Abstract
Objectives:
To determine the effect of Clinpro 5000, Clinpro Tooth Crème, and MI-Paste Plus on the formation of white spot lesions in patients undergoing orthodontic treatment.
Materials and Methods:
Three prospective groups with 40 patients undergoing orthodontic treatment in each group were evaluated (total recruitment = 120 subjects). The selected product was brushed on for 2 minutes twice daily for 4 months. Subjects were reviewed for 4 months on a monthly basis. The Enamel Decalcification Index (EDI) was used to determine the number of white spot lesions per surface at each visit.
Results:
100 subjects (35 using Clinpro 5000, 32 using Clinpro Tooth Crème, and 33 using MI Paste Plus) completed the study. The data lend strong support for Clinpro 5000 providing superior protection against enamel decalcification when compared to Clinpro Crème, and mixed support when compared to MI Paste Plus.
Conclusions:
The use of Clinpro 5000, Clinpro Crème, and MI paste Plus all have a reduction effect on white spot lesions when compared to studies reported previously. Clinpro 5000 has a marginally better effect than the two other test pastes. The results of this study can be used by clinicians when deciding the effectiveness of using fluoride dentifrice products to prevent white spot lesions in their orthodontic practice (ClinicalTrials.gov ID: NCT03440996).
Keywords: Tooth demineralization, Fluorides, Toothpastes, Dental caries, Randomized controlled trial, Enamel decalcification
INTRODUCTION
During orthodontic treatment, practitioners normally face two common iatrogenic treatment side effects: root resorption and enamel decalcification, with the latter occurring at a much higher frequency. Progression to clinically detectable white spot lesions (WSLs) may occur as early as one month after the placement of orthodontic appliances.1 Although the processes that lead to enamel demineralization are well understood, methods to diminish or perhaps eliminate degradation of enamel surfaces are being sought. Previous studies have reported the effectiveness of different means of fluorides on preventing WSLs in patients undergoing orthodontic treatment.2–7
Two new anticavity toothpastes, Clinpro 5000 with 1.1% Sodium Fluoride and Clinpro Tooth Crème with 0.21% Sodium Fluoride (3M ESPE, St. Paul, Minn, USA), are currently available and have been shown in some initial case reports to be useful in the reduction of WSLs. Both the Clinpro products are advanced formulas containing an innovative tricalcium phosphate ingredient. During the manufacturing process, a protective barrier is created around the calcium allowing it to coexist with the fluoride ions. As the toothpaste comes in contact with saliva during brushing, the barrier breaks down and makes the calcium, phosphate, and fluoride readily available to the tooth. The tooth naturally absorbs these components, helping to prevent the initiation and further progression of demineralization and allowing remineralization to occur.
MI Paste Plus (GC America, Alsip, Ill, USA), which contains casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), has been shown to be useful in the reduction of WSLs.8–10 It is reported that the casein phosphopeptide stabilizes the amorphous calcium phosphate in solution, maintaining high concentration gradients of calcium and phosphate in the white spot lesion, thus effecting high rates of enamel remineralization.9 However, there are no studies in the current literature in which these products are tested together during orthodontic treatment. Thus, the aim of the present study was to determine and compare the preventive effect of Clinpro 5000, Clinpro Tooth Crème, and MI Paste Plus on the formation of WSLs for patients undergoing orthodontic treatment.
