Abstract
Background and aims
Peripheral artery disease (PAD) is a systemic manifestation of atherosclerosis that is associated with a high risk of major adverse cardiovascular events (MACE). LDL aggregation contributes to atherosclerotic plaque progression and may contribute to plaque instability. We aimed to determine if LDL aggregation is associated with MACE in patients with PAD undergoing lower extremity revascularization (LER).
Methods
Two hundred thirty-nine patients with PAD undergoing LER had blood collected at baseline and were followed prospectively for MACE (myocardial infarction, stroke, cardiovascular death) for one-year. 19 age, sex and LDL-C-matched control subjects without cardiovascular disease also had blood drawn. Subject LDL was exposed to sphingomyelinase and LDL aggregate size measured via dynamic light scattering.
Results
Mean age was 72.3±10.9 years, 32.6% were female, and LDL-cholesterol was 68±25mg/dL. LDL aggregation was inversely associated with triglycerides, but not associated with demographics, LDL-cholesterol or other risk factors. Maximal LDL aggregation occurred significantly earlier in subjects with PAD than in control subjects.
15.9% of subjects experienced MACE over one year. The 1st tertile (shortest time to maximal aggregation) exhibited significantly higher MACE (25% vs. 12.5% in tertile 2 and 10.1% in tertile 3, p=0.012). After multivariable adjustment for demographics and CVD risk factors, the hazard ratio for MACE in the 1st tertile was 4.57 (95% CI 1.60 – 13.01; p=0.004) compared to tertile 3. Inclusion of LDL aggregation in the Framingham Heart Study risk calculator for recurrent coronary heart disease events improved the c-index from 0.57 to 0.63 (p=0.01).
Conclusions
We show that in the setting of very well controlled LDL-cholesterol, patients with PAD with the most rapid LDL aggregation had a significantly elevated MACE risk following LER even after multivariable adjustment. This measure further improved the classification specificity of an established risk prediction tool. Our findings support broader investigation of this assay for risk stratification in patients with atherosclerotic CVD.
Keywords: peripheral artery disease, low-density lipoprotein, major adverse cardiovascular events, risk
Introduction
Low-density lipoprotein (LDL) is both a key risk factor and causative factor in atherosclerosis – contributing to lipid-laden plaques with its retention in the arterial intima.1 Despite robust lowering of LDL-cholesterol, cardiovascular events remain frequent in patients with atherosclerotic cardiovascular disease (ASCVD).2, 3 Peripheral artery disease (PAD) is a systemic manifestation of atherosclerosis and those with PAD are at particularly high risk of major adverse cardiovascular events (MACE).4 Improved risk stratification and the identification of additional modifiable risk factors for adverse events are needed to improve outcomes in this population.5
We previously developed an ex vivo assay of LDL aggregation – a process that occurs within and contributes to atherosclerotic plaques.6, 7 Our work has shown that LDL aggregation differs among individuals independent of plasma LDL-cholesterol concentration and LDL particle size and is predictive of death in coronary artery disease (CAD).8 The aims of the present study were to determine if LDL aggregation is associated with MACE in patients with PAD undergoing lower extremity revascularization (LER) and may help identify those at increased risk for adverse outcomes.
Patients and methods
Clinical study design
Men and women scheduled for LER at NYU Tisch Hospital or Bellevue Hospital Center (New York, NY) provided informed consent and were enrolled into the Platelet Activity in Vascular Surgery and Cardiovascular Events study (NCT02106429) under an IRB-approved protocol conforming to the Declaration of Helsinki. Fasting blood samples were collected prior to LER, plasma and serum isolated and immediately frozen at −80°C until analysis. As part of a separate, ongoing protocol, we recruit individuals without known ASCVD from our hospitals to undergo clinical evaluation and blood sampling. Prior to LDL-aggregation analysis, we identified 19 individuals within this cohort who were of similar sex, race/ethnicity and had similar LDL-C levels as the PAD patients. Samples from these control subjects were processed and analyzed along with samples from PAD subjects in a blinded fashion.
