Abstract
BACKGROUND
Previous reports have suggested that the p38 mitogen-activated protein kinase signaling pathway is involved in the development of severe acute pancreatitis (SAP)-related acute lung injury (ALI). Inhibition of p38 by SB203580 blocked the inflammatory responses in SAP-ALI. However, the precise mechanism associated with p38 is unclear, particularly in pulmonary microvascular endothelial cell (PMVEC) injury.
AIM
To determine its role in the tumor necrosis factor-alpha (TNF-α)-induced inflammation and apoptosis of PMVECs in vitro. We then conducted in vivo experiments to confirm the effect of SB203580-mediated p38 inhibition on SAP-ALI.
METHODS
In vitro, PMVEC were transfected with mitogen-activated protein kinase kinase 6 (Glu), which constitutively activates p38, and then stimulated with TNF-α. Flow cytometry and western blotting were performed to detect the cell apoptosis and inflammatory cytokine levels, respectively. In vivo, SAP-ALI was induced by 5% sodium taurocholate and three different doses of SB203580 (2.5, 5.0 or 10.0 mg/kg) were intraperitoneally injected prior to SAP induction. SAP-ALI was assessed by performing pulmonary histopathology assays, measuring myeloperoxidase activity, conducting arterial blood gas analyses and measuring TNF-α, interleukin (IL)-1β and IL-6 levels. Lung microvascular permeability was measured by determining bronchoalveolar lavage fluid protein concentration, Evans blue extravasation and ultrastructural changes in PMVECs. The apoptotic death of pulmonary cells was confirmed by performing a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analysis and examining the Bcl2, Bax, Bim and cle-caspase3 levels. The proteins levels of P-p38, NFκB, IκB, P-signal transducer and activator of transcription-3, nuclear factor erythroid 2-related factor 2, HO-1 and Myd88 were detected in the lungs to further evaluate the potential mechanism underlying the protective effect of SB203580.
RESULTS
In vitro, mitogen-activated protein kinase (Glu) transfection resulted in higher apoptotic rates and cytokine (IL-1β and IL-6) levels in TNF-α-treated PMVECs. In vivo, SB2035080 attenuated lung histopathological injury, decreased inflammatory activity (TNF-α, IL-1β, IL-6 and myeloperoxidase) and preserved pulmonary function. Furthermore, SB203580 significantly reversed changes in the bronchoalveolar lavage fluid protein concentration, Evans blue accumulation, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cell numbers, apoptosis-related proteins (cle-caspase3, Bim and Bax) and endothelial microstructure. Moreover, SB203580 significantly reduced the pulmonary P-p38, NFκB, P-signal transducer and activator of transcription-3 and Myd88 levels but increased the IκB and HO-1 levels.
CONCLUSION
p38 inhibition may protect against SAP-ALI by alleviating inflammation and the apoptotic death of PMVECs.
Keywords: Acute pancreatitis, Acute lung injury, Pulmonary microvascular endothelial cells, P38, SB203580, Apoptosis
Core Tip: We explored the role of the p38 mitogen-activated protein kinase pathway in the tumor necrosis factor-alpha-induced inflammation and apoptosis of pulmonary microvascular endothelial cells in vitro and verified the effect of SB203580-mediated p38 inhibition on severe acute pancreatitis-related acute lung injury in rats. p38 mitogen-activated protein kinase overactivation promoted tumor necrosis factor-alpha-induced inflammatory cytokine expression and pulmonary microvascular endothelial cell apoptosis in vitro. SB203580 improved severe acute pancreatitis-related acute lung injury by downregulating P-p38, NFκB, P-SATA3 and Myd88 and upregulating IκB and HO-1, which alleviated inflammation and apoptotic cell death in rats.
INTRODUCTION
Acute pancreatitis (AP), a common gastrointestinal disease, ranges from a self-limiting disease to a severe condition with high mortality. The overall mortality rate is approximately 2%[1], while the mortality rate of severe AP (SAP) with distant organ dysfunction increases to 37%-50%[2]. To date, the pathogenesis of SAP has not been completely clarified, and no specific pharmacological therapies for SAP or its associated organ failure are available. The management of AP is mainly limited to supportive care. The lung is the most vulnerable organ in patients with SAP. Acute lung injury (ALI) or its more severe form, acute respiratory distress syndrome, is reported to occur in 10%-25% of patients with AP and accounts for up to 60% of AP-associated deaths[3,4]. Therefore, the identification of an effective therapy for AP-associated ALI is still urgently needed to decrease AP mortality.
ALI secondary to SAP is clinically and histologically indistinguishable from other indirect (sepsis, trauma or burns) or direct (e.g., toxins or infection) insults to the lungs[5]. ALI is characterized by disruption of the alveolar capillary barrier, which increases endothelial and epithelial permeability, decreases lung compliance and impairs gas exchange[6]. Multiple biological processes are involved in the mechanisms of endothelial permeability, such as cytoskeletal rearrangements, assembly/ disassembly of cell junctions and survival/death[7-9]. Pulmonary microvascular endothelial cells (PMVECs) line the interior of blood vessels and are an essential part of the blood and tissue microenvironment. They are major actors responding to insults related to ALI[10,11]. PMVECs are the main targets of humoral and cellular mediators during injury[12] and actively produce proinflammatory cytokines, increase the levels of adhesion molecules and secrete prothrombotic substances, promoting injury by transforming to a prothrombotic and proinflammatory phenotype to further activate the immune and hemostatic systems[4,8]. PMVECs are likely to be the first-line responders in efforts to prevent the development of SAP-associated ALI. Therefore, modulation of pulmonary vascular homeostasis is crucial for the successful treatment of AP-associated ALI.
p38 mitogen-activated protein kinase (p38 MAPK), a member of the serine/ threonine kinase family, is ubiquitously expressed. It plays an important role in diverse processes including endocytosis, gene transcription, cell proliferation, cytokine production, apoptosis and cytoskeletal redistribution[8]. Therefore, unsurprisingly, p38 MAPK is involved in many cascades that result in the development of mild AP with limited pancreatic injury to SAP with ALI. In an experimental AP model, p38 MAPK activation was observed in the lungs as early as 2 h after model establishment[13], while p38 MAPK inhibitors (SB203580 or CNI-1493) alleviated pancreatic and pulmonary injury, limited cytokine increases and improved survival[14-16]. Currently, the precise mechanism by which the p38 MAPK signaling pathway regulates SAP-related ALI is unclear, particularly PMVEC injury. In this study, we overactivated p38 MAPK to define its role in the tumor necrosis factor-alpha (TNF-α)-induced inflammation and apoptosis of rat PMVECs in vitro and then investigated the effect of SB203580, a specific p38 MAPK inhibitor, on SAP rats with ALI in vivo.
MATERIALS AND METHODS
Cell culture
Rat PMVECs were obtained from the BeNa Culture Collection (338210, Beijing, China). Cells were identified according to staining with rat anti–factor VIII and binding of FITC-labeled lectin (Sigma, United States)[17]. The cells were cultured in DMEM (Thermo Fisher, United States) supplemented with 100 mL/L fetal bovine serum (BI, Australia), 50 U/mL penicillin and 50 µg/mL streptomycin (Thermo Fisher, United States). PMVECs were maintained at 37 °C in a humidified atmosphere with 950 mL/L O2 and 50 mL/L CO2.
Mitogen-activated protein kinase kinase 6 (Glu) recombinant adenovirus construction and transduction
The MAPK pathway is comprised of three sequential dual-specificity kinases, and MAPK kinase (MKK) 6 is highly selective for p38 activation. Overexpression of activated MKK 6 (Glu) constitutively activates p38 MAPK[18,19]. Lentiviruses carrying either no inserted sequence (null) or the MKK 6 (Glu) gene were constructed by Wuhan PeptBio company limited China. PMVECs were infected with the lentivirus at a multiplicity of infection of 10 in the presence of 5 μg/mL polybrene (Sigma, United States). After lentivirus infection, MKK 6 (Glu)-overexpressing cells and control cells were obtained via puromycin (2 μg/mL) screening and analyzed using western blotting. When the cells reached 70%–80% confluence, they were treated with TNF-α (20 ng/mL) for the indicated times and then used for the subsequent analyses.
