Abstract
Introduction:
Evidence from model systems implicates long-chain acyl-CoA synthetases (ACSLs) as key regulators of skeletal muscle fat oxidation and fat storage; however, such roles remain underexplored in humans.
Purpose:
We sought to determine protein expression of ACSL isoforms in skeletal muscle at rest and in response to acute exercise, and identify relationships between skeletal muscle ACSLs and measures of fat metabolism in humans.
Methods:
Sedentary adults (n=14 [4M/10F], BMI 22.2±2.1 kg/m2, VO2max 32.2±4.5 ml/kg/min) completed two study visits. Trials were identical other than completing 1 hour of cycling exercise (65% VO2max) or remaining sedentary. Vastus lateralis biopsies were obtained 15-minutes post-exercise (or rest) and 2-hours post-exercise to determine ACSL protein abundance. Whole-body fat oxidation was assessed at rest and during exercise using indirect calorimetry. Skeletal muscle triacylglycerol (TAG) was measured via lipidomic analysis.
Results:
We detected protein expression for 4 of the 5 known ACSL isoforms in human skeletal muscle. ACSL protein abundances were largely unaltered in the hours following exercise aside from a transient increase in ACSL5 15-minutes post-exercise (P=0.01 vs. Rest). Skeletal muscle ACSL1 protein abundance tended to be positively related with whole-body fat oxidation during exercise (P=0.07, r=0.53), when skeletal muscle accounts for the majority of energy expenditure. No such relationship between ACSL1 and fat oxidation was observed at rest. Skeletal muscle ACSL6 protein abundance was positively associated with muscle TAG content at rest (P=0.05, r=0.57).
Conclusion:
Most ACSL protein isoforms can be detected in human skeletal muscle, with minimal changes in abundance following acute exercise. Our findings agree with those from model systems implicating ACSL1 and ACSL6 as possible determinants of fat oxidation and fat storage within skeletal muscle.
Keywords: Long Chain Acyl-CoA Synthetase, ACSL, Skeletal Muscle, Lipid Metabolism, Acute Exercise, Human
Introduction
Understanding the regulation of skeletal muscle fat oxidation and fat storage remains an area of high interest in the context of both exercise performance and disease prevention (1). Acute exercise is known to influence skeletal muscle fat metabolism, including both fat oxidation and fat storage (2–6). Mounting evidence from model systems indicates different long chain acyl-coenzyme A synthetase isoforms (ACSLs) may play an integral role in the regulation of fat oxidation and fat storage within skeletal muscle (7–11). Previous findings in humans have primarily focused on identifying potential racial differences in ACSL activity (12–14). However, the current understanding regarding the role of specific ACSL isoforms in skeletal muscle fat metabolism is limited and changes in ACSLs with acute exercise in humans are unknown. Identifying the protein abundance and potential roles of different ACSL isoforms within human skeletal muscle at rest and in response to acute exercise may provide novel insight into the regulation of skeletal muscle fat metabolism in humans.
ACSLs modify fatty acids entering muscle cells by catalyzing the formation of long-chain acyl-CoA, thereby enabling further metabolic processing. For example, such “activation” of fatty acids by ACSLs is required before oxidation or storage can occur (15,16). Much of the current understanding regarding the function and expression of ACSLs in various tissues was pioneered by the laboratory of Dr. Rosalind A. Coleman. For example, there are 5 known ACSL isoforms that vary in expression, function, and localization among tissues (7,17), with growing interest in the isoform-specific roles in metabolically active tissues such as skeletal muscle (18,19). Genetically modified mouse and cell models have elucidated potential functions of some ACSL isoforms in skeletal muscle (8,9,11,20). Skeletal muscle-specific knock out of ACSL1 in mice resulted in attenuated fat oxidation at rest and exercise intolerance compared with wild type animals (8). Overexpression of ACSL5 in cultured human muscle cells increased complete oxidation of palmitate, as well as basal and maximal uncoupled mitochondrial respiration (20). Such findings suggest ACSL1 and ACSL5 may facilitate skeletal muscle fat oxidation. Conversely, knockdown of ACSL6 in cultured human and rat muscle cells attenuated lipid storage, as evidenced by decreased lipid droplet size and lower triacylglycerol (TAG) content (9,11). Overexpression of ACSL6 increased phospholipid accumulation and decreased fat oxidation (9). These findings demonstrate the potential role for ACSL6 in skeletal muscle fat storage. Taken together, genetic modification models suggest distinct roles for different ACSL isoforms in skeletal muscle fat metabolism.
We recently investigated roles of skeletal muscle ACSL isoforms in the regulation of fat metabolism using wild type C57BL/6J mice, including physiologic interventions such as high fat diet (HFD)-induced obesity and aerobic exercise training (10). We demonstrated skeletal muscle ACSL1 protein abundance was increased in response to the HFD intervention (10), which aligns with other adaptive responses we have reported in this model to increase capacity for fat oxidation within skeletal muscle (21,22). We also reported skeletal muscle ACSL6 protein abundance was greater following both HFD and aerobic exercise training, and was positively associated with skeletal muscle fat storage (10). To what extent ACSLs may play similar regulatory roles in human skeletal muscle remains largely underexplored.
The overall objective of this study was to provide insight into skeletal muscle ACSLs in humans. Our first aim was to determine protein expression of known ACSL isoforms in human skeletal muscle and identify effects of acute exercise on skeletal muscle ACSL protein abundance. Our second aim was to identify relationships between skeletal muscle ACSL isoforms and measures of fat metabolism. Herein we demonstrate detection of protein expression for 4 of 5 known ACSL isoforms at rest and after exercise, and provide evidence indicating ACSL isoforms 1 and 6 may play integral roles in human skeletal muscle fat metabolism.
Methods
Participants
Participants were generally healthy and free of any major medical conditions. Inclusion criteria included 18–45 years old and body mass index (BMI) between 18–26 kg/m2. All participants were nonsmokers, weight stable (± 2 kg) for ≥ 6 months, and sedentary (less than 60 minutes of purposeful exercise per week) ≥ 6 months. Exclusionary criteria consisted of structured physical activity (>60 minutes per week), changes in body weight (>2 kg in previous 6 months), hyperglycemia (fasting glucose >126 mg/dl), hypertension, cancer, heart disease, pregnancy, un-treated hypo- or hyperthyroid, and allergy to lidocaine. Exclusionary medications included insulin, metformin, thiazolidinediones, statins, chronic non-steroidal anti-inflammatory medications, hypertensive treatments and hyperlipidemic treatments. Regular use of oral contraceptive medications was permitted. Previous findings indicate that fluctuations in sex hormone levels can alter fat oxidation (23). We therefore studied female participants during the early follicular phase of the menstrual cycle for each metabolic study day.
