Abstract
Cell migration, critical to numerous biological processes, can be guided by surface topography. However, fabrication limitations constrain topography studies to geometries that may not adequately mimic physiological environments. Direct Laser Writing (DLW) provides the necessary 3D flexibility and control to create well-defined waveforms at length scales that are similar to those found in physiological settings. We find that endothelial cells migrate fastest along square waves, intermediate along triangular waves, and slowest along sine waves and that directional cell migration on sine waves decreases as sinusoid wavelength increases. Interestingly, inhibition of Rac1 decreases directional migration on 3D sine waves but not on flat surfaces with micropatterned lines, suggesting that cells may utilize different molecular pathways to sense curved topographies. Our study demonstrates that DLW can be employed to investigate directional migration on a wide array of surfaces with curvatures that are challenging to fabricate using conventional manufacturing techniques.
Keywords: Direct Laser Writing (DLW), directional cell migration, topography, Rac1, contact guidance
Introduction
Cell migration plays a central role in a large variety of biological processes including early embryonic development, immune cell trafficking and surveillance, angiogenesis and blood vessel remodeling, cancer metastasis, and tissue repair.[1–3] When cells migrate, they typically move over and across extracellular matrix (ECM), the polymerized fibrous scaffolds that provide the physical structure of our bodies. Interestingly, the structural organization of the ECM influences how cells adhere and migrate. To study these processes in a more controlled setting, researchers generated surfaces patterned with aligned ridges, where it was observed that cells preferentially orient and migrate parallel to the ridges, a phenomenon termed contact guidance. [4–7] Modulating cell migration speed and direction using topography could be valuable for designing biomedical devices where controlled cell repopulation is critical, such as in vascular stents, where an endothelial cell monolayer is necessary for proper vascular function. A major advantage of using topography as a cellular control mechanism for medical implants is that it is purely physical and does not change the biochemistry of the implant environment.[8]
Cells exhibit contact guidance on a wide variety of substrate materials and feature sizes ranging from tens of nanometers to hundreds of microns.[9–12] In general, cells tend to migrate faster on patterned surfaces compared to flat surfaces and migration speed decreases at longer wavelengths.[10,13] To fabricate these surfaces, researchers have predominantly employed lithography-based approaches to produce square-shaped waveforms on which cells generally elongate less as the square wave wavelength becomes longer.[4,10,14] However, square wave topographies are composed of features such as flat surfaces and sharp right-angle edges that are rarely encountered in vivo.
How cells respond to and migrate on curved topographies has not been well studied, primarily due to limitations in the ability to fabricate such curved surfaces. Physiological curvatures are present across a range of length scales such as collagen fibers in the ECM with a diameter of 30–100 nm or the lumen of a blood vessel that can range in diameter from tens to hundreds of microns.[15] Researchers have used methods including spin-coating, hydrogel swelling, or polymer deformation to fabricate curved surface topographies.[5,16–21] Although these studies show that aligned curved surfaces are able to elicit the contact guidance response, many of the fabrication methods are limited in their ability to produce well-defined curved structures with controllable dimensions. Given the prominence of curved features in native tissues, a deeper understanding of how cells sense and respond to such features is important to further elucidate.
To study how endothelial cells migrate on curved topography, we employed an advanced fabrication technique known as Direct Laser Writing (DLW). DLW offers complete 3D spatial control to produce virtually any user-programmed surface with sub-micron resolution. The excellent 3D spatial control of DLW has made it an increasingly popular tool to study the effects of 3D microenvironments in biology and cell migration.[22–24] We used a commercial DLW system (Nanoscribe Photonic Professional GT, 780 nm) and photoresist (IP-Dip, Nanoscribe GmbH) to generate customizable 3D cell-adhesive surfaces to explore the effects of varying waveform, amplitude, and wavelength on Human Umbilical Vein Endothelial Cell (HUVEC) migration. Endothelial cell migration has previously been studied on square wave topography, but not on curved surfaces resembling the lumen of blood vessels where they reside physiologically.[12,25–28] We then examined both the effects of curved sine wave surfaces on cell migration and the differences in the molecular regulation of directional migration on topography compared to flat chemically-micropatterned surfaces.
Results
DLW-printed structures were designed to have an aligned wave topography spanning an area of 3 × 3 mm2 and to contain a defined starting location for cells in the center of the pattern. The cell starting location consisted of a “coliseum-like” circular wall structure, which was designed to confine a single droplet of cells suspended in media. The structure had a diameter of 2 mm, a height of 200 μm, and a wall thickness of 15 μm, (Figure 1 A). Within several minutes, cells settled and adhered to the surface inside of the circular walls. Importantly, 50 μm diameter semi-circular arches were inserted in the base of the circular wall to allow cells to migrate out of the central region following attachment. To ensure a uniform substrate material for cell attachment and migration, a flat surface was printed inside the coliseum walls and all surfaces were coated with 50 μg/mL fibronectin (FN) prior to seeding HUVECs.