Flow cytometry
PMVEC apoptosis was assessed using an Annexin V-FITC/PI kit (4A Biotech, China). Briefly, PMVECs were detached using trypsin, washed with cold PBS, pelleted and resuspended in binding buffer. Then, 10 μL of Annexin V was added, and they were incubated for 5 min in the dark. After mixing with 5 μL of propidium iodide, the cells were analyzed using a flow cytometer (BD Biosciences, United States).
Animals
Adult male Sprague-Dawley rats (n = 25, 210-230 g) were purchased from the Experimental Animal Center of Sichuan University (Chengdu, China). The animals were housed at 22-25 °C on a 12 h day-night cycle and fed standard rodent chow and tap water ad libitum for 1 wk of acclimation. The animal experiments performed in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was designed to minimize pain or discomfort to the animals and approved by the Animal Ethics Committee of West China Hospital of Sichuan University.
Induction of the SAP model and experimental groups
After fasting for 12 h, the SAP model was established by biliary-pancreatic duct injection of 50 g/L sodium taurocholate (Sigma, United States, 1 mL/kg) using a micropump at a speed of 0.2 mL/min[20]. In the sham group, the rats received the same volume of saline instead of sodium taurocholate. All rats received a saline injection subcutaneously to compensate for fluid loss during the surgery (20 mL/kg body weight). The rats were randomly divided into the SO group, SAP group and SAP + SB203580 group. The SAP + SB203580 group was further divided into low-dose (SB2.5 group), middle-dose (SB5 group) and high-dose (SB10 group) subgroups according to the dose of SB203580 (2.5 mg/kg, 5.0 mg/kg and 10.0 mg/kg, respectively, n = 5). SB203580 (Selleck, United States) was intraperitoneally administered before model establishment. The rats were sacrificed 12 h after model induction, and samples were harvested rapidly for subsequent analysis.
Arterial blood gas analysis
At the end of the experiment, arterial blood samples were collected by cardiac puncture. A blood gas analysis was performed to detect the partial pressure of oxygen (PaO2) and oxygen saturation (SaO2, Cobas b 123 Analyzer, Roche, Switzerland).
Bronchoalveolar lavage fluid collection
After opening the chest, the right lung was ligated. Bronchoalveolar lavage fluid (BALF) was collected through slow and careful lavage of the left lung with 2 mL of 4 °C PBS. Then, the fluid was centrifuged at 500 g for 5 min. The supernatant was collected and stored at -80 °C until the analyses of the protein concentration and cytokine levels.
Histological examination and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining
The pancreas and lung were fixed with 40 g/L formaldehyde for 24 h. The tissues were then embedded in paraffin, and continuously sliced at a 5 mm thickness for staining with hematoxylin and eosin. The pancreatic and pulmonary histological assessment was performed and scored by two pathologists using a microscope at 200 × magnification[21,22]. For the assessment of the apoptosis of lung tissue, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed with a commercially available In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions.
Measurements of cytokines and BALF protein concentrations
Concentrations of TNF-α, interleukin (IL)-1β and IL-6 in the serum and BALF were evaluated using ELISA kits according to the manufacturer’s instructions (R and D Systems, United States). The BALF protein concentration was determined using a BCA kit (Thermo, United States).
Lung microvascular permeability analysis
Evans blue extravasation was used to quantify the capillary permeability in the lungs. A 10 g/L Evans Blue solution (20 mg/kg, Sigma-Aldrich, United States) was injected into the tail vein 1 h before exsanguination. PBS was used to flush the remaining Evans blue from the pulmonary vascular system. After the complete removal of blood, the left lung was removed, weighed, homogenized in formamide and incubated at 37 °C for 24 h. Finally, the supernatant was quantified spectrophotometrically at 620 nm.
Myeloperoxidase activity
Pulmonary myeloperoxidase (MPO) activity was measured using the method described by Dawra et al[23]. Briefly, lung tissue was homogenized in phosphate buffer (100 mmol/L, pH 7.4) at 4 °C and centrifuged to pellet the tissues. The samples were then resuspended in phosphate buffer (100 mmol/L, pH 5.4) containing protease inhibitors, 5 g/L hexadecyl trimethyl ammonium bromide and 10 mmol/L EDTA and subjected to three rapid freeze-thaw cycles. The suspension was sonicated and centrifuged to obtain the supernatant. Twenty microliters of the supernatant were incubated with 200 μL of phosphate buffer (pH 5.4) containing 5 g/L hexadecyl trimethyl ammonium bromide and 2 mmol/L 3,3,5,5-tetramethylbenzidine for 3 min at room temperature followed by the addition of 50 μL H2O2. The difference in the absorbance of this assay solution between 0 min and 3 min was measured at 655 nm at 37 °C with a microplate reader (BMG LABTECH, Germany). MPO activity was calculated from a human MPO standard curve, normalized to the protein concentration and reported as mU/mg protein.
Western blotting
Lysates from PMVECs or lung tissues were extracted with RIPA lysis buffer (Abcam, United Kingdom) supplemented with protease inhibitors (Roche, Germany). The total protein concentration was quantified using a BCA kit (Thermo, United States). The same amounts of protein were separated on 10% or 12% SDS-PAGE gels and transferred to a polyvinylidene fluoride membrane. Membranes were blocked with 50 g/L nonfat milk for 2 h at room temperature and subsequently incubated with the following primary antibodies overnight at 4 °C: P-p38 (1:2000), NFκB (1:1000), IκB (1:1000), cleaved caspase-3 (cle-casp3, 1:1000), P-signal transducer and activator of transcription-3 (P-STAT3, 1:1000), myeloid differentiation factor 88 (Myd88, 1:1000), nuclear factor erythroid 2-related factor 2 (Nrf2, 1:1000), Bax (1:1000) and Bim (1:1000) from CST, United States; IL-1β (1:1000), heme oxygenase-1 (HO-1, 1:1000) and Bcl2 (1:1000) from Abcam, United States; and TNF-α (1:1000), IL-6 (1:1000) and β-actin (1:1000) from Proteintech, China. After rinsing with Tris-buffered saline containing Tween 20 (10 min, × 3), the membranes were incubated with a secondary goat anti-rabbit (1:5000, Proteintech, China) or a goat anti-mouse IgG-HRP antibody (1:10000, Proteintech, China) for 1-2 h at room temperature. Finally, after washing, the membranes were imaged using a chemiluminescence detection system (Bio-Rad, United States) and analyzed with Image Lab software.
Transmission electron microscopy
A 2 mm3 peripheral specimen was fixed with 25 mL/L glutaraldehyde for 2 h and then washed with 0.1 mol/L phosphate buffer (PH 7.4, 10 min × 3). After fixing the samples with 10 mL/L osmium tetroxide for 2 h, the specimens were dehydrated through a graded series of ethanol solutions and acetone and embedded in Epon 812 (Sigma, United States). Ultrathin sections were cut with an ultramicrotome (Leica, Germany). Finally, the sections were stained with uranyl acetate and lead citrate and examined using a JEM-1230 transmission electron microscope (JEOL, Japan).
Statistical analysis
All statistical tests were performed using SPSS 20.0 (SPSS, United States) by a biomedical statistician. Data are presented as the mean ± SE for continuous variables or frequencies and percentages for categorical variables. Data were analyzed using one-way analysis of variance or the Kruskal-Wallis test. A P value of < 0.05 was considered statistically significant.