MATERIALS AND METHODS
Study Design
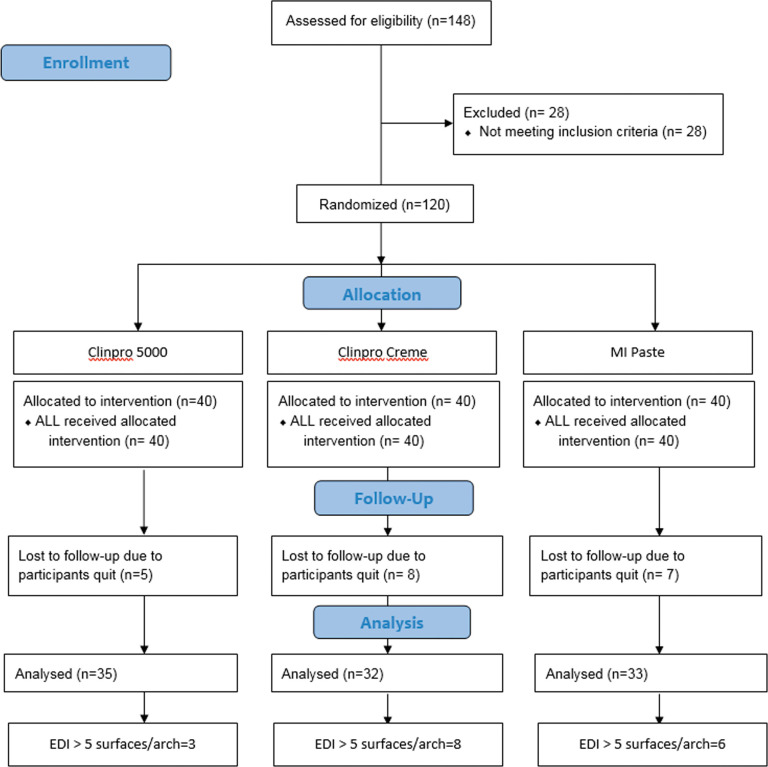
The study was approved by the Western Institutional Review Board (WIRB) (WIRB reference number: 20102132). The trial named “Comparison of Clinpro™ 5000 1.1% Sodium Fluoride Anti-Cavity Toothpaste, Clinpro™ Tooth Crème, and MI-Paste Plus for the Prevention and Reduction of White Spot Lesions in Orthodontic Treatment” was registered in the Registry of Clinical Trials run by the United States National Library of Medicine (ClinicalTrials.gov identifier: NCT03440996). The present study followed the CONSORT (Consolidated Standards of Reporting Trials) checklist to ensure transparent and standardized reporting of the trial. Three prospective groups (Clinpro 5000, Clinpro Tooth Crème, MI-Paste Plus) with 40 randomly assigned participants who were undertaking routine orthodontic treatment in each group were evaluated (total recruitment = 120 subjects) (Figure 1). Sample size calculation was based on the results of a previous study by Robertson et al. with similar groups of subjects.9 In their study, the control group (0.67 ± 0.44) showed a higher mean Enamel Decalcification Index (EDI) score compared to the MI paste group (0.30 ± 0.30). With a sample size of 30 to 35 per group, there was a greater than 0.85 power with a 5% statistical significant level to detect a difference of 1.0 unit change in the decalcification index.
Figure 1.
Flow diagram of participants' progress during the study.
Inclusion criteria were: (1) patients 12 years and older with permanent dentition, (2) patients did not use extensive fluoride regimes, and (3) patients who agreed to use a nonfluoridated toothpaste (such as Tom's of Maine) for a 1-week period prior to starting the trial. Informed consent was obtained from the patient or the parent/legal guardian if under the age of 18. Patients were excluded when they had any medical or dental condition that could impact study results, were using any investigational drug, planned to relocate or move within 6 months of enrollment, had or were currently undergoing fluoride treatment for WSLs, had IgE Casein allergy or known allergies to fluoride or other components of the test materials, or were pregnant women.
Clinical Procedures
The following test products were used: Clinpro 5000 1.1% Sodium Fluoride Anti-Cavity Toothpaste with Tri-Calcium Phosphate that contains 5000 ppm fluoride and an innovative tricalcium phosphate ingredient (3M ESPE); Clinpro Tooth Crème 0.21% Sodium Fluoride Anti-Cavity Paste with Tri-Calcium Phosphate, which contains 950 ppm fluoride ion (like regular toothpaste) and a functionalized tricalcium phosphate ingredient (fTCP); and GC America MI Paste Plus, a reference and a positive control that contains 0.2% sodium fluoride. Subjects were randomly assigned to use a given product by drawing individual slips of paper from an envelope on which 40 of each study product (ie, Clinpro 5000, Clinpro Tooth Crème, or MI-Paste Plus) were written.