Figure 1:
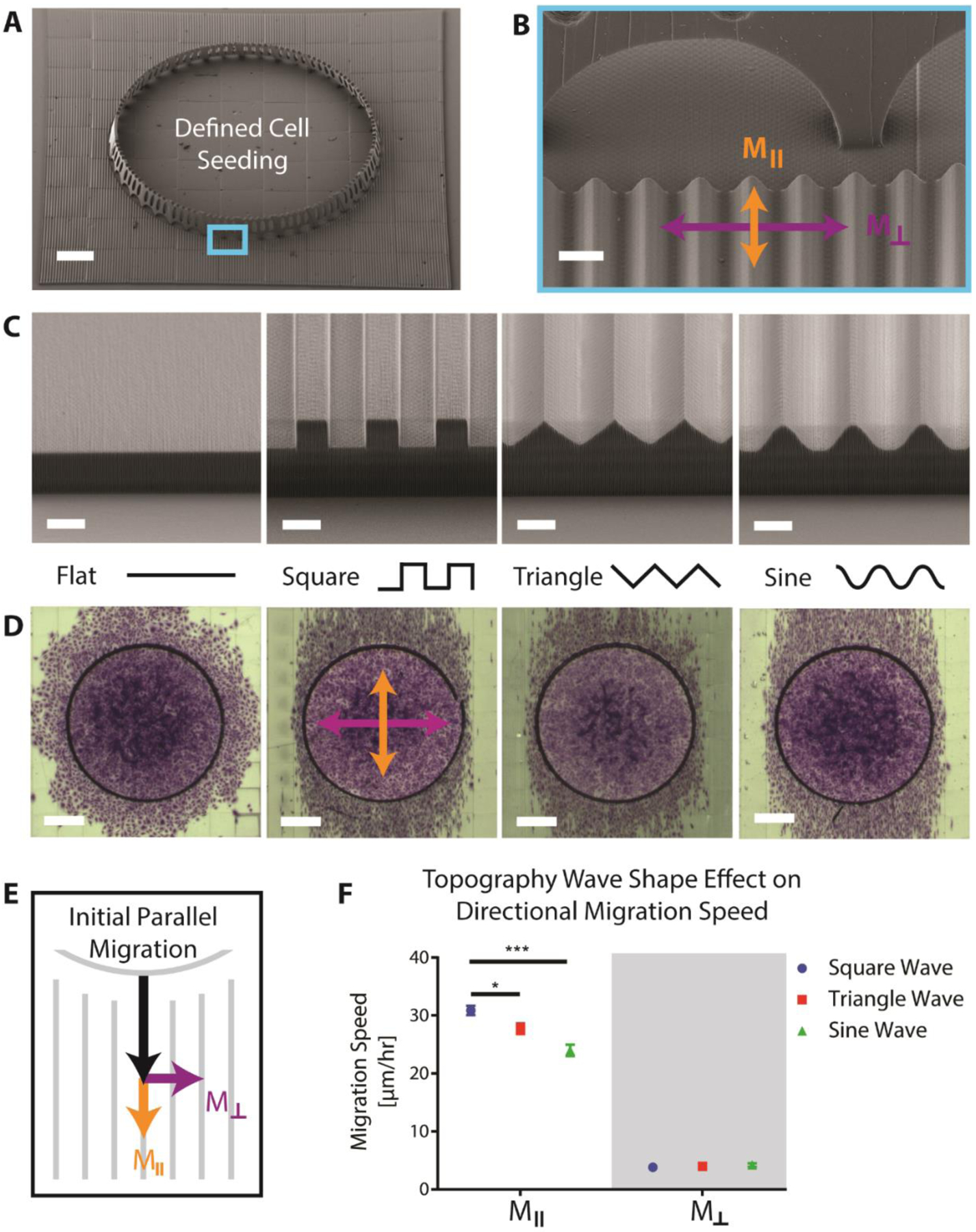
SEM images of A) a single printed test structure with defined cell seeding location surrounded by aligned square wave topography, scale bar 300 μm, and B) magnified image of wall arches that allow cells to migrate out following initial seeding, scalebar 20 μm. C) SEM images of the outer edges of different topography waveforms with 10 μm peak-to-peak amplitude and 20 μm wavelength, scale bar 10 μm. D) Optical images of the cell migration fronts stained with crystal violet after 24 hours on the different topography shapes, scale bar 500 μm. E) Schematic of cell migration speed broken into its orthogonal components, Mǁ and M⊥. F) Plot showing values of Mǁ and M⊥ for different waveform shapes (error bars represent mean ± SEM).
To investigate whether wave shape, amplitude, or wavelength had significant effects on cell migration, we printed patterns with peak-to-peak amplitudes of 3, 5 or 10 μm, wavelengths of 5, 10, or 20 μm, and three different waveforms: square, triangle, or sinusoid (Figure 1 B). The values for wavelength were chosen to be similar in diameter to a spread cell on a flat surface and amplitude values were chosen to span the average values utilized in past studies.[9] Instead of using a full combinatorial experiment requiring 27 unique patterns, we employed a three-level Taguchi L9 factorial design to collapse the number of patterns to nine. This partial factorial experimental design was used to initially screen if there is a significant effect on cell migration when any of the three parameters (amplitude, wavelength, and waveform) are varied. Cells were seeded in the center of each pattern and allowed to migrate for 24 hours prior to fixation and staining with crystal violet to visualize the migration front (Figure 1 C). On flat surfaces, cells appeared to migrate out of the walls isotropically, whereas cells on topographies migrated predominantly parallel to the waves independent of the waveform.
