Abstract
Objective
This study examined the initial impact of a national BNT162b2 vaccine rollout on SARS-CoV-2 infections in Qatar.
Methods
All individuals who had completed ≥14 days of follow-up by 16 March 2021 after receiving the BNT162b2 vaccine were included. This study calculated incidence rates (IR) and their 95% confidence intervals (CI) during days 1–7, 8–14, 15–21, 22–28, and >28 days post-vaccination. Poisson regression was used to calculate incidence rate ratios (IRR) relative to the first 7-day post-vaccination period.
Results
A total of 199,219 individuals with 6,521,124 person-days of follow-up were included. SARS-CoV-2 infection was confirmed in 1877 (0.9%), of which 489 (26.1%) were asymptomatic and 123 (6.6%) required oxygen support. The median time from first vaccination to SARS-CoV-2 confirmation was 11.9 days (IQR 7.7–18.2). Compared with the first 7-day post-vaccination period, SARS-CoV-2 infections were lower by 65.8–84.7% during 15–21, 22–28, and >28 days (P < 0.001 for each). For severe COVID-19, the incidence rates were 75.7–93.3% lower during the corresponding time periods (P < 0.001 for each).
Conclusion
The results were consistent with an early protective effect of BNT162b2 vaccine against all degrees of SARS-CoV-2 severity.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Vaccine, BNT162b2, mRNA, Qatar
Introduction
A two-dose regimen of BNT162b2, the Pfizer-BioNTech COVID-19 mRNA vaccine, was shown to reduce the risk of SARS-CoV-2 by around 95% in a randomized clinical trial and in a mass national vaccination program (Dagan et al., 2021, Polack et al., 2020). On 23 December 2020, Qatar started a national BNT162b2 rollout programme, in addition to existing COVID-19 public health control measures. The rollout initially prioritised healthcare workers, individuals aged ≥50 years, and those with chronic or immunosuppressive medical conditions. This study reports the initial impact of BNT162b2 on SARS-CoV-2 infections in Qatar.
Methods
SARS-CoV-2 infections were confirmed using real-time polymerase chain reaction (PCR) on upper or lower respiratory samples. BNT162b2 was supplied in multidose 0.45 mL vials, and stored, prepared and administered according to the manufacturer’s instructions (Pfizer-BioNTech, 2021). The COVID-19 database at the Communicable Disease Center, Hamad Medical Corporation, was used to retrospectively collate clinical and outcome data for all individuals who by 16 March 2021 had completed ≥14 days of follow-up after the receipt of at least one dose of BNT162b2. Confirmed SARS-CoV-2 infection, symptomatic COVID-19, and severe COVID-19 were defined according to the Food and Drug Administration criteria (Polack et al., 2020).
SARS-CoV-2 infection incidence rates (IR) and their 95% confidence intervals (CI) were calculated per 100,000 person-days during five time periods: <7, 8–14, 15–21, 22–28, and >28 days from receipt of the first BNT162b2 dose. Individuals stopped contributing person-days once SARS-CoV-2 infection was confirmed or at the study end date, whichever came first. Poisson regression was used to calculate incidence rate ratios (IRR) and their 95% CI for the latter four time periods relative to IR during the first 7 days from the first BNT162b2 dose. Statistical analyses were performed using Stata Statistical Software, Release 16.1 (StataCorp., College Station, Texas).
Results
A total of 199,219 individuals contributed 6,521,124 person-days of follow-up; SARS-CoV-2 infection was confirmed in 1877 (0.9%). The median time from first vaccination to SARS-CoV-2 confirmation was 11.9 days (IQR 7.7–18.2). A total of 242 (12.9%) individuals had confirmed COVID-19 after a second BNT162b2 dose, of which 28 (1.5%) had confirmed COVID-19 > 14 days after the second dose.
Compared with those without SARS-CoV-2 infection, infected individuals were significantly older and more likely to have co-existing medical conditions (Table 1 ). Cough (1018, 54.2%) and fever (745, 39.7%) were the most frequent presenting symptoms, while infections were asymptomatic in 489 (26.1%). High-flow nasal oxygen or non-invasive ventilation was required for 28 (1.5%) individuals, invasive mechanical ventilation for 11 (0.6%), and oxygen via face mask or nasal cannula for 84 (4.5%). Five individuals (median age 81 years, range 52–93) had fatal COVID-19, all with multiple comorbidities, and most with infected household contacts. For fatal COVID-19 cases, symptoms started after a median of 12 days (range 7–25) after the first BNT162b2 dose.
Table 1.
