Abstract
Background
High levels of the tumor necrosis factor alpha (TNF-α) induce apoptosis and pro-inflammatory effects for primary degeneration of tendon and development of tendinopathy. The aim of this study was to investigate the association between the TNF-α polymorphisms and tendinopathy in athletes.
Methods
Two hundred and seventy athletes (135 tendinopathy cases and 135 controls) were included and genotyped (TNF-α -1031T > C; -857 C > T; -308G > A) using TaqMan validated assays. The association of the polymorphisms with tendinopathy was evaluated by a multivariate logistic regression model, using odds ratios (OR) and 95 % confidence intervals (CI).
Results
The variant allele − 308 A was significantly associated with patellar (OR: 1.9; 95 % CI: 1.01–3.6) or Achilles tendinopathies (OR: 2.7; 95 % CI: 1.1–6.7). No significant differences were found in allele or genotype distributions of the − 1031T > C and − 857 C > T polymorphisms between cases and controls. TNF-α TCA haplotype was associated with increased tendinopathies risk, either considering all cases (OR: 2.6, 95 % CI: 1.3–5.3), patellar (OR: 3.3, 95 % CI: 1.5–7.3), rotator cuff (OR: 3.1, 95 % CI: 1.4–7.2) or Achilles tendinopathies (OR: 3.8, 95 % CI: 1.1–12.7).
Conclusions
These results suggest that the TNF-α polymorphisms could influence the susceptibility to developing tendinopathy among athletes. Knowledge of the TNF-α polymorphisms associated to tendinopathy in athletes can further understanding of the inflammatory role in the early stages of the disease and contribute for sports injury surveillance programmes, in which athletes with TNF-α TCA haplotype could be early subjected to cryotherapy after training and competition to avoid tendinopathy development.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13102-021-00276-2.
Keywords: Tendinopathy, Polymorphism, TNF-α, Athletes
Background
Tendinopathy is characterized by pain, swelling, structural change and functional limitation of the tendon due to overuse [1, 2]. It is the main reason for clinical musculoskeletal complaint in athletes (15–50 %) [3], which can lead to reduced level of performance or end of one’s sport career [4]. The commonly identified risk factor associated with tendinopathy in athletes are age, sex, metabolic and hormonal concentrations, and high physical load during training and matches according to each sport modality [4, 5].
Single nucleotide polymorphisms (SNPs), a variation of the nucleotide at a single position in DNA sequence, involved with inflammatory process were associated as non-modifiable factors for developing tendinopathies [6–8]. Pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), and growth factors have been implicated as a mechanism in early stages of tendinopathies [1, 9]. Macrophages, mast cells, fibroblasts and endothelial cells synthesized and released TNF-α cytokine, which is the main chemokine inducer when tendons are mechanically overloaded [1]. TNF-α signaling is mediated by two functionally distinct receptors: TNF-α receptor-1 (TNFR1) and TNF-α receptor-2 (TNFR2). The ligand receptor interaction between TNF-α–TNFR1 is responsible for induce apoptosis and proinflammatory effects, while the interaction between TNF-α–TNFR2 regulates tissue growth and repair [10]. An experimental tendinopathy model produced by overuse shown that TNF-α mRNA was increased 11-fold in torn supraspinatus tendon compared to controls [11]. In addition, TNF-α and its receptors were expressed in peritendinous tissue [12], and in rounded/enlarged nucleus human tenocytes, a typical characteristic of tendinopathy [13].
TNF-α is encoded by the gene of the same name, located on chromosome 6p21.3 between the human leukocyte antigen-B (HLA-B) and human leukocyte antigen-DR (HLA-DR) genes at the major histocompatibility complex class III region [14]. The SNPs in the promoter region of TNF-α gene, such as -1031T > C (rs1799964), -857 C > T (rs1799724) and − 308G > A (rs1800629), have shown potential to alters the binding of transcription factors in the DNA, regulating TNF-a expression [15, 16]. The choice of TNF-α SNPs was due to their biological relevance in altering the expression of the gene and for have relatively high frequency in different populations [15]. In addition, previous studies associated these TNF-α SNPs with some diseases, such as inflammatory bowel diseases [17], Congenital Zika syndrome [18], and cystic fibrosis [19].
We hypothesized that the TNF-α SNPs could be associated with a risk of developing tendinopathy in athletes; since high TNF-α expression could be modulated by polymorphisms and TNF-α induce apoptosis and pro-inflammatory effects for primary degeneration of tendon. As far as we know, there are no studies evaluating the influence of TNF-α SNPs as possible risk factors involved in the inflammatory molecular mechanism leading to tendinopathy. Thus, this study aimed to investigate the association between the TNF-α polymorphisms and tendinopathy in athletes.
Methods
Study design and population
This case-control study was approved by the Human Ethics Committee of the Instituto Nacional de Traumatologia e Ortopedia Jamil Haddad (protocol number 2.455.630/2017). All participating athletes provided written informed consent and answered a questionnaire about their epidemiological, clinical, sport and training characteristics, as well as tendon injury history and their specific information such as type, location and number of tendinopathy episodes, as previously described [20]. At the end of data collection, a trained observer checked the questionnaire with each athlete, and the database was double-checked by different trained researchers.
The inclusion criteria were Brazilian competitive levels athletes aged 18–45 years old who were recruited between March 2018 and September 2019 at different sports training centres and competitions.