While the overall ellipsoidal shape of the migration front on patterned surfaces demonstrated preferential migration, it was not clear how individual cells were behaving. To characterize individual cell migration on the topographies, the nuclei of the HUVECs were tagged by expressing histone H2B-RFP via a lentiviral vector and single cell migration tracks were recorded using time lapse microscopy. Directional migration of individual cells was quantified by splitting total migration speed into its orthogonal components: parallel (Mǁ) or perpendicular (M⊥) migration relative to the aligned wave patterns (Figure 1 D). We found that varying wave shape had a significant effect on total migration speed and Mǁ, but no significant effect on M⊥ (Figure 1 E). Cells migrated fastest on square waves, followed by triangle waves, and lastly sine waves with average speeds of 31.6 ± 1.4, 28.7 ± 1.5, and 25.1 ± 1.7 μm/hour respectively. We also observed a significant effect of varying wavelength on M⊥ (Table 4.S1). In contrast, varying amplitude did not precipitate significant differences within our tested range for any of the response metrics.
Since the factorial results indicated that topography waveform and wavelength significantly affect migratory behavior of endothelial cells, we decided to study migration as a function of wavelength on the least-studied waveform, the sine wave. Whereas a number of publications have shown that square wave features with dimensions as small as 35 nm can direct cell migration, the upper limit of feature sizes that a cell will respond to is not well defined, especially for curved topographies.[15] We proceeded to explore the effects of longer wavelength sine wave topographies with a fixed 10 μm peak-to-peak amplitude and wavelengths of 20, 50, 100, or 150 μm (Figure 2 A). It was observed that as wavelength increased, the shape of the cell migration front transitioned from visibly elliptical to a more isotropic migration pattern resembling that of a flat surface (Figure 2 B). These findings were corroborated by single-cell analysis, which showed slower Mǁ and faster M⊥ migration speeds as wavelength increased (Figure 2 C,E). Variability of the angle of migration, visualized by rose plot histograms, also increased at longer wavelengths (Figure 2 D). In comparison with flat printed patterns, Mǁ was only significantly faster on the 20 μm wavelength and M⊥ significantly decreased across all wavelengths (Figure 2 E). Even though the magnitude of the effect of topography on cell migration decreased at longer wavelengths, cells were still able to sense and respond to sinusoidal topographies with 150 μm wavelength, roughly three to four times the diameter of a single cell.
Figure 2:
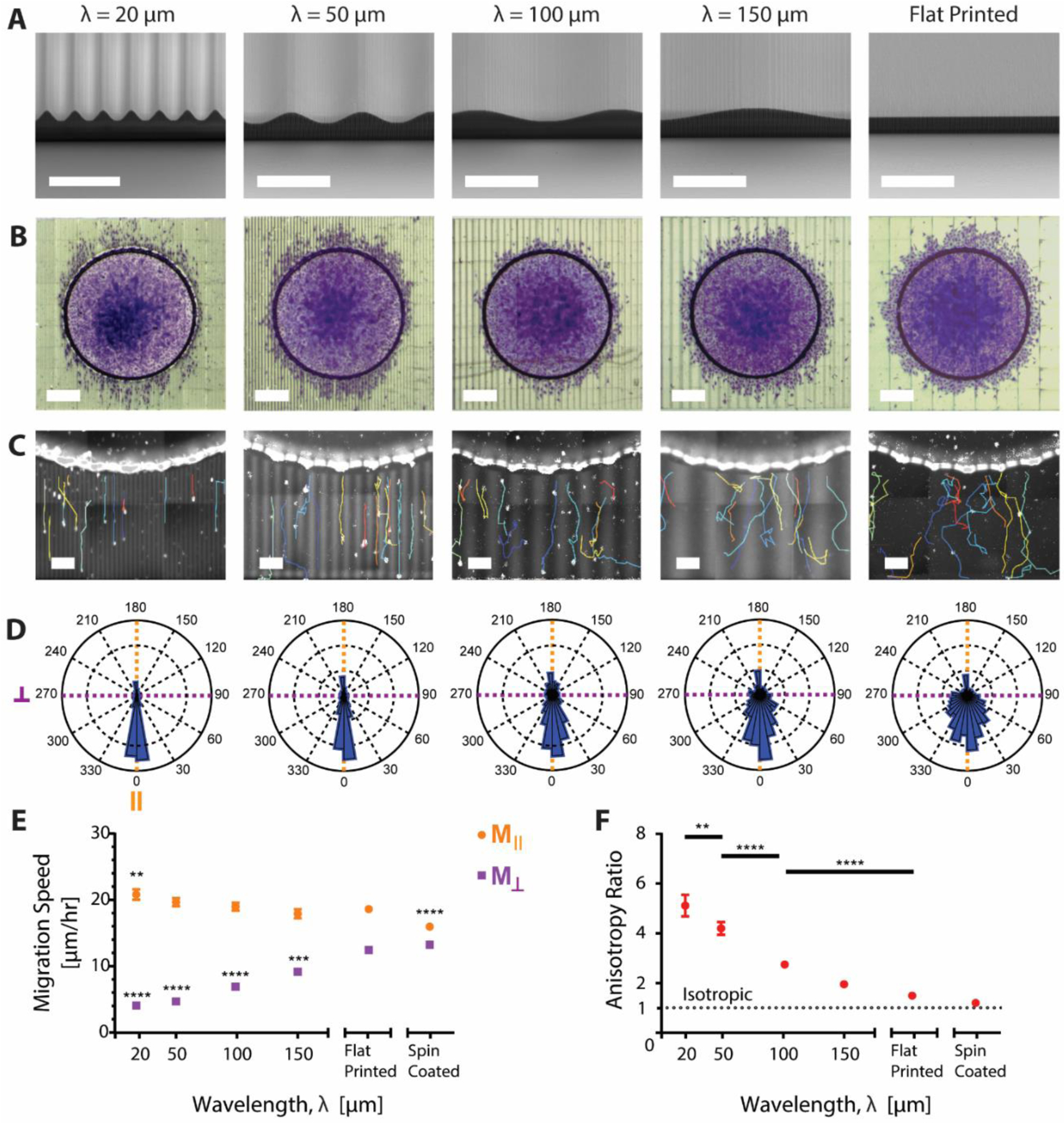
A) SEM images of sine-wave topography prints with varying wavelength. Scale bar, 50 μm. B) Optical images of the cell migration front on the varied wavelength (λ) sine waves. Cells were fixed and stained with crystal violet. Scale bar, 300 μm. C) Representative single cell tracks output from Trackmate overlaid onto an image of H2B-RFP tagged cell nuclei. Printed patterns are autofluorescent and thus visible along with cell nuclei. Higher intensity areas denote the peaks of the sine waves while lower intensity areas denote the troughs. Scale bar, 50 μm. D) Rose plot histograms of the angle of cell migration over one-hour time intervals for each wavelength. Angle of