Abstract
Purpose:
Controversy exists regarding the best method for biopsy of uveal melanoma (UM). We describe our transvitreal technique, and evaluate the safety of this technique as well as the efficacy for obtaining sample for prognostic genetic profiling.
Methods:
Description of surgical technique and retrospective case series. Medical records for UM patients who underwent transvitreal biopsy using our described technique were analyzed for tumor size, location, primary treatment, method of biopsy and any complications thereof. Characteristics of tumors that underwent transvitreal biopsy were noted including tumor size, location, or presence of subretinal fluid, to see if these affected surgeon preference for biopsy modality. A cohort of contemporaneous UM patients who underwent biopsy via a transscleral technique served as a comparator group for these patient, tumor, and complication factors.
Results:
A total of 27 patients aged 27.2–88.6 years (mean 64.8) underwent transvitreal biopsy using our described technique between 2013–2016. There were 15 small, 10 medium, and 2 large tumors at diagnosis with the majority (n=17) posterior to the equator. Intraoperative complications included a clot or small trickle of blood at the biopsy site in 20 (74.1%) of patients, small localized subretinal hemorrhage in 8 (29.6%), small vitreous hemorrhage in 4 (14.8%), and small transient choroidal detachments in 1 patient (3.6%). When subretinal hemorrhage occurred, it was almost always into a pre-existing pocket of subretinal fluid (p=0.0093). However, the presence of subretinal fluid was not associated with the decision to proceed with any biopsy (p=0.36) or transvitreal biopsy specifically (p=1.00). By 3 months, subretinal and/or vitreous hemorrhage resolved in essentially all cases. There were no cases of iatrogenic retinal detachment or extraocular tumor spread over a mean follow-up of 41.7 (range: 20–62.1) months. Adequate tissue for gene expression profiling was obtained from each biopsy. The comparator group of patients undergoing transscleral biopsy including 21 UMs in 20 patients (one eye had 2 melanomas). Transvitreal biopsies were more common in patients with small (n=15; p<0.0001), posterior (n=17; p<0.0001) tumors, compared to patients who underwent transscleral biopsy during the same period.
Conclusions:
This technique can be used for small or posterior tumors, or for small anterior tumors where a transscleral approach would risk tumor perforation. Complications were minor, transient, and self-limited. Biopsy yields for molecular prognosis were adequate in all cases. The presence of subretinal fluid may be considered a relative contraindication, because it may lead to subretinal hemorrhage in the fluid pocket, but did not dissuade us from utilizing this transvitreal technique for patients who would benefit from it.
Keywords: biopsy, choroidal melanoma, uveal melanoma, retina surgery
Brief Summary:
We describe a technique for biopsy of uveal melanomas using the BIOM system, which allows for excellent visualization and control. All of the manipulations performed are geared toward minimizing the known complications of transvitreal tumor biopsy. We include data from the first 27 patients who underwent biopsy at our institution.
INTRODUCTION
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults.1 Despite effective local therapies with excellent local control rates, approximately 50% of patients develop liver metastases, which are almost uniformly fatal1,2. Clinical risk factors for metastasis include clinical and histologic characteristics such as large tumor basal diameter and thickness, extrascleral extension, epithelioid cell type, and high mitotic index3. Several molecular methods, including gene expression profiling (GEP) and multiplex ligation-dependent probe amplification (MLPA), exist to aid with prognostication and stratify metastatic risk for uveal melanoma,4–6 and these molecular tests have been shown to be superior to clinical and pathologic characteristics.7,8 Unlike histopathologic risk factors, which can only be discerned on enucleation specimens, samples for molecular testing can be obtained by fine needle aspiration biopsy (FNAB). Since prognostic testing is now potentially available to all patients, not just those undergoing enucleation, this has become more important both for patient counseling and possibly for metastatic surveillance to allow early detection of metastasis in patients at high risk7,9–13.
However, FNAB does potentially involve certain risks. Reported risks of FNAB include hemorrhage, retinal detachment, and extraocular extension of tumor through the needle track.14–16 In particular, risk of complications is greatest with posterior tumors that must be approached through a transvitreal route, because a trans-retinal needle pass is necessary and increases the risk of retinal tear and detachment, and because the plaque does not cover and sterilize the scleral needle track. Controversy exists regarding the best method for biopsy of UM, particularly when a transvitreal approach is indicated. We describe an optimized transvitreal biopsy technique, which benefits from BIOM-assisted visualization but without the time required for full vitrectomy and without the costs associated with a full vitrectomy pack. We evaluate the indications, efficacy and safety profile of this technique.
METHODS
Patient Population and Inclusion Criteria
This is a retrospective study that was performed in accordance with the tenets of the Declaration of Helsinki and with the Health Insurance Portability and Accountability ACT (HIPAA). This study was approved by the Institutional Review Board at Vanderbilt University Medical Center. Written, informed consent was provided by all patients before undergoing any of the described procedures.