Overall Study Design
The overall study design is summarized in Figure 1A. The study protocol was approved by the Institutional Review Board at Oregon State University (IRB #7605) and registered at clinicaltrials.gov (#NCT02987491). The current evaluation of ACSL isoforms was performed using a sub-set of vastus lateralis muscle biopsies collected during metabolic study days that investigated glucose metabolism following acute exercise. All study procedures were performed at the Samaritan Athletic Medical Center on the campus of Oregon State University. The four study visits were for medical screening, maximal aerobic capacity (VO2max) and two metabolic study days. During the medical screening visit, a member of the study team explained all procedures to the participant prior to obtaining written consent. A fasting blood draw was collected for clinical blood panel (analyzed at Samaritan Regional Medical Center). Anthropometrics were collected including height, weight, waist circumference, and body composition was assessed by dual energy x-ray absorptiometry (Hologic). Females were not pregnant as verified using point-of-care urine testing. Eligible participants returned for a graded exercise test on a cycle ergometer with 12-lead electrocardiogram. Participants then completed study visits three and four, which consisted of two separate metabolic study visits (i.e., one no-exercise “rest” trial and one exercise trial), in a randomized crossover design separated by ≥ 1 week.
Figure 1: Study Design.
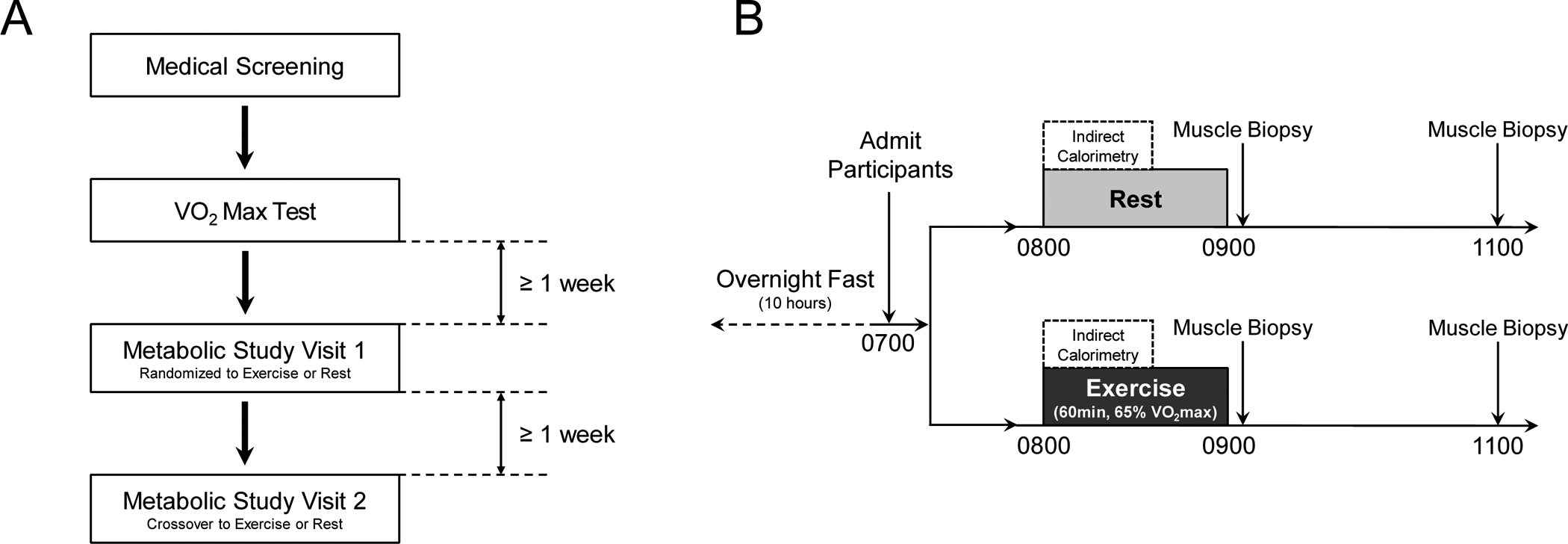
A) The overall study design was 4 study visits which included a medical screening, VO2max testing, and two metabolic study visits separated by at least 1 week. Participants completed metabolic study visits in a randomized crossover design. B) Metabolic study visits were identical other than completing 1 h of moderate intensity cycling exercise (65% VO2max) or remaining sedentary. Muscle biopsy samples obtained at 1100 h during the rest trial were not analyzed for this study to eliminate duplication of resting measures.
VO2max Test
Upon arrival, participants were fitted with a 12-lead electrocardiogram (Nasiff Associates, Inc., Central Square, NY). The VO2max test was performed on an electronically braked stationary cycle ergometer (Lode Corival) with indirect calorimetry (Parvo Medics, Sandy, UT) at least 1 week prior to the first metabolic study visit. The protocol consisted of a 2-minute warm up at 50 W intensity then increased 25 W each minute for males and increased 15 W each minute for females until volitional fatigue (~8–12 minutes). Participants were verbally encouraged throughout the test. Ratings of perceived exertion (6–20 on Borg scale) and blood pressure were collected every 3 minutes. VO2max was defined as peak oxygen consumption during 30-second averaging achieved with 1) failure to maintain pedaling cadence of at least 60 revolutions per minute, 2) reaching approximately 10% of age-predicted maximal heart rate, and 3) respiratory exchange ration greater than 1.1.
Experimental Protocol
The experimental protocol for metabolic study visits is presented in Figure 1B. Each participant completed two separate metabolic study visits separated by at least 1 week in randomized order. Participants recorded a 24-hour diet log the day prior to their first metabolic study visit which they were then asked to repeat prior to their second metabolic study visit. Participants were admitted to the Samaritan Athletic Medicine Center at 0700 h following an overnight fast. Between 0800 and 0900 hours, participants either rested in bed or completed a single session of exercise on a stationary cycle ergometer (Lode Corival) at 65% of their predetermined VO2max. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured at 0800 h by indirect calorimetry (Parvo Medics TrueOne 2400) during both trials for calculation of whole-body substrate oxidation and to verify exercise intensity. Resting indirect calorimetry was measured while participants lay in a quiet room with low lights for 30 minutes using a ventilated-hood, whereas exercise indirect calorimetry was measured during the first 20 minutes of moderate-intensity exercise using a two-way valve mouthpiece. Vastus lateralis skeletal muscle biopsies were obtained at 0915 h (15-minutes post rest and exercise) and 1100 h (120-minutes post-exercise) during both trials. A local anesthetic (2% lidocaine) was used to numb the biopsy site before a 6–8 mm incision was made through the skin and fascia. The muscle biopsy sample was obtained using a 5 mm Bergström needle with light suction, with the 0915 and 1100 h biopsies obtained from contralateral legs. For this study, only the 1100 h muscle biopsy sample from the exercise trial was used to measure ACSL protein abundance 120 minutes post-exercise to preserve human muscle sample by eliminating duplication of resting measures. Muscle biopsy samples were dissected free of adipose and connective tissue, blotted dry, frozen in liquid nitrogen, and stored at −80°C until further analysis.