Baseline characteristics of the study cohort.
| Total study cohort | Group without SARS-CoV-2 infection | Group with SARS-CoV-2 infection | P-value | |
|---|---|---|---|---|
| (n = 199,219) | (n = 197,342) | (n = 1,877) | ||
| BNT162b2 doses received, n (%) | ||||
| One dose | 129,462 (65%) | 129,950 (65%) | 1512 (80.5%) | |
| Two doses | 69,757 (35%) | 69,392 (35%) | 365 (19.5%) | |
| Female, n (%) | 83,034 (41.7%) | 82,214 (41.7%) | 820 (43.7%) | 0.076 |
| Age (years) | 42 (33–55) | 42 (33–55) | 44 (35–54) | <0.001 |
| Age groups (years), n (%) | ||||
| 16–24 | 13,826 (6.9%) | 13,764 (7%) | 62 (3.3%) | |
| 25–34 | 43,302 (21.7%) | 42,934 (21.8%) | 368 (19.6%) | |
| 35–44 | 51,934 (26.1%) | 51,384 (26%) | 550 (29.3%) | |
| 45–54 | 39,274 (19.7%) | 38,823 (19.7%) | 451 (24%) | |
| 55–64 | 31,257 (15.7%) | 30,978 (15.7%) | 279 (14.9%) | |
| ≥65 | 19,626 (9.9%) | 19,459 (9.9%) | 167 (8.9%) | |
| Co-existing medical conditions | ||||
| Diabetes mellitus | 39,994 (20.1%) | 39,550 (20%) | 444 (23.7%) | <0.001 |
| Hypertension | 42,994 (21.6%) | 42,512 (21.5%) | 482 (25.7%) | <0.001 |
| Chronic heart disease | 10,308 (5.2%) | 10,190 (5.2%) | 118 (6.3%) | <0.029 |
| Chronic lung disease | 19,477 (9.8%) | 19,270 (9.8%) | 207 (11%) | 0.067 |
| Chronic kidney disease | 4272 (2.1%) | 4209 (2.13%) | 63 (3.4%) | <0.001 |
| Chronic liver disease | 1781 (0.9%) | 1754 (0.9%) | 27 (1.4%) | 0.012 |
| Malignant disease | 3724 (1.9%) | 3691 (1.9%) | 33 (1.8%) | 0.72 |
| Organ transplant recipient | 653 (0.33%) | 640 (0.32%) | 13 (0.69%) | 0.005 |
Data are presented as number (%) or median (interquartile range). P-values were derived from Pearson’s chi-squared or Wilcoxon rank-sum test, as appropriate.
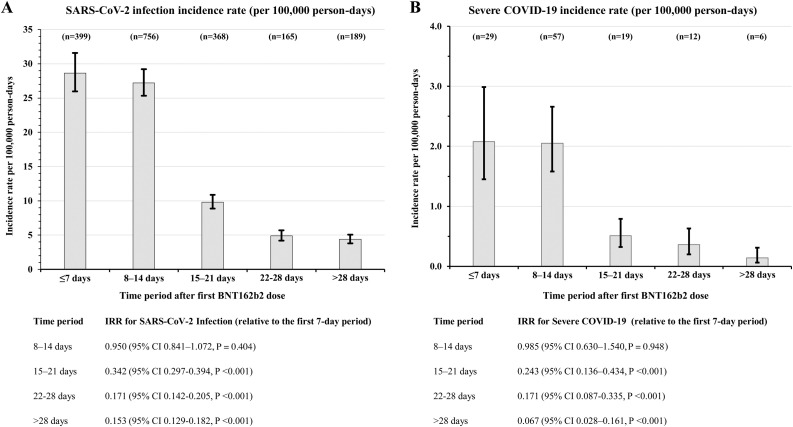
SARS-CoV-2 IR was 28.63/100,000 person-days (95% CI 25.96–31.58) during the first 7 days post-vaccination, and 27.19/100,000 person-days (95% CI 25.32–29.2) during days 8–14. Compared with the first 7-day post-vaccination period, the IR was significantly lower during days 15–21 (IRR 0.342, 95% CI 0.297–0.394, P < 0.001), days 22–28 (IRR 0.171, 95% CI 0.142–0.205, P < 0.001), and >28 days (IRR 0.153, 95% CI 0.129–0.182, P < 0.001). Similarly, in comparison with the first 7-day post-vaccination period, the IRR for severe COVID-19 decreased significantly during days 15–21 (IRR 0.243, 95% CI 0.136–0.434, P < 0.001), days 22–28 (IRR 0.171, 95% CI 0.087–0.335, P < 0.001), and >28 days (IRR 0.067, 95% CI 00.028–0.161, P < 0.001) (Figure 1 ).