One hundred thirty-five athletes had tendinopathy clinically diagnosed by medical practitioners and confirmed with magnetic resonance image examination (MRI). All tendinopathy diagnoses were confirmed by two blinded specialized orthopaedic surgeons, as described in previous studies [6, 21]. The control group (N = 135) consisted of athletes without previous imaging diagnosis of tendinopathy and who were matched with tendinopathy cases for age (difference of ± 2 years), sex and sport modality. The sample size was calculated using Epi Info 7, version 7.1.3. (http://wwwn.cdc.gov/epiinfo/html/downloads.htm) to detect a difference between case and control groups, assuming an odds ratio (OR) of 2.0 with a power of 0.8 and 5 % type I error. The OR was based on previous evidence [22–24], and at least 128 athletes per group was necessary.
Genotyping of polymorphisms
Genomic DNA was obtained from oral mucosa collected from each athlete by swab. The TNF-α -1031T > C (rs1799964), -857 C > T (rs1799724) and − 308G > A (rs1800629) polymorphisms were genotyped using a TaqMan allelic discrimination assay obtained from Applied Biosystems (C___7514871_10, C__11918223_10 and C___7514879_10, respectively). For all polymorphisms real-time polymerase chain reaction (PCR) reactions were performed on a 7500 Real-Time System (Applied Biosystems, Foster City, CA, USA), and the genotypes were then determined directly. To assure genotyping quality, in each reaction two standardized positive controls of each polymorphism genotype were used.
Statistical analysis
The normally distribution of studied population was determined by the Shapiro-Wilk test. Comparisons of continuous variables between tendinopathy cases and controls groups were performed using the Student’s t test, and data were presented as mean ± standard deviation (SD). According to distribution and clinical significance the continuous variables (height, age at the beginning of sport practice, years of training and weekly training hours) were divided into quartiles. Categorical data were shown in proportions and differences between the two groups were evaluated using the Chi-squared (χ2) statistic test or Fischer exact test, when applicable.
Deviations from Hardy–Weinberg equilibrium (HWE) were assessed by the goodness-of-fit χ2 test. TNF-α (-1031T > C, -857 C > T, -308G > A) allele frequency and genotype distribution were derived by gene counting and frequencies between the two groups were compared using the χ2 test or, when appropriate, the Fisher’s exact test. The haplotype patterns and linkage disequilibrium coefficients (D’ is degree of imbalance in module and R2 is degree of correlation) were inferred using Haploview, as previously described [25].
Multivariate logistic regression analyses model were performed to evaluate the possible associations between epidemiological, clinic, sport and training characteristics as much as of the polymorphisms with tendinopathy, which was estimated by the OR with a 95 % confidence interval (95 % CI). As a final regression model used to control possible confounding factors, each variable was introduced considering the biological and statistical significance of the univariate analysis, which a input significance level less than 0.25 (P ≤ 0.25) and output significance was 0.05 (P ≤ 0.05) at the regression model. The difference was statistically significant when P < 0.05. All analyses were performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA, version 20.0).
Results
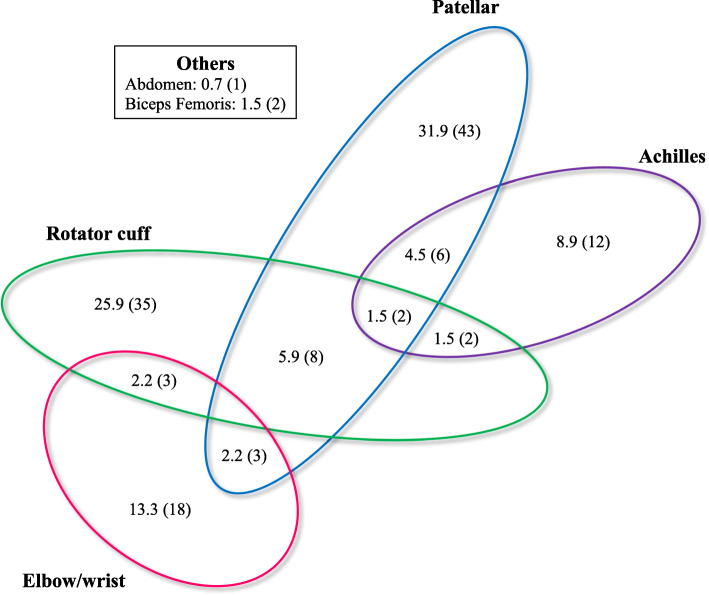
Of the 135 tendinopathy cases, 24 athletes (17.8 %) reported more than one diseased tendon. The cases reported tendinopathies of the patellar (N = 62, 45.9 %), rotator cuff (N = 50, 37.0 %), Achilles (N = 22, 16.3 %), wrist (N = 15, 11.1 %) and elbow tendinopathy (N = 9, 6.7 %) (Fig. 1).
Fig. 1.