Inclusion criteria were patients at least 18 years of age with a diagnosis of uveal melanoma who underwent FNAB via the described technique at Vanderbilt University Medical Center between November 2013 and March 2017. Each patient was staged according to both the collaborative ocular melanoma study classification and the guidelines of the 7th edition of the American Joint Committee on Cancer (AJCC) TNM (Tumor-Node-Metastasis) staging system.17 Records were analyzed for tumor size, location, treatment modality, method of biopsy and any complications thereof. Tumor thickness, diameter, location, and the presence of subretinal fluid were recorded, to analyze if there was any correlation between these tumor factors and the decision to proceed with biopsy or the particular biopsy technique selected (transvitreal or transscleral).
Fine Needle Aspiration Biopsy Technique
The specific method, and the rationale for specific surgical maneuvers and choice of implements, is demonstrated and described in the attached video (see Video 1). Briefly, a localized peritomy was created and episcleral hemostasis was obtained. The direction of approach (temporal or nasal) was decided based on tumor location. An off-side 25-gauge valved cannula was inserted in a uniplanar fashion 3.5 mm posterior to the limbus (regardless of lens status) for the lightpipe. A second 25-gauge non-valved cannula was then inserted in a uniplanar fashion on the side from which the biopsy was to be performed. The BIOM wide-angle operative viewing system was used, and no vitrectomy was performed. A 25- or 27-gauge light pipe was used via the valved cannula to illuminate the tumor while a 27-gauge, 1.25 inch needle (attached via a short segment of IV tubing to a 10 cc syringe) (see Figure 1) was inserted through the non-valved cannula. Once the insertion site was selected and the needle inserted, bevel up, into the tumor near the tumor apex under direct visualization. Any visible retinal vessels or pockets of subretinal fluid were avoided. Sample was gently aspirated under constant manual pressure by the assistant to 1.5mL. During aspiration, the needle tip was rotated and also advanced and withdrawn slightly within the tumor to sample multiple locations along the needle track. The needle was withdrawn while maintaining the plunger position at the 1.5mL mark, and without additional suction. Care was taken to avoid relieving traction on the plunger once the needle tip was removed from the tumor to avoid expulsion of needle contents back into the vitreous cavity. The syringe was then detached and filled with air. The sample was expressed with air from the needle into a tube. The needle was then washed with RNA extraction buffer and combined with the original sample, and the tube was immediately placed on dry ice and then sent for gene expression profile analysis. Avastin was injected intravitreally. Both sclerotomies were inspected for evidence of tumor/pigment, and were then sutured with 7–0 vicryl suture. Plaque placement then proceeded as per usual.
Figure 1:
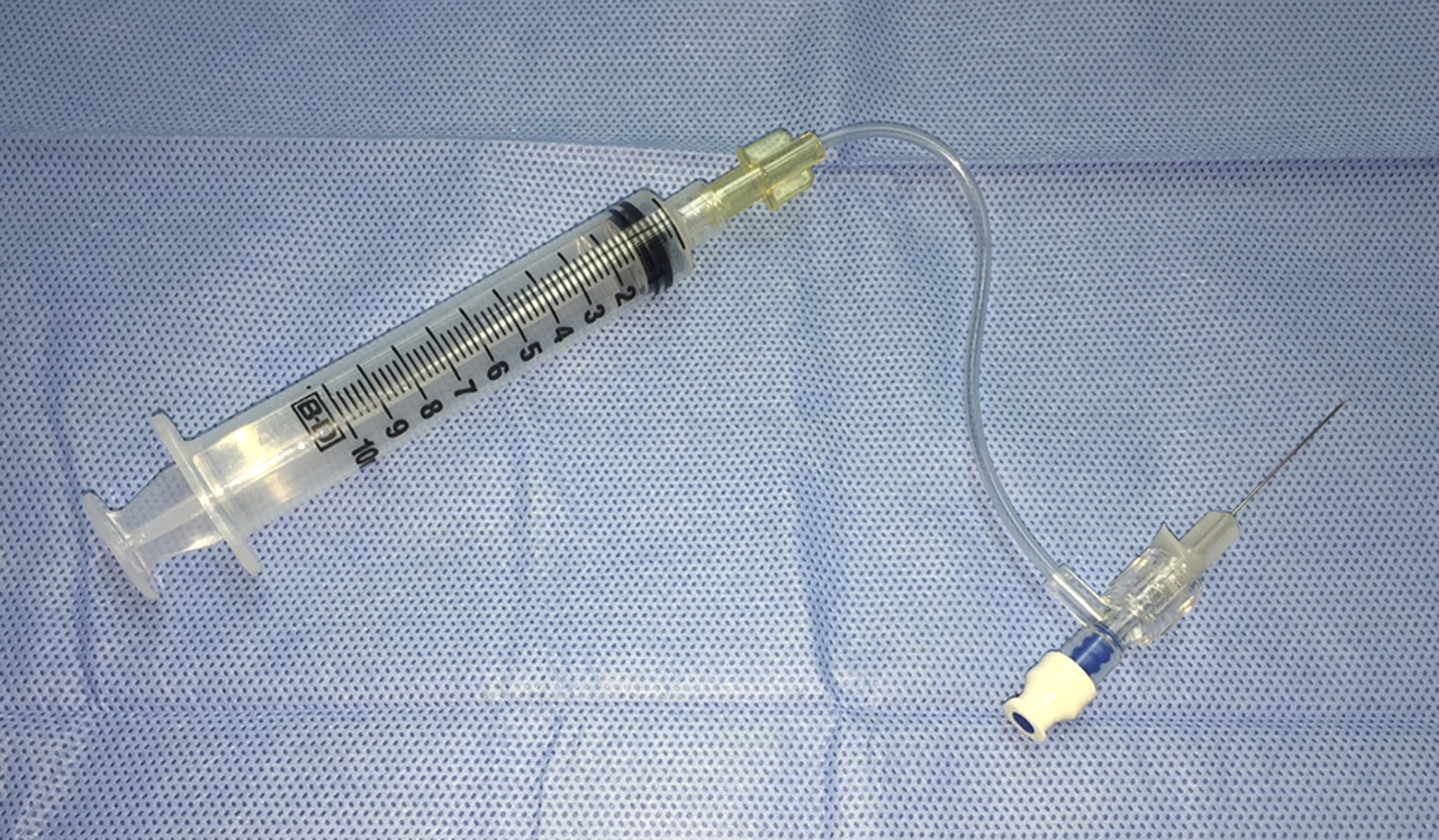
Biopsy Setup