Figure 1.
SARS-CoV-2 incidence rate by time-period following the first dose of BNT162b2 vaccination.
Panel A, SARS-CoV-2 infection incidence rate (per 100,000 person-days), Panel B, Severe COVID-19 incidence rate (per 100,000 person-days).
COVID-19, Coronavirus Disease 2019; CI, confidence interval; IRR, incidence rate ratio; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
I bars indicate 95% confidence intervals.
n, the absolute number of individuals in the corresponding time-period.
Discussion
These findings are consistent with those previously shown in a community setting and in healthcare workers, but this report is the first to include an entire national cohort of BNT162b2 recipients (Dagan et al., 2021, Hall et al., 2021). This study also demonstrated a significant reduction in risk of severe COVID-19. This is particularly important, given its potential to reduce COVID-19-associated morbidity and mortality, and decrease its impact on healthcare resource utilization (Hodgson et al., 2021).
This study found relatively high SARS-CoV-2 IR during the first two weeks following receipt of the first BNT162b2 dose. While the vaccine’s protective effect may not be apparent during the first two weeks after BNT162b2 vaccination (Polack et al., 2020), recipients may wrongly perceive themselves to be at a reduced risk of SARS-CoV-2 infection and become less adherent to nonpharmacological preventive measures such as social distancing and face covering (Trogen and Caplan, 2021). Careful education and counselling during the vaccination process could help efforts to minimise such risk-compensation behaviour.
SARS-CoV-2 variants of concerns (VOC) such as B.1.1.7 lineage (first reported in the United Kingdom) and B.1.351 lineage (first reported in South Africa) are known to have been circulating in Qatar during the study’s follow-up period (The Peninsula Qatar, 2021). There has been concern that VOC may reduce the effectiveness of some COVID-19 vaccines (Abdool Karim and de Oliveira, 2021); however, BNT162b2 elicits high neutralising antibodies titres against B.1.1.7 and B.1.351 lineages (Liu et al., 2021). Moreover, recently announced results from an ongoing phase 3 BNT162b2 randomised trial suggest that it is highly effective against the B.1.351 lineage (Pfizer and BioNTech, 2021).
Deaths in this study mostly occurred in older individuals with multiple comorbidities; vaccine effectiveness is generally lower in such groups (Dagan et al., 2021). Notably, infected household contacts were identified in three out of the five fatal COVID-19 cases, reinforcing the importance of wide vaccine rollout to maximise protection of the most vulnerable (Hodgson et al., 2021).
The limitations of this study included its observational nature and the lack of a non-vaccinated control group. The first 7-day post-vaccination period, during which no vaccine effectiveness is expected, was used as a reference to assess the vaccine’s protective benefits in later time-periods. Overall, these results are consistent with an early protective effect of BNT162b2 against all degrees of SARS-CoV-2 severity. It is anticipated that, in addition to ongoing nonpharmacological interventions, broader vaccine coverage will contribute to the national and global pandemic control efforts.
Ethical issues
The study was approved by Hamad Medical Corporation’s Institutional Review Board with a waiver of informed consent (MRC-01-21-207).
Conflict of interests
The authors declare no conflict of interests in relation to this manuscript.
Funding
The publication of this report was funded by Qatar National Library. No other funding was required.
Acknowledgments
We would like to thank Hussam Alsoub and Faraj S. Howady for their support during the preparation of this report.
References
- Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021;384:1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer and BioNTech . 2021. Pfizer and BioNTech confirm high efficacy and no serious safety concerns through up to six months following second dose in updated topline analysis of landmark COVID-19 vaccine study.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious [Google Scholar]
- Pfizer-BioNTech . 2021. Pfizer-BioNTech Covid-19 vaccine emergency use authorization combined full prescribing information and fact sheet.http://labeling.pfizer.com/ShowLabeling.aspx?id=14471 [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Peninsula Qatar . 2021. We are seeing positive COVID-19 cases in Qatar with new UK strain variant.https://www.thepeninsulaqatar.com/article/10/03/2021/We-are-seeing-positive-COVID-19-cases-in-Qatar-with-new-UK-strain-variant-Dr-Khal [Google Scholar]
- Trogen B., Caplan A. Risk compensation and COVID-19 vaccines. Ann Intern Med. 2021 doi: 10.7326/M20-8251. [DOI] [PMC free article] [PubMed] [Google Scholar]