Distribution of the tendinopathy locations in the study athletes (N = 135). The values are expressed in % (N)
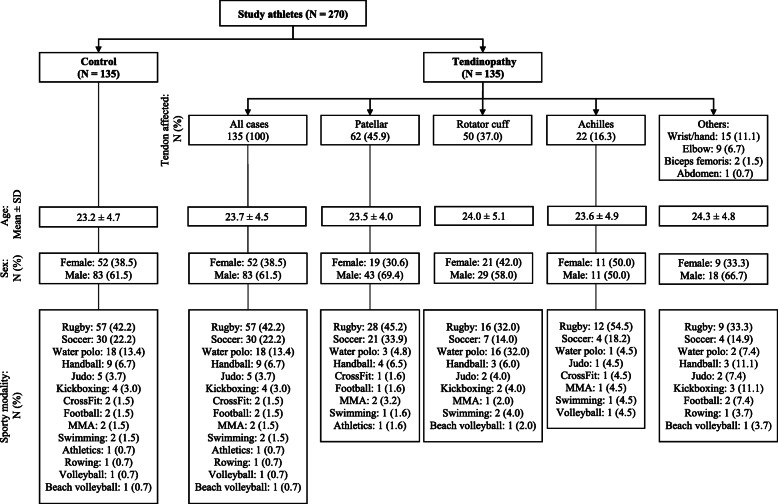
Age, sex, sport modality of the controls and all cases, as well as cases divided into affected tendon groups, is summarized in Fig. 2. There was no significant difference of the age, sex and sports modality between tendinopathies subgroups (patellar, rotator cuff and Achilles) and control; however, these variables entered the multivariate model for stratified association analyzes, according to the biological importance for the tendinopathy development.
Fig. 2.
Flowchart of the study population
The demographic, clinical, sport and training characteristics variables of all tendinopathy cases and controls were presented in Table 1. In summary, all variables were analyzed to identify possible confounding variables of the true association between SNPs and tendinopathy. Initially, the variables BMI (P = 0.09), alcohol consumption (P = 0.25), nutritional follow-up by a specialist during a sports career (P = 0.002), declared preference member (P = 0.21) and weekly training hours (P = 0.15) were inserted in the logistic regression model. After multivariate analysis, only BMI and nutritional follow-up remained in this model.
Table 1.
Epidemiological, clinical, sport and training characteristics of all athlete’s tendinopathy cases and controls (N = 270)
| Variables | Control (N = 135) |
Tendinopathy (N = 135) |
P-valuea,b | Unadjusted OR (CI 95%) |
Adjusted ORb (CI 95%) |
|---|---|---|---|---|---|
| N (%) | |||||
| Height (centimeters)d | |||||
| ≤ 166 | 36 (26.9) | 34 (25.2) | 0.75 | 1c | 1c |
| 167 – 175 | 38 (28.4) | 40 (29.6) | 1.11 (0.58 – 2.13) | 0.91 (0.35 – 1.56) | |
| 176 – 181 | 29 (21.6) | 25 (18.5) | 0.91 (0.45 – 1.86) | 0.66 (0.52 – 2.00) | |
| ≥ 182 | 31 (23.1) | 36 (26.7) | 1.23 (0.63 – 2.40) | 0.90 (0.44 – 1.83) | |
| BMI (Kg/m2)e | |||||
| < 25 | 87 (65.4) | 73 (54.5) | 0.04 | 1c | 1c |
| 25 – 29.99 | 41 (30.8) | 49 (36.6) | 1.42 (0.35 – 2.39) | 1.44 (0.85 – 2.46) | |
| ≥ 30 | 5 (3.8) | 12 (8.9) | 2.86 (0.93 – 2.50) | 3.65 (1.20 – 11.16) | |
| Level of schoolingf | |||||
| Middle school | 6 (4.5) | 3 (2.2) | 0.49 | 1c | 1c |
| High school | 60 (45.1) | 56 (41.5) | 1.87 (0.44 – 7.82) | 1.80 (0.39 – 8.31) | |
| University education | 67 (50.4) | 76 (56.3) | 2.27 (0.55 – 9.43) | 2.19 (0.48 – 10.02) | |
| Alcohol consumption | |||||
| No | 53 (39.3) | 44 (32.6) | 0.36 | 1c | 1c |
| Yes | 82 (60.7) | 91 (67.4) | 1.38 (0.81 – 2.20) | 1.27 (0.76 – 2.14) | |
| Smokingd | |||||
| No | 125 (93.3) | 122 (90.4) | 0.38 | 1c | 1c |
| Yes | 9 (6.7) | 13 (9.6) | 1.48 (0.61 – 3.59) | 0.44 (0.57 – 3.59) | |
| Nutritional follow-up | |||||
| No | 78 (57.8) | 52 (38.5) | 0.001 | 1c | 1c |
| Yes | 57 (42.2) | 83 (61.5) | 2.18 (1.34 – 3.55) | 2.31 (1.40 – 3.80) | |
| Side of dominance | |||||
| Right | 110 (81.5) | 104 (77.0) | 0.27 | 1c | 1c |
| Left | 11 (8.1) | 20 (14.8) | 1.92 (0.88 – 4.21) | 1.67 (0.74 – 3.76) | |
| Bilateral | 14 (10.4) | 11 (8.2) | 0.83 (0.36 –1.91) | 0.69 (0.29 – 1.63) | |