BMI was calculated based on pre-operative measures of height and weight. Measurements of circulating lipoproteins were made from serum samples using NMR spectroscopy (LabCorp Inc., Morrisville, NC).9
Clinical follow-up of subjects with PAD occurred prospectively at 30-days, 6 months and 12 months from the time of operation. Three blinded reviewers (2 cardiologists and 1 vascular surgeon) adjudicated the composite endpoint of MACE (all cause death, myocardial infarction (MI), stroke (both ischemic and hemorrhagic) and major adverse cardiovascular and limb events (MACLE; MACE + acute limb ischemia, major amputation, and repeat revascularization).
LDL aggregation assay
LDL aggregation was assessed blinded and as previously described.10 First, LDL was isolated by sequential ultracentrifugation in D2O-based buffers as described,11 with the exception that samples were first centrifuged twice at a density of 1.019 g/ml for efficient removal of all chylomicrons, VLDL, and IDL from the samples. LDL was isolated at a density of 1.063 g/ml and, exposed to human recombinant sphingomyelinase produced in-house10 and LDL aggregate size was measured every 30 minutes for 5 hours via dynamic light scattering (Wyatt DynaPro Plate Reader II; Wyatt Technology, CA). Time-size curves were constructed based on all data points for an individual subject and the inflection point (mid-point of the sigmoidal curve – time point when the aggregation curve has the steepest upward slope) was identified. This inflection point was used for between-subject comparisons.8 The intra-assay variability in the LDL aggregation inflection point was 6.8% (range 5.9% – 8.2%) and inter-assay variability was 8.1 %. The inter-operator variability was 9.6%.
Statistical analyses
Comparisons between PAD and healthy control subjects were made with two sample t- and Mann-Whitney tests. Multivariable linear regression modeling included age, sex, BMI, statin use, insulin use, clopidogrel use, LDL-C, ApoB, triglycerides, smoking, diabetes, hyperlipidemia and hypertension as covariates. Comparisons between tertiles of subjects with PAD were performed with Kruskal Wallis tests and Jonckheere-Terpstra and Cochrane-Armitage tests for trend. Prediction models were generated using Cox proportional hazards regression. The relation of LDL aggregation inflection point with MACE and MACLE was presented as Kaplan-Meier curves with inflection point categorized by tertile. The cumulative risk of MACE and MACLE were calculated in each group and compared using the log-rank test.
We tested the capacity of the Framingham Risk Calculator for secondary cardiovascular events12 to predict 1-year MACE within this PAD cohort and compared the associated Harrell c-statistic with and without the addition of LDL aggregation inflection point using the z-score test. A two-tailed p-value less than 0.05 was set a priori as the criterion for statistical significance.
Results
Two hundred thirty-nine patients with PAD had LDL aggregation assessed. The mean age was 72.3±10.9 years and 32.6% were female. The cohort had a high burden of ASCVD risk factors (Table 1) with more than half having known CAD. LDL aggregation inflection point was not associated with clinical variables, LDL parameters, or other hematologic measures, other than serum triglyceride concentrations (Table 2).
Table 1.
Descriptive characteristics of subjects with peripheral artery disease and control subjects without known atherosclerotic cardiovascular disease.
| No ASCVD | PAD | p = | |
|---|---|---|---|
| n=19 | n=239 | ||
| Age, mean (SD), years | 63.2 (6.3) | 72.3 (10.9) | <0.001 |
| Female sex, n (%) | 7 (37%) | 78 (32.6%) | 0.81 |
| BMI, mean (SD), kg/m2 | 29.9 (5.1) | 26.7 (5.3) | 0.01 |
| LDL-cholesterol, mean (SD), mg/dL | 77 (42) | 68 (25) | 0.47 |
| HDL-cholesterol, mean (SD), mg/dL | 55 (17) | 43 (13) | 0.006 |
| Triglycerides, median [range], mg/dL | 116 [35, 259] | 94 [31, 349] | 0.20 |
| Hypertension, n (%) | 11 (57.9) | 205 (85.8) | 0.002 |
| Hyperlipidemia, n (%) | 12 (63.2) | 177 (74.1) | 0.34 |
| Diabetes, n (%) | 3 (15.8) | 118 (49.4) | 0.002 |
| Insulin use, n (%) | 0 (0.0) | 75 (31.4) | - |
| Coronary artery disease, n (%) | 0 (0.0) | 133 (55.6) | - |
| Prior myocardial infarction, n (%) | 0 (0.0) | 55 (23.0) | - |
| Current smokers, n (%) | 1 (5.3) | 38 (15.9) | 0.20 |
| Statin use, n (%) | 11 (57.9) | 193 (80.7) | 0.02 |
| Aspirin use, n (%) | 10 (52.6) | 208 (87.0) | <0.001 |
| Clopidogrel use, n (%) | 0 (0.0) | 90 (37.7) | - |
SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259.
