include low sensitivity, less accuracy, time taking, difficulty in sample preparation and purification, high maintenance cost, accessories and complex operational mechanics, not suitable for mass data analysis and on site rapid detection [70]. Therefore, to overcome the limitations of traditional viral detection methods, there is a need of rapid and efficient methods that can ensure easy handling, accuracy and large scale availability, and takes versatility of virus into consideration [71]. In the following subsections we have discussed about various kind of biosensors for the fast diagnosis of SARS-CoV-2 which can be applied for mass population to control the spread of COVID-19.
5.1. Nano-biosensors for COVID-19
Currently, nanoparticles (NPs) are gaining huge interest in viral detection because of their fast sensing and biological activity. Recent advancements in nanotechnology offer the utilization of nanomaterials for the development of biosensors which results into significant upgradation in the performance of these devices [[72], [73], [74], [75],100]. Nanomaterials are known to enhance the efficiency and sensitivity of biosensors due to their good conductance, excellent photoelectrochemical features and the miniaturization platform [76,77,101]. Nano-biosensors are crucial tools for specific and fast detection. A sensor was developed by coupling clustered regularly interspaced short palindromic repeats (CRISPR)-Chip with a graphene-based field effect transistor (FET) [78]. This coupled biosensor does not need amplification and detects DNA and RNA at very low concentrations with swift response. This technique uses the coating of FET graphene sheets with monoclonal antibody for COVID-19 surface spike protein [79]. Similarly, various electrochemical reactions based nano-biosensors are being developed for viral detection. Mahari et al., developed an in-house biosensor device (eCovSens) coated with fluorine doped tin oxide (FTO) electrode along with gold nanoparticles (AuNPs) and nCOVID-19 antibody against COVID-19 spike protein [80]. Recently, optical nano-biosensors based on localized surface plasmon resonance (LSPR) phenomena were developed for COVID-19 detection. This sensor can quantify the concentration of virus in real time [81]. Similarly, there are other nano-biosensors which are also present for the detection of virus and are divided into various categories like chemical, electrical, optical, electrochemical, piezoelectric, magnetic thermal, and biological detection on the basis of detection strategy. Table 1 enlists nano-biosensor based detection in COVID-19 infection.
Table 1.
Nano-biosensor based detection systems for COVID-19 infection.
| S. No |
Biological Sample |
Target |
Detection |
Nanomaterial |
References |
|---|---|---|---|---|---|
| Electrochemical Nano-biosensors | |||||
| 1. | Human nasopharyngeal swab samples, Cultured virus from COVID-19 patients | SARS-CoV-2 spike protein | Field-Effect Transistor (FET) | Graphene sheet | [82] |
| 2. | Green Monkey Kidney Cell Culture |
COVID-19 S1 Spike Protein Antigen |
Bioelectric Recognition Assay (BERA) | Membrane-Engineered Vero Cells (Vero/Anti-S1) | [83] |
| 3. | Spiked saliva samples | COVID-19 S1 Spike Protein Antigen |
Differential Pulse Voltammetry (DPV), Cyclic Voltammetry (CV) | Fluorine tin oxide (FTO) electrode with gold nanoparticle (AuNPs) | [80] |
| Optical Nano-biosensors | |||||
| 4. | Upper respiratory tract (URT) Samples/Respiratory secretions |
SARS-CoV-2 Nucleic acid | Plasmonic photo-thermal (PPT) and localized surface plasmon resonance (LSPR | Gold nanoislands functionalized (AuNIs) having complementary DNA receptors |
[81] |
| 5. | Clinical samples | RNA sample of COVID-19 | Fluorescent detection | [84] | |
| 6. | Isolated RNA samples | RNA sample of COVID-19 | Colorimetric assay | Gold nanoparticles | [85] |
| 7. | Blood samples procured from PCR tested COVID-19 positive patients (397) and negative patients (128). | COVID-19 IgG-IgM combined antibody | Colorimetric assay | Gold nanoparticle (AuNP) colloids | [86] |
| Magneto optical Nano-biosensors | |||||
| 8. | COVID-19 pseudovirus in 200 μL of serum samples | Viral RNA extraction of COVID-19 | Fluorescence and convectional RT-PCR protocol | Poly (amino ester) having carboxyl groups (PC)-coated magnetic nanoparticle (pcMNPs) | [87] |
| 9. | Fetal bovine serum (FBS) | COVID-19 RdRp coding sequences |