| Coachd | |||||
| Certified athletic trainer | 92 (68.1) | 81 (60.4) | 0.54 | 1c | 1c |
| Former professional athlete | 31 (23.0) | 34 (25.4) | 1.25 (0.70 – 2.20) | 1.20 (0.66 – 2.17) | |
| Both | 12 (8.9) | 19 (14.2) | 1.80 (0.82 – 3.93) | 1.53 (0.68 – 3.46) | |
| Age at the beginning of sport practice (years)d | |||||
| ≤ 10 | 41 (30.3) | 41 (30.6) | 0.80 | 1c | 1c |
| 11 – 14 | 32 (23.7) | 29 (21.6) | 0.91 (0.47 – 1.76) | 0.97 (0.48 – 1.95) | |
| 15 – 19 | 31 (23.0) | 37 (27.6) | 1.19 (0.63 – 2.27) | 1.33 (0.66 – 2.66) | |
| ≥ 20 | 31 (23.0) | 27 (20.1) | 0.87 (0.44 – 1.71) | 0.99 (0.48 – 2.04) | |
| Years of trainingd | |||||
| ≤ 5 | 49 (36.3) | 38 (28.4) | 0.84 | 1c | 1c |
| 6 – 8 | 25 (18.5) | 27 (20.1) | 1.39 (0.70 – 2.77) | 1.28 (0.63 – 2.61) | |
| 9 – 12 | 32 (23.7) | 35 (26.1) | 1.41 (0.74 – 2.67) | 1.29 (0.65 – 2.57) | |
| ≥ 13 | 29 (21.5) | 34 (25.4) | 1,51 (0.79 – 2.90) | 1.28 (0.65 – 2.52) | |
| Weekly training hours | |||||
| ≤ 7 | 38 (28.1) | 32 (23.7) | 0.46 | 1c | 1c |
| 8 – 12 | 49 (36.3) | 38 (28.1) | 0.92 (0.49 – 1.73) | 0.86 (0.44 – 1.68) | |
| 13 – 17 | 22 (16.3) | 24 (17.8) | 1.29 (0.61 – 2.73) | 1.22 (0.56 – 2.66) | |
| ≥ 18 | 26 (19.3) | 41 (30.4) | 1.87 (0.95 – 3.70) | 1.45 (0.70 – 2.99) | |
OR Odds ratio; CI confidence interval. aP-value ≤ 0.05 was obtained through the Chi-squared Test (Pearson p-value) or Fisher’s exact test. bOR adjusted by BMI and nutritional follow-up. cReference value. dInformation was obtained from 269 athletes. eInformation was obtained from 267 athletes. fInformation was obtained from 268 athletes.
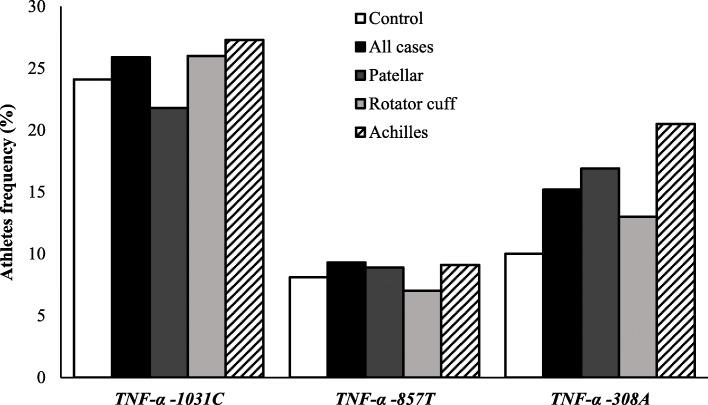
The distribution of TNF-α (-1031T > C, -857 C > T and − 308G > A) SNPs was in Hardy–Weinberg equilibrium. The minor allele frequencies of the TNF-α SNPs in the study population are shown in Fig. 3. After adjustment by co-factors of the logistic regression model (age, sex, sport modality, BMI and nutritional follow-up) the TNF-α-308 A allele was significantly associated with patellar and Achilles tendinopathies. Moreover, the TNF-α -308AA genotype was only present in the tendinopathy cases, either considering all cases, patellar, rotator cuff or Achilles tendinopathies. Considering the recessive co-dominance model (TNF-α -308GG + GA versus AA) the TNF-α -308AA genotype was significantly associated with tendinopathy cases, either considering all cases, patellar and Achilles tendinopathies (Table 2). Despite of the TNF-α -308AA genotype suggests a more likely of having tendinopathy nis more than one tendon, when to compared only tendinopathy cases group (one versus 2 or more affected tendons) there was not statistical power due to the decrease of the sample size (data not shown). In addition, no significant differences were found in allele or genotype distributions of the TNF-α -1031T > C and TNF-α -857 C > T polymorphisms between tendinopathy cases and controls (data not shown).
Fig. 3.
The minor allele frequencies of the SNPs in study population. P-value ≤ 0.05 was obtained through the Chi-squared Test (Pearson P-value) or Fisher’s exact test
Table 2.