ASCVD – atherosclerotic cardiovascular disease
BMI – body mass index
LDL – low density lipoprotein
HDL – high density lipoprotein
PAD – peripheral artery disease
SD – standard deviation
Table 2.
Correlations of clinical and hematologic variables with LDL aggregation inflection point.
| r = | p = | |
|---|---|---|
| Age | −0.03 | 0.69 |
| BMI | −0.08 | 0.21 |
| White blood cells | 0.02 | 0.83 |
| Neutrophils | −0.002 | 0.98 |
| Monocytes | −0.08 | 0.22 |
| Hemoglobin A1c | −0.03 | 0.73 |
| Total cholesterol | 0.004 | 0.96 |
| LDL-cholesterol | −0.05 | 0.44 |
| LDL-particles | 0.03 | 0.65 |
| LDL-particle size | −0.08 | 0.22 |
| Triglycerides | 0.14 | 0.03 |
| HDL-cholesterol | −0.02 | 0.79 |
| Creatinine | 0.08 | 0.25 |
| Systolic blood pressure | −0.03 | 0.35 |
BMI – body mass index
LDL – low density lipoprotein
HDL – high density lipoprotein
In comparison to control subjects recruited from the same hospitals without known ASCVD, patients with PAD exhibited similar LDL-cholesterol and triglyceride levels, but were older, had slightly lower BMI and a greater prevalence of cardiovascular disease risk factors (Table 1). Maximal LDL aggregation occurred significantly earlier in subjects with PAD than in control subjects (Figure 1). This difference persisted after adjustment for demographics, ASCVD risk factors and statin use (β=−0.55 (0.25), p=0.03).
Figure 1. LDL-aggregation in subjects with peripheral artery disease.
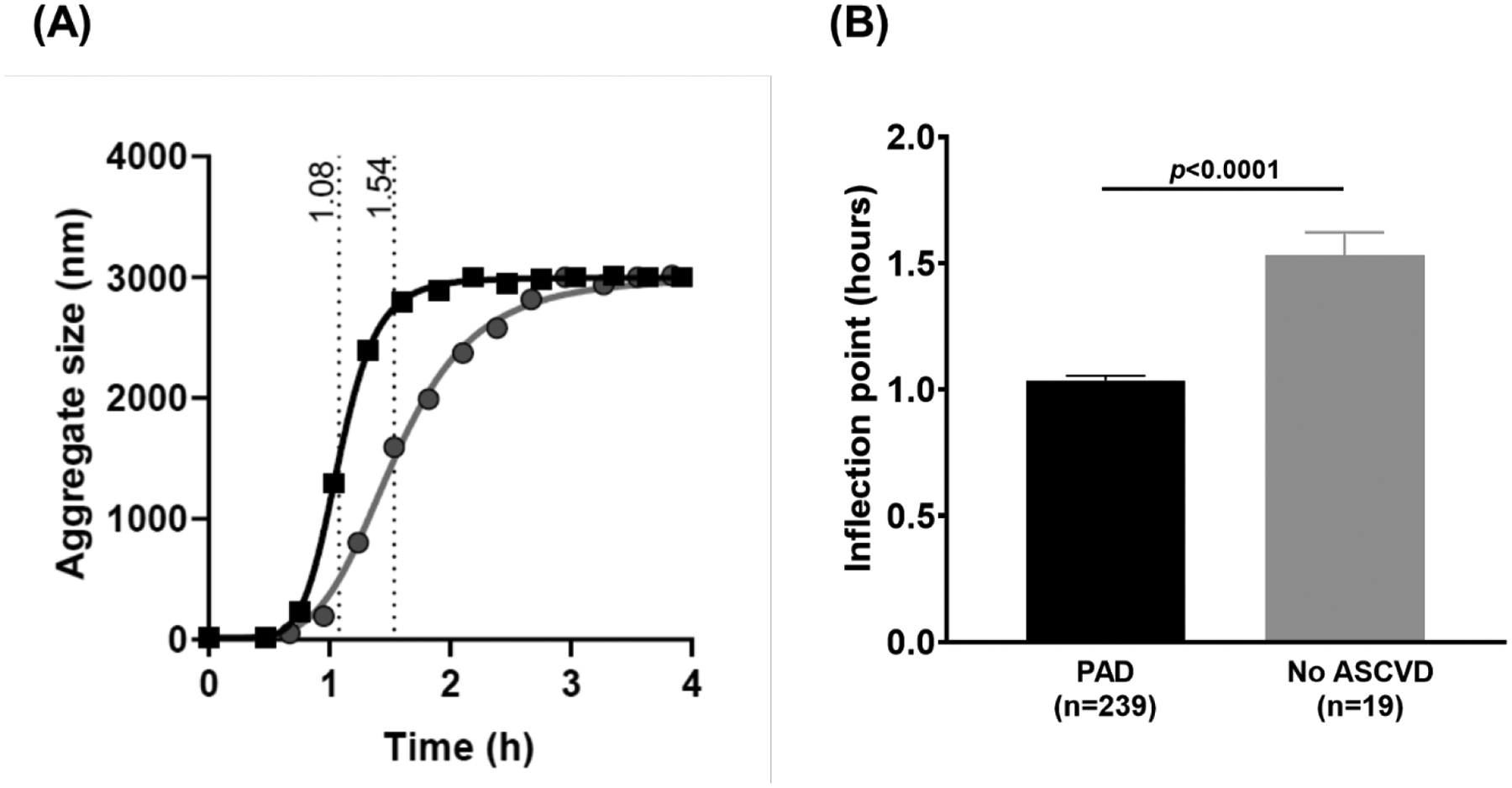
(A) Representative aggregation curves of a PAD subject (black squares) and healthy control subject (gray circles) with inflection point highlighted. (B) LDL aggregation inflection point in all subjects with peripheral artery disease (n=239) and control subjects without known atherosclerotic cardiovascular disease (n=19). Comparison with two sample t-tests.
We divided subjects with PAD into tertiles based on LDL aggregation inflection point. There was a linear trend of higher triglycerides across LDL aggregation tertiles (Table 3). However, there were no other differences in demographics, LDL quantity or particle size, medication use or other ASCVD risk factors across LDL aggregation tertiles.