A 27 gauge 1 ¼” needle attached to flexible tubing connected to a 10 mL syringe allows for adequate suction and good surgical control.
Patient Follow Up
The first follow up visit was scheduled on post-operative day 1, followed by 1 month after the treatment and then every 3–6 months. These follow up visits involved comprehensive ophthalmic examinations (visual acuity, slit-lamp biomicroscopy, dilated fundus examination) along with ultrasonography, optical coherence tomography (OCT), and fundus photography.
During the follow up period, all adverse events occurring after FNAB were recorded including: retinal detachment, extent of vitreous hemorrhage (if present), subretinal hemorrhage, endophthalmitis, epibulbar dissemination of tumor, local recurrence of tumor, and need for additional surgery. Vitreous hemorrhage was classified as localized or diffuse. Subretinal hemorrhage extent and location relative to preoperative subretinal fluid location was recorded.
Statistical Analysis
All statistical analyses were performed using Microsoft Excel 2010. The means and medians have been provided with their respective range. Proportions were compared by the X2 test or Fisher exact test. P values less than 5% (p<0.05) were considered statistically significant.
RESULTS
A total of 27 UMs (of 27 patients) underwent transvitreal biopsy using the described technique. Patient demographics are summarized in Table 1. The study group included 12 men (44.4%) and 15 women (55.6%) with a median age 68.1 (range 27.2–88.6) years. The mean follow up was 41.7 (range: 20–62.1) months. The mean tumor diameter was 11.0 mm (4.86–18.2 mm) and the mean thickness was 3.16 mm (1.65–8.7mm). According to the COMS criteria there were 15 small, 10 medium, and 2 large tumors at diagnosis. Of the 27 choroidal or ciliochoroidal tumors: 3 were anterior to the equator, 17 were posterior to the equator not involving the macula, and 7 were macular tumors. All 27 tumors were treated with plaque radiotherapy.
Table 1:
Patient Demographics
| Transvitreal Biopsy Group n (%) | Transscleral Biopsy Comparator Group n (%) | |
|---|---|---|
| Subjects n | 27 | 21 |
| Female n (%) | 15 (55.6%) | 12 (57.1%) |
| Median Age years (range) | 68.1 (27.2 – 88.6) | 66.1 (24.6 – 87.3) |
| Average Follow Up months (range) | 41.7 (20 – 62.1) | 40.1 (11.1 – 60.8) |
| Size n (%) | ||
| Small | 15 (55.6%) | 0 (0%) |
| Medium | 10 (37.0%) | 13 (61.9%) |
| Large | 2 (7.4%) | 8 (38.1%) |
| Location n (%) | ||
| Anterior to Equator | 3 (11.1%) | 15 (21.4%) |
| Posterior to Equator | 17 (63.0%) | 5 (23.8%) |
| Macular | 7 (25.9%) | 1 (4.8%) |
We included a group of UM patients against which to compare the patient and tumor characteristics of the patients undergoing our transvitreal biopsy technique. This comparator group consisted of all patients undergoing transscleral tumor biopsy during the same time period. This included 21 tumors in 20 patients (one patient had two distinct melanomas in one eye18, see Table 1). Transvitreal biopsies were more common in patients with small tumors (n=15/27; p<0.05), and tumors posterior to the equator (n=24/27; p<0.0001). A transcleral approach was typically used for more anterior tumors (n=15/21; p<0.05).