Whole-body Fat Oxidation
Rates of whole-body fat oxidation were calculated from VO2 and VCO2 measures using the equations of Frayn and normalized to body weight (24). At minimum, the first 5 minutes of data acquisition were not used for calculations to allow for equilibration. The average values for VO2 and VCO2 measures were obtained over the course of ~10 minutes once steady state was achieved. Exercise intensity was adjusted via cycle ergometer resistance to achieve the desired 65% of VO2max. We recognize measures of whole-body fat oxidation are not specific to skeletal muscle. However, skeletal muscle comprises ~40% of total body mass and is known to be a principal determinant of energy expenditure at rest (25). During moderate-intensity exercise, whole-body substrate oxidation is largely representative of metabolism within skeletal muscle (26,27).
Skeletal Muscle Fat Storage
Skeletal muscle lipids were extracted and analyzed as previously reported by our group (21,28). Vastus lateralis muscle biopsies (~10 mg) were homogenized in 900 μl ddH2O, and an aliquot was taken for protein concentration (Pierce BCA). Then, 750 μl of homogenized sample was transferred to a glass screw cap tube with methanol (MeOH), methyl tert-butyl ether (MTBE), and internal standards. The internal standard cocktail included TAG-d5 (14:0/16:1/14:0) and TAG (17:0/17:0/17:0), among many other reference lipids. Samples were vortexed, rotated for 5 min at room temperature, and centrifuged at 2500 × g for 5 min to separate phases. The upper phase containing lipids was transferred to new glass culture tubes. Residual lipids in the lower phase were repeat extracted with additional MTBE. The combined extracts were dried under nitrogen gas and low heat (~30°C). The total lipid extract was transferred to autosampler vials using 2:1 chloroform:methanol and re-dried under nitrogen gas. Lipids were resuspended in 95:5:0.1 hexane:dichloromethane:actic acid for analysis. Triacylglycerols (TAG) were analyzed by a Sciex API 2000 triple quadrupole mass spectrometer. TAG species were separated by reverse phase chromatography (Phenomenex C8, using Solvent A - Acetonitrile/water (60/40) with 10 mM ammonium acetate, Solvent B – Isopropanol/Acetonitrile (900/100) with 10 mM ammonium acetate. Standard curves were generated with reference standards combined with the same quantity of internal standard cocktail added to samples upon extraction. Concentration was determined by comparing ratios of unknowns to internal standards, and compared to standard curves representing typical lipid species. TAG species were quantified using MultiQuant software (Sciex, Framingham, MA).
Skeletal Muscle ACSL Abundance
Vastus lateralis muscle biopsy samples (~30 mg) were homogenized using glass-glass homogenizers as described previously by our group (10,28). In brief, samples were homogenized 1:10 wt/vol in lysis buffer with protease inhibitors (20 mM Tris HCL, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1x Sigma Protease Inhibitor Cocktail no. P8340). Homogenates rotated at 4°C for 20 minutes and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was stored at −80°C until further analysis. Approximately 30 μg protein was separated on bis-tris gels and transferred to nitrocellulose membranes. Each gel was loaded with the same internal control sample in 2 lanes, the average density of both lanes was used to normalize band density between gels. Ponceau staining of membranes was used to verify equal loading and transfer of protein. Membranes were blocked in 5% bovine serum albumin (BSA) in tris-buffered saline with tween (TBST) and incubated in primary antibodies at 4°C. Following primary incubation, membranes were washed in TBST and incubated in secondary antibody diluted in blocking buffer at room temperature. Images were generated using infrared detection (LI-COR Odyssey). Primary antibodies used included ACSL1 (product no. 4047, Cell Signaling Technology), ACSL3 (product no. 166374, Santa Cruz), ACSL4 (product no. PA5-27137, Invitrogen), ACSL5 (product no. 365478, Santa Cruz) and ACSL6 (product no. PA5-30465, Invitrogen). All primary antibodies were derived using recombinant fragments of human origin and are recommended for detection of their respective ACSL isoform in human tissue. Each primary antibody was diluted 1:1000 in blocking buffer (5% BSA in TBST). Primary antibodies for ACSL isoforms 1 (cited 23 times), 4 (cited 3 times), and 5 (cited 2 times) have been previously verified by knockdown and/or overexpression models. Internal testing by the manufacturer verified primary antibodies for ACSL6 (cited 1 time) by measuring relative expression in various tissues to ensure that the antibody binds to the antigen stated. Primary antibodies for ACSL3 (cited 5 times) have not been verified by knockdown or overexpression; however, internal testing by the manufacturer verified expression of ACSL3 in mouse muscle cell line (C2C12). The secondary antibodies used were anti-rabbit-700 (product no. 926-68071) and anti-mouse-800 (product no. 926-32212) from LICOR diluted 1:10,000.
Statistical analysis
This study was a repeated cross-over design. The effect of acute moderate-intensity exercise on skeletal muscle ACSL protein abundance was analyzed by repeated measure one-way analysis of variance (ANOVA) model with Dunnett’s posthoc analysis comparing post exercise time-points with rest. Although our study was not powered to investigate differences between males and females, we investigated potential sex-based differences in ACSL isoform protein abundance by unpaired two-tailed student’s t-tests. Relationships between skeletal muscle ACSL isoform protein abundances (measured during the rest trial) and measures of fat oxidation and fat storage were analyzed by Pearson’s correlational analysis. Statistical significance was set as P ≤ 0.05. Statistical analysis and generation of figures was performed using Prism version 8 (GraphPad Software) and R Studio version 3.3.1 (R Studio, Inc). Data are presented as mean and standard deviation, with individual data points shown, in accordance with recommendations for the field (29).
Results
Participant characteristics and exercise intensity
A total of 14 sedentary women and men (female/male: 10/4) completed the study. A summary of participant characteristics, substrate oxidation at rest and during exercise, and skeletal muscle fat storage at rest are presented in Table 1. Study participants were younger (28 ± 7 years of age) relatively lean (body mass index 22 ± 2 kg/m2) adults. All participants successfully completed the 1-hour moderate-intensity exercise session as planned (mean intensity 63 ± 3% of VO2max).
Table 1.