The selected product was brushed on for 2 minutes twice daily for 4 months. After brushing on the product, patients were instructed not to rinse their mouths with water. Rather, they were requested to expectorate so they didn't clear out the actives from the product. Patients were instructed not to eat or drink for 30 minutes following the treatment. Subjects were examined every 4 weeks when they came for their appointment. Case Report Forms (CRF) were designed to record data pertinent to each subject. In addition to this form, the patient also filled a brushing diary visit by visit. At each visit, three intraoral photos were taken (frontal and buccal views) to determine the presence of the WSLs (Figure 2) in each study group. The Serious Adverse Event/Serious Adverse Drug Reaction Form was used to record serious adverse events.
Figure 2.
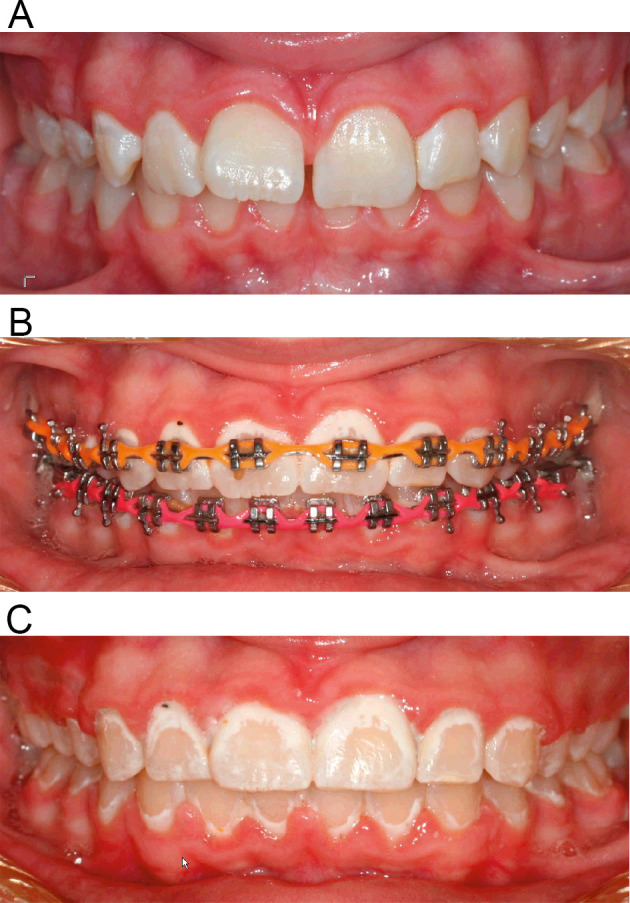
Representative clinical photographs: (A) before orthodontic treatment, (B) during orthodontic treatment, and (C) after orthodontic treatment in a patient exhibiting severe enamel demineralization.
The EDI was recorded following Banks and Richmond's method.11 The facial surfaces of these teeth were divided into four areas: gingival, mesial, distal, and occlusal. Each area was scored 0 (no decalcification), 1 (decalcification covering <50% of the area), or 2 (decalcification covering >50% of the area) at each time period. A baseline measurement of decalcification was taken after consenting to study participation (referred to as time 0). The resulting dataset followed a deeply nested hierarchy: facial surface areas within a tooth, teeth within arches, arches within patients, across four time periods. Analysis was done at the tooth level, aggregating the EDI scores from all four areas, creating an EDI for each tooth ranging potentially from 0 to 8.
Statistical Analysis
Three-way analysis of variance (ANOVA) (StatView; SAS Institute, Cary, NC) was used to analyze the EDI scores of the maxillary and mandibular arches from the right first premolar to the left first premolar at 0.05 level of significance. Fisher-protected least significant difference intervals were used to compare mean EDI scores.
Two operators scored the photographs independently. Their scores were compared by using the t-test; no statistically significant difference (P ≥ .05) was found between the operators. Results of each product were analyzed individually and among themselves.
RESULTS
A total of 120 subjects were recruited into the study. However, only 100 subjects were successfully recalled over the four study periods (Figure 1). No severe adverse effect was found in the present study.
Each patient had the facial surfaces of up to 20 teeth (10 per arch) evaluated for enamel decalcification. The maximum EDI in the data was 6. Table 1 displays distribution statistics for EDI scores of the three groups across the four time periods, and at study consent (T0).
Table 1.