Of the 27 patients who had transvitreal biopsy, intraoperative complications included a clot or small trickle of blood at the biopsy site in 20 (74.1%) of patients, small localized subretinal hemorrhage in 9 (33.3%), small vitreous hemorrhage in 4 (14.8%), and small transient choroidal detachments in 1 patient (3.7%) (see Table 2 and Figures 2 and 3). Six of the nine cases of subretinal hemorrhage occurred in cases in which subretinal fluid was already present over the tumor (p=0.0093), and 6/11 tumors with preexisting subretinal fluid experienced subretinal hemorrhage. However, the presence of subretinal fluid was not associated with the decision to proceed with any biopsy (p=0.36) or transvitreal biopsy specifically (p=1.00), versus the comparator group of patients undergoing transscleral biopsy. By 3 months, almost all cases of subretinal or vitreous hemorrhage had resolved. There were no cases of endophthalmitis, iatrogenic retinal detachment, epibulbar extraocular tumor spread, or local recurrence.
Table 2:
Complications of Transvitreal Biopsy
| Intra-operative | ≥ 3 months | |
|---|---|---|
| Clot at Biopsy Site | 20 (71.4%) | 0 (0.0%) |
| Vitreous Hemorrhage | 4 (14.8%) | 2 (7.4%) |
| Subretinal Hemorrhage | 9 (33.3%) | 3 (11.1%) |
| Choroidal Formation | 1 (3.6%) | 0 (0.0%) |
| Retinal Detachment | 0 (0.0%) | 0 (0.0%) |
| Endophthalmitis | 0 (0.0%) | 0 (0.0%) |
| Epibulbar tumor spread | 0 (0.0%) | 0 (0.0%) |
| Recurrence | 0 (0.0%) | 0 (0.0%) |
| None | 1 (3.6%) | 23 (85.2%) |
Figure 2:
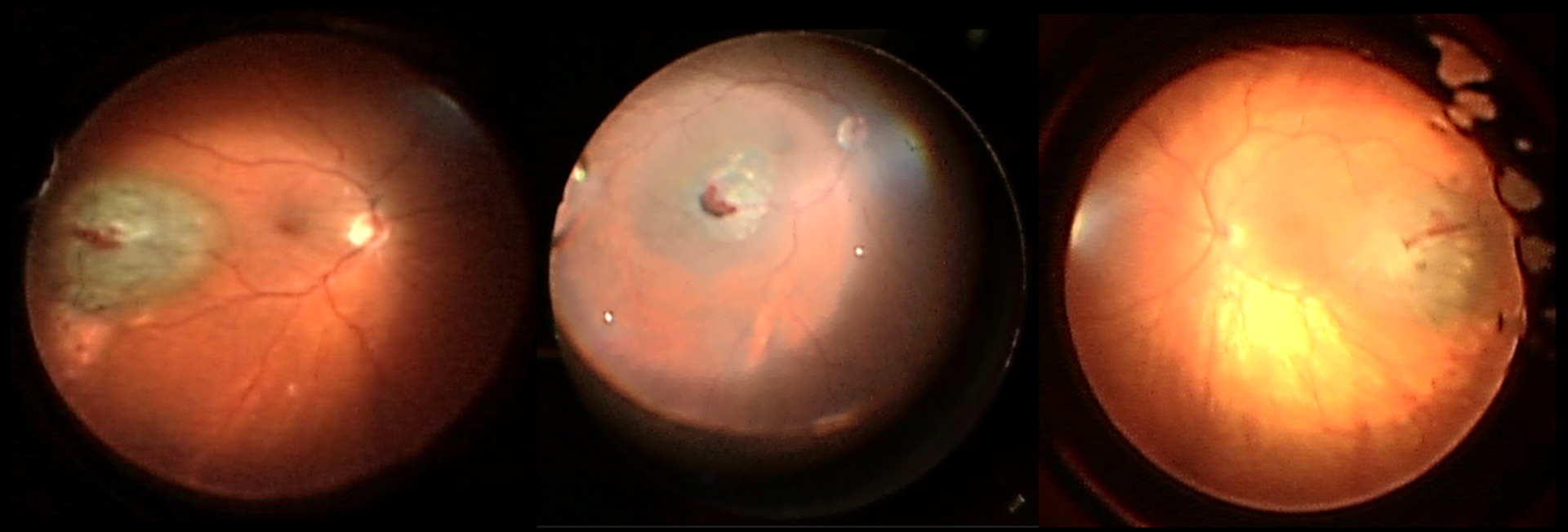
Typical small hemorrhage seen intraoperatively immediately after biopsy
Intraoperative photos of three different eyes that underwent transvitreal biopsy for malignant melanomas posterior to the equator using the described technique. Each shows the typical appearance of a small clot at the biopsy site immediately after the sample was taken.