Participant characteristics and measures of fat oxidation and fat storage
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (years) | 28 ± 7 | 19 – 44 |
| BMI (kg/m2) | 22 ± 2 | 19 – 26 |
| Waist circumference (cm) | 77 ± 8 | 67 – 93 |
| Systolic blood pressure (mmHg) | 113 ± 9 | 99 – 127 |
| Diastolic blood pressure (mmHg) | 74 ± 7 | 64 – 90 |
| Body mass (kg) | 61.6 ± 10.3 | 49.4 – 86.3 |
| Fat free mass (kg) | 44.9 ± 8.2 | 34.3 – 62.9 |
| Fat mass (kg) | 16.6 ± 4.4 | 8.4 – 24.0 |
| Body fat (%) | 27.0 ± 5.3 | 13.0 – 34.4 |
| Absolute VO2max (L/min) | 2.01 ± 0.35 | 1.44 – 2.82 |
| Relative VO2max (ml/kg/min) | 32.2 ± 4.5 | 23.6 – 40.0 |
| Basal RER | 0.78 ± 0.08 | 0.70 – 0.85 |
| Exercise RER | 0.94 ± 0.04 | 0.88 – 0.97 |
| Basal fat oxidation (μmol FA/kg/min) | 4.37 ± 0.95 | 2.87 – 6.43 |
| Exercise fat oxidation (μmol FA/kg/min) | 8.26 ± 3.65 | 2.96 – 14.81 |
| Skeletal muscle triacylglycerol (pmol/μg) | 31.8 ± 26.2 | 7.9 – 101.9 |
Data are presented as mean ± SD (standard deviation). RER, respiratory exchange ratio; FA, fatty acid.
Skeletal muscle ACSL protein abundance at rest and after exercise
We successfully measured 4 of the 5 ACSL isoforms in human vastus lateralis muscle samples via western blotting, including ACSL isoforms 1, 4, 5, and 6, with notable variance among individuals (Figure 2). ACSL3 was below limits of detection (data not shown). We also identified the effects of acute exercise on skeletal muscle ACSL protein abundance 15- and 120-minutes post-exercise. ACSL5 protein abundance was significantly increased 15-minutes post-exercise (+26% vs. Rest, P=0.01, Figure 2C) but returned to basal levels by 120-minutes (Figure 2C). ACSL isoforms 1, 4 and 6 were not significantly altered 15- or 120-minutes post-exercise (Figure 2). Although our study was not designed to identify differences between males and females, we investigated the potential for sex-based differences of skeletal muscle ACSL protein abundance at rest. Female participants tended to have greater total protein abundance for all measured ACSL isoforms compared with males, with sex-based differences in ACSL1 and ACSL6 achieving statistical significance (P≤0.01 for females vs. males, Figure 3). The effect of acute exercise on skeletal muscle ACSL isoform protein abundance was not different on the basis of sex (data not shown). Collectively, we demonstrate 4 of the 5 known ACSL isoforms are readily detectable in human skeletal muscle, with only transient changes in ASCL5 protein abundance following acute exercise, and the potential for sex-based differences in skeletal muscle ACSL protein abundance.
Figure 2: Skeletal muscle ACSL isoform protein abundance at rest and following acute exercise.
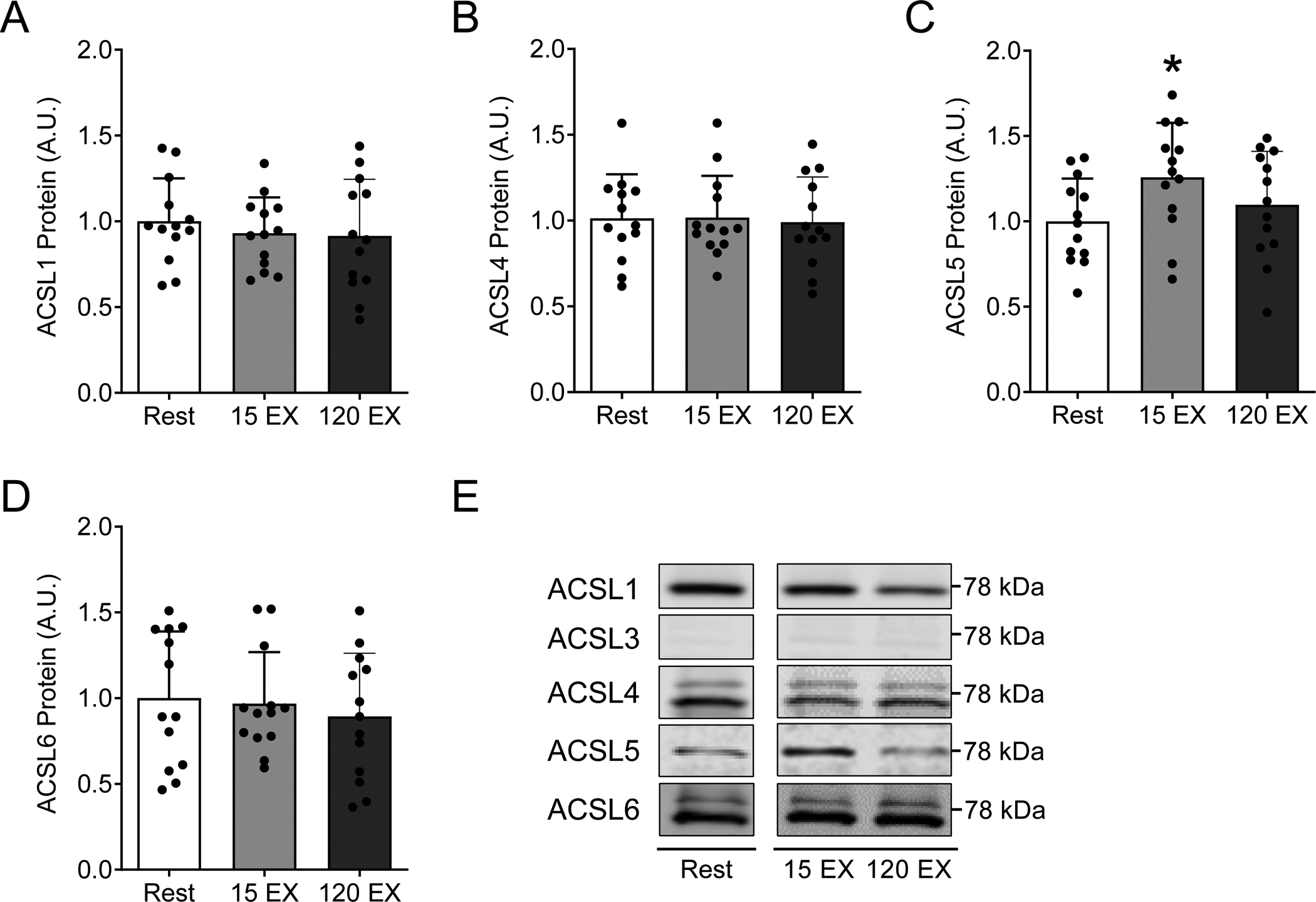
ACSL isoform protein abundance in vastus lateralis muscle biopsy samples at rest, 15 minutes after acute exercise (15 EX), and 120 minutes after acute exercise (120 EX). Total protein abundance for A) ACSL1, B) ACSL4, C) ACSL5, and D) ACSL6, with E) representative western blot images. Each representative image was spliced from the same blot/image for the purpose of highlighting samples reported in this study, with no alterations to the images. Full blot and ponceau images for each representative image were made available during the review process. The effects of acute exercise on skeletal muscle ACSL protein abundance were analyzed by repeated measures one-way analysis of variance models, with Dunnett’s posthoc analysis comparing post exercise time-points to rest. Data are presented as mean and standard deviation with individual data points shown. *P≤ 0.05 vs. Rest. n=14 (female/male: 10/4).