RESULTS
Overactivation of p38 MAPK aggravates TNF-α-induced apoptosis and inflammatory cytokine expression in PMVECs
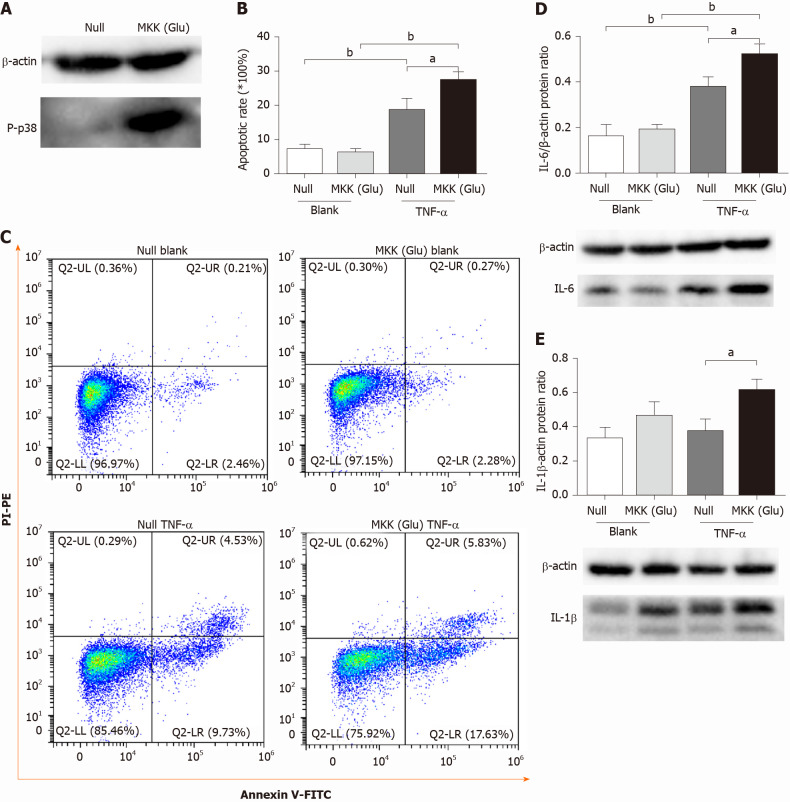
We overexpressed a constitutively activated MKK6 (Glu) in PMVECs, which was confirmed to over activate p38 MAPK (Figure 1A). Then, the cells were incubated in the absence or presence of TNF-α. Flow cytometry analysis showed TNF-α induced apoptosis in both null and MKK (Glu) cells. Compared with the null groups, the apoptotic rate was significantly higher in the MKK (Glu) group treated with TNF-α (Figure 1B and 1C). Meanwhile, the levels of inflammatory cytokines (IL-6 and IL-1β) were also significantly upregulated in the MKK (Glu) cells (Figure 1D and 1E).
Figure 1.
p38 mitogen-activated protein kinase overactivation aggravates tumor necrosis factor-alpha-induced apoptosis and inflammatory cytokine expression in pulmonary microvascular endothelial cells. A: Western blotting confirmed that mitogen-activated protein kinase kinase (Glu) transfection constitutively activated p38 mitogen-activated protein kinase in pulmonary microvascular endothelial cells; B: Apoptotic rate in null and mitogen-activated protein kinase kinase (Glu) pulmonary microvascular endothelial cells stimulated with or without tumor necrosis factor-alpha for 24 h; C: Representative images of flow cytometry analysis for apoptosis; D: The expression level of interleukin-6 in each group; E: The expression level of interleukin-1β in each group. Data are expressed as mean ± SE of 3-4 samples per group; aP < 0.05; bP < 0.01. IL: Interleukin; MKK: Mitogen-activated protein kinase kinase; TNF-α: Tumor necrosis factor-alpha.
SB203580 attenuates pancreatic damage in SAP rats
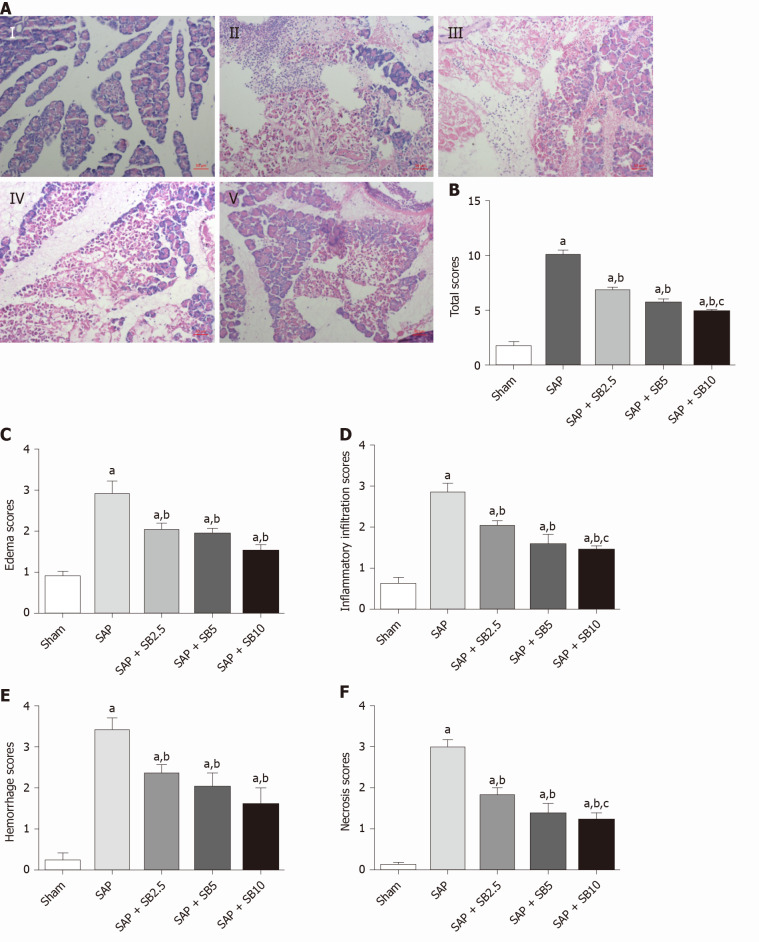
Representative images of hematoxylin and eosin staining of the pancreas were shown in Figure 2A. Except for slight pancreatic edema, no obvious changes indicative of AP were observed in sham-operated animals. Sodium taurocholate induced typical histopathological changes associated with AP, including diffuse edema, marked neutrophil infiltration, intrapancreatic hemorrhage and acinar cell necrosis. The histopathological scores were all significantly increased (Figure 2B-F). All three tested doses of SB203580 (2.5, 5.0 and 10.0 mg/kg) alleviated injury to the pancreatic tissues (all P < 0.05). We explored the difference in the therapeutic effect among the three different doses; compared to the SB2.5 group, 10.0 mg/kg SB203580 further significantly decreased the histopathological inflammatory infiltration, necrosis and total scores (Figure 2B, 2D and 2F).
Figure 2.
Effects of SB203580 on pancreatic histopathological changes and histopathological severity scores. A: Representative hematoxylin and eosin images of pancreatic sections (magnification 200 ×); I: Sham group; II: Severe acute pancreatitis (SAP) group; III: SAP + SB2.5 group; IV: SAP + SB5 group; V: SAP + SB10 group; B: Total scores; C: Edema scores; D: Inflammatory infiltration scores; E: Hemorrhage scores; F: Necrosis scores. B-F: Pancreatic histopathology scores in different groups; Data were expressed as mean ± SE; aP < 0.05 vs sham group; bP < 0.05 vs SAP group; cP < 0.05 vs SAP + SB2.5 group. SAP: Severe acute pancreatitis.