Total Number of Teeth in Each Group and the EDI Scores
| EDI 0, % |
EDI 1, % |
EDI 2, % |
EDI 3, % |
EDI 4, % |
EDI 5, % |
EDI 6, % |
|
| Clinpro 5000 (N: 643 teeth over 35 patients) | |||||||
| T0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| T1 | 99.8 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| T2 | 95.2 | 3.0 | 1.1 | 0.8 | 0.0 | 0.0 | 0.0 |
| T3 | 87.7 | 6.5 | 3.4 | 1.7 | 0.3 | 0.0 | 0.3 |
| T4 | 86.8 | 7.2 | 3.7 | 1.7 | 0.3 | 0.0 | 0.3 |
| Clinpro Crème (N: 604 teeth over 32 patients) | |||||||
| T0 | 97.7 | 2.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| T1 | 91.9 | 6.0 | 1.2 | 0.2 | 0.8 | 0.0 | 0.0 |
| T2 | 87.4 | 9.1 | 2.3 | 0.3 | 0.7 | 0.0 | 0.2 |
| T3 | 79.1 | 14.9 | 3.8 | 0.8 | 1.2 | 0.0 | 0.2 |
| T4 | 74.7 | 18.4 | 4.5 | 1.2 | 1.2 | 0.0 | 0.2 |
| MI Paste (N: 638 teeth over 33 patients) | |||||||
| T0 | 99.8 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| T1 | 94.5 | 4.9 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| T2 | 88.7 | 8.2 | 1.9 | 0.6 | 0.6 | 0.0 | 0.0 |
| T3 | 83.4 | 11.0 | 3.4 | 1.6 | 0.6 | 0.0 | 0.0 |
| T4 | 79.6 | 13.9 | 3.4 | 2.2 | 0.6 | 0.2 | 0.0 |
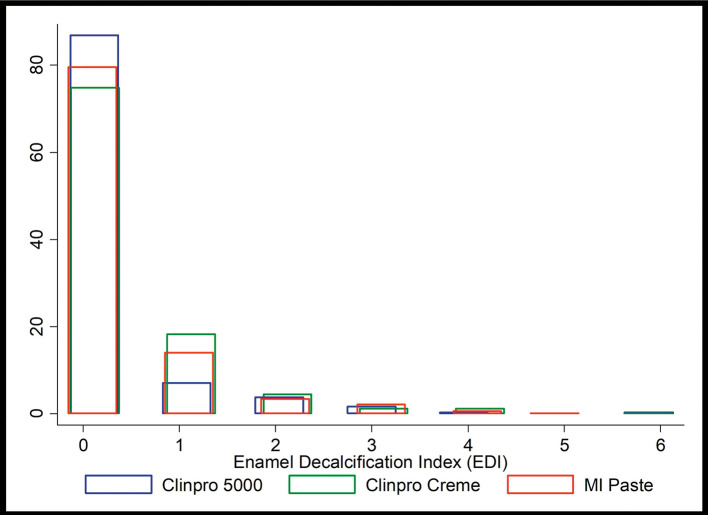
Figure 3 shows the distribution of the EDI score data at Time 4 by treatment. Multilevel mixed-effects Poisson regression was used to model the data. Table 1 highlights that the baseline EDI scores were different across the three groups of interest, with Clinpro 5000 showing a lower initial average EDI score than the other two groups. The regression method used allowed control for the fact that the baseline EDI states were different across the three treatment groups. All models included lagged EDI scores (EDI scores from the previous time period) as an independent control variable to adjust for such differences and properly model incremental changes in EDI. Lowess curves presented in Figure 4 show smoothed fit lines for the three treatment groups.
Figure 3.
Percentage distribution histograms of EDI scores at Time 4.
Figure 4.