Figure 3:
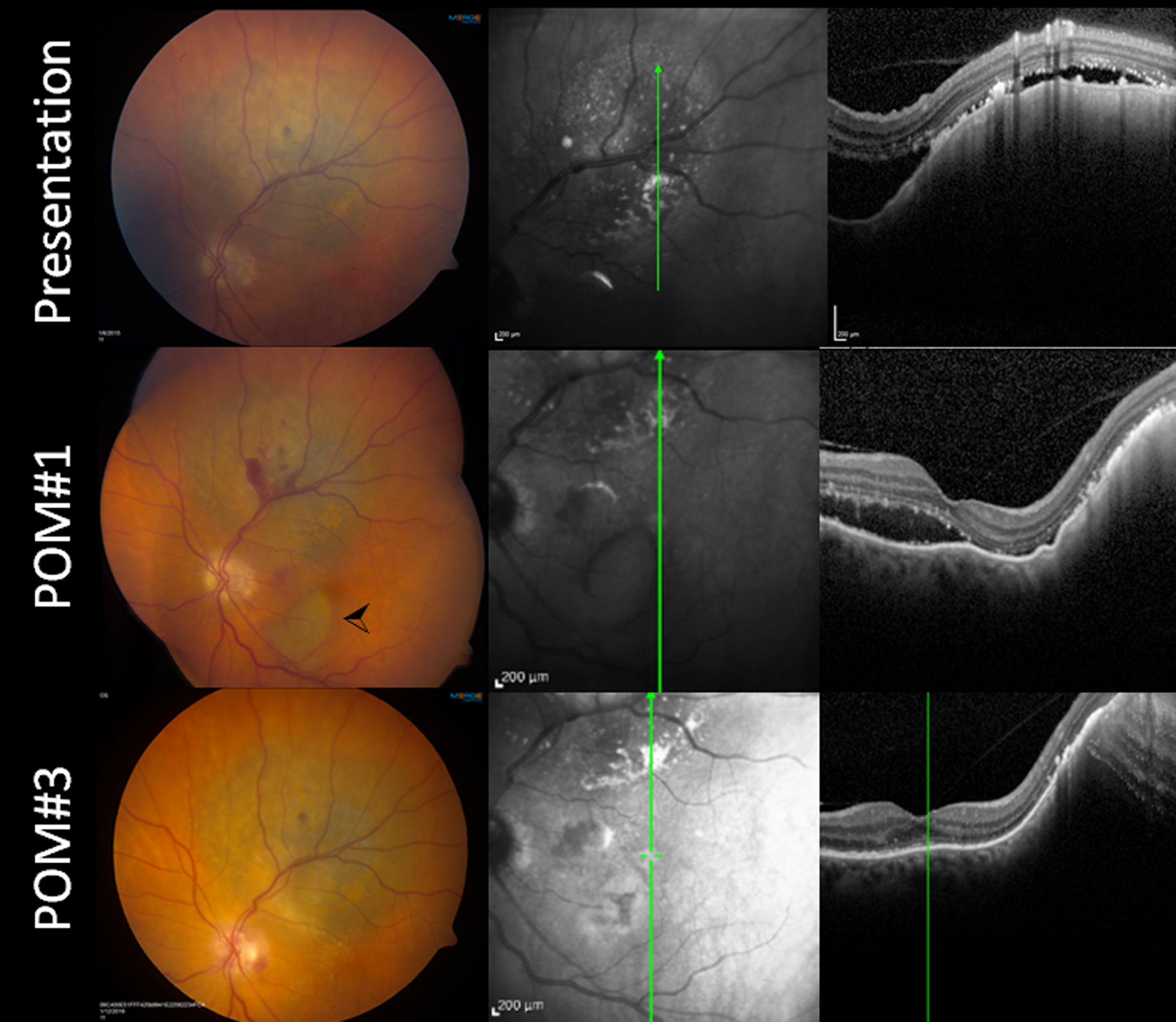
Subretinal hemorrhage into area of existing exudative retinal detachment.
A patient with a medium juxtapapillary melanoma (A) with adjacent exudative detachment with subretinal fluid extending into the macula elected to undergo transvitreal biopsy prior to plaque placement. The patient was on aspirin, and this was not discontinued. The extent of the subretinal fluid can be seen on optical coherence tomography (B, C). At the time of biopsy, there was subretinal hemorrhage extending into the macula. (D-F) One-month postoperative images. D) Fundus photograph demonstrating residual minor hemorrhage at the biopsy site and a pocket of dehemoglobinized blood in the inferior macula (arrowhead). (E-F) The OCT shows the subretinal fluid resolving, though still present into the macula. (G-I) Three-month postoperative images. G) Fundus photograph demonstrating resolution of the subretinal hemorrhage. The subretinal fluid similarly resolved, and none was visible on optical coherence tomography at that point (H-I). The hemorrhage and the subretinal fluid had resolved both clinically and on OCT. There were minor pigmentary changes best visible on infrared imaging (H) in the macula in the location of the previous subretinal blood.