SB203580 alleviates lung injury in SAP rats
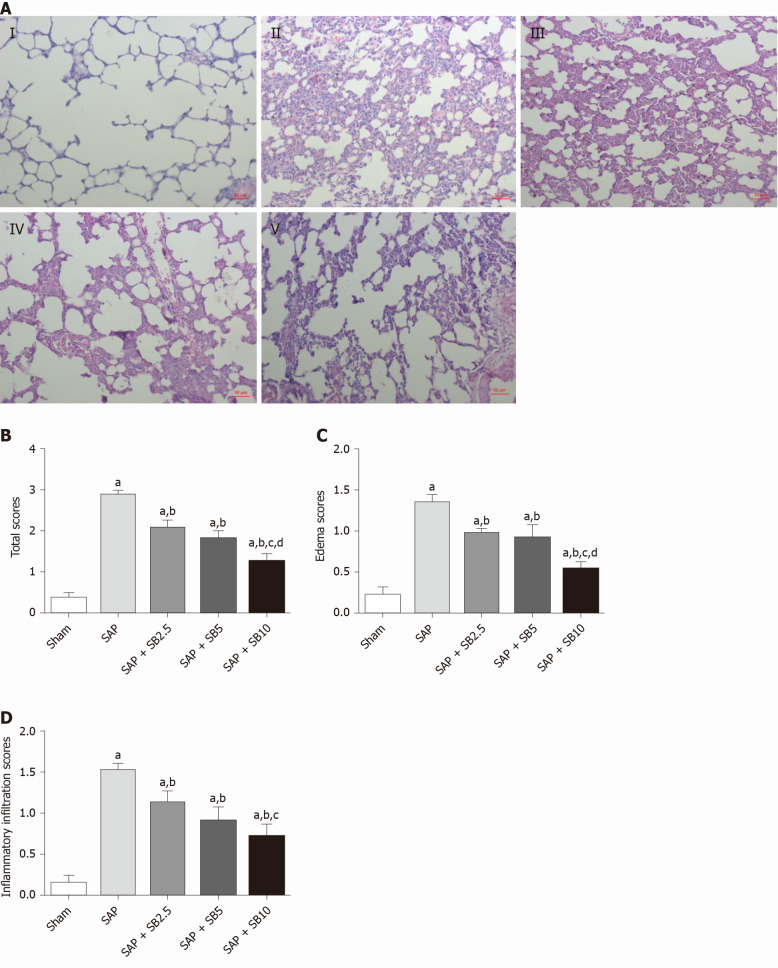
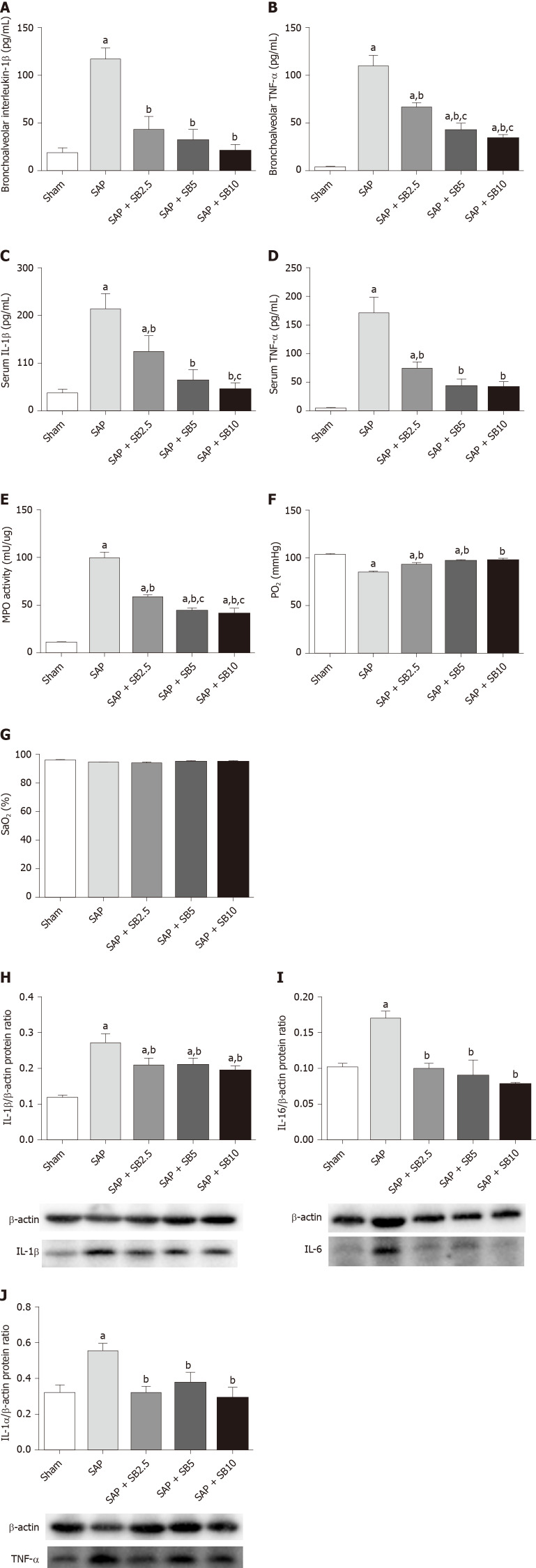
The histopathological changes in the pulmonary tissues were shown in Figure 3A. In SAP rats, lung damage was characterized by neutrophil infiltration, thickened alveolar walls, intra-alveolar hemorrhaging and alveolar collapse. The histopathological scores were also significantly increased (Figure 3B-D). These changes were accompanied by significantly increased IL-1 and TNF-α levels in the BALF and serum (Figure 4A-D) and pulmonary MPO activity (Figure 4E) and a decreased PaO2 (Figure 4F). There was no change of SO2 in the SAP rats (Figure 4G). At the same time, western blotting analysis also confirmed increased levels of inflammatory cytokines (IL-1, TNF-α, IL-6) in the SAP group compared with the sham group (Figure 4H-J). SB203580 treatment alleviated the histopathological injuries and scores, decreased the levels of inflammatory factors (IL-1, TNF-α, IL-6 and MPO) and preserved PaO2. Among the three doses tested, SB203580 at 10.0 mg/kg consistently and significantly reduced all histopathological and biochemical severity indices with the highest efficiency.
Figure 3.
Effects of SB203580 on pulmonary histopathological changes and histopathological severity scores. A: Representative hematoxylin and eosin images of pulmonary sections (magnification 200 ×); I: Sham group; II: Severe acute pancreatitis (SAP) group; III: SAP + SB2.5 group; IV: SAP + SB5 group; V: SAP + SB10 group; B: Total scores. C: Edema scores; D: Inflammatory infiltration scores. B-D: Pulmonary histopathology scores in different groups; Data are expressed as mean ± SE of the mean; aP < 0.05 vs sham group; bP < 0.05 vs SAP group; cP < 0.05 vs SAP + SB2.5 group; dP < 0.05 vs SAP + SB5 group. SAP: Severe acute pancreatitis.
Figure 4.
Effects of SB203580 on pulmonary severity indices of severe acute pancreatitis in rats. A: Bronchoalveolar interleukin-1β (IL-1β); B: Bronchoalveolar tumor necrosis factor alpha (TNF-α); C: Serum IL-1β; D: Serum TNF-α; E: Lung myeloperoxidase activity; F: Partial pressure of oxygen; G: Oxygen saturation; H: Representative western blotting analysis results for IL-1β in lung tissues; I: Representative western blotting analysis results for IL-6 in lung tissues; J: Representative western blotting analysis results for TNF-α in lung tissues. Data are expressed as mean ± SE of the mean; aP < 0.05 vs sham group; bP < 0.05 vs severe acute pancreatitis group; cP < 0.05 vs severe acute pancreatitis + SB2.5 group. IL: Interleukin; MPO: Myeloperoxidase; PaO2: Pressure of oxygen; SaO2: Oxygen saturation; SAP: Severe acute pancreatitis; TNF-α: Tumor necrosis factor-alpha.