migration was rotated so that the initial direction of migration out of the walls was in the 0° direction. Each plot shows combined data with n=8. E) Mǁ (orange) and M⊥ (purple) cell migration speed with varying wavelength. * indicates significance as compared to the flat printed condition. F) Migration speed anisotropy ratio: the ratio of Mǁ / M⊥ migration across the various wavelengths. An anisotropy ratio of 1 indicates isotropic migration. *, **, ***, or **** indicate p-value less than 0.05, 0.01, 0.001, and 0.0001 respectively. All error bars represent mean ± SEM.
To quantify the anisotropy of the observed migration, we calculated the anisotropy ratio, defined as Mǁ / M⊥. The anisotropy ratio was highest for the 20 μm wavelength (~5) and decreased at longer wavelengths, approaching isotropy (1) (Figure 2 F). Although the anisotropy ratio was lowest for the flat printed patterns, migration was not isotropic. We compared flat patterns printed using DLW with those with a flat spin coated surface and found differences in Mǁ between the two (Figure 2 E,F). SEM imaging of the flat printed topographies showed the presence of aligned nanoscale topography formed from incomplete voxel overlap between printed lines (Figure S1). These small aligned grooves were approximately 100 nm deep and spaced at the width of one voxel (300 nm) from each other and were enough to significantly increase Mǁ speed on “flat” printed patterns when compared to the spin coated migration (Figure 2 E). Nonetheless, the topographies of all substrates were printed in the same orientation using the same printing parameters, allowing us to make controlled comparisons within our system framework.
Directional cell migration arises from cell polarization, where cells have a protrusive front and a contractile rear. Some of the molecular pathways involved in cell polarization have previously been revealed by 2D cell migration studies that used microcontact printing to pattern adhesive ligands. Three major molecular drivers identified in directional migration are non-muscle myosin II (NMMII), which forms contractile actomyosin bundles at the cell rear and locally inhibits protrusion initiation, phosphoinositide 3-kinase (PI3K), which regulates a number of pathways involved in actin cytoskeletal remodeling in cell migration, and Rac1, which locally concentrates actin polymerization and lamellipodial protrusion to the cell front.[1,29] However, it is unclear how the data from these 2D migration studies translates to curved topographies, and if the molecular pathways are similar.
To explore whether directional migration induced by topography uses the same molecular mechanisms as those previously described for flat substrates, we added inhibitors of NMMII, PI3K, and Rac1 to cells on the 20 μm wavelength sine waves. Cell migration on these sine waves was compared to the migration of cells treated with the same inhibitors on flat substrates patterned with fibronectin-coated lines. Alternating 10 μm wide lines of cell-adhesive fibronectin and non-adhesive Pluronic were microcontact-printed onto glass (Figure 3 A). The flat lines induced cell elongation and directional migration in the parallel direction analogous to our topographies and similar previous reports (Figure 3 B).[13,30] Cell seeding density was kept constant and seeding was confined using PDMS rings to keep conditions as similar as possible to that of cells on the sine waves.