Genotypic distributions of the TNF-α -308 G>A polymorphism and their association with tendinopathy
| TNF-α -308G>A | Control | Tendinopathy | P-valuea | Adjusted OR (CI 95%) |
|---|---|---|---|---|
| N (%) | ||||
| All tendinopathy casesb | N = 135 | N = 135 | ||
| GG | 108 (80.0) | 97 (71.9) | 0.06 | 1d |
| GA | 27 (20.0) | 35 (25.9) | 1.45 (0.80 – 2.64) | |
| AA | 0 (0.0) | 3 (2.2) | - | |
| GG+GA | 135 (100.0) | 132 (97.8) | 0.04 | 1d |
| AA | 0 (0.0) | 3 (2.2) | - | |
| G | 243 (90.0) | 229 (84.8) | 0.007 | 1d |
| A | 27 (10.0) | 41 (15.2) | 1.63 (0.95 – 2.81) | |
| Patellar tendinopathyc | N = 135 | N = 62 | ||
| GG | 108 (80.0) | 44 (71.0) | 0.01 | 1d |
| GA | 27 (20.0) | 15 (24.2) | - | |
| AA | 0 (0.0) | 3 (4.8) | ||
| GG+GA | 135 (100.0) | 59 (95.2) | 0.01 | 1d |
| AA | 0 (0.0) | 3 (4.8) | - | |
| G | 243 (90.0) | 103 (83.1) | 0.04 | 1d |
| A | 27 (10.0) | 21 (16.9) | 1.92 (1.02 – 3.66) | |
| Rotator cuff tendinopathyc | N = 135 | N = 50 | ||
| GG | 108 (80.0) | 38 (76.0) | 0.34 | 1d |
| GA | 27 (20.0) | 11 (22.0) | 1.27 (0.56 – 2.89) | |
| AA | 0 (0.0) | 1 (2.0) | - | |
| GG+GA | 135 (100.0) | 49 (98.0) | 0.17 | 1d |
| AA | 0 (0.0) | 1 (2.0) | - | |
| G | 243 (90.0) | 87 (87.0) | 0.35 | 1d |
| A | 27 (10.0) | 13 (13.0) | 1.42 (0.69 – 2.95) | |
| Achilles tendinopathyc | N = 135 | N = 22 | ||
| GG | 108 (80.0) | 15 (68.2) | 0.01 | 1d |
| GA | 27 (20.0) | 5 (22.7) | 1.50 (0.45 – 4.93) | |
| AA | 0 (0.0) | 2 (9.1) | - | |
| GG+GA | 135 (100.0) | 20 (90.9) | 0.004 | 1d |
| AA | 0 (0.0) | 2 (9.1) | - | |
| G | 243 (90.0) | 35 (79.5) | 0.03 | 1d |
| A | 27 (10.0) | 9 (20.5) | 2.74 (1.12 – 6.75) | |
OR Odds ratio; CI confidence interval. aP-value ≤ 0.05 was obtained through the Chi-squared Test (Pearson P-value) or Fisher’s exact test to compared control and tendinopathy cases. b OR adjusted by BMI and nutritional follow-up. c OR adjusted by Age, sex, sport modality, BMI and nutritional follow-up. d Reference value.
Seven haplotypes of the TNF-α (-1031T > C, -857 C > T, -308G > A) SNPs were inferred, which account 100 % of the study population. The TCG haplotype was considered wild-type/reference haplotype due to present the highest frequency in the study population (N = 320, 59.2 %). After adjusting for confounding variables (age, sex, sport modality, BMI and nutritional follow-up), the TNF-α TCA haplotype was associated with increased tendinopathies risk, either considering all cases (P = 0.006), patellar (P = 0.004), rotator cuff (P = 0.008) or Achilles tendinopathies (P = 0.03) (Table 3).
Table 3.
Haplotype distributions of TNF-α in athletes and their association with tendinopathy
|
TNF-α Haplotypes – 1031T>C, – 857C>T and – 308G>A |
Control | Tendinopathy | P-valuea | Adjusted OR (CI 95%) |
|---|---|---|---|---|
| N (%) | ||||
| All tendinopathy casesb | N = 270 | N = 270 | ||
| TCG | 172 (63.7) | 148 (54.8) | 0.11 | 1d |
| TCA | 15 (5.6) | 31 (11.5) | 2.65 (1.32 – 5.30) | |
| TTG | 14 (5.2) | 18 (6.7) | 1.74 (0.80 – 3.80) | |
| TTA | 4 (1.5) | 3 (1.1) | 0.79 (0.17 – 3.71) | |
| CCG | 53 (19.5) | 59 (21.8) | 1.35 (0.86 – 2.11) | |
| CCA | 8 (3.0) | 7 (2.6) | 1.03 (0.35 – 2.97) | |
| CTG | 4 (1.5) | 4 (1.5) | 1.50 (0.36 – 6.26) | |
| Patellar tendinopathyc | N = 270 | N = 124 | ||
| TCG | 172 (63.7) | 71 (57.3) | 0.10 | 1d |
| TCA | 15 (5.6) | 17 (13.7) | 3.28 (1.47 – 7.31) | |
| TTG | 14 (5.2) | 8 (6.5) | 2.09 (0.76 – 5.71) | |
| TTA | 4 (1.5) | 1 (0.8) | 0.51 (0.05 – 4.81) | |
| CCG | 53 (19.5) | 22 (17.7) | 0.59 (0.59 – 1.98) | |
| CCA | 8 (3.0) | 3 (2.4) | 0.25 (0.25 – 3.92) | |
| CTG | 4 (1.5) | 2 (1.6) | 0.30 (0.30 – 9.81) | |
| Rotator cuff tendinopathyc | N = 270 | N = 100 | ||
| TCG | 172 (63.7) | 55 (55.0) | 0.01 | 1d |
| TCA | 15 (5.6) | 13 (13.0) | 3.14 (1.36 – 7.24) | |
| TTG | 14 (5.2) | 6 (6.0) | 1.88 (0.63 – 5.00) | |
| TTA | 4 (1.5) | 0 (0.0) | - | |
| CCG | 53 (19.5) | 25 (25.0) | 1.39 (0.77 – 2.51) | |
| CCA | 8 (3.0) | 0 (0.0) | - | |
| CTG | 4 (1.5) | 1 (1.0) | 0.67 (0.07 – 6.32) | |
| Achilles tendinopathyc | N = 270 | N = 44 | ||
| TCG | 172 (63.7) | 23 (52.3) | 0.26 | 1d |
| TCA | 15 (5.6) | 5 (11.4) | 3.79 (1.14 – 12.68) | |
| TTG | 14 (5.2) | 3 (6.8) | 1.99 (0.47 – 8.38) | |
| TTA | 4 (1.5) | 1 (2.3) | 0.93 (0.09 – 9.90) | |
| CCG | 53 (19.5) | 9 (20.5) | 1.33 (0.55 – 3.23) | |
| CCA | 8 (3.0) | 3 (6.8) | 3.83 (0.86 – 17.04) | |
| CTG | 4 (1.5) | 0 (0.0) | - | |
OR Odds ratio; CI confidence interval. aP-value ≤ 0.05 was obtained through the Chi-squared Test (Pearson P-value) or Fisher’s exact test. bOR adjusted by BMI and nutritional follow-up. cOR adjusted by Age, sex, sport modality, BMI and nutritional follow-up. dReference value
Discussion
Tendinopathy is a serious public health care problem and the knowledge of molecular mechanisms involved in its etiology remains an active area of ongoing research [26]. Any tendon can undergo a tendinopathy process and some modifiable and non-modifiable risk factors are common for the disease in different affected tendon [27]. Recent studies have challenged the “degenerative process” paradigm, suggesting that tendon overload is linked to a complex role of inflammation on tendon homeostasis dysregulation [6, 9, 26, 28]. Although the role of the inflammatory process is not clear, the dysregulation of the proinflammatory cytokines expression and release may contribute to chronic inflammatory responses [9, 26].