Optomagnetic sensing | Magnetic nanoparticle (MNP) | [88] |
| Piezoelectric Nano-biosensors | |||||
| 10. | Oral swab samples | COVID-19 spike protein | Quartz crystal microbalance (QCM) | Nanoparticles | [89] |
5.2. Cell based biosensors
Cell-based biosensors (CBBs) are generally developed by immobilizing living cells onto the biosensor platform and they are capable to carry out real time bioassay swiftly and positively. Mavrikou et al., presented a cell based biosensor that can recognize the COVID-19 S1 spike protein which is present over the surface of the virus. The designing of the biosensor is done by engineering mammalian cell membrane carrying human chimeric S1 spike antibody. They showed the binding of spike protein with membrane bound antibodies which results in considerable and selective alterations in cellular bioelectric parameters calculated via bioelectric recognition assay. They presented this biosensor as ultra-rapid, ready to use and can be operated by smartphones [83]. These type of cell based biosensors do not need sample processing and can be used for mass screening of COVID-19 antigens. Therefore, CBBs offer a plausible solution for the detection of virus and eventually aid in controlling the global COVID-19 pandemic.
5.3. Digital wearable sensors
Currently, for the identification of COVID-19 a fairly good number of wearable sensors are in use. They are used in the form of watches, smartwatches and bio-harness. These digital sensors maintain a track on patient health and also trace the symptoms, infection and recovery [90]. As it is investigated that COVID-19 affects the upper respiratory system, these digital sensors monitor the respiration process in an individual in most effective way [91]. Additionally, these sensors can detect other symptoms of COVID-19 as well like cough, cold and fever [92]. Various advancements of wearable sensors are available in market such as Huami device which is utilized for indicating fever, cardiac rhythm and heart rate [93]. Fit bit watches detect heart rate along with other physiological health parameters. Smartphone uses a RADAR system for monitoring normal health conditions along with CNS alterations and multiple sclerosis. Temperature guns are used widely in communal areas to check body temperature [94]. Apart from health monitoring, these digital devices proved useful in following social distancing, and the inbuilt GPS helps in contact tracing. Countries like Germany are using these advanced digital sensor devices for collecting health data of their population to reduce health risks [95].
6. Conclusion
Our review presents the summary of neurological manifestations linked with COVID-19 and secondary factors such as smoking and alcoholism which augments the vulnerability of infection. Though the neurological manifestations are not the hallmark of early detection and treatment, and the fraction of patients having neurological manifestations is less in comparison to that of respiratory symptoms. However, the neurological manifestations are not unusual and cannot be neglected as they may proceed during the course of the disease and can lead to severe complications if not identified and managed early. Nevertheless, the progressing pandemic, and the assumption that 50–70% of the world's population may be infected before developing herd immunity, propose that the overall proportion of patients with neurological manifestations could become large. Patients with COVID-19 can exhibit a diverse scale of neurological manifestations which is because of peripheral and CNS injury via cytokine storm or direct damage by COVID-19. Neurological manifestations mainly encephalitis and hyper-inflammation by cytokine storm can cause lifelong inability associated with potentially economic, social and health costs. Therefore, patients with COVID-19 should be examined early for neurological symptoms like headache, anosmia, ageusia, consciousness inability and other pathological signs. Smoking is also most commonly linked with adverse progression and negative outcomes of the COVID-19 infection. The effective management of COVID-19 pandemic requires timely diagnosis and advanced imaging techniques like MRI and CT scanning are playing a vital role. Further, innovative sensor based products are emerging and offer novel means for effective prevention, diagnosis, and therapy of COVID-19.