Figure 3:
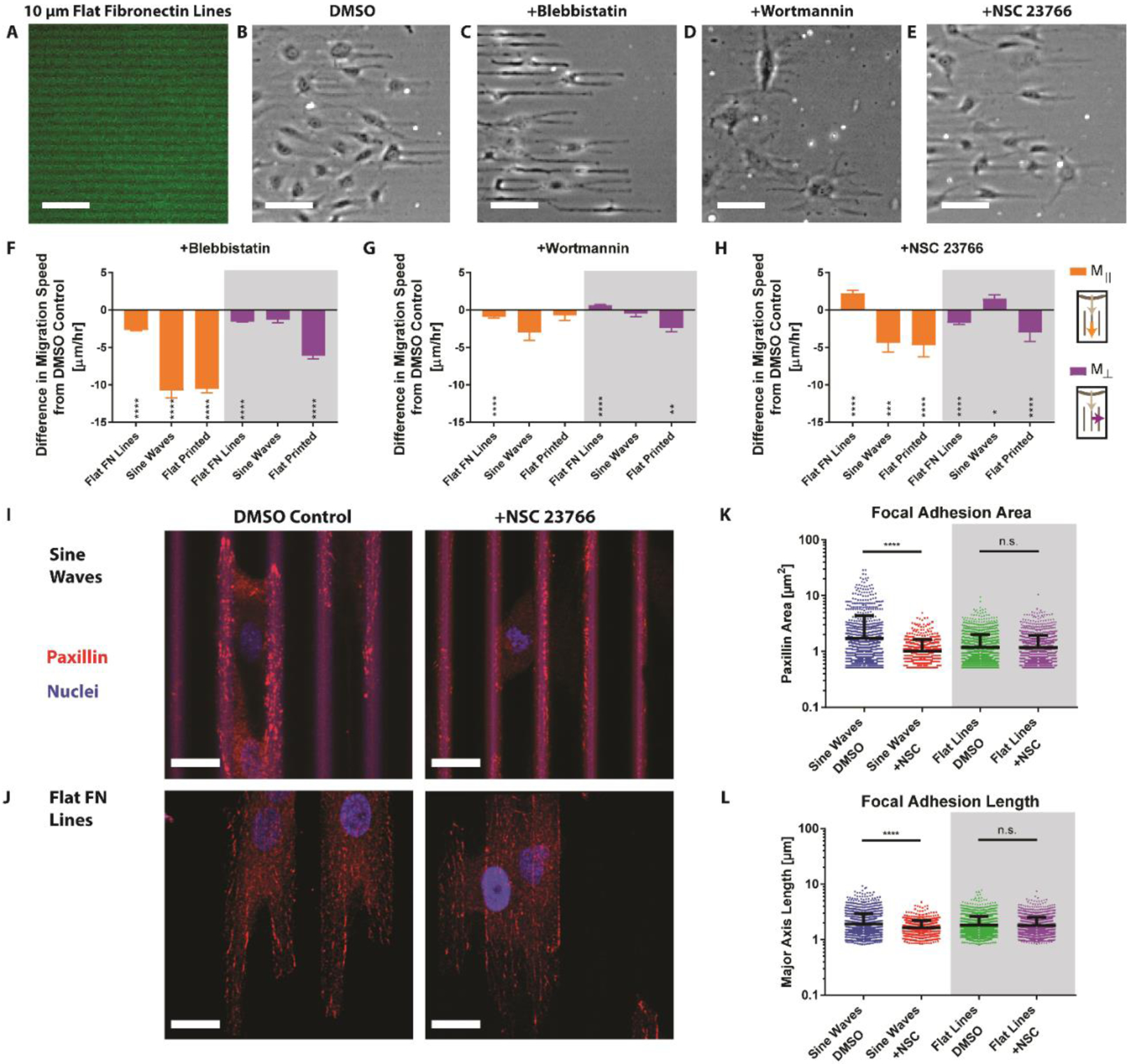
A) Microcontact-printed flat fluorescent green fibronectin lines of 10 μm width. B) Cells were seeded in a circle then migrated out along the lines, left to right. Scale bar, 100 μm. B) DMSO, C) blebbistatin, D) wortmannin, or E) NSC 23766 was added 6 hours after seeding. Cell migration on flat fibronectin (FN) lines, sine waves, or flat printed patterns was recorded and compared to the DMSO control for F) blebbistatin, G) wortmannin, or H) NSC (error bars represent mean ± SEM). I) Confocal imaging of cells migrating on sine waves stained for paxillin and cell nuclei with either DMSO (left) or NSC (right) added. Scale bar, 20 μm. J) Cells migrating on flat FN lines stained for paxillin and cell nuclei with either DMSO (left) or NSC (right) added. Scale bar, 20 μm. Quantification of K) focal adhesion area and L) focal adhesion length for each condition (error bars represent mean ± SD). *,**,***, or **** indicate p-value less than 0.05, 0.01, 0.001, and 0.0001 respectively.
Two out of the three selected inhibitors, NMMII and PI3k, elicited a similar response in both surface types. The NMMII inhibitor blebbistatin decreased Mǁ and M⊥ on both sine waves and flat lines (Figure 3 C,F). The PI3k inhibitor wortmannin reduced cell elongation and modestly decreased directional cell migration (decreased Mǁ, increased M⊥) for both the sine waves and flat lines (Figure 3 D,G). Interestingly, the Rac1 inhibitor NSC23766 increased directional migration of cells on the flat lines, consistent with previous reports, but had the opposite effect for cells on the sine waves.[30] On flat lines NSC23766 increased Mǁ and decreased M⊥, but on sine waves NSC23766 decreased Mǁ and increased M⊥ (Figure 3 E,H). These findings suggest that endogenous Rac1 may suppress directional migration on flat surfaces, but is in fact important for mediating topography-induced directional migration (Figure 3 H).