Figure 3: Resting skeletal muscle ACSL isoform protein abundance in males and females.
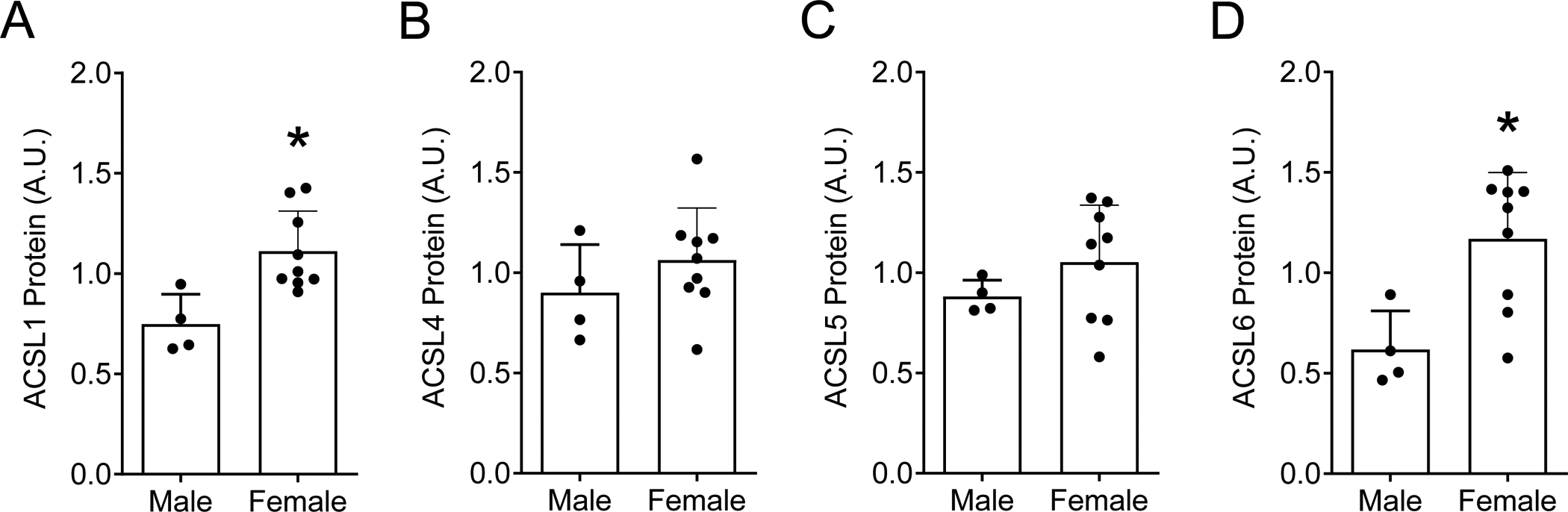
Sex-based differences in ACSLs were evaluated by comparing resting measures of ACSL protein abundances between female and male participants (using resting ACSL data presented in Figure 2). Sex-based evaluation of resting skeletal muscle ACSL abundance includes: A) ACSL1, B) ACSL4, C) ACSL5, and D) ACSL6. The effect of sex on skeletal muscle ACSL protein abundance was analyzed by unpaired two-tailed student’s t-tests. Data are presented as mean and standard deviation with individual data points shown. *P≤ 0.05 vs. Rest. n=14 (female/male: 10/4).
Relationships between skeletal muscle ACSLs and fat metabolism
We next investigated relationships between skeletal muscle ACSL isoforms and measures of fat metabolism in humans. Previous evidence in model systems demonstrates ACSL1 and ACSL5 may direct fatty acids toward oxidation whereas ACSL6 may be important for fatty acid synthesis and storage; less is known regarding the role of other ACSL isoforms in skeletal muscle fat metabolism. We therefore performed unbiased correlation analysis between ACSL protein abundances and measures of fat oxidation and skeletal muscle TAG concentration (Figure 4A). Whole-body fat oxidation at rest was not related to skeletal muscle ACSL1 protein abundance (P=0.64, r=0.15, Figure 4B). During exercise, however, when skeletal muscle metabolism constitutes the vast majority of total substrate oxidation (26,27), whole-body fat oxidation tended to positively relate to ACSL1 protein abundance (P=0.07, r=0.53, Figure 4C). ACSL5 protein abundance was not related to measures of whole-body fat oxidation at rest or during exercise (P=0.44 and P=0.39, Figure 4D and 4E, respectively). Resting skeletal muscle TAG concentration (i.e., fat storage) was positively related to ACSL6 protein abundance (P=0.05, r=0.57, Figure 4F), which agrees with previous findings suggesting a role for ACSL6 in the trafficking of fatty acids toward synthesis and storage (9–11). Skeletal muscle TAG content was also positively related to skeletal muscle ACSL1 protein abundance (P=0.01, r=0.67). Taken together, these findings suggest ACSL1 and/or ACSL6 may be important for fat oxidation during exercise and skeletal muscle fat storage.
Figure 4: Relationships between skeletal muscle ACSL protein abundance and measures of fat metabolism.