Funding source
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sintl.2021.100098.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fehr A.R., Perlman S. In: Coronaviruses: Methods and Protocols. Maier H.J., Bickerton E., Britton P., editors. Springer New York; New York, NY: 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [Google Scholar]
- 2.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kundu A., et al. The COVID-19 paradox: impact on India and developed nations of the world. Sensors International. 2020;1:100026. doi: 10.1016/j.sintl.2020.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurana I., et al. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med. Hypotheses. 2021;149:110534. doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetti N.P., et al. Invasion of novel corona virus (COVID-19) in Indian territory. Sensors International. 2020;1:100012. doi: 10.1016/j.sintl.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S., et al. Current treatment protocol for COVID-19 in India. Sensors International. 2020;1:100013. doi: 10.1016/j.sintl.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. J. Am. Med. Assoc. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 8.Belouzard S., et al. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C.-k., et al. Recent insights into the development of therapeutics against coronavirus diseases by targeting N protein. Drug Discov. Today. 2016;21(4):562–572. doi: 10.1016/j.drudis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y., et al. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133(1):4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prajapat M., et al. Drug targets for corona virus: a systematic review. Indian J. Pharmacol. 2020;52(1):56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Q., Yang Y., Gao J. Vol. 56. EBioMedicine; 2020. Infectivity of human coronavirus in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Q., Yang Y., Gao J. Infectivity of human coronavirus in the brain. EBioMedicine. 2020;56:102799. doi: 10.1016/j.ebiom.2020.102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butowt R., Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11(9):1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 17.Scoppettuolo P., Borrelli S., Naeije G. Neurological involvement in SARS-CoV-2 infection: a clinical systematic review. Brain, Behavior, & Immunity - Health. 2020;5:100094. doi: 10.1016/j.bbih.2020.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iroegbu J.D., Ifenatuoha C.W., Ijomone O.M. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. 2020;41(6):1329–1337. doi: 10.1007/s10072-020-04469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 20.Vaira L.A., et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42(6):1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam S.B., Willows S. Severe acute respiratory syndrome coronavirus 2 may be an underappreciated pathogen of the central nervous system. 2020;27(11):2348–2360. doi: 10.1111/ene.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaira L.A., et al. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. International Forum of Allergy & Rhinology. 2020;10(9):1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellul M.A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhalla V., Blish C.A., South A.M. A historical perspective on ACE2 in the COVID-19 era. J. Hum. Hypertens. 2020 doi: 10.1038/s41371-020-00459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nepal G., et al. Neurological manifestations of COVID-19: a systematic review. Crit. Care. 2020;24(1):421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benameur K., et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, atlanta, Georgia, USA. Emerging Infectious Disease journal. 2020;26(9) doi: 10.3201/eid2609.202122. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steardo L., Steardo L., Verkhratsky A. Psychiatric face of COVID-19. Transl. Psychiatry. 2020;10(1):261. doi: 10.1038/s41398-020-00949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzet M., Vieau D., Day R. Crosstalk between nervous and immune systems through the animal kingdom: focus on opioids. Trends Neurosci. 2000;23(11):550–555. doi: 10.1016/s0166-2236(00)01642-8. [DOI] [PubMed] [Google Scholar]
- 30.Wybran J. Federation Proceedings. 1985. Enkephalins and endorphins as modifiers of the immune system: present and future. [PubMed] [Google Scholar]
- 31.Mørch H., Pedersen B.K. β-Endorphin and the immune system-possible role in autoimmune diseases. Autoimmunity. 1995;21(3):161–171. doi: 10.3109/08916939509008013. [DOI] [PubMed] [Google Scholar]
- 32.Weigent D., Blalock J. Production of peptide hormones and neurotransmitters by the immune system. Chem. Immunol. 1997;69:1–30. doi: 10.1159/000058652. [DOI] [PubMed] [Google Scholar]
- 33.Kowalski J. Immunologic action of [Met5] enkephalin fragments. Eur. J. Pharmacol. 1998;347(1):95–99. doi: 10.1016/s0014-2999(98)00079-x. [DOI] [PubMed] [Google Scholar]