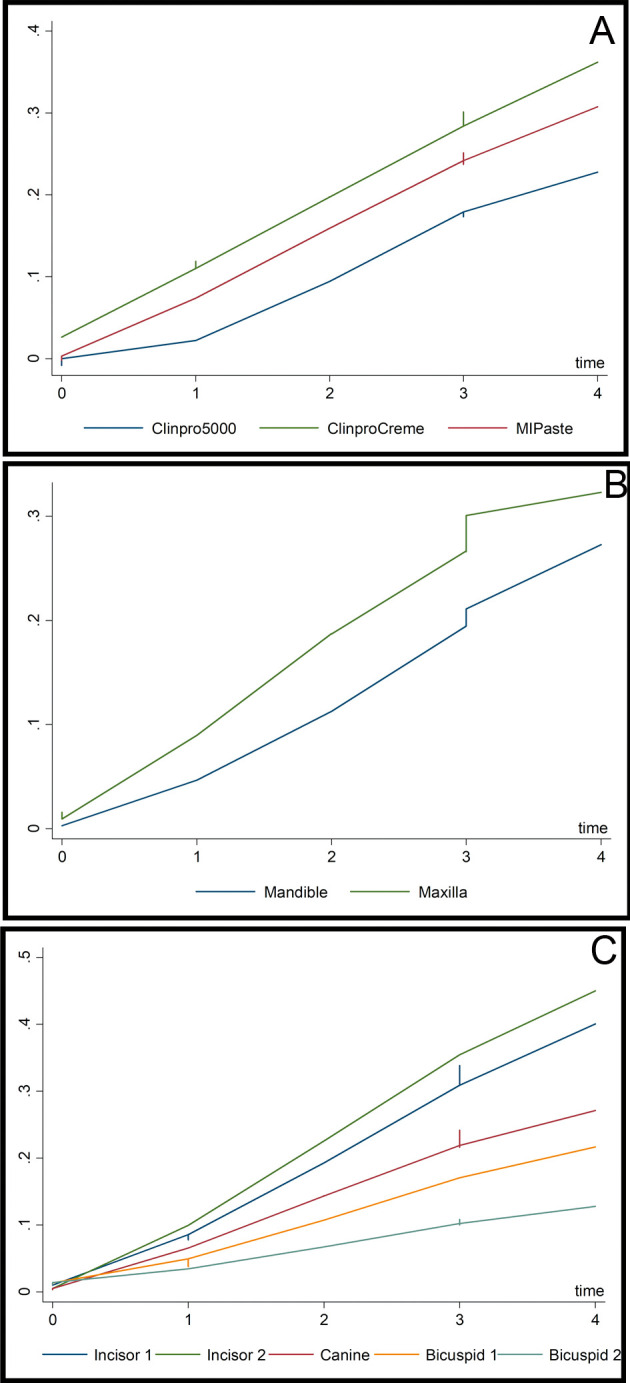
(A) Lowess curves of mean EDI scores across time by treatment group. (B) Lowess curves of mean EDI scores across time by arch. (C) Lowess curves of mean EDI scores across time by tooth type.
Five models were used to develop the analysis. The first four models made use of all four one-period lagged time spans (time 0 to time 1, time 1 to time 2, time 2 to time 3, and time 3 to time 4), providing an analysis set of 7540 total tooth observations. The results are presented in Table 2.
Table 2.
Statistical Analysis Results of Five Models
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
|
| Lagged EDI (1 time period) | 0.454 (0.029) | 0.234 (0.034) | 0.234 (0.034) | 0.209 (0.034) | |
| Lagged EDI (4 time periods) | 1.320 (0.367) | ||||
| Maxilla | 0.548 (0.194) | 0.591 (0.207) | 0.589 (0.207) | 0.595 (0.208) | 0.256 (0.188) |
| Incisor 2 | 0.136 (0.155) | 0.149 (0.177) | 0.148 (0.177) | 0.150 (0.180) | 0.127 (0.132) |
| Canine | −0.191 (0.164) | −0.217 (0.186) | −0.217 (0.186) | −0.219 (0.189) | −0.298 (0.149) |
| Bicuspid 1 | −0.558 (0.174) | −0.634 (0.197) | −0.634 (0.197) | −0.643 (0.200) | −0.692 (0.160) |
| Bicuspid 2 | −1.255 (0.203) | −1.423 (0.227) | −1.423 (0.227) | −1.445 (0.230) | −1.242 (0.188) |
| Time | 0.344 (0.031) | 0.344 (0.031) | 0.544 (0.065) | ||
| Clinpro Crème | 1.892 (0.841) | 2.730 (0.875) | 1.495 (0.697) | ||
| MI Paste | 1.505 (0.838) | 2.113 (0.873) | 1.006 (0.697) | ||
| Clinpro Crème X Time | −0.274 (0.072) | ||||
| MI Paste X Time | −0.197 (0.074) | ||||
| Constant | −4.718 (0.412) | −5.782 (0.446) | −6.892 (0.698) | −7.527 (0.727) | −3.961 (0.579) |
| Patient R.E. Constant | 1.044 (0.126) | 1.089 (0.126) | 1.053 (0.126) | 1.059 (0.126) | 0.842 (0.131) |
| Maxilla R.E. Constant | −0.265 (0.168) | −0.221 (0.168) | −0.222 (0.168) | −0.217 (0.168) | −0.312 (0.190) |
| Tooth Number R.E. Constant | −0.238 (0.119) | −0.050 (0.101) | −0.052 (0.101) | −0.028 (0.099) | −0.940 (0.238) |
| N | 7540 | 7540 | 7540 | 7540 | 1885 |
| Degrees of Freedom | 6 | 7 | 9 | 11 | 8 |
| Wald Chi-square | 362.918 | 458.440 | 464.131 | 464.840 | 87.765 |
| Prob > Chi-square | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Model 1 served as a baseline model, controlling for one-period lagged EDI, arch, and tooth type. The upper arch exhibited higher enamel decalcification, as did the premolars when compared to the central incisor. Model 2 introduced time, which was also positively associated with higher EDI. Model 3 brought in the treatment variables, Clinpro Crème and MI Paste, in comparison to the omitted treatment group, Clinpro 5000. The results provided support that treatment effects did exist, above and beyond the other variables controlled for. Relative to Clinpro 5000, Clinpro Crème exhibited higher levels of enamel decalcification (significant at the 95% level) whereas MI Paste showed marginal significance (at the 90% level) in having higher EDI than Clinpro 5000.
Model 4 introduced interaction terms of treatment by time. Significance is displayed for the base effects as well as the interaction terms with combined results, suggesting that Clinpro 5000 was associated with lower EDI relative to the other treatments, though the magnitude of the effect fell off with the progression of time as indicated by the negative interaction terms. This finding was somewhat in tandem with the Lowess curves illustrated in Figure 4 Appendix, with Clinpro 5000 exhibiting a steep departure from the other