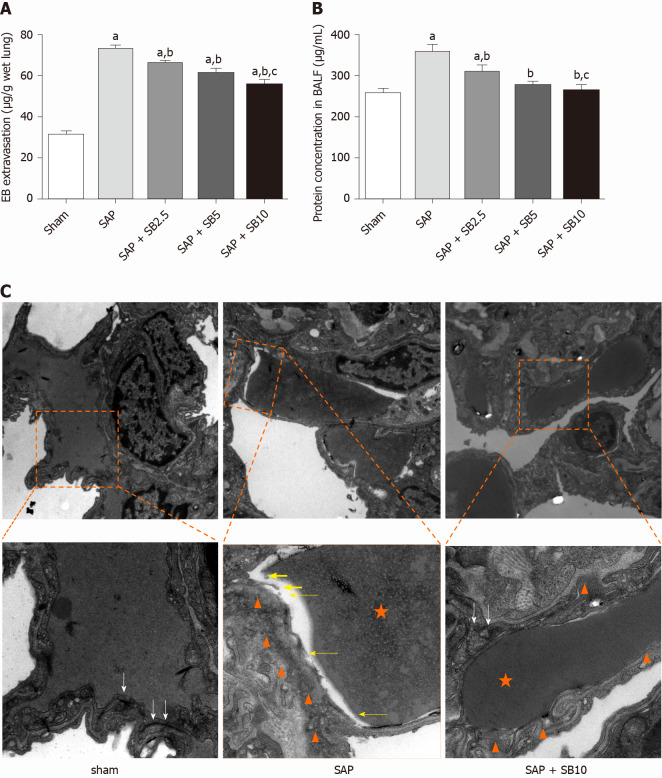
Protective effects of SB203580 on capillary injury
The permeability of the PMVECs was evaluated in vivo by measuring the BALF protein concentration and Evans blue accumulation in the lung tissue. Quantitative analysis showed a significant increase in these two parameters in SAP rats, which were suppressed by SB203580 cotreatment (Figure 5A and 5B). They further decreased after treatment with increasing SB203580 doses. The microstructure and integrity of the endothelium were evaluated using transmission electron microscopy. In the sham group, the lung tissues showed a normal capillary endothelium, as evidenced by an intact capillary basal membrane, dense endothelial cell-cell junctions and continuous and clear delineation between cells (Figure 5C). In the SAP group, capillary endothelial dissolution and rupture were noted. In addition, low electron density clouds with an irregular thickness indicating basal membrane edema, increased vacuolization, loose cellular junctions, swollen cellular organelles and nuclear degeneration were often observed. In contrast, the swelling and injury of endothelial cells were partially alleviated in lungs from SB203580-treated rats. After treatment with 10.0 mg/kg SB203580, no obvious rupture of endothelial cells or red cells extravasating from the vessels were observed.
Figure 5.
Effects of SB203580 on pulmonary capillary injury of severe acute pancreatitis rats. A: Evans blue extravasation in lung tissues; B: Bronchoalveolar protein concentration; C: Representative electron microscope photomicrographs of pulmonary microvascular endothelial cells in lung tissues; Sham group: Magnification 1500 × and 4000 ×, respectively; Severe acute pancreatitis (SAP) group: Magnification 1500 × and 5000 ×, respectively; SAP + SB10 group: Magnification 1500 × and 5000 ×, respectively; White arrows: Dense endothelial cell–cell junctions; White arrowheads: Low electron density clouds with an irregular thickness indicating basal membrane edema; Yellow arrows: Dissolution, rupture and debris of capillary endothelial; Orange star: Red blood cell. Data were expressed as mean ± SE; aP < 0.05 vs sham group; bP < 0.05 vs SAP group; cP < 0.05 vs SAP + SB2.5 group. SAP: Severe acute pancreatitis.
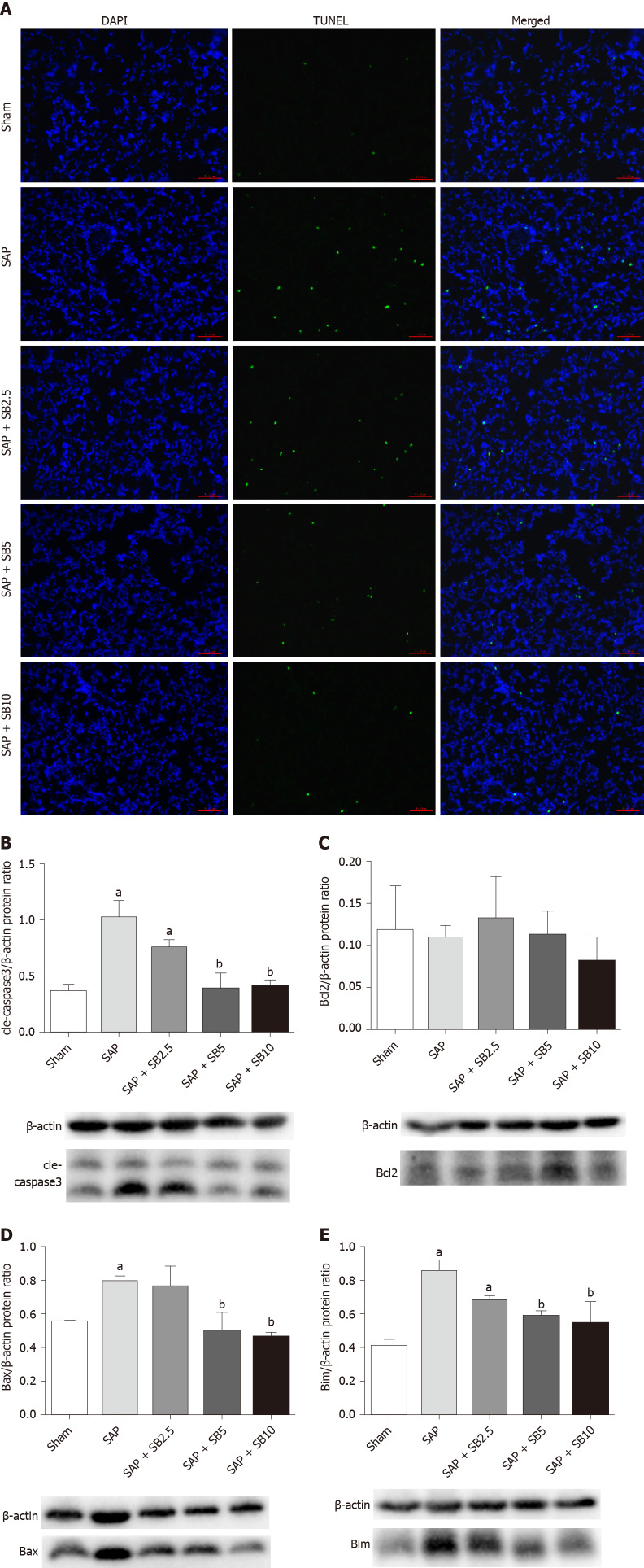
SB203580 inhibits apoptosis in the lungs of SAP rats
TUNEL staining was used to examine cell death in lung tissues. Cell apoptosis in the lung tissues was assessed by the colocalization of TUNEL-green and DAPI-blue staining. A prominent increase in the number of positively stained cells was observed in the SAP group compared to the sham group, indicating a higher apoptotic rate (Figure 6A). Treatment of SAP rats with SB203580 significantly decreased the number of apoptotic cells, and the greatest inhibitory effect was observed in rats treated with 10.0 mg/kg SB203580. Proteins regulating the apoptotic signaling pathways were examined using western blotting to further confirm the cell death pathway. As shown in Figure 6B-E, although no change in the antiapoptotic protein Bcl2 was observed, the levels of the proapoptotic proteins Bax, Bim and cle-caspase3 were upregulated in the SAP group compared to the sham group. SB203580 treatment reversed the changes in the levels of these proteins in the SAP group in a dose-dependent manner.
Figure 6.
Effect of SB203580 on apoptosis of lung tissues in severe acute pancreatitis. A: Representative terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling images of pulmonary sections (200 ×); B: Cle-caspase3; C: Bcl2; D: Bax; E: Bim. B-E: Representative western blotting analysis results for apoptosis-related proteins in lung tissues; Data were expressed as mean ± SE; aP < 0.05 vs sham group; bP < 0.05 vs severe acute pancreatitis group. SAP: Severe acute pancreatitis.