Table 3.
Descriptive characteristics of tertiles of LDL aggregation in subjects with peripheral artery disease undergoing lower extremity revascularization.
| LDL-aggregation Tertile 1 | LDL-aggregation Tertile 2 | LDL-aggregation Tertile 3 | p-value for trend | |
|---|---|---|---|---|
| n=80 | n=79 | n=80 | ||
| Age, mean (SD), years | 73.5(12.2) | 72.7 (9.8) | 70.3 (11.0) | 0.15 |
| Female sex, n (%) | 28 (35.0) | 28 (35.4) | 22 (27.5) | 0.31 |
| BMI, mean (SD), kg/m2 | 26.8 (5.6) | 27.2 (5.1) | 26.3 (5.4) | 0.58 |
| LDL-cholesterol, mean (SD), mg/dL | 71 (27) | 75 (24) | 68 (23) | 0.97 |
| ApoB, mean (SD), mg/dL | 109 (27) | 107 (24) | 107 (22) | 0.93 |
| LDL-particles, mean (SD), nmol/L | 1147 (379) | 1232 (358) | 1145 (323) | 0.56 |
| LDL-particle size, mean (SD), nm | 20.5 (0.6) | 20.5 (0.6) | 20.3 (0.6) | 0.10 |
| HDL-cholesterol, mean (SD), mg/dL | 41 (11) | 43 (12) | 41 (10) | 0.23 |
| Triglycerides, median [range], mg/dL | 83 [31, 290] | 97 [35, 236] | 118 [41, 349] | <0.01 |
| Hypertension, n (%) | 68 (85.0) | 69 (87.3) | 68 (85.0) | 0.85 |
| Hyperlipidemia, n (%) | 63 (78.8) | 55 (69.6) | 59 (73.8) | 0.42 |
| Diabetes, n (%) | 36 (45.0) | 36 (45.6) | 46 (57.5) | 0.08 |
| Insulin use, n (%) | 28 (35.0) | 26 (32.9) | 21 (26.3) | 0.25 |
| Coronary artery disease, n (%) | 48 (60.0) | 43 (54.4) | 42 (52.5) | 0.43 |
| Prior myocardial infarction, n (%) | 16 (20.0) | 20 (25.3) | 19 (23.8) | 0.57 |
| Current smokers, n (%) | 9 (11.3) | 12 (15.2) | 17 (21.3) | 0.09 |
| Statin use, n (%) | 65 (81.5) | 62 (78.5) | 66 (82.5) | 0.65 |
| Aspirin use, n (%) | 63 (78.8) | 66 (83.5) | 69 (86.3) | 0.15 |
| Clopidogrel use, n (%) | 30 (37.5) | 26 (32.9) | 34 (42.5) | 0.58 |
SI conversion factors: To convert cholesterol to mmol/L, multiply values by 0.0259.
ASCVD – atherosclerotic cardiovascular disease
BMI – body mass index
LDL – low density lipoprotein