To gain a better understanding of the observed Rac1 inhibitor behavior, we examined focal adhesion (FA) formation between treated and untreated cells on both the patterned FN lines and 3D sine waves. One of the hypothesized underlying causes of contact guidance is the regulation of FA area by physical topography, where FA elongation is constrained in the perpendicular direction, leading to proportionally more parallel FAs.[31–33] Results showed that cells on the sine waves had significantly less FA formation when treated with NSC (Figure 3 I). Quantification revealed significantly higher average FA area and length for control versus NSC23766 conditions (Figure 3 K,L). In contrast, cells on flat fibronectin lines had the same amount of FA formation in the control and NSC conditions (Figure 3 J,K,L). Thus, the reduction in directional migration caused by NSC23766 may be mediated by a reduction of FA area and length leading to diminished cell polarization. This finding agrees with the theory that the underlying cause of contact guidance is the regulation of FA area by the physical topography.[21,31,32,34]
Discussion
This study highlights the importance of studying cell migration on relevant 3D surfaces. HUVEC migration is different on curved topographies compared to the traditional square wave topographies used for topography studies. Song et al. have previously used X-ray lithography to fabricate well-defined sinusoidal waves to study T-cell migration and found that cells migrated preferentially within the troughs of the waves, more so on shorter wavelength waves.[35] Although X-ray lithography proves to be an acceptable fabrication method for creating various sine wave features, it may not be ideal for users to produce a new X-ray mask for every new structure they would like to test. DLW is well suited for producing various curved topographies since it can provide the resolution of a photolithography process without having to use a mask. Using DLW-printed curved topographies, we found that endothelial cells had decreased directional migration on sine wave topographies with longer wavelengths. Furthermore, the upper limit of a cell’s ability to sense changes in topography was tested. HUVECs continued to exhibit directional migration on sine waves with wavelengths of 150 μm.
While the field has largely presumed that contact guidance by adhesive patterns such as parallel lines and by 3D topographic features would occur via a common mechanism, our results suggest fundamental differences between the two processes, specifically emphasizing an important role for the Rac1 GTPase in topography-induced guidance of cell migration. Analysis of FA area on both flat FN-patterned and sine wave surfaces supports the hypothesis that contact guidance is influenced by the topographic constraints on FA formation. FA proteins mechanically link the cell’s actin cytoskeleton to the extracellular environment, and are critical for force transduction that occurs in the cell during migration.[36] As cells spread and elongate parallel to topography, FAs align within the cell, stabilizing cellular forces along the axis of elongation. As FAs mature they elongate, primarily in the direction of actin stress fibers in the cell, allowing them to bear higher cellular tension forces. FA alignment and elongation parallel to the topography may lead to anisotropic force distribution and to cell polarization.[11,21,32,37] The observation that Rac1 inhibition leads to both decreased directional migration and reduced FA length supports the hypothesis that FA area regulation is linked to the contact guidance effect, and illuminates a potential pathway by which Rac1 inhibition could lead to decreased directional migration on the sine wave topography.
In conclusion, we’ve demonstrated that DLW is an excellent tool for producing curved topographies at biologically relevant length scales. It has enabled us to rapidly iterate through different designs that were previously difficult to fabricate using traditional microfabrication techniques in order to identify how parameters such as waveform, wavelength, and amplitude impact contact guidance of endothelial cells. However, there are several drawbacks to this fabrication method for topography studies; along with the high resolution that DLW offers comes relatively small writing areas, making it difficult to print uniform topography patterns over large surface areas. The user can stitch together these writing areas to form structures that can span large regions (~cm), but trade-offs include long print times as well as sample tilt complications. Although there are some technical tradeoffs for DLW as a fabrication method, it can be used to produce high resolution structures for a variety of biological studies. Going forward, DLW can be used to systematically study the effect of novel geometries on cell migration and behavior.
Materials and Methods
Printing:
Structures were programmed in MATLAB with 500 nm z-steps and 300 nm steps in the x-y plane. Structures were printed using IP-Dip photoresist (Nanoscribe GmbH) onto PET substrates (Melinex 561 1000 gauge, Dupont) to ensure surface adhesion in aqueous solutions for duration of the testing time. After printing, structures were soaked in PGMEA for 40 min. to dissolve any remaining unpolymerized photoresist, then briefly rinsed in NOVEC 7100 (3M) solvent. To ensure complete structure polymerization, prints were exposed to UV light for 20 seconds. The minimum feature sizes, or voxel dimensions for the IP-Dip photoresist with the 25x magnification, 1.4 NA objective used in this study are approximately 500 nm in the x-y plane and 1.5 um along the z axis.
Cell Culture and Seeding:
HUVECs (Lonza) were cultured in EGM-2 media (Lonza) at 37° C in a humidified 5% CO2 incubator. Prior to seeding, the surfaces were coated with 50 μg/mL of human fibronectin (FisherSci) for 1 hour then dried using compressed air. To seed cells, a 2 μL droplet of cells (P510) at a concentration of 3.3 million cell/mL (6,600 total cells) in EGM-2 was pipetted into the circular wall and allowed to adhere for thirty minutes at 37° C in a humidified dish. Surface tension caused the liquid droplet, and therefore the cells, to remain confined within the walls. After 30 minutes, media was added to the entire dish and cells began to migrate out of the walls through the arches. Cell migration fronts were visualized using phase contrast imaging or fixed with 4% paraformaldehyde and stained with crystal violet (FisherSci).