two treatments earlier on. The last model showed the change in EDI between the beginning of treatment (time 0) and the end of treatment (time 4) (Figure 4B). Thus, Model 5 used a quarter of the observations as the first models rather than aggregate four short time periods. Model 5 used the data once across the span of the study, from start state to end state. The results were slightly different than in the previous models; arch differences were no longer significant, canine teeth also exhibited lower levels of EDI than the central incisor (Figure 4B and C), and MI Paste was no longer even significant at the 90% level. Additional models were created changing the omitted treatment group to test for differences between MI Paste and Clinpro Crème; no significant differences were found. Altogether, the results strongly supported that Clinpro 5000 provided superior protection against enamel decalcification when compared to Clinpro Tooth Crème, and mixed support when compared to MI Paste.
DISCUSSION
Previous studies reported a similar beneficial effect from either supplemental fluoride or CPP-ACP.2,4,12 A previous study using synchrotron radiation micro computed tomography to access the densities of bovine enamel WSL suggested Clinpro 5000, which contains 1.1% NaF, delivered remineralization benefits at the surface of the WSLs better than the 0.21% Clinpro Tooth Crème.13 Since penetration of fluoride into enamel is limited and decreases exponentially with enamel depth, it might be possible that the functionalized TCP present in the Clinpro dentifrices helps extend the depth of fluoride penetration, and therefore lesion remineralization.14 Notably, several in vitro studies have demonstrated the beneficial effect of fTCP on WSLs prevention and remineralization.15–17 In an in vitro study, the same products used in the current study (5000 ppm sodium fluoride; GC MI paste plus and Clinpro tooth crème) were compared and evaluated under a scanning Electron Microscope for calculation of the percentage of occluded tubules. Similar to this study, they concluded that sodium fluoride showed the greatest remineralization and dentinal tubule occlusion property when compared with GC MI paste plus and Clinpro tooth crème.16 Similar results were found in an in vitro study where CPP-ACP, casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and tricalcium phosphate fluoride (TCP-F) were compared. They found that remineralization efficacy was TCP-F > CPP-ACPF > CPP-ACP.17 Also, results indicated that combining fluoride with TCP could provide more anticaries benefits compared to using fluoride alone.18
In the present study, when analyzing the results, the comparison of the facial surface areas within a tooth showed that, even though the EDI percentage increased from time points T0–T4, the final result at T4 had overall the lowest percentage for Clinpro 5000 followed by the MI Paste Plus and Clinpro Tooth Crème. Also, the baseline (EDI 0) was maintained highest for Clinpro 5000. The results were further analyzed in different hierarchies across the four time points; comparing the teeth within the arches, it showed that the maxilla seemed to be more affected than the mandible. This result can be explained by the fact that the maxilla has more tooth surface than the mandible. The comparison of arches within patients, however, showed an interesting result in the order of the most to the least affected teeth. There was no consistent explanation for the specific order.