HDL – high density lipoprotein
PAD – peripheral artery disease
SD – standard deviation
Overall, MACE occurred in 15.9% of PAD subjects at 1-year. The 1st tertile (shortest time to maximal aggregation rate), exhibited a significantly higher rate of MACE (25% vs. 12.5% in tertile 2 and 10.1% in tertile 3, Figure 2A). Data were consistent for each component of the MACE endpoint. After multivariable adjustment for age, sex, race, BMI, smoking status, LDL-cholesterol, ApoB, triglycerides, diabetes, insulin use, statin use, clopidogrel use and history of prior MI, the hazard ratio for MACE in the 1st tertile was 4.57 (95% confidence interval (CI) 1.60 – 13.01; p=0.004) compared to the 3rd tertile. In a secondary analysis, we also assessed the association of LDL aggregation inflection point with MACLE. A trend similar to MACE was present (Figure 2B), but the hazard ratio was no longer statistically significant at 1.67 (0.98, 2.86; p = 0.06).
Figure 2. Major adverse cardiovascular events following lower extremity revascularization stratified by tertile of LDL aggregation.
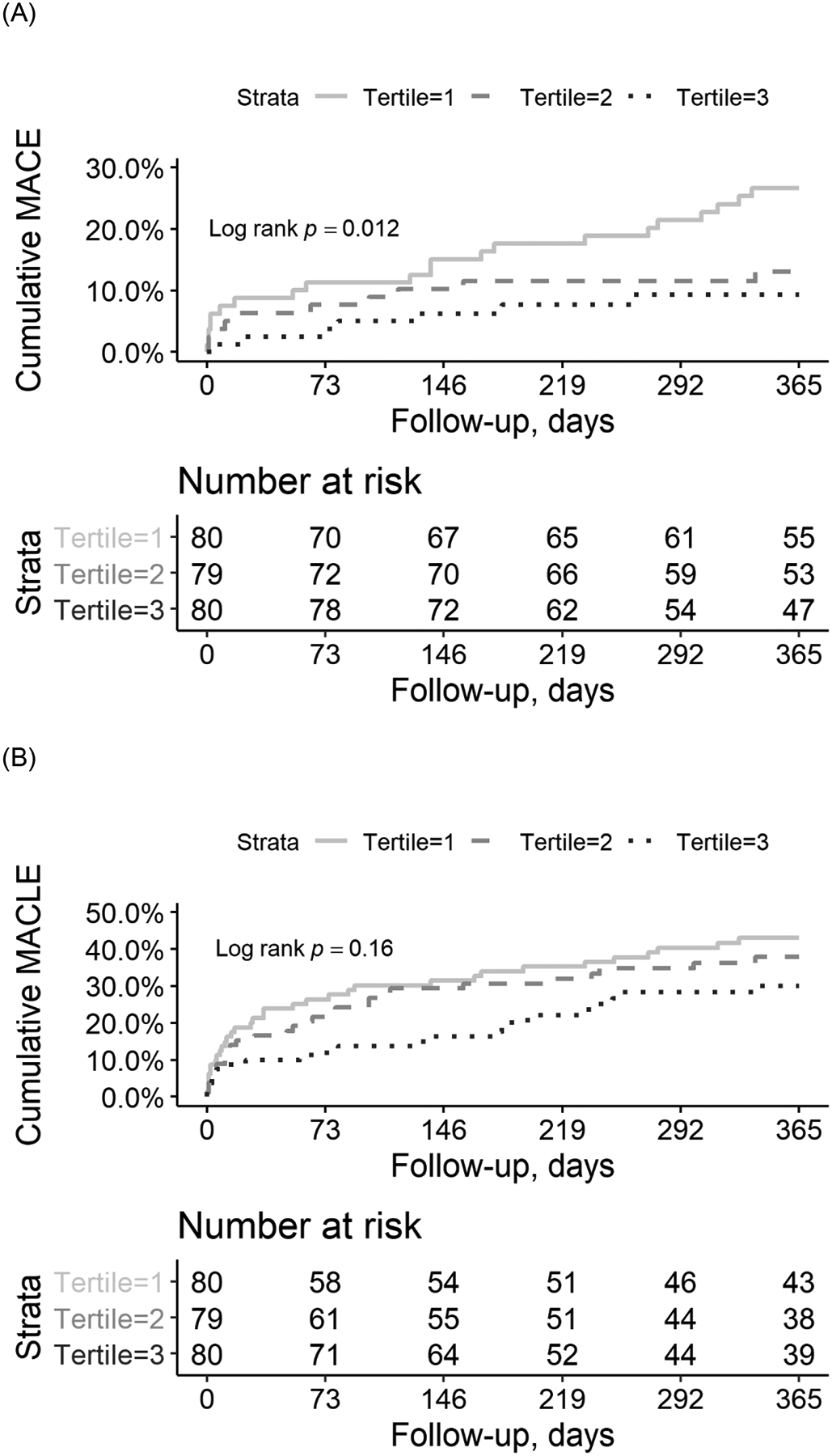
Kaplan-Meier curves for (A) major adverse cardiovascular events and (B) major adverse cardiovascular and limb events over one year following lower extremity revascularization in 239 subjects stratified by tertiles of LDL aggregation inflection point. Cumulative risk of MACE and MACLE between tertiles was compared using the log-rank test.
The Framingham Heart Study risk equation for recurrent coronary heart disease events had a c-index of 0.57 (95% CI 0.48, 0.67) for 1-year MACE in our cohort of patients with PAD undergoing LER. The addition of LDL aggregation inflection point to this equation increased the c-statistic to 0.63 (95% CI 0.53, 0.73; p=0.01).
Discussion