To reuse DLW structures, cells were removed with 0.05% trypsin and substrates were subsequently cleaned using 10% sodium dodecyl sulfate (SDS) solution left on an orbital shaker for at least 24 hours followed by multiple washes with 70% ethanol and distilled water.
Fractional factorial experimental design:
We used JMP statistical software package to design a fractional factorial experimental design to screen for significant effects of varying wave shape, amplitude, or wavelength on cell migration. Instead of using a full combinatorial experiment requiring 27 unique patterns, we employed a three-level Taguchi L9 factorial design to collapse the number of patterns to nine while preserving the power to determine the main effect of each parameter on cell migration. We used peak-to-peak amplitudes of 3, 5 or 10 μm, wavelengths of 5, 10, or 20 μm, and three different waveforms: square, triangle, or sinusoid, creating nine combinations with the three set points for each of the three variables. The initial amplitude and wavelength values were taken as the range of dimensions from previous literature that showed the highest magnitude and most robust effect on cell migration speed from topography.[10,13,38,39]
Single-cell tracking experiments:
Cells were recorded using live fluorescence imaging, then cell position tracks were output using the FIJI plugin Trackmate.[40,41] Cell tracks were filtered to only keep those with good fidelity, as measured through Trackmate’s “quality” filter. A custom MATLAB script was used to analyze the tracks. Average migration speeds were calculated for each cell by calculating the total distance traveled for each hour time interval, then averaging all the time intervals together for each single cell track. To get the final value shown in figures, we averaged values for all cell tracks together over multiple experiments. The angle of migration was calculated for each one-hour interval for each cell and plotted as rose plot histograms.
Microcontact Printing:
We used microcontact printing to fabricate coverslips with alternating 10 μm wide parallel lines of cell-adhesive fibronectin and 10 μm wide lines of non cell-adhesive Pluronic F-127. 10 μm width raised lines were molded in PDMS from silicon wafers. 50 mg mL−1 fibronectin with 1% Alexa-Fluor-488 conjugated fibronectin was adsorbed to PDMS stamps for one hour before inverting onto a UV-ozone treated glass coverslip. Coverslips were then treated with 0.2% Pluronic F-127 solution and subsequently washed in PBS. A 2 μL droplet of cells at a concentration of 3.3 million cells/mL (6,600 total cells) in EGM-2 was seeded in a 2 mm diameter PDMS ring. The PDMS ring was removed after 30 minutes, allowing cells to migrate outward.
Inhibitor Studies:
NSC23766 (50 μM), blebbistatin (50 μM), and wortmannin (50 nM) (Tocris Bioscience), were reconstituted and stored in DMSO. Drugs were added in cell media 6 hours after initial cell seeding and left for 24 hours during imaging.
Focal Adhesion Quantification:
Cells were stained using anti-paxillin antibody (diluted 1:100, BD Biosciences #610568) and an Alexa-647 goat anti-mouse secondary antibody (1:400, Invitrogen #A21236). Confocal imaging was taken using 0.8 μm slice thickness. Four image slices (total 3.2 μm height) encompassing the peaks of the sine waves were stacked using max projection, processed using FIJI’s threshold and watershed functions, then analyzed using the “analyze particles” function in FIJI. Data from 10 image stacks was averaged, encompassing 20–30 cells total. To quantify focal adhesion length, particles were fitted with an ellipsoid and the major axis length was measured.
Statistics:
The fractional factorial experimental design was set up and analyzed using JMP statistical software package (SAS) using a least squares fit model. Statistical testing and graphing was done using GraphPad Prism software. Single-cell statistics were conducted using two-way ANOVA with a post-hoc Dunnett test. Focal adhesion quantification statistics were conducted using students t-test to compare DMSO and NSC conditions.
Supplementary Material
Acknowledgements
Daniel Cheng and Rachael Jayne contributed equally to this work. This research was supported in part by the Boston University College of Engineering, the Boston University Photonics Center, the National Science Foundation Graduate Research Fellowship Program (DC), the Clare Boothe Luce Graduate Research Fellowship (RKJ), and by the NSF CELL-MET ERC award no. 1647837.
Contributor Information
Daniel Cheng, Department of Biomedical Engineering, Boston University, Boston, MA 02215, USA.