Metabolic diseases related to increased adiposity has been identified as important potentially modifiable risk factor for the onset and progression of a variety of tendinopathies [28]. Adipose tissue is tightly associated with tendon inflammation and early tissue degeneration [29]. High BMI and nutritional follow-up by a specialist during a sports career were non-modifiable risk factors for tendinopathy in our athletes. The increased BMI in athletes can result in nutritional monitoring for muscle mass gain, which optimizes the athlete’s performance and physical ability [30]; however, nutritional supplements may be a key component in the etiology of various diseases [31, 32], and diet can contribute negatively with tendon homeostasis [28].
Despite the different risk factors different types of tendinopathies, overloading and mechanical stress may induce the secretion of TNF-α by tenocytes and cause change cellular proliferation, onset of pain and ECM degradation [1]. Under normal physiological conditions TNF-α is not detectable in tendon; however, TNF-α was detect in human tenocytes of Achilles tendinopathy samples, suggesting association with onset tissue apoptosis and in mechanotransduction failure to adapt tendon load [13]. The variation in TNF-α cytokines production is tightly regulated by genetic variants [33]. The present results indicate a positive association between TNF-α TCA haplotype and the risk of developing tendinopathy (2-4-fold), which is observed when analyzing only the patellar, rotator cuff or Achilles subgroups. The TNF-α TCA (-1031T > C, -857 C > T and − 308G > A) haplotype characterized by the presence of the variant allele of TNF-α – 308 A, which promotes loss of transcription factors like activator protein-2 binding, increasing the level of gene transcription [34]. The TNF-α SNPs in the promoter region site are strongly in linkage disequilibrium and creates established haplotypes that affect differently gene expression and activity than those of each SNP evaluated separately [33, 34]. This may explain the increased level of TNF-α mRNA found in the degenerate tendon [11, 13]; and consequently, contribute to inter-individual variation in tendinopathy development.
Within the Brazilian population the TNF-α-308AA genotype is rarer (approximately 0–2 %) [23, 35, 36], and was only observed in the tendinopathy cases (~ 2 %). The total sample size was adequate to detect significant associations with 80 % statistical power; the small number of athletes with different locations of tendinopathies was the main limitation of this study. However, the strength of this study included the control group was matched with all tendinopathy case for age, sex, and sport modality to minimize the influence of the confounding factors. The results can be used to build a database from different populations to identify modifiable and non-modifiable risk factors associated with tendinopathy development in athletes.
It is essential to understand the molecular mechanism involved in the etiology of the disease and for control mechanical stress on the tendon of athletes most likely to develop overuse injuries [37]. The changes in the cytokine production due different genotypes can have significant influence in the tendinopathy, which can impair early tissue regeneration. Identifying genetic changes may improve the prognosis of the disease and clarify new therapeutic targets or personalized training for the athlete, avoiding movement limitations, loss of physical performance and sports ability. Athletes with TNF-α TCA haplotype could be early subjected to cryotherapy after training and competition to avoid tendinopathy development. Whole-body cryotherapy decreased serum TNF-α (around 60 %) 24 h following exercise [38]. Thus, this finding can be used in future studies to better understand the influence of genetic factors in the tendinopathy susceptibility and contribute to create sports injury surveillance programmes using genetic information aim reduce cases of the illness in athletes.
Conclusions
The TNF-α -308G > A SNP was potential non-modifiable risk associated with development of disease.
Supplementary Information
Acknowledgements
The authors thank the support of Sports Trauma Center of National Institute of Traumatology and Orthopaedics (INTO), Sports Training Center and National or State Sports Championships for an opportunity to recruit athletes. And the technical assistance of Ana Carolina Leocadio de Souza, Camili Gomes Pereira and Jade Pires do Nascimento from Laboratório de Pesquisa de Ciências Farmacêuticas, Centro Universitário Estadual da Zona Oeste (UEZO) and Research Division of INTO. This study was partially supported by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) - Finance Code 001.