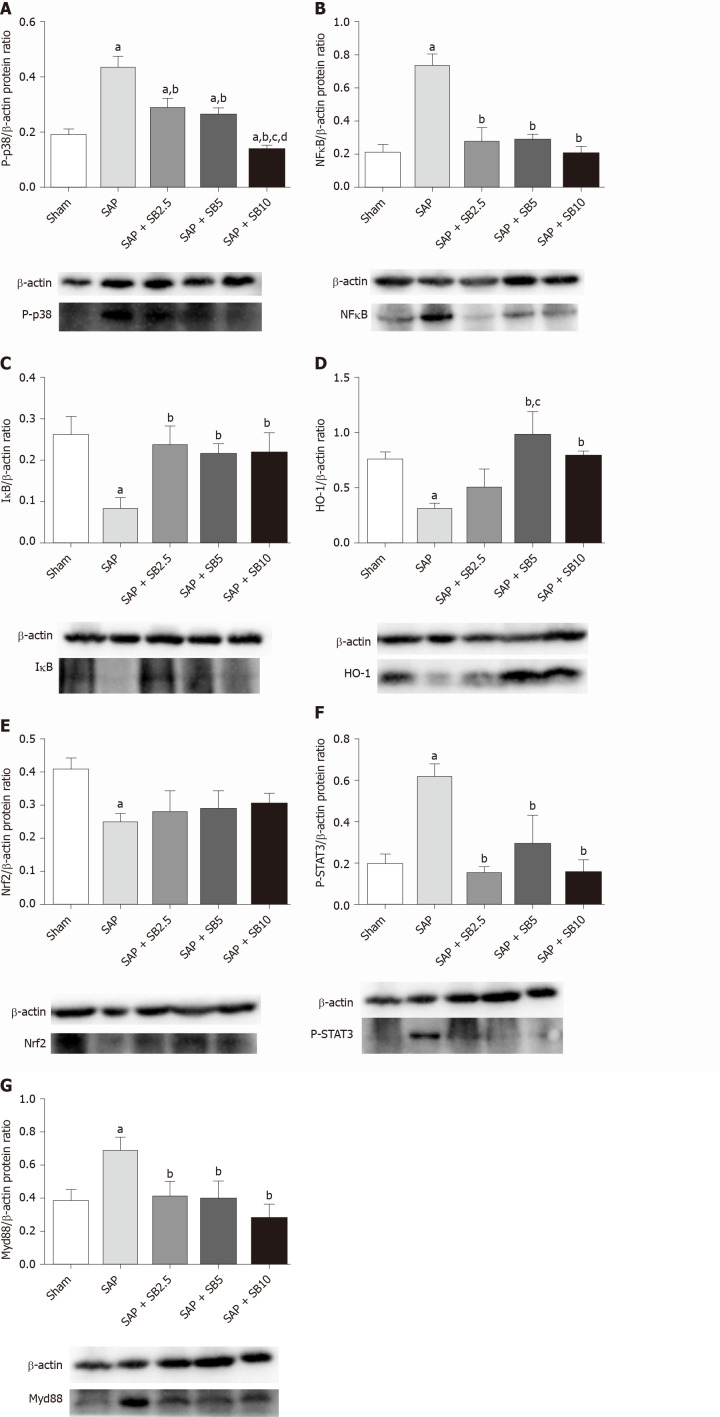
SB203580 protects against ALI by regulating proinflammatory and proapoptotic signaling pathways
Levels of the P-p38, NFκB, IκB, Nrf2, HO-1, P-SATA3 and Myd88 proteins were detected in the lungs for further evaluation of the protective mechanism of SB203580. Figure 7 showed increased levels of P-p38, NFκB, P-SATA3 and Myd88 but decreased levels of IκB, Nrf2 and HO-1 in the SAP group compared with the sham group. Intriguingly, SB203580 reversed the changes in the levels of these proteins, reducing P-p38, NFκB, P-SATA3 and Myd88 levels but increasing IκB and HO-1 levels compared with the SAP group. However, except for P-p38 and HO-1, the improvement of these parameters was not positively related to the dose of SB203580. In addition, SB203580 slightly increased the level of Nrf2, but the difference was not statistically significant (Figure 7E).
Figure 7.
Effect of SB203580 on proinflammatory and proapoptotic signaling pathways. Representative Western blotting analysis results for protein expression in pulmonary tissue; A: P-p38; B: NFκB; C: IκB; D: HO-1; E: Nrf2; F: P-STAT3; G: Myd88. Data are expressed as mean ± SE; aP < 0.05 vs sham group; bP < 0.05 vs severe acute pancreatitis group; cP < 0.05 vs severe acute pancreatitis + SB2.5 group; dP < 0.05 vs severe acute pancreatitis + SB5 group. Nrf2: Nuclear factor erythroid 2-related factor 2; STAT3: Signal transducer and activator of transcription-3.
DISCUSSION
Although the mechanisms of ALI/acute respiratory distress syndrome following SAP have not been completely elucidated, an increasing number of studies have described PMVEC injury and dysfunction as prerequisites for ALI[24]. The PMVECs are activated, participate in host defenses and result in increased microvascular permeability and damaged lung function[8,25]. Regardless of the etiology, an exaggerated inflammatory response is widely accepted to play a vital role in the transition of AP into a systemic disease[26]. Various inflammatory mediators have been related to AP severity and they induce their own or other mediators’ secretion through a positive feedback mechanism, leading to amplification of the inflammatory cascade[27]. TNF-α was discovered to play a role in the early phase and cause the activation and injury of endothelial cells, functioning as a key promoter of the development of SAP-ALI[28]. In the present study, TNF-α was used to mimic SAP-related PMVEC injury in vitro.
p38 is activated by various insults, including cytokines, while the activation of p38 MAPK inevitably induces the expression of proinflammatory cytokines[29]. In our study, TNF-α induced higher levels of inflammatory cytokines (IL-1β and IL-6) in PMVECs with p38 overactivation (Figure 1D and 1E). These results further confirmed a positive role for the p38 MAPK signaling pathway in regulating cytokine expression.
According to previous studies, p38 MAPK is involved in both survival and apoptosis pathways. It promotes anti- or proapoptotic effects in a cell type and stimulus-specific manner in endothelial cells. For example, one group reported that SB203580 inhibited p38 MAPK and then decreased the endothelial cell apoptosis stimulated by TNF-α and cycloheximide[30], while another group illustrated that SB203580 enhanced endothelial cell apoptosis triggered by TNF-α alone[31]. It was reported that endothelial cells are relatively resistant to TNF-α, which induce only modest or no cell damage[31,32]. Then, we chose to overexpress activated MKK 6 (Glu) to constitutively activate p38 MAPK in PMVECs. Flow cytometry analysis revealed apoptotic cell death in null PMVECs stimulated with TNF-α, and MKK 6 (Glu) transfection resulted in a significantly higher apoptotic rate (Figure 1B). This finding indicated that persistent activation of p38 MAPK promotes TNF-α-induced PMVEC apoptosis. However, SB203580 pretreatment conversely aggravated PMVEC injury in this study (data not shown) and did not alleviate cell apoptosis. This discrepancy may be attributed to the extent or duration of p38 MAPK activation[33]. Rapid and transient p38 activation modulates inhibitors of the apoptosis protein family to prevent TNF-α-induced apoptosis[31], while sustained activation leads to apoptosis through the phosphorylation and downregulation of Bcl-xL[30]. Therefore, SB203580 may exert a beneficial effect to maintain an appropriate level of p38 MAPK activity.
Many small-molecule inhibitors targeting p38 MAPK, such as SB203850, CNI-1493[34], SB202190[35], RWJ-67657[36] and UM101[37], have been tested in various cell injury or disease models. In AP, the p38 MAPK signaling pathway is involved not only in pancreatic acinar injury[20,38] but also in injury to distant organs (lung, liver, kidney, gut and placenta)[39-43]. SB203850 is a pyridinyl imidazole derivative that competes for ATP binding, and the inhibition of p38 MAPK has been shown to attenuate the severity of AP and its related distant organ injury[14,15,39,44]. In the present study, we treated SAP rats with SB203580 to investigate its protective effect on SAP-associated lung injury and the potential underlying mechanisms. As shown in Figure 2, SB203580 significantly alleviated pancreatic histopathological damage in SAP rats. Furthermore, it alleviated lung histopathological injuries (Figure 3), decreased the levels of inflammatory factors (IL-1β, TNF-α, IL-6 and MPO, Figure 4) and preserved PaO2, consistent with previous reports[14,15]. Based on these results, SB203580 effectively decreased the inflammatory response in rats with SAP-ALI. In addition, by analyzing three different doses of SB203580, we observed a dose-dependent improvement in the therapeutic effects, as 10.0 mg/kg was the most effective.