Gene expression profile class could be determined in all transvitreal biopsy samples (n=27), giving a success rate of 100%. Of these patients, 22 (81.5%) were class 1A, 4 (14.8%) were 1B, and 1 (3.7%) was class 2. Normal discriminant values were reported for all GEP results.
During follow up, no patients who underwent plaque radiotherapy had any evidence of recurrence, or required enucleation for treatment failure.
DISCUSSION
We demonstrate that our transvitreal approach, utilizing a 27-gauge needle under BIOM visualization without prior vitrectomy, has excellent clinical yield with minimal complications, while being simpler and less costly than performing a full vitrectomy. This technique has several benefits: 1) it is simple to perform and does not require vitreoretinal surgical training, 2) it allows excellent tumor visualization, 3) it incorporates many surgical variations that likely reduce the risk of both intraocular complications and also epibulbar tumor spread, 4) it does not require specialized vitreoretinal equipment in the operating room such as for vitrectomy, 5) it is quick to perform and minimizes operative time, 6) intraocular complications are lower than for all previously-reported techniques/series, and 7) sample yield is higher than for all previously-reported techniques/series. Our experience using this technique shows that it may be most advantageous for patients with posterior tumors of any size, or small anterior tumors. These tumors are generally more difficult tumors to approach transclerally compared to larger, more anterior tumors.
Our technique includes several variations that we feel minimize the usual risks of transvitreal biopsy, including the risks of retinal detachment, epibulbar tumor spread, and diffuse vitreous hemorrhage, that may decrease postoperative vision and may preclude subsequent transpupillary thermotherapy. Specifically, we advocate for the following maneuvers: 1) epibulbar hemostasis with cautery to allow for better visualization of any tumor tissue/pigment on the scleral surface, 2) non-beveled trocar entry to minimize passage length through the sclera and to allow for better visualization of any tumor tissue/pigment in the cannula track, and 3) suturing of the wound to again minimize the risk of epibulbar seeding. Similarly, we advocate the following choice of implements: 1) the use of cannulas, so the outside of the needle, which was in contact with the tumor tissue, does not come in contact with the sclera, 2) a non-valved cannula (for the biopsy side) so any tumor tissue clinging to the outside of the needle is not wiped off by the valve leaflets into the cannula and back into the eye, and 3) a 27-gauge needle in a 25-gauge cannula, again to minimize tumor tissue being wiped off by the inner cannula rim into the eye. Singh et al recently examined cells remaining on the inside of a 25-gauge valved cannula after FNAB for melanoma, and found that 4 of 20 samples showed either melanoma cells or atypical cells.19 Presumably, without the valves, any cells clinging to the exterior of the needle after biopsy would stay on the needle as it is withdrawn from the eye and removed from the field, thus decreasing the theoretical risk of epibulbar tumor extension, as compared to clinging to the valve leaflet near the scleral surface, as was found in their study.
In this study, the most common complication involved minor hemorrhage at the biopsy site. The degree of hemorrhage seen with this technique was significantly less than that reported with other techniques, such as needle biopsy under indirect ophthalmoscopy guidance or vitrector biopsies20–24, owing to the precise needle placement and retinal vessel avoidance possible under BIOM visualization, and the small gauge, pointed tip, and atraumatic entry of a 27-gauge needle. The needle also has the benefit of ubiquitous availability and low cost as compared to a vitrector or a full vitrectomy setup, and does not require a vitrectomy machine to be available in the OR. Furthermore, without the need for a vitrectomy, the surgery is more efficient, the patient is exposed to less anesthesia, and the overall operating room time is minimized.
In order to minimize bleeding during surgery, other practitioners have advocated for transiently increasing intraocular pressure, using an endolaser to assist with hemostasis, or stopping anticoagulant medications prior to surgery. Using our transvitreal technique, we do not find that any of these measures is necessary. As we do not perform a vitrectomy with our FNAB technique, no infusion line is used, and although external pressure could be theoretically used to increase the intraocular pressure, the bleeding is so minimal and hemostasis is achieved spontaneously that this is unnecessary. Similarly, we do not advocate using an endolaser in an eye that has not undergone vitrectomy, because of the risk of introducing iatrogenic tears.
In our hands, choroidal tumor biopsies with the 27-gauge vitrector have led to more hemorrhage than the needle-based technique presented here. However, we acknowledge that, for very thin tumors, the vitrector has the advantage of eliminating the risk of piercing the sclera under the tumor. This is particularly true for tumors less than 2 mm in thickness, since the standard 27-gauge needle has a bevel 2 mm in length. We have found that an angled entry in these cases allows extra leeway by increasing the intratumoral distance through which the needle can pass safely. It should be noted, however, that using vitrectomy cannulas does not allow the surgeon to pre-bend the needle to allow a more angled approach into the tumor mass, as can be done when no cannulas are used. For the reasons described in the previous paragraph, we feel that the benefits of cannulas outweigh this potential disadvantage.