Abbreviations
- 95 % CI
: 95 % Confidence interval
- χ2
Chi-square
- BMI
Body mass index
- D’
Degree of imbalance in module
- HLA-B
Human leukocyte antigen-B
- HLA-DR
Human leukocyte antigen-DR
- HWE
Hardy–Weinberg equilibrium
- MRI
Magnetic resonance image
- mRNA
Messenger RNA
- OR
Odds ratio
- PCR
Polymerase chain reaction
- R²
Degree of correlation
- SD
Standard deviation
- SNP
Single nucleotide polymorphisms
- SPSS
Statistical Package for Social Sciences
- TNF-α
Tumor necrosis factor alpha
- TNFR1
TNF-α receptor-1
- TNFR2
TNF-α receptor-2
Authors’ contributions
JAP participated in conception and design of study. LRL, VARM, GGAS, VWS, RAG and JAP collated the data and developed the database. LRL, VSW, and JAP helped to experiments. LRL performed the statistical analysis. LRL, VARM, GGAS, JAMG, JAGN and JAP analysis and interpretation of data. LRL, VARM and JAP wrote the manuscript. JAMG, JAGN, RAG and JAP critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was supported by the Brazilian agency Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ, Brazil. Funding body contributed to acquisition of research inputs.
Availability of data and materials
Original data are available as Supplementary file 1.
Declarations
Ethics approval and consent to participate
This study was approved by the Human Research Ethics Committee of the Instituto Nacional de Traumatologia e Ortopedia, Rio de Janeiro, Brazil (protocol number 2.455.630/2017). All participating provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Addona A, Maffulli N, Formisano S, et al. Inflammation in tendinopathy. Surgeon. 2017;15(5):297–302. doi: 10.1016/j.surge.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Kaux JF, Forthomme B, Goff CL, et al. Current opinions on tendinopathy. J Sports Sci Med. 2011;10(2):238–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins C, Fu SC, Chua E, et al. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2016;4:9–20. doi: 10.1016/j.asmart.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docking SI, Rio E, Cook J, et al. The prevalence of Achilles and patellar tendon injuries in Australian football players beyond a time-loss definition. Scand J Med Sci Sports. 2018;28(9):2016–22. doi: 10.1111/sms.13086. [DOI] [PubMed] [Google Scholar]
- 5.Abate M, Schiavone C, Salini V, et al. Occurrence of tendon pathologies in metabolic disorders. Rheumatology. 2013;52(4):599–608. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- 6.Salles JI, Lopes LR, Duarte MEL, et al. Fc receptor-like 3 (-169T > C) polymorphism increases the risk of tendinopathy in volleyball athletes: a case control study. BMC Med Genet. 2018;19(1):119. doi: 10.1186/s12881-018-0633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diniz-Fernandes T, Godoy-Santos AL, Santos MC, et al. Matrix metalloproteinase-1 (MMP-1) and (MMP-8) gene polymorphisms promote increase and remodeling of the collagen III and V in posterior tibial tendinopathy. Histol Histopathol. 2018;33(9):929–36. doi: 10.14670/HH-11-982. [DOI] [PubMed] [Google Scholar]
- 8.Posthumus M, Collins M, Cook J, et al. Components of the transforming growth factor-beta family and the pathogenesis of human Achilles tendon pathology–a genetic association study. Rheumatology. 2010;49(11):2090–7. doi: 10.1093/rheumatology/keq072. [DOI] [PubMed] [Google Scholar]
- 9.Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13(2):110–22. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 10.Ihnatko R, Kubes M. TNF signaling: early events and phosphorylation. Gen Physiol Biophys. 2007;26(3):159–67. [PubMed] [Google Scholar]
- 11.Millar NL, Wei AQ, Molloy TJ, et al. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91(3):417–24. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 12.Spang C, Renström L, Alfredson H, et al. Marked expression of TNF receptors in human peritendinous tissues including in nerve fascicles with axonal damage - Studies on tendinopathy and tennis elbow. J Musculoskelet Neuronal Interact. 2017;17(3):226–36. [PMC free article] [PubMed] [Google Scholar]
- 13.Gaida JE, Bagge J, Purdam C, et al. Evidence of the TNF-α system in the human Achilles tendon: expression of TNF-α and TNF receptor at both protein and mRNA levels in the tenocytes. Cells Tissues Organs. 2012;196(4):339–52. doi: 10.1159/000335475. [DOI] [PubMed] [Google Scholar]
- 14.Campbell RD, Trowsdale J. Map of the human MHC. Immunol Today. 1993;14(7):349–52. doi: 10.1016/0167-5699(93)90234-C. [DOI] [PubMed] [Google Scholar]
- 15.Zhang BB, Liu XZ, Sun J, et al. Association between TNF α gene polymorphisms and the risk of duodenal ulcer: a meta-analysis. PLoS One. 2013;8(2):e57167. doi: 10.1371/journal.pone.0057167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajeer AH, Hutchinson IV. TNF-alpha gene polymorphism: clinical and biological implications. Microsc Res Tech. 2000;50(3):216–228. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Nourian M, Chaleshi V, Pishkar L, et al. Evaluation of tumor necrosis factor (TNF)-α mRNA expression level and the rs1799964 polymorphism of the TNF-α gene in peripheral mononuclear cells of patients with inflammatory bowel diseases. Biomed Rep. 2017;6(6):698–702. doi: 10.3892/br.2017.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos CNO, Ribeiro DR, Cardoso Alves J, et al. Association Between Zika Virus Microcephaly in Newborns With the rs3775291 Variant in Toll-Like Receptor 3 and rs1799964 Variant at Tumor Necrosis Factor-α Gene. J Infect Dis. 2019;220(11):1797–801. doi: 10.1093/infdis/jiz392. [DOI] [PubMed] [Google Scholar]