- 34.Gilmore W., Moradzadeh D.S. β-Endorphin protects mice from neurological disease induced by the murine coronavirus MHV-JHM. J. Neuroimmunol. 1993;48(1):81–90. doi: 10.1016/0165-5728(93)90061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda N. Vol. 108. Epilepsy & Behavior; 2020. Epilepsy and COVID-19: associations and important considerations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohal S., Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L., et al. medRxiv; 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a Retrospective Case Series Study; p. 2020. [Google Scholar]
- 38.Sheraton M., et al. A review of neurological complications of COVID-19. Cureus. 2020;12(5) doi: 10.7759/cureus.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollono C., et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quartuccio L., et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J. Med. Virol. 2020;92(11):2852–2856. doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg R. Spectrum of neurological manifestations in covid-19: a review. Neurol. India. 2020;68(3):560–572. doi: 10.4103/0028-3886.289000. [DOI] [PubMed] [Google Scholar]
- 44.Berlin I., et al. Nicotine & Tobacco Research; 2020. COVID-19 and Smoking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patanavanich R., Glantz S.A. Nicotine Tob Res; 2020. Smoking is associated with COVID-19 progression: a meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J.-j., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 49.Guan W.-j., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuki N., Hartung H.-P. Guillain–barré syndrome. N. Engl. J. Med. 2012;366(24):2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 51.Hughes R.A. Springer Science & Business Media; 2012. Guillain-Barré Syndrome. [Google Scholar]
- 52.Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann. Neurol.: Official Journal of the American Neurological Association and the Child Neurology Society. 1990;27(S1):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 53.Ottaviani D., et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol. Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes R.A., Cornblath D.R. Guillain-barre syndrome. Lancet. 2005;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 55.Abdullahi A., et al. Is guillain–barré syndrome associated with COVID-19 infection? A systemic review of the evidence. Front. Neurol. 2021;11(1670) doi: 10.3389/fneur.2020.566308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uncini A., Kuwabara S. The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin. Neurophysiol. 2018;129(12):2586–2593. doi: 10.1016/j.clinph.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 57.Toscano G., et al. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virani A., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh M.-d., et al. Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea. Kor. J. Intern. Med. 2018;33(2):233. doi: 10.3904/kjim.2018.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau K.-K., et al. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10(2):342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katal S., Gholamrezanezhad A. Neuroimaging findings in COVID-19: a narrative review. Neurosci. Lett. 2020:135529. doi: 10.1016/j.neulet.2020.135529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chougar L., et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kremer S., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katal S., et al. Reopening the country: recommendations for nuclear medicine departments. World J. Nucl. Med. 2021;20(1):1. doi: 10.4103/wjnm.WJNM_73_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katal S., Amini H., Gholamrezanezhad A. PET in the diagnostic management of infectious/inflammatory pulmonary pathologies: a revisit in the era of COVID-19. Nucl. Med. Commun. 2020 doi: 10.1097/MNM.0000000000001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delorme C., et al. COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur. J. Neurol. 2020;27(12):2651–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grimaldi S., et al. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from 18F-FDG PET imaging and neuronal autoantibodies. J. Nucl. Med. 2020;61(12):1726–1729. doi: 10.2967/jnumed.120.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karimi-Galougahi, M., et al., 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad. Radiol., 2020. 27(7): p. 1042-1043. [DOI] [PMC free article] [PubMed]
- 69.Hamid S., Mir M.Y., Rohela G.K. New microbes and new infections; 2020. Noval Coronavirus Disease (COVID-19): A Pandemic (Epidemiology, Pathogenesis and Potential Therapeutics) p. 100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samson R., Navale G.R., Dharne M.S. Biosensors: frontiers in rapid detection of COVID-19. 3 Biotech. 2020;10(9):1–9. doi: 10.1007/s13205-020-02369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen T., Duong Bang D., Wolff A. Novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2019;11(3):306. doi: 10.3390/mi11030306. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iravani S. Nano-and biosensors for the detection of SARS-CoV-2: challenges and opportunities. Materials Advances. 2020;1(9):3092–3103. [Google Scholar]