This clinical study had some limitations. One was that the patients' compliance could not be ideally controlled. An attempt to overcome the compliance barrier was made by giving the patients brushing technique instructions, standardizing the time and frequency for brushing, and using the brushing diary as a control for their actual compliance with the protocol. However, plaque accumulation and gingival inflammation were still observed in a considerable number of patients. A more serious limitation was the duration of this study, which was designed to be 4 months based on some previous studies.9,19,20 It is found that development of clinically visible WSLs in orthodontic patients can occur within 4 weeks or less.21,22 In addition, the prevalence of white spots in orthodontic treatment was reported to reach 38% in 6 months, and increased slightly to 46% after 12 months.23 However, the longer the treatment is, the more likely the WSLs will develop. Additionally, patients' oral hygiene may also worsen with treatment time. Thus, the results of this study only showed the effect of the three fluoride dentifrices in the early stage of orthodontic treatment.
Therefore, in the future, it would be useful to conduct a similar study over a longer experimental period to evaluate more fully the preventive effect of fluoride dentifrices during orthodontic treatment. It would also be desirable to detect the long-term effect of using different fluoride dentifrices in preventing WSLs, especially after orthodontic treatment. Clinical studies to explore the mechanisms underlying how these different fluoride dentifrices function based on the previous in vitro studies would also be meaningful.
CONCLUSIONS
Overall, the results of this study can help clinicians to decide the effectiveness of using fluoride dentifrice products to prevent WSLs in their orthodontic practice. The study found that the use of Clinpro 5000, Clinpro Crème, and MI Paste Plus could prevent WSL formation during orthodontic treatment, with Clinpro 5000 showing a marginally better effect than the two other test pastes. This information could lead to more appropriate therapeutic decisions.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by an Industry Grant by 3M Espe.
REFERENCES
- 1.Melrose CA, Appleton J, Lovius BB. A scanning electron microscopic study of early enamel caries formed in vivo beneath orthodontic bands. Br J Orthod. 1996;23(1):43–47. doi: 10.1179/bjo.23.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Benson PE, Parkin N, Dyer F, Millett DT, Furness S, Germain P. Fluorides for the prevention of early tooth decay (demineralised white lesions) during fixed brace treatment. The Cochrane Library. 2013. [DOI] [PubMed]
- 3.Ogaard B, Larsson E, Henriksson T, Birkhed D, Bishara SE. Effects of combined application of antimicrobial and fluoride varnishes in orthodontic patients. Am J Orthod Dentofacial Orthop. 2001;120(1):28–35. doi: 10.1067/mod.2001.114644. [DOI] [PubMed] [Google Scholar]
- 4.Baeshen HA, Lingstrom P, Birkhed D. Effect of fluoridated chewing sticks (Miswaks) on white spot lesions in postorthodontic patients. Am J Orthod Dentofacial Orthop. 2011;140(3):291–297. doi: 10.1016/j.ajodo.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Singh SP, Goyal A, Utreja AK, Jena AK. Effects of various remineralizing agents on the outcome of post-orthodontic white spot lesions (WSLs): a clinical trial. Prog Orthod. 2016;17(1):25. doi: 10.1186/s40510-016-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrini F, Lombardo L, Arreghini A, Medori S, Siciliani G. Caries prevention during orthodontic treatment: In-vivo assessment of high-fluoride varnish to prevent white spot lesions. Am J Orthod Dentofacial Orthop. 2016;149(2):238–243. doi: 10.1016/j.ajodo.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo M, Bussaneli D, Jeremias F, et al. Control of white spot lesions with use of fluoride varnish or chlorhexidine gel during orthodontic treatment a randomized clinical trial. J Clin Pediatr Dent. 2016;40(4):274–280. doi: 10.17796/1053-4628-40.4.274. [DOI] [PubMed] [Google Scholar]