- 19.Hassanzad M, Farnia P, Ghanavi J, et al. TNFα -857 C/T and TNFR2 + 587 T/G polymorphisms are associated with cystic fibrosis in Iranian patients. Eur J Med Genet. 2019;62(11):103584. doi: 10.1016/j.ejmg.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Goes RA, Lopes LR, Cossich VRA, et al. Musculoskeletal injuries in athletes from five modalities: a cross-sectional study. BMC Musculoskelet Disord. 2020;21(1):122. doi: 10.1186/s12891-020-3141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salles JI, Duarte ME, Guimarães JM, et al. Vascular Endothelial Growth Factor Receptor-2 Polymorphisms Have Protective Effect against the Development of Tendinopathy in Volleyball Athletes. PLoS One. 2016;11(12):e0167717. doi: 10.1371/journal.pone.0167717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomelí-Nieto JA, Muñoz-Valle JF, Baños-Hernández CJ, et al. TNFA – 308G > A and – 238G > A polymorphisms and risk to systemic sclerosis: impact on TNF-α serum levels, TNFA mRNA expression, and autoantibodies. Clin Exp Med. 2019;19(4):439–47. doi: 10.1007/s10238-019-00569-4. [DOI] [PubMed] [Google Scholar]
- 23.Rocha Loures MA, Macedo LC, Reis DM, et al. Influence of TNF and IL17 Gene Polymorphisms on the Spondyloarthritis Immunopathogenesis, Regardless of HLA-B27, in a Brazilian Population. Mediators Inflamm. 2018;2018:1395823. doi: 10.1155/2018/1395823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Tang X, Song K, et al. Association of -308G/A and – 238G/A polymorphisms of TNF-α and osteosarcoma risk. Int J Clin Exp Pathol. 2015;8(4):4177–81. [PMC free article] [PubMed] [Google Scholar]
- 25.Perini JA, Cardoso JV, Berardo PT, et al. Role of vascular endothelial growth factor polymorphisms (-2578 C > A, -460 T > C, -1154G > A, + 405G > C and + 936 C > T) in endometriosis: a case-control study with Brazilians. BMC Womens Health. 2014;14:117. doi: 10.1186/1472-6874-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dakin SG, Newton J, Martinez FO, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52(6):359–67. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675–92. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 28.Loiacono C, Palermi S, Massa B, et al. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina. 2019;55(8):447. doi: 10.3390/medicina55080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pingel J, Petersen MC, Fredberg U, et al. Inflammatory and Metabolic Alterations of Kager’s Fat Pad in Chronic Achilles Tendinopathy. PLoS One. 2015;10(5):e0127811. doi: 10.1371/journal.pone.0127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulens M, Vansant G, Lysens R, et al. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25(5):676–81. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- 31.DePhillipo NN, Aman ZS, Kennedy MI, et al. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop J Sports Med. 2018;6(10):2325967118804544. doi: 10.1177/2325967118804544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott A, Nordin C. Do Dietary Factors Influence Tendon Metabolism? Adv Exp Med Biol. 2016;920:283–9. doi: 10.1007/978-3-319-33943-6_27. [DOI] [PubMed] [Google Scholar]
- 33.Mandal RK, Khan MA, Hussain A, et al. A trial sequential meta-analysis of TNF-α -308G > A (rs800629) gene polymorphism and susceptibility to colorectal cancer. Biosci Rep. 2019;39(1):BSR20181052. doi: 10.1042/BSR20181052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimi M, Goldie LC, Cruickshank MN, et al. A critical assessment of the factors affecting reporter gene assays for promoter SNP function: a reassessment of -308 TNF polymorphism function using a novel integrated reporter system. Eur J Hum Genet. 2009;17(11):1454–62. doi: 10.1038/ejhg.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oki E, Norde MN, Carioca AAF, et al. Polymorphisms of the TNF-α gene interact with plasma fatty acids on inflammatory biomarker profile: a population-based, cross-sectional study in São Paulo, Brazil. Br J Nutr. 2017;117(12):1663–73. doi: 10.1017/S0007114517001416. [DOI] [PubMed] [Google Scholar]
- 36.Patente TA, Monteiro MB, Vieira SM, et al. Linkage disequilibrium with HLA-DRB1-DQB1 haplotypes explains the association of TNF-308G > A variant with type 1 diabetes in a Brazilian cohort. Gene. 2015;568(1):50–4. doi: 10.1016/j.gene.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Maffulli N, Margiotti K, Longo UG, et al. The genetics of sports injuries and athletic performance. Muscles Ligaments Tendons J. 2013;3(3):173–89. [PMC free article] [PubMed] [Google Scholar]
- 38.Ziemann E, Olek RA, Kujach S, et al. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J Athl Train. 2012;47(6):664–72. doi: 10.4085/1062-6050-47.6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available as Supplementary file 1.