Although apoptosis is regarded as a protective mechanism, excessive apoptosis is harmful to the alveolar capillary membrane and pulmonary function. As the first-line responder to systemic injurious factor insults, damage to endothelial cells is detected earlier than damage to epithelial cells[45]. PMVEC damage results in lung injury, which in turn promotes the secretion of inflammatory mediators to destroy PMVECs in individuals with SAP[46]. In the present study, increased Evans blue extravasation and BALF protein concentrations revealed increased capillary permeability in SAP rats. Moreover, transmission electron microscopy analysis directly confirmed capillary endothelial dissolution and rupture in the lung (Figure 5). Finally, the results of the TUNEL and western blot analyses (Figure 6) suggested that an increased number of apoptotic cells was involved in the pathological process of SAP-ALI. However, SB203580 significantly reversed the changes in Evans blue accumulation, BALF protein concentration, TUNEL-positive cells, apoptosis-regulated proteins and endothelial microstructure, providing evidence for the underlying protective effects of p38 MAPK inhibitors on endothelial cell apoptosis.
Because p38 MAPK is a proximal initiator of signal transduction, various substrates are directly or indirectly activated by p38 MAPK, permitting it to perform a wide range of functions[29,47]. The biological consequences of p38 MAPK inhibition are complicated by interactions with other signaling pathways. p38 MAPK was reported to have a dual function in regulating Nrf2/HO-1 signaling[48,49]. HO-1 possesses cytoprotective properties, including anti-inflammatory, antioxidant and antiapoptotic activities. NFκB, which is downstream of p38 MAPK, represses Nrf2 transcriptional activity[50,51], while HO-1 inhibits proinflammatory mediators through the inactivation of NFκB[52]. STAT3 is activated by p38 MAPK, transferring signals to the nucleus and regulating gene expression[53,54]. MyD88 is considered a classical proinflammatory and proapoptotic upstream signaling adaptor molecule of p38 MAPK[55]. While the mechanism underlying the effects of SB203580 must be further elucidated, the levels of these key proteins involved in inflammatory, oxidative and apoptotic signaling pathways were measured. In the present study, SB203580 treatment reversed the increased levels of P-p38, NFκB, P-STAT3 and Myd88 and decreased levels of IκB and HO-1 in the SAP group (Figure 7). Therefore, the protective effects of p38 inhibition via SB203580 were likely partially attributed to the inhibition of pulmonary proinflammatory and proapoptotic signaling pathways.
CONCLUSION
In conclusion, p38 MAPK overactivation promoted TNF-α-induced inflammatory cytokine expression and PMVEC apoptosis in vitro. The inhibition of p38 MAPK with SB203580 protected against SAP-ALI by alleviating inflammation and apoptotic cell death in rats. Therefore, SB203580 may alleviate SAP-ALI by inhibiting the proinflammatory and proapoptotic effects of the p38 MAPK pathway on PMVECs. These results suggest the possibility of applying p38 MAPK inhibitors as treatments for SAP-ALI in the clinical setting.
ARTICLE HIGHLIGHTS
Research background
Acute lung injury (ALI) is the main reason for the high mortality of patients with severe acute pancreatitis (SAP). Injury and dysfunction of pulmonary microvascular endothelial cells (PMVEC) are considered prerequisites for ALI. The p38 mitogen-activated protein kinase (p38 MAPK) signaling pathway is involved in the development of SAP-related ALI. However, the precise mechanism by which p38 MAPK regulates PMVEC injury in SAP-related ALI is unclear.
Research motivation
To date, no specific pharmacological therapies for SAP associated ALI are available. Elucidating the mechanism of PMVEC injury regulated by p38 MAPK is expected to help identify new treatments for SAP-associated ALI.
Research objectives
To determine the role of p38 MAPK in the tumor necrosis factor-alpha-induced injury of PMVECs in vitro and to explore the effect of SB203580-mediated p38 inhibition on SAP-ALI in vivo.
Research methods
In vitro, PMVECs were transfected with MAPK kinase 6 (Glu) and stimulated with tumor necrosis factor-alpha to detect cell apoptosis and inflammatory cytokine levels. In vivo, SAP-ALI rats were treated with three different doses of SB203580 (2.5, 5.0 or 10.0 mg/kg). Blood, bronchoalveolar lavage fluid and tissue samples were harvested to assess cytokine levels, blood gas analyses, histopathological changes, myeloperoxidase activity, bronchoalveolar protein concentration, Evans blue extravasation, apoptosis and ultrastructural changes of PMVECs. Then, the mechanism underlying the effects of SB203580 was also detected in the lungs.
Research results
In vitro, MAPK kinase (Glu) transfection resulted in higher apoptotic rates and cytokine levels in TNF-α-treated PMVECs. In vivo, SB2035080 attenuated lung histopathological injury, decreased inflammatory activity and preserved pulmonary function. Furthermore, SB203580 significantly reversed the microvascular permeability, the increase in the number of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cells, the increased expression of apoptosis-related proteins and the endothelial microstructure changes. Moreover, SB203580 significantly reduced pulmonary P-p38, NFκB, P-SATA3 and Myd88 levels but increased the IκB and HO-1 levels.
Research conclusions
p38 inhibition may protect against SAP-ALI by alleviating inflammation and the apoptotic death of PMVECs.
Research perspectives
In this study, p38 MAPK overactivation promoted PMVEC injury in vitro, while p38 inhibition protected against SAP-ALI in vivo. These results suggest the potential of applying p38 MAPK inhibitors as treatments for SAP-ALI in the clinical setting.
Footnotes
Institutional animal care and use committee statement: The study was performed according to the Guidelines of Animal Care and Use Committee of West China Hospital of Sichuan University (protocol number, 2017082A).
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
ARRIVE guidelines statement: The manuscript has been prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: December 26, 2020
First decision: January 29, 2021
Article in press: March 29, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kozarek R S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Xiao-Xin Zhang, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Hao-Yang Wang, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Xue-Fei Yang, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Zi-Qi Lin, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Na Shi, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Chan-Juan Chen, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Lin-Bo Yao, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Xin-Min Yang, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Jia Guo, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Qing Xia, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China.
Ping Xue, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China. xueping@wchscu.cn.
Data sharing statement
No additional data are available.