The present study, the largest to date employing the LDL aggregation assay, is the first study to assess LDL aggregation in patients with PAD and the first prospective study to evaluate the capacity of this measure in risk stratification. We show that in the setting of very well controlled LDL-cholesterol (mean LDL-cholesterol was 68±25mg/dL), and after correction for other ASCVD risk factors, patients with the most rapid LDL aggregation had a significantly elevated risk of MACE over one year following LER. This measure further improved the classification specificity within this cohort of an established risk prediction tool. Our findings support broader investigation of this assay for risk stratification in patients with ASCVD.
Patients with PAD undergoing LER are at very high risk for MACE,4 as evidenced by the 15.9% event rate in our cohort. Understanding potential mechanisms and identifying those at increased risk may help improve outcomes in this population. We and others previously showed that aggregated LDL is present in human atherosclerotic plaques and that sphingomyelinase catalyzes the aggregative process.6, 13 We subsequently developed a highly reliable assay of ex vivo LDL aggregation to human recombinant sphingomyelinase8 and demonstrated a difference in LDL aggregation between subjects with stable CAD and those who would die from CAD over an average of 2.5 years of follow-up.8 Beyond stimulating LDL retention and overall plaque burden, LDL aggregation may also contribute to plaque rupture and adverse outcomes. Aggregated LDL isolated from human atherosclerotic lesions activates the inflammasome,6 and MMP-7 secretion from macrophages is induced by LDL treated with sphingomyelinase.8 Both of these activities are associated with plaque instability and rupture and may mechanistically link elevated LDL aggregation and atherothrombotic events. Accordingly, the tertile of most rapid LDL aggregation in our cohort experienced a 3-fold elevated risk of MACE over one year even after correction for traditional risk factors. Incorporation of LDL aggregation inflection point into an established risk equation improved the c-statistic by more than 10%, demonstrating the potential capacity of this assay to discriminate those at elevated risk.