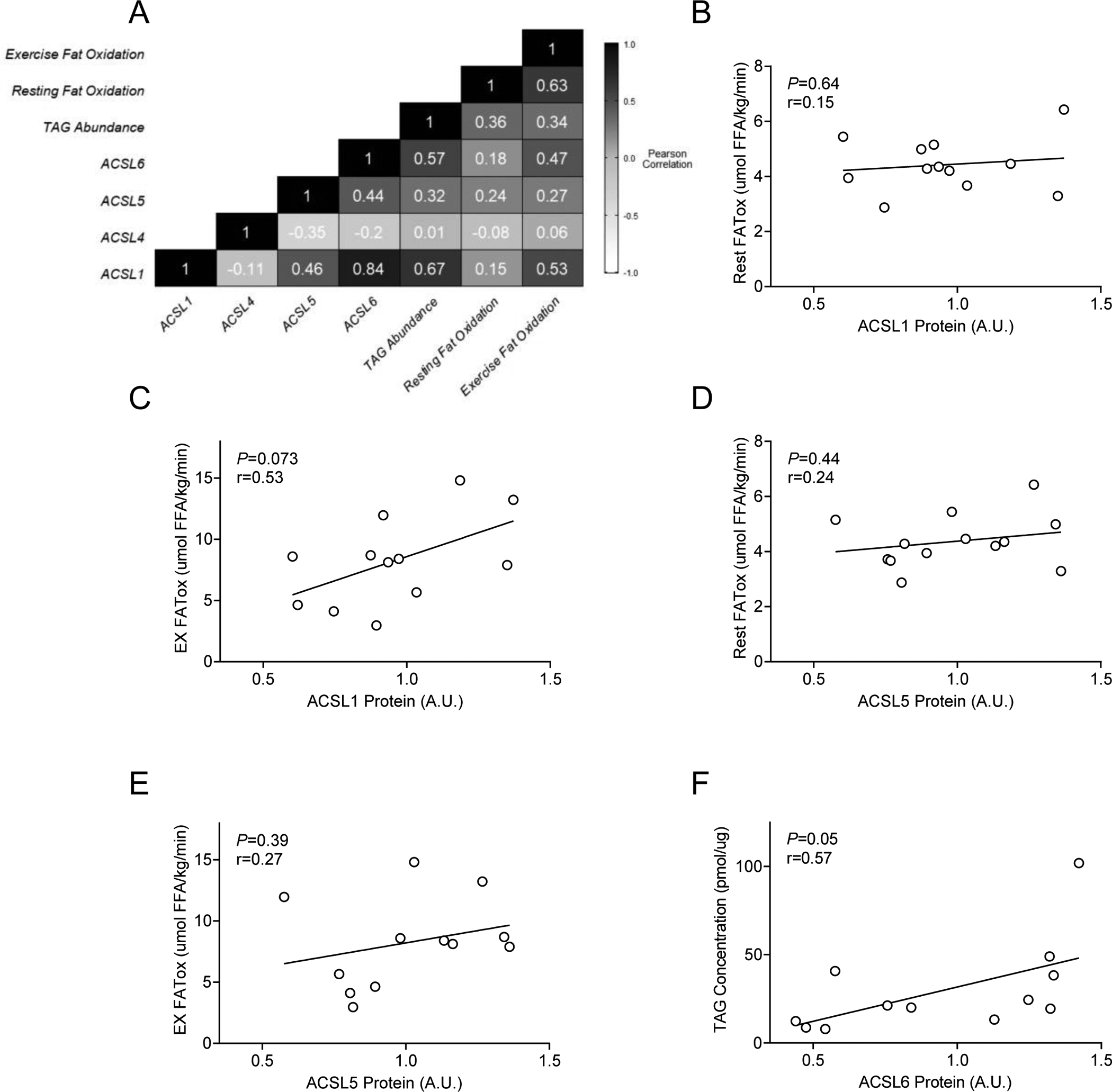
A) Unbiased correlation analysis to identify potential relationships between ACSLs and measures of fat oxidation and storage. B-F) Relationships of interest determined a priori on the basis of other reported findings. The predetermined relationships of interest included B) skeletal muscle ACSL1 protein abundance and whole-body fat oxidation at rest (Rest FATox), C) skeletal muscle ACSL1 protein abundance and whole-body fat oxidation during moderate-intensity exercise (EX FATox), D) skeletal muscle ACSL5 protein abundance and Rest FATox, E) skeletal muscle ACSL5 protein abundance and EX FATox, and F) skeletal muscle ACSL6 protein abundance and skeletal muscle triacylglycerol (TAG) concentration. n=14 (female/male: 10/4).
Discussion
The primary aims of this study were to measure known ACSL isoforms in human skeletal muscle, determine the effects of acute exercise on skeletal muscle ACSL protein abundance, and identify relationships between ACSL isoforms and measures of fat metabolism. Protein abundance for ACSL isoforms 1, 4, 5, and 6 was readily detected in human vastus lateralis muscle, whereas ACSL3 was below limits of detection. Even among a relatively homogeneous population of sedentary lean adults, there was notable variability in skeletal muscle ACSL abundance among participants, including strong evidence for possible sex-based differences in ACSL abundance. Skeletal muscle ACSL protein abundance was largely unchanged in the hours after acute moderate-intensity exercise, the one exception being a modest increase in ACSL5 measured 15 minutes post-exercise that returned to resting levels by 120 minutes post-exercise. Skeletal muscle ACSL1 protein abundance tended to be positively related to whole-body fat oxidation during exercise, whereas ACSL6 protein abundance was positively related to resting measures of fat storage (i.e., TAG content). We interpret these findings to indicate skeletal muscle ACSL1 and ACSL6 may be key determinants of skeletal muscle fat oxidation and fat storage.
ACSLs have emerged as potential critical regulators in fat metabolism; however, the protein expression and roles of ACSL isoforms in human skeletal muscle are currently underexplored. The five known ACSL isoforms exhibit differences in substrate preference and intracellular location (7,30,31), suggesting distinct functions for each isoform. Isoform localization within skeletal muscle remains to be fully elucidated, though evidence suggests skeletal muscle ACSL1 and ACSL5 may localize to mitochondria whereas ACSL6 may localize to sarcoplasmic reticulum (8,9,20). Additionally, ACSL isoform mRNA expression varies by tissue in rodents, further suggesting isoform-specific roles (7). For example, skeletal muscle mRNA content for ACSL isoforms 1, 3, and 6 are more abundant than isoforms 4 and 5 (7). We recently published evidence of protein expression for ACSL isoforms 1, 4, 5, and 6 in mouse skeletal muscle, demonstrating mRNA may not be indicative of protein abundance (10). In light of this, we focused on protein abundance, and not transcriptional control. We add to this literature by demonstrating similar detection of ACSL isoforms 1, 4, 5, and 6 in human skeletal muscle. Protein abundance of ACSL3 was below limits of detection in our samples. This is consistent with our previous findings in mice, yet unexpected on the basis of high reported mRNA expression in rodent skeletal muscle (7). Our findings also demonstrate potential sex-based differences in skeletal muscle ACSL isoform protein expression between male and female participants. Skeletal muscle ACSL1 and ACSL6 were greater in females compared with male participants which may, in part, contribute to sex-dependent differences in skeletal muscle fat metabolism (32). Our study was not designed to investigate such sex-based differences, yet we see this as an important area for continued investigation. Another consideration is the extent by which ACSL expression may vary by muscle fiber type and therefore among skeletal muscles. Skeletal muscle lipid metabolism differs by fiber type (33,34), and thus muscle-specific expression of ACSLs remains as an area of interest for future directions.