The most common complications seen were a small localized clot of blood over the needle entry site in 20 (74.1%) biopsies and subretinal hemorrhage in 8 (29.6%) biopsies, which was seen predominantly trickling into pre-existing pockets of subretinal fluid overlying the tumor. Importantly, subretinal hemorrhage was seen only rarely when subretinal fluid was not pre-existing, and the hemorrhage did not extend beyond the area of pre-existing subretinal fluid. Significant vitreous hemorrhage was seen in 4 (14.8%) biopsies. Almost all of our patients had spontaneous resolution of this localized vitreous hemorrhage and of any subretinal hemorrhage by three months postoperatively. Only 5 (18.5%) patients had old vitreous hemorrhage or very small areas of subretinal hemorrhage still remaining at 3 months. Of these, 1 patient was on coumadin, 3 were on aspirin, and 1 was not on any anticoagulation. This suggests that hemorrhage may persist in anticoagulated patients, but given the minor nature and rarity of persistent hemorrhage, and a lack of significant visual impact, our practice does not include holding any anticoagulants, especially since many UM patients are older, and may require continued anticoagulation for cardiac comorbidities.
This rate is similar or less than previously reported rates of vitreous hemorrhage following transvitreal biopsy under indirect ophthalmoscopy, which ranged from 46–80%5,9,13,25. However, in a series using a 25-gauge vitreous cutter to perform a transvitreal biopsy, the vitreous hemorrhage was so great that 80% of patients required subsequent vitrectomy to clear visually significant vitreous hemorrhage.25 In a separate series of biopsies performed with a 27-gauge vitrector, 72% of eyes had vitreous hemorrhage, and 46% of eyes required limited vitrectomy at the time of biopsy.20 In contrast, in our present series, essentially all cases of vitreous hemorrhage resolved spontaneously by 3 months, and no patients had vitreous hemorrhage that necessitated vitrectomy.
We did not observe any cases of iatrogenic retinal detachment with our technique. This is in contrast to rates of retinal detachment reported to be as high as 11% when a 27-gauge vitrector is used.20 There were likewise no cases of extraocular tumor spread or local recurrence, consistent with the very low rates in previous published series.5,9,13,14,16,25 Very few cases of endophthalmitis have been published after FNAB, and we did not experience any in our series either.14,24,26,27
The risk of any procedure must balance the success of the procedure and any potential complications. Our described approach offers the advantage of lower complication rates and high efficacy in obtaining sufficient material for GEP analysis and class categorization (100% of samples were sufficient using our transvitreal technique). Other published success rates for transvitreal biopsy with other techniques range from 71 – 97%.5,13,28
Strengths of our study include long-term follow-up (>3 years in all patients, and almost 6 years for many of them), and performance of biopsy by a single vitreoretinal- and ocular oncology-trained surgeon using the exact same transvitreal technique in each case. Potential limitations are the retrospective nature of the study, the absence of a comparator group in which an alternative transvitreal technique was utilized (although a transscleral biopsy comparator group is included), and the absence of any randomization.
Supplementary Material
Funding:
This work was funded by NIH/NEI 5K08EY027464-02 [ABD], Research to Prevent Blindness Career Development Award [ABD], and an unrestricted grant from Research to Prevent Blindness to the Vanderbilt Department of Ophthalmology and Visual Sciences.
Footnotes
A portion of this work was presented at the Vail Vitrectomy meeting, Vail, Colorado, February 20–23, 2016, and at the Association for Research in Vision and Ophthalmology Annual Meeting, Baltimore, Maryland, May 7–11, 2017.
None of the authors has any financial disclosures or conflicts of interests to report.