Given the mechanistic rationale for the association of LDL aggregation with higher rates of atherothrombosis, this measure is potentially a modifiable causative factor, in addition to a marker of risk. While a recent randomized trial did not find that dietary supplementation with alpha-linoleic acid or increased fish consumption affected LDL aggregation,14 other studies suggest that adopting a Nordic-style diet, adding plant stanols to the diet, or the use of a PCSK9 inhibitor reduce LDL aggregation in association with altered LDL particle sphingomyelin content.8, 15 Notably, PCSK9 inhibition has been shown to reduce MACE in individuals with PAD16 and we have demonstrated a strong association of dietary quality with PAD.17, 18 Whether PCSK9 inhibition and/or intensive dietary modification might improve outcomes in patients with PAD with rapid LDL aggregation who are at increased risk for MACE, despite well controlled LDL-cholesterol, will require randomized trials.
In agreement with our previous reports, LDL aggregation was not associated with LDL-cholesterol or LDL particle size in the present study. LDL aggregation inflection point did inversely correlate with serum triglycerides, also in accordance with prior studies.8 The reasons for this seemingly paradoxical association are unclear. Serum triglyceride level reflects triglyceride content of LDL particles and greater LDL triglyceride content has been shown to associate with reduced aggregation-susceptibility of LDL particles. Increases in LDL-triglycerides are associated with greater proportions of phosphatidylcholines and lower proportions of sphingomyelins in the surface monolayer of LDL particles. We previously showed that changes in the proportions of these two types of phospholipids control the aggregation of LDL particles.8 LDL from human APOB transgenic/LDLr−/−/Soat2−/− mice exhibiting increased LDL-triglyceride content is extremely resistant to sphingomyelinase-induced LDL aggregation.8 Whether LDL triglyceride content independently affects LDL aggregative potential is the focus of ongoing studies.
Our study has several limitations. Our cohort only contains individuals with severe PAD requiring LER. Whether LDL aggregation provides similar risk prediction in a broader population of patients with PAD will require additional investigation. Additionally, while our subjects came from two separate hospitals, they represented a single locale and were predominantly Caucasian males. We recently reported differences in LDL aggregation between races, with South Asians exhibiting more rapid aggregation than Caucasians.10 Further studies of more diverse cohorts, including greater numbers of women – in whom PAD is most prevalent19, 20 – are necessary before extrapolating our findings.
In conclusion, we show that patients with PAD undergoing LER with more rapid LDL aggregation have a significantly elevated risk of MACE over one year and that this measure improves risk prediction in this group. These findings support broader investigation of this assay for risk stratification and potential intervention in patients with ASCVD.
Acknowledgements
We are grateful for the technical assistance of Maija Atuegwu. Portions of the graphical abstract were created using BioRender.com.
Financial support
SPH was supported by NIH (HL135398). KÖ was supported by The Academy of Finland (#315568), the Finnish Foundation for Cardiovascular Research, and Aarne Koskelo Foundation. JSB was supported by NIH (HL139909 and HL144993).
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- LER
lower extremity revascularization
- MACE
major adverse cardiovascular events
- MACLE
major adverse cardiovascular and limb events
- MI
myocardial infarction
- PAD
peripheral artery disease
Footnotes
Conflict of interest
KÖ and MKR have applied for a patent on the LDL aggregation assay. The authors have no other disclosures to report.