Acute exercise has been shown to increase rates of fat oxidation (2,3) and intramuscular triacylglycerol synthesis (i.e., fat storage) (4–6). Due to the potential role of ACSLs facilitating oxidation and storage of fatty acids within skeletal muscle, we hypothesized that observed changes in skeletal muscle fat metabolism following exercise may be due, in part, to changes in skeletal muscle ACSL protein abundance. In support of this hypothesis, ACSL1 and ACSL6 expression can be upregulated by peroxisome proliferator-activated receptor alpha (PPARα) and sterol regulatory binding element-1c (SREBP-1c) (9,35–37), respectively, and both transcription factors are upregulated by exercise (5,38,39). In contrast to our hypothesis, protein abundance of ACSL isoforms was largely unchanged in the 2 hours following acute moderate intensity-exercise, with the exception of a transient increase in ACSL5. The functional significance of such transient changes in ACSL5 remain to be determined; however, overexpression of ACSL5 in cultured human muscle cells provide evidence that this isoform may be important for fat oxidation (20). Our previous findings in mice demonstrated isoform-specific differences in ACSL protein abundance within skeletal muscle following aerobic exercise training, whereby ACSL4 was lower and ACSL6 was greater compared with remaining sedentary (10). It remains possible that acute exercise-induced changes in ACSL protein abundance may require longer to manifest (i.e., more than 2 hours), repeated exercise stimuli (i.e., training) or perhaps exercise of a greater duration and/or intensity. Nevertheless, we interpret our current findings to indicate ACSL isoform protein abundance is largely unchanged in the first few hours following an acute bout of moderate-intensity exercise.
Genetically altered model systems have helped elucidate the potential role of ACSL isoforms 1, 5 and, 6 in skeletal muscle fat metabolism (8,9,11,20). Previous evidence demonstrates ACSL1 is critical for normal fat oxidation within skeletal muscle (8). We therefore anticipated that ACSL1 protein abundance may be related to measures of whole-body fat oxidation. Consistent with our previous findings in mice, however (10), ACSL1 protein abundance was not related to resting measures of whole-body fat oxidation in humans. These findings highlight the need for direct measurement of skeletal muscle fat oxidation at rest, given our current evidence demonstrates ACSL1 is positively related with whole-body fat oxidation during exercise, when skeletal muscle is a major determinant of whole-body substrate oxidation (26,27). Neither skeletal muscle ACSL4 nor ACSL5 were related to any measures of fat metabolism in the current study. Several reports, including our previous study in mice (9–11), have demonstrated ACSL6 may be a critical determinant for skeletal muscle fat synthesis and storage. Our current findings further support this conclusion and demonstrate ACSL6 positively relates to resting measures of skeletal muscle TAG concentration (i.e., fat storage) in humans. We also note that our unbiased analysis identified a strong correlation between ACSL1 and ACSL6 protein abundance. This may be due to the fact that the ACSL1 and ACSL6 isoforms belong to the same subfamily of ACSLs (30,31), and perhaps reflects similar overall regulation of their expression. Collectively, we interpret our findings to further support the importance of ACSL1 and ACSL6 in regulation of skeletal muscle fat metabolism.
Although our current findings provide more insight into potential isoform-specific roles of skeletal muscle ACSLs in humans, our measures of protein abundance may not reflect ACSL activity. Critical next steps will include identifying functional consequence of identified post-translational modifications to ACSL proteins (40), determining the effects of exercise training on isoform-specific ACSL activity (12), transcriptional regulation of ACSLs, and investigating ACSL isoforms in more diverse study populations which may provide further insight into roles for ACSLs in health and disease. For example, previous findings indicate lower measures of fat oxidation in African-American women when compared with Caucasian women (14), which may be due, in part, to lower ACSL activity (13). However, the potential racial differences in skeletal muscle ACSL isoform expression is unknown and therefore remains an area of interest for future research. Additionally, skeletal muscle TAG synthesis across various populations is positively associated with insulin sensitivity and it is unknown if ACSL6 is a contributing mechanism to observed changes in TAG synthesis within skeletal muscle of different populations (41). Lastly, altered skeletal muscle fat oxidation is associated with various disease states which could be, in part, due to changes in ACSL1 function (42,43). Overall, there is much that still remains unknown in regard to the role and regulation of ACSL isoforms within skeletal muscle and this is an area ripe for future research in both human performance and the prevention of metabolic disease.
In conclusion, protein abundance for 4 of 5 known ACSL isoforms was readily detected in human vastus lateralis muscle. Skeletal muscle ACSL protein abundance was largely unchanged following a single session of acute exercise; however, ACSL5 abundance transiently increased 15-minutes post-exercise. ACSL1 protein abundance tended to be positively related to measures whole-body fat oxidation during moderate-intensity exercise, indicating potential contribution to fat oxidation with increased energetic demand within skeletal muscle. ACSL6 protein abundance was positively related to measures of skeletal muscle TAG content, suggesting contribution to regulation of lipid storage. Collectively, we interpret our evidence to further support the role of ACSLs in skeletal muscle fat oxidation and fat storage in humans.
Acknowledgements
We thank Kathleen Harrison, Ph.D., Karin Zemski Berry, Ph.D., and Bryan Bergman, Ph.D. at the University of Colorado Anschutz Medical Campus for their skilled assistance with lipidomic measures. We also thank Anthony Bouranis for his skilled assistance with R studio in generating the correlation matrix figure. Lastly, we would like to thank the participants and the helpful staff at Samaritan Athletic Medicine Center, including Drs. Nicholas Phillips, Craig Graham and Joshua Lenhof. The results of the present investigation do not constitute endorsement by the American College of Sports Medicine. We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Grants
This project was partly supported by the ACSM Foundation Doctoral Student Research Grant from the American College of Sports Medicine Foundation awarded to H.D.S. This project was also supported by KL2TR002370 awarded to S.A.N. as part of the Oregon Clinical & Translational Research Institute’s Clinical and Translational Science Award from the National Institutes of Health, the Collins Medical Trust awarded to S.A.N. and M.M.R., and the John C. Erkkila, M.D. Endowment for Health and Human Performance awarded to S.A.N. and M.M.R. M.M.R. was supported by DK103829 from the National Institutes of Health. H.D.S. and S.E.E. are supported by fellowships from Oregon State University.
Footnotes
Disclosures
The authors have no conflict of interest to declare.