Supplemental Content:
Transvitreal Biopsy Technique Final.wmv
REFERENCES:
- 1.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Archives of ophthalmology (Chicago, Ill : 1960). 2005;123(12):1639–1643. [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Investigative ophthalmology & visual science. 2003;44(11):4651–4659. [DOI] [PubMed] [Google Scholar]
- 3.Gamel JW, McCurdy JB, McLean IW. A comparison of prognostic covariates for uveal melanoma. Investigative ophthalmology & visual science. 1992;33(6):1919–1922. [PubMed] [Google Scholar]
- 4.Cassoux N, Rodrigues MJ, Plancher C, et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. The British journal of ophthalmology. 2014;98(6):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields CL, Ganguly A, Materin MA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases. Transactions of the American Ophthalmological Society. 2007;105:43–52; discussion 52–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Schopper VJ, Correa ZM. Clinical application of genetic testing for posterior uveal melanoma. Int J Retina Vitreous. 2016;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols EE, Richmond A, Daniels AB. Tumor Characteristics, Genetics, Management, and the Risk of Metastasis in Uveal Melanoma. Semin Ophthalmol. 2016;31(4):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CL, Ganguly A, Bianciotto CG, Turaka K, Tavallali A, Shields JA. Prognosis of uveal melanoma in 500 cases using genetic testing of fine-needle aspiration biopsy specimens. Ophthalmology. 2011;118(2):396–401. [DOI] [PubMed] [Google Scholar]
- 10.Damato B, Eleuteri A, Taktak AF, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. 2011;30(5):285–295. [DOI] [PubMed] [Google Scholar]
- 11.Voelter V, Schalenbourg A, Pampallona S, et al. Adjuvant intra-arterial hepatic fotemustine for high-risk uveal melanoma patients. Melanoma Res. 2008;18(3):220–224. [DOI] [PubMed] [Google Scholar]
- 12.Lane AM, Egan KM, Harmon D, Holbrook A, Munzenrider JE, Gragoudas ES. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology. 2009;116(11):2206–2212. [DOI] [PubMed] [Google Scholar]
- 13.Sellam A, Desjardins L, Barnhill R, et al. Fine Needle Aspiration Biopsy in Uveal Melanoma: Technique, Complications, and Outcomes. American journal of ophthalmology. 2016;162:28–34.e21. [DOI] [PubMed] [Google Scholar]
- 14.Caminal JM, Sanz S, Carreras M, Catala I, Arruga J, Roca G. Epibulbar seeding at the site of a transvitreal fine-needle aspiration biopsy. Archives of ophthalmology (Chicago, Ill : 1960). 2006;124(4):587–589. [DOI] [PubMed] [Google Scholar]
- 15.Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87(6):588–601. [DOI] [PubMed] [Google Scholar]
- 16.Schefler AC, Gologorsky D, Marr BP, Shields CL, Zeolite I, Abramson DH. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA ophthalmology. 2013;131(9):1220–1224. [DOI] [PubMed] [Google Scholar]
- 17.Kivela T, Kujala E. Prognostication in eye cancer: the latest tumor, node, metastasis classification and beyond. Eye (London, England). 2013;27(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MM, Papakostas TD, Malcolm AW, et al. Multiple simultaneous choroidal melanomas arising in the same eye: globe salvage by radiotherapy. Acta Ophthalmol. 2016;94(8):e799–e802. [DOI] [PubMed] [Google Scholar]
- 19.Singh AD, Aziz HA, Pelayes D, Biscotti CV. TWENTY-FIVE-GAUGE CANNULA-ASSISTED FINE-NEEDLE ASPIRATION BIOPSY OF CHOROIDAL MELANOMA: Cytopathological Analysis. Retina. 2017;37(9):1674–1677. [DOI] [PubMed] [Google Scholar]
- 20.Grewal DS, Cummings TJ, Mruthyunjaya P. Outcomes of 27-Gauge Vitrectomy-Assisted Choroidal and Subretinal Biopsy. Ophthalmic Surg Lasers Imaging Retina. 2017;48(5):406–415. [DOI] [PubMed] [Google Scholar]
- 21.Sen J, Groenewald C, Hiscott PS, Smith PA, Damato BE. Transretinal choroidal tumor biopsy with a 25-gauge vitrector. Ophthalmology. 2006;113(6):1028–1031. [DOI] [PubMed] [Google Scholar]
- 22.Sellam A, Desjardins L, Barnhill R, et al. Fine Needle Aspiration Biopsy in Uveal Melanoma: Technique, Complications, and Outcomes. American journal of ophthalmology. 2016;162:28–34 e21. [DOI] [PubMed] [Google Scholar]
- 23.Bagger M, Tebering JF, Kiilgaard JF. The ocular consequences and applicability of minimally invasive 25-gauge transvitreal retinochoroidal biopsy. Ophthalmology. 2013;120(12):2565–2572. [DOI] [PubMed] [Google Scholar]
- 24.Eide N, Syrdalen P, Walaas L, Hagmar B. Fine needle aspiration biopsy in selecting treatment for inconclusive intraocular disease. Acta Ophthalmol Scand. 1999;77(4):448–452. [DOI] [PubMed] [Google Scholar]
- 25.Grixti A, Angi M, Damato BE, et al. Vitreoretinal surgery for complications of choroidal tumor biopsy. Ophthalmology. 2014;121(12):2482–2488. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner-Jones BE, Foster WJ, Harbour JW, Smith ME, Davila RM. Fine needle aspiration biopsy with adjunct immunohistochemistry in intraocular tumor management. Acta cytologica. 2005;49(3):297–308. [DOI] [PubMed] [Google Scholar]
- 27.Cohen VM, Dinakaran S, Parsons MA, Rennie IG. Transvitreal fine needle aspiration biopsy: the influence of intraocular lesion size on diagnostic biopsy result. Eye (London, England). 2001;15(Pt 2):143–147. [DOI] [PubMed] [Google Scholar]
- 28.Chang MY, McCannel TA. Comparison of uveal melanoma cytopathologic sample retrieval in trans-scleral versus vitrectomy-assisted transvitreal fine needle aspiration biopsy. The British journal of ophthalmology. 2014;98(12):1654–1658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