- 73.Zhang X., Guo Q., Cui D. Recent advances in nanotechnology applied to biosensors. Sensors. 2009;9(2):1033–1053. doi: 10.3390/s90201033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khurana A., et al. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 75.Khurana A., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antiochia R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: from past to perspectives. Microchimica Acta. 2020;187(12):1–13. doi: 10.1007/s00604-020-04615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandra P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sensors International. 2020;1:100019. doi: 10.1016/j.sintl.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alhalaili B., et al. Nanobiosensors for the detection of novel coronavirus 2019-nCoV and other pandemic/epidemic respiratory viruses: a review. Sensors. 2020;20(22):6591. doi: 10.3390/s20226591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hajian R., et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nature biomedical engineering. 2019;3(6):427–437. doi: 10.1038/s41551-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahari S., et al. BioRxiv; 2020. eCovSens-Ultrasensitive Novel In-House Built Printed Circuit Board Based Electrochemical Device for Rapid Detection of nCovid-19 Antigen, a Spike Protein Domain 1 of SARS-CoV-2. [Google Scholar]
- 81.Qiu G., et al. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 82.Seo G., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 83.Mavrikou S., et al. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors. 2020;20(11):3121. doi: 10.3390/s20113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Z., et al. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020;164:112316. doi: 10.1016/j.bios.2020.112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moitra P., et al. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14(6):7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Z., et al. BioRxiv; 2020. A Simple Magnetic Nanoparticles-Based Viral RNA Extraction Method for Efficient Detection of SARS-CoV-2. [Google Scholar]
- 88.Tian B., et al. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens. Bioelectron. 2020;165:112356. doi: 10.1016/j.bios.2020.112356. [DOI] [PubMed] [Google Scholar]
- 89.Pandey L.M. Design of engineered surfaces for prospective detection of SARS-CoV-2 using quartz crystal microbalance-based techniques. Expet Rev. Proteonomics. 2020;17(6):425–432. doi: 10.1080/14789450.2020.1794831. [DOI] [PubMed] [Google Scholar]
- 90.Seshadri D.R., et al. Wearable sensors for COVID-19: a call to action to harness our digital infrastructure for remote patient monitoring and virtual assessments. Frontiers in Digital Health. 2020;2:8. doi: 10.3389/fdgth.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ke G., et al. Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining. 2019. DeepGBM: a deep learning framework distilled by GBDT for online prediction tasks. [Google Scholar]
- 92.Behera S., et al. Biosensors in diagnosing COVID-19 and recent development. Sensors International. 2020:100054. [Google Scholar]
- 93.Ranjan Y., et al. RADAR-base: open source mobile health platform for collecting, monitoring, and analyzing data using sensors, wearables, and mobile devices. JMIR mHealth and uHealth. 2019;7(8):e11734. doi: 10.2196/11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stewart C.L., et al. Proceedings of the 2018 ACM International Joint Conference and 2018 International Symposium on Pervasive and Ubiquitous Computing and Wearable Computers. 2018. RADAR-base: major depressive disorder and epilepsy case studies. [Google Scholar]
- 95.Gui Q., et al. The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors. 2017;17(7):1623. doi: 10.3390/s17071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allawadhi P., Khurana A., Allwadhi S., Joshi K., Packirisamy G., Bharani K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35:100982. doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allawadhi P., Khurana A., Allwadhi S., Navik U.S., Joshi K., Banothu A.K., Bharani K.K. potential of electric stimulation for the management of COVID-19. Medical Hypotheses. 2020;144:110259. doi: 10.1016/j.mehy.2020.110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allawadhi P., et al. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phytotherapy Research. 2018;32(10):1908–1932. doi: 10.1002/ptr.6152. [DOI] [PubMed] [Google Scholar]
- 99.Khurana A., et al. It’s all about the spaces between cells: role of extracellular matrix in liver fibrosis. Ann Transl Med. 2020 doi: 10.21037/atm-20-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khurana A., et al. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–1825. doi: 10.2217/nnm-2018-0318. [DOI] [PubMed] [Google Scholar]
- 101.Khurana A., et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomedicine: Nanotechnology, Biology and Medicine. 2019;18:54–65. doi: 10.1016/j.nano.2019.02.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.