References
- 1.Kiens B Skeletal Muscle Lipid Metabolism in Exercise and Insulin Resistance. Physiol Rev. 2006;86(1):205–43. [DOI] [PubMed] [Google Scholar]
- 2.Kimber NE, Heigenhauser GJF, Spriet LL, Dyck DJ. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J Physiology. 2003;548(3):919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Votruba SB, Atkinson RL, Schoeller DA. Prior Exercise Increases Dietary Oleate, but Not Palmitate Oxidation. Obes Res. 2003;11(12):1509–18. [DOI] [PubMed] [Google Scholar]
- 4.Décombaz J, Schmitt B, Ith M, et al. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am J Physiology-regulatory Integr Comp Physiology. 2001;281(3):R760–9. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda S, Miyazaki H, Nakatani T, et al. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Bioph Res Co. 2002;296(2):395–400. [DOI] [PubMed] [Google Scholar]
- 6.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid–induced insulin resistance. J Clin Invest. 2007;117(6):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mashek DG, Li LO, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res. 2006;47(9):2004–10. [DOI] [PubMed] [Google Scholar]
- 8.Li LO, Grevengoed TJ, Paul DS, et al. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes. 2014;64(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teodoro BG, Sampaio IH, Bomfim LHM, et al. Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. J Physiology. 2016;595(3):677–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stierwalt HD, Ehrlicher SE, Robinson MM, Newsom SA. Diet and Exercise Training Influence Skeletal Muscle Long-Chain acyl-CoA Synthetases. Medicine Sci Sports Exerc. 2020;52(3):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung YH, Bu SY. Suppression of long chain acyl-CoA synthetase blocks intracellular fatty acid flux and glucose uptake in skeletal myotubes. Biochimica Et Biophysica Acta Bba - Mol Cell Biology Lipids. 2020;1865(7):158678. [DOI] [PubMed] [Google Scholar]
- 12.Cortright RN, Sandhoff KM, Basilio JL, et al. Skeletal Muscle Fat Oxidation Is Increased in African-American and White Women after 10 days of Endurance Exercise Training*. Obesity. 2006;14(7):1201–10. [DOI] [PubMed] [Google Scholar]
- 13.Privette JD, Hickner RC, MacDonald KG, Pories WJ, Barakat HA. Fatty acid oxidation by skeletal muscle homogenates from morbidly obese black and white American women. Metabolis. 2003;52(6):735–8. [DOI] [PubMed] [Google Scholar]
- 14.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol. 2001;90(6):2319–24. [DOI] [PubMed] [Google Scholar]
- 15.Digel M, Ehehalt R, Stremmel W, Füllekrug J. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol Cell Biochem. 2008;326(1–2):23–8. [DOI] [PubMed] [Google Scholar]
- 16.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21(3):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashek DG, Bornfeldt KE, Coleman RA, et al. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family: TABLE 1. J Lipid Res. 2004;45(10):1958–61. [DOI] [PubMed] [Google Scholar]
- 18.Watt MJ, Hoy AJ. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function. Am J Physiol-endoc M. 2012;302(11):E1315–28. [DOI] [PubMed] [Google Scholar]
- 19.Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernández-Fernández C, Mouriño-Bayolo D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion. 2018;46:73–90. [DOI] [PubMed] [Google Scholar]
- 20.Kwak H-B, Woodlief TL, Green TD, et al. Overexpression of Long-Chain Acyl-CoA Synthetase 5 Increases Fatty Acid Oxidation and Free Radical Formation While Attenuating Insulin Signaling in Primary Human Skeletal Myotubes. Int J Environ Res Pu. 2019;16(7):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasari S, Newsom SA, Ehrlicher SE, Stierwalt HD, Robinson MM. Remodeling of skeletal muscle mitochondrial proteome with high-fat diet involves greater changes to β-oxidation than electron transfer proteins in mice. Am J Physiology Endocrinol Metabolism. 2018;315(4):E425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newsom SA, Miller BF, Hamilton KL, Ehrlicher SE, Stierwalt HD, Robinson MM. Long-term rates of mitochondrial protein synthesis are increased in mouse skeletal muscle with high fat feeding regardless of insulin sensitizing treatment. Am J Physiology Endocrinol Metabolism. 2017;313(5):ajpendo.00144.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane DA, Lin C-T, Anderson EJ, et al. Progesterone increases skeletal muscle mitochondrial H2O2 emission in nonmenopausal women. Am J Physiology Endocrinol Metabolism. 2010;300(3):E528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–34. [DOI] [PubMed] [Google Scholar]
- 25.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86(5):1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol-endoc M. 1993;265(3):E380–91. [DOI] [PubMed] [Google Scholar]
- 27.van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiology. 2001;536(1):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stierwalt HD, Ehrlicher SE, Bergman BC, Robinson MM, Newsom SA. Insulin-stimulated Rac1-GTP binding is not impaired by palmitate treatment in L6 myotubes. Physiological Reports. 2018;6(24):e13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran-Everett D Explorations in statistics: standard deviations and standard errors. Adv Physiol Educ. 2008;32(3):203–8. [DOI] [PubMed] [Google Scholar]
- 30.Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2(4):465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA Metabolism and Partitioning. Annu Rev Nutr. 2014;34(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundsgaard A-M, Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol. 2014;5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Watkins S, Kelley DE. Skeletal Muscle Lipid Content and Oxidative Enzyme Activity in Relation to Muscle Fiber Type in Type 2 Diabetes and Obesity. Diabetes. 2001;50(4):817–23. [DOI] [PubMed] [Google Scholar]
- 34.Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic Characteristics of Fibre Types in Human Skeletal Muscle. Acta Physiol Scand. 1975;95(2):153–65. [DOI] [PubMed] [Google Scholar]
- 35.Durgan DJ, Smith JK, Hotze MA, et al. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol-heart C. 2006;290(6):H2480–97. [DOI] [PubMed] [Google Scholar]
- 36.Schoonjans K, Watanabe M, Suzuki H, et al. Induction of the Acyl-Coenzyme A Synthetase Gene by Fibrates and Fatty Acids Is Mediated by a Peroxisome Proliferator Response Element in the C Promoter. J Biol Chem. 1995;270(33):19269–76. [DOI] [PubMed] [Google Scholar]
- 37.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biological Chem. 1997;272(45):28210–7. [DOI] [PubMed] [Google Scholar]
- 38.Cresci S, Wright LD, Spratt JA, Briggs FN, Kelly DP. Activation of a novel metabolic gene regulatory pathway by chronic stimulation of skeletal muscle. Am J Physiol-cell Ph. 1996;270(5):C1413–20. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiology Endocrinol Metabolism. 2000;279(2):E348–55. [DOI] [PubMed] [Google Scholar]
- 40.Frahm JL, Li LO, Grevengoed TJ, Coleman RA. Phosphorylation and Acetylation of Acyl-Coa Synthetase- I. J Proteom Bioinform. 2011;04(07):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergman BC, Perreault L, Strauss A, et al. Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. Am J Physiol-endoc M. 2018;314(2):E152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turcotte LP, Swenberger JR, Tucker MZ, Yee AJ. Increased Fatty Acid Uptake and Altered Fatty Acid Metabolism in Insulin-Resistant Muscle of Obese Zucker Rats. Diabetes. 2001;50(6):1389–96. [DOI] [PubMed] [Google Scholar]
- 43.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]