- 8.Rollings S, Greene L, Borrie F, Lamont T. Small trial finds beneficial effect for MI Paste in preventing white spot lesions during orthodontic treatment. Evid Based Dent. 2012;13(4):117–118. doi: 10.1038/sj.ebd.6400899. [DOI] [PubMed] [Google Scholar]
- 9.Robertson MA, Kau CH, English JD, Lee RP, Powers J, Nguyen JT. MI. Paste Plus to prevent demineralization in orthodontic patients: a prospective randomized controlled trial. Am J Orthod Dentofacial Orthop. 2011;140(5):660–668. doi: 10.1016/j.ajodo.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Karlinsey RL, Mackey AC, Stookey GK, Pfarrer AM. In vitro assessments of experimental NaF dentifrices containing a prospective calcium phosphate technology. Am J Dent. 2009;22(3):180–184. [PubMed] [Google Scholar]
- 11.Banks P, Richmond S. Enamel sealants: a clinical evaluation of their value during fixed appliance therapy. Eur J Orthod. 1994;16(1):19–25. doi: 10.1093/ejo/16.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Bailey DL, Adams GG, Tsao CE, et al. Regression of post-orthodontic lesions by a remineralizing cream. J Dent Res. 2009;88(12):1148–1153. doi: 10.1177/0022034509347168. [DOI] [PubMed] [Google Scholar]
- 13.Makoto A, Uesugi K, Masato H, Tomoaki K, Allen CM, Robert LK. In vitro assessments of white-spot lesions treated with NaF plus tricalcium phosphate (TCP) toothpastes using synchrotron radiation micro computed tomography (SR micro-CT) Academic Journals. 2014;6(1):10–21. [Google Scholar]
- 14.Karlinsey RL, Mackey AC, Schwandt CS, Walker TJ. SEM evaluation of demineralized dentin treated with professional-strength NaF topical pastes. Am J Dent. 2011;24(6):357–362. [PubMed] [Google Scholar]
- 15.Jo S-Y, Chong H-J, Lee E-H, et al. Effects of various toothpastes on remineralization of white spot lesions. Korean J Orthod. 2014;44(3):113–118. doi: 10.4041/kjod.2014.44.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prabhakar AR, Manojkumar AJ, Basappa N. In vitro remineralization of enamel subsurface lesions and assessment of dentine tubule occlusion from NaF dentifrices with and without calcium. J Indian Soc Pedod Prev Dent. 2013;31(1):29–35. doi: 10.4103/0970-4388.112403. [DOI] [PubMed] [Google Scholar]
- 17.Patil N, Choudhari S, Kulkarni S, Joshi SR. Comparative evaluation of remineralizing potential of three agents on artificially demineralized human enamel: an in vitro study. J Conserv Dent. 2013;16(2):116–120. doi: 10.4103/0972-0707.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaechi BT, Ramalingam K, Mensinkai PK, Chedjieu I. In situ remineralization of early caries by a new high-fluoride dentifrice. Gen Dent. 2012;60(4):e186–192. [PubMed] [Google Scholar]
- 19.Derks A, Frencken J, Bronkhorst E, Kuijpers-Jagtman AM, Katsaros C. Effect of chlorhexidine varnish application on mutans streptococci counts in orthodontic patients. Am J Orthod Dentofacial Orthop. 2008;133(3):435–439. doi: 10.1016/j.ajodo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman DA, Clark AE, Rody WJ, McGorray SP, Wheeler TT. A prospective randomized clinical trial into the capacity of a toothpaste containing NovaMin to prevent white spot lesions and gingivitis during orthodontic treatment. Prog Orthod. 2015;16(1):25. doi: 10.1186/s40510-015-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92(1):33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 22.Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94(1):68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 23.Artun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod. 1986;8(4):229–234. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.