References
- 1.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ and Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL and Cooney MT. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS and Pedersen TR. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. The New England journal of medicine. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 4.Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, Woodward M and Rahimi K. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ (Clinical research ed). 2015;351:h4865–h4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vascular health and risk management. 2007;3:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehti S, Nguyen SD, Belevich I, Vihinen H, Heikkilä HM, Soliymani R, Käkelä R, Saksi J, Jauhiainen M, Grabowski GA, Kummu O, Hörkkö S, Baumann M, Lindsberg PJ, Jokitalo E, Kovanen PT and Öörni K. Extracellular Lipids Accumulate in Human Carotid Arteries as Distinct Three-Dimensional Structures and Have Proinflammatory Properties. The American journal of pathology. 2018;188:525–538. [DOI] [PubMed] [Google Scholar]
- 7.Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen MR, van de Sluis B, Wiklund O, Tokgozoglu L, Catapano AL and Ginsberg HN. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruuth M, Nguyen SD, Vihervaara T, Hilvo M, Laajala TD, Kondadi PK, Gisterå A, Lähteenmäki H, Kittilä T, Huusko J, Uusitupa M, Schwab U, Savolainen MJ, Sinisalo J, Lokki M-L, Nieminen MS, Jula A, Perola M, Ylä-Herttula S, Rudel L, Öörni A, Baumann M, Baruch A, Laaksonen R, Ketelhuth DFJ, Aittokallio T, Jauhiainen M, Käkelä R, Borén J, Williams KJ, Kovanen PT and Öörni K. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. European heart journal. 2018;39:2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manmadhan A, Lin BX, Zhong J, Parikh M, Berger JS, Fisher EA and Heffron SP. Elevated GlycA in severe obesity is normalized by bariatric surgery. Diabetes, obesity & metabolism. 2019;21:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruuth M, Janssen LGM, Äikäs L, Tigistu-Sahle F, Nahon KJ, Ritvos O, Ruhanen H, Käkelä R, Boon MR, Öörni K and Rensen PCN. LDL aggregation susceptibility is higher in healthy South Asian compared with white Caucasian men. J Clin Lipidol. 2019;13:910–919.e2. [DOI] [PubMed] [Google Scholar]
- 11.Hallberg C, Hådén M, Bergström M, Hanson G, Pettersson K, Westerlund C, Bondjers G, Ostlund-Lindqvist AM and Camejo G. Lipoprotein fractionation in deuterium oxide gradients: a procedure for evaluation of antioxidant binding and susceptibility to oxidation. Journal of lipid research. 1994;35:1–9. [PubMed] [Google Scholar]
- 12.D’Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW and Hartz SC. Primary and subsequent coronary risk appraisal: new results from the Framingham study. American heart journal. 2000;139:272–81. [DOI] [PubMed] [Google Scholar]
- 13.Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ and Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manninen S, Lankinen M, Erkkilä A, Nguyen SD, Ruuth M, de Mello V, Öörni K and Schwab U. The effect of intakes of fish and Camelina sativa oil on atherogenic and anti-atherogenic functions of LDL and HDL particles: A randomized controlled trial. Atherosclerosis. 2019;281:56–61. [DOI] [PubMed] [Google Scholar]
- 15.Ruuth MAL, Tigistu-Sahle F, Kakela R, Lindholm H, Simonen P, Kovanen PT, Gylling H, Oorni K. Plant stanol esters reduce LDL aggregation by altering LDL surface lipids. The BLOOD FLOW randomized intervention study. Arteriosclerosis Thrombosis and Vascular Biology. 2020. [DOI] [PubMed] [Google Scholar]
- 16.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR and Sabatine MS. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 17.Heffron SP, Rockman CB, Gianos E, Guo Y and Berger JS. Greater frequency of nut consumption is associated with lower prevalence of peripheral arterial disease. Prev Med. 2015;72:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heffron SP, Rockman CB, Adelman MA, Gianos E, Guo Y, Xu JF and Berger JS. Greater Frequency of Fruit and Vegetable Consumption Is Associated With Lower Prevalence of Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. 2017;37:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E and Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–91. [DOI] [PubMed] [Google Scholar]
- 20.Zheng ZJ, Rosamond WD, Chambless LE, Nieto FJ, Barnes RW, Hutchinson RG, Tyroler HA and Heiss G. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Prev Med. 2005;29:42–9. [DOI] [PubMed] [Google Scholar]