Rachael K. Jayne, Department of Mechanical Engineering, Boston University, Boston, MA 02215, USA
Alice E. White, Department of Mechanical Engineering, Boston University, Boston, MA 02215, USA
Christopher S. Chen, Department of Biomedical Engineering, Boston University, Boston, MA 02215, USA
References
- [1].Petrie RJ, Doyle AD, Yamada KM, Nat. Rev. Mol. Cell Biol 2009, 10, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Friedl P, Wolf K, Nat. Rev. Cancer 2003, 3, 362. [DOI] [PubMed] [Google Scholar]
- [3].Daley WP, Yamada KM, Curr. Opin. Genet. Dev 2013, 23, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD, Development 1990, 108, 635. [DOI] [PubMed] [Google Scholar]
- [5].Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT, Biomaterials 2006, 27, 2558. [DOI] [PubMed] [Google Scholar]
- [6].Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, Mclauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P, Biochem. J 2007, 408, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Den Braber ET, De Ruijter JE, Smits HTJ, Ginsel LA, Von Recum AF, Jansen JA, Biomaterials 1996, 17, 1093. [DOI] [PubMed] [Google Scholar]
- [8].Miyoshi H, Adachi T, Ju J, Lee SM, Cho DJ, Ko JS, Uchida G, Yamagata Y, Biomaterials 2012, 33, 395. [DOI] [PubMed] [Google Scholar]
- [9].Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A, Biomaterials 2012, 33, 5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaiser J-P, Reinmann A, Bruinink A, Biomaterials 2006, 27, 5230. [DOI] [PubMed] [Google Scholar]
- [11].Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ, Biomaterials 2006, 27, 3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Biela SA, Su Y, Spatz JP, Kemkemer R, Acta Biomater. 2009, 5, 2460. [DOI] [PubMed] [Google Scholar]
- [13].Li S, Bhatia S, Hu Y, Shiu Y, Li Y, Usami S, Chien S, Biorheology 2001, 38, 101. [PubMed] [Google Scholar]
- [14].Lu X, Leng Y, J. Biomed. Mater. Res 2003, 66A, 677. [DOI] [PubMed] [Google Scholar]
- [15].Loesberg WA, te Riet J, van Delft FCMJM, Schön P, Figdor CG, Speller S, van Loon JJWA, Walboomers XF, Jansen JA, Biomaterials 2007, 28, 3944. [DOI] [PubMed] [Google Scholar]
- [16].Guvendiren M, Yang S, Burdick JA, Adv. Funct. Mater 2009, 19, 3038. [Google Scholar]
- [17].Yang P, Baker RM, Henderson JH, Mather PT, Soft Matter 2013, 9, 4705. [Google Scholar]
- [18].Lam MT, Sim S, Zhu X, Takayama S, Biomaterials 2006, 27, 4340. [DOI] [PubMed] [Google Scholar]
- [19].Lee J, Chung S, Song H, Kim S, Hong Y, J. Phys. D. Appl. Phys 2013, 46, 105305. [Google Scholar]
- [20].Brau F, Vandeparre H, Sabbah A, Poulard C, Boudaoud A, Damman P, Nat. Phys 2011, 7, 56. [Google Scholar]
- [21].Saito AC, Matsui TS, Ohishi T, Sato M, Deguchi S, Exp. Cell Res 2014, 327, 1. [DOI] [PubMed] [Google Scholar]
- [22].Jeon H, Hidai H, Hwang DJ, Healy KE, Grigoropoulos CP, Biomaterials 2010, 31, 4286. [DOI] [PubMed] [Google Scholar]
- [23].Tayalia P, Mendonca CR, Baldacchini T, Mooney DJ, Mazur E, Adv. Mater 2008, 20, 4494. [Google Scholar]
- [24].Richter B, Hahn V, Bertels S, Claus TK, Wegener M, Delaittre G, Barner-Kowollik C, Bastmeyer M, Adv. Mater 2017, 29, 1604342. [DOI] [PubMed] [Google Scholar]
- [25].Franco D, Klingauf M, Bednarzik M, Cecchini M, Kurtcuoglu V, Gobrecht J, Poulikakos D, Ferrari A, Soft Matter 2011, 7, 7313. [Google Scholar]
- [26].Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ, J. Biomed. Mater. Res. -Part A 2005, 75, 668. [DOI] [PubMed] [Google Scholar]
- [27].Franco D, Milde F, Klingauf M, Orsenigo F, Dejana E, Poulikakos D, Cecchini M, Koumoutsakos P, Ferrari A, Kurtcuoglu V, Biomaterials 2013, 34, 1488. [DOI] [PubMed] [Google Scholar]
- [28].Morgan JT, Wood JA, Shah NM, Hughbanks ML, Russell P, Barakat AI, Murphy CJ, Biomaterials 2012, 33, 4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiménez C, Portela RA, Mellado M, Rodríguez-Frade JM, Collard J, Serrano A, Martínez-A C, Avila J, Carrera AC, J. Cell Biol 2000, 151, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Doyle AD, Wang FW, Matsumoto K, Yamada KM, J. Cell Biol 2009, 184, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ventre M, Natale CF, Rianna C, Netti PA, J. R. Soc. Interface 2014, 11, 20140687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ray A, Lee O, Win Z, Edwards RM, Alford PW, Kim DH, Provenzano PP, Nat. Commun 2017, 8, 14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kubow KE, Conrad SK, Horwitz AR, Curr. Biol 2013, 23, 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ohara PT, Buck RC, Exp. Cell Res 1979, 121, 235. [DOI] [PubMed] [Google Scholar]
- [35].Song KH, Park SJ, Kim DS, Doh J, Biomaterials 2015, 51, 151. [DOI] [PubMed] [Google Scholar]
- [36].Brown MC, Turner CE, Physiol. Rev 2004, 84, 1315. [DOI] [PubMed] [Google Scholar]
- [37].Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B, Nat. Cell Biol 2001, 3, 466. [DOI] [PubMed] [Google Scholar]
- [38].Kim D-H, Han K, Gupta K, Kwon KW, Suh K-Y, Levchenko A, Biomaterials 2009, 30, 5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dalton BA, Walboomers XF, Dziegielewski M, Evans MDM, Taylor S, Jansen JA, Steele JG, J. Biomed. Mater. Res 2001, 56, 195. [DOI] [PubMed] [Google Scholar]
- [40].Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW, Methods 2017, 115, 80. [DOI] [PubMed] [Google Scholar]
- [41].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Nat. Methods 2012, 9, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