References
- 1.Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Elder AS, Saccone GT, Dixon DL. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12:49–56. doi: 10.1016/j.pan.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 4.De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surg Infect (Larchmt) 2007;8:107–120. doi: 10.1089/sur.2006.011. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 7.Fang M, Zhong WH, Song WL, Deng YY, Yang DM, Xiong B, Zeng HK, Wang HD. Ulinastatin Ameliorates Pulmonary Capillary Endothelial Permeability Induced by Sepsis Through Protection of Tight Junctions via Inhibition of TNF-α and Related Pathways. Front Pharmacol. 2018;9:823. doi: 10.3389/fphar.2018.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Sakata K, Mizuno N, Palikhe S, Yamashita S, Hattori K, Matsuda N, Hattori Y. Different involvement of the MAPK family in inflammatory regulation in human pulmonary microvascular endothelial cells stimulated with LPS and IFN-γ. Immunobiology. 2018;223:777–785. doi: 10.1016/j.imbio.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Li Q, Deng Z, Zhang Z, Xu J, Qian G, Wang G. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway. Mol Biol Rep. 2011;38:3615–3622. doi: 10.1007/s11033-010-0473-8. [DOI] [PubMed] [Google Scholar]
- 13.de Campos T, Deree J, Martins JO, Loomis WH, Shenvi E, Putnam JG, Coimbra R. Pentoxifylline attenuates pulmonary inflammation and neutrophil activation in experimental acute pancreatitis. Pancreas. 2008;37:42–49. doi: 10.1097/MPA.0b013e3181612d19. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Xia H, Zhao L, Mei F, Li M, You Y, Zhao K, Wang W. SB203580 attenuates acute lung injury and inflammation in rats with acute pancreatitis in pregnancy. Inflammopharmacology. 2019;27:99–107. doi: 10.1007/s10787-018-0522-9. [DOI] [PubMed] [Google Scholar]
- 15.Cao MH, Xu J, Cai HD, Lv ZW, Feng YJ, Li K, Chen CQ, Li YY. p38 MAPK inhibition alleviates experimental acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int. 2015;14:101–106. doi: 10.1016/s1499-3872(15)60327-7. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Denham W, Tracey KJ, Wang H, Kramer AA, Salhab KF, Norman J. The physiologic consequences of macrophage pacification during severe acute pancreatitis. Shock. 1998;10:169–175. doi: 10.1097/00024382-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 17.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Hu J, He T, Zhang Q, Yang X, Lan X, Zhang D, Mei H, Chen B, Huang Y. P38/MAPK contributes to endothelial barrier dysfunction via MAP4 phosphorylation-dependent microtubule disassembly in inflammation-induced acute lung injury. Sci Rep. 2015;5:8895. doi: 10.1038/srep08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JY, Chu ZG, Han J, Dang YM, Yan H, Zhang Q, Liang GP, Huang YS. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010;67:321–333. doi: 10.1007/s00018-009-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren HB, Li ZS, Xu GM, Tu ZX, Shi XG, Jia YT, Gong YF. Dynamic changes of mitogen-activated protein kinase signal transduction in rats with severe acute pancreatitis. Chin J Dig Dis. 2004;5:123–125. doi: 10.1111/j.1443-9573.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 21.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawra R, Ku YS, Sharif R, Dhaulakhandi D, Phillips P, Dudeja V, Saluja AK. An improved method for extracting myeloperoxidase and determining its activity in the pancreas and lungs during pancreatitis. Pancreas. 2008;37:62–68. doi: 10.1097/MPA.0b013e3181607761. [DOI] [PubMed] [Google Scholar]
- 24.Ye W, Zheng C, Yu D, Zhang F, Pan R, Ni X, Shi Z, Zhang Z, Xiang Y, Sun H, Shi K, Chen B, Zhang Q, Zhou M. Lipoxin A4 Ameliorates Acute Pancreatitis-Associated Acute Lung Injury through the Antioxidative and Anti-Inflammatory Effects of the Nrf2 Pathway. Oxid Med Cell Longev. 2019;2019:2197017. doi: 10.1155/2019/2197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi L, Huang X, Guo F, Zhou Z, Chang M, Huan J. GSK-3Beta-Dependent Activation of GEF-H1/ROCK Signaling Promotes LPS-Induced Lung Vascular Endothelial Barrier Dysfunction and Acute Lung Injury. Front Cell Infect Microbiol. 2017;7:357. doi: 10.3389/fcimb.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Zhang X, Lin Z, Guo J, Yang X, Yao L, Wang H, Xue P, Xia Q. Chaiqin chengqi decoction alleviates severe acute pancreatitis associated acute kidney injury by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Biomed Pharmacother. 2020;125:110024. doi: 10.1016/j.biopha.2020.110024. [DOI] [PubMed] [Google Scholar]
- 27.Sandoval J, Pereda J, Pérez S, Finamor I, Vallet-Sánchez A, Rodríguez JL, Franco L, Sastre J, López-Rodas G. Epigenetic Regulation of Early- and Late-Response Genes in Acute Pancreatitis. J Immunol. 2016;197:4137–4150. doi: 10.4049/jimmunol.1502378. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Xie J, Xiang Y, Dai S, Yu D, Sun H, Chen B, Zhou M. Downregulation of TNF-α/TNF-R1 Signals by AT-Lipoxin A4 May Be a Significant Mechanism of Attenuation in SAP-Associated Lung Injury. Mediators Inflamm. 2019;2019:9019404. doi: 10.1155/2019/9019404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viemann D, Goebeler M, Schmid S, Klimmek K, Sorg C, Ludwig S, Roth J. Transcriptional profiling of IKK2/NF-kappa B- and p38 MAP kinase-dependent gene expression in TNF-alpha-stimulated primary human endothelial cells. Blood. 2004;103:3365–3373. doi: 10.1182/blood-2003-09-3296. [DOI] [PubMed] [Google Scholar]
- 30.Grethe S, Ares MP, Andersson T, Pörn-Ares MI. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L) Exp Cell Res. 2004;298:632–642. doi: 10.1016/j.yexcr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Furusu A, Nakayama K, Xu Q, Konta T, Kitamura M. MAP kinase-dependent, NF-kappaB-independent regulation of inhibitor of apoptosis protein genes by TNF-alpha. J Cell Physiol. 2007;210:703–710. doi: 10.1002/jcp.20881. [DOI] [PubMed] [Google Scholar]
- 32.Roulston A, Reinhard C, Amiri P, Williams LT. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Murphy C, Denham W, Botchkina G, Tracey KJ, Norman J. Evidence of a central role for p38 map kinase induction of tumor necrosis factor alpha in pancreatitis-associated pulmonary injury. Surgery. 1999;126:216–222. [PubMed] [Google Scholar]
- 35.Lüschen S, Scherer G, Ussat S, Ungefroren H, Adam-Klages S. Inhibition of p38 mitogen-activated protein kinase reduces TNF-induced activation of NF-kappaB, elicits caspase activity, and enhances cytotoxicity. Exp Cell Res. 2004;293:196–206. doi: 10.1016/j.yexcr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Kuldo JM, Westra J, Asgeirsdóttir SA, Kok RJ, Oosterhuis K, Rots MG, Schouten JP, Limburg PC, Molema G. Differential effects of NF-{kappa}B and p38 MAPK inhibitors and combinations thereof on TNF-{alpha}- and IL-1{beta}-induced proinflammatory status of endothelial cells in vitro. Am J Physiol Cell Physiol. 2005;289:C1229–C1239. doi: 10.1152/ajpcell.00620.2004. [DOI] [PubMed] [Google Scholar]
- 37.Shah NG, Tulapurkar ME, Ramarathnam A, Brophy A, Martinez R 3rd, Hom K, Hodges T, Samadani R, Singh IS, MacKerell AD Jr, Shapiro P, Hasday JD. Novel Noncatalytic Substrate-Selective p38α-Specific MAPK Inhibitors with Endothelial-Stabilizing and Anti-Inflammatory Activity. J Immunol. 2017;198:3296–3306. doi: 10.4049/jimmunol.1602059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twait E, Williard DE, Samuel I. Dominant negative p38 mitogen-activated protein kinase expression inhibits NF-kappaB activation in AR42J cells. Pancreatology. 2010;10:119–128. doi: 10.1159/000290656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang J, Zhang ZH, Zhou YX, Niu WC, Zhou F, Shen CB, Chen RG, Li X. Up-regulation of Tight-Junction Proteins by p38 Mitogen-Activated Protein Kinase/p53 Inhibition Leads to a Reduction of Injury to the Intestinal Mucosal Barrier in Severe Acute Pancreatitis. Pancreas. 2016;45:1136–1144. doi: 10.1097/MPA.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 40.Fan HN, Chen W, Fan LN, Wu JT, Zhu JS, Zhang J. Macrophages-derived p38α promotes the experimental severe acute pancreatitis by regulating inflammation and autophagy. Int Immunopharmacol. 2019;77:105940. doi: 10.1016/j.intimp.2019.105940. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Zhao KL, Hu WJ, Zuo T, Ding YM, Wang WX. Macrophage Migration Inhibitor Promoted the Intrahepatic Bile Duct Injury in Rats with Severe Acute Pancreatitis. Dig Dis Sci. 2019;64:759–772. doi: 10.1007/s10620-018-5379-7. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Yu J, Zhao L, Mei FC, Zhou Y, Hong YP, Zuo T, Wang WX. Inhibition of macrophage migration inhibitory factor attenuates inflammation and fetal kidney injury in a rat model of acute pancreatitis in pregnancy. Int Immunopharmacol. 2019;68:106–114. doi: 10.1016/j.intimp.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 43.Zuo T, Yu J, Wang WX, Zhao KL, Chen C, Deng WH, He XB, Wang P, Shi Q, Guo WY. Mitogen-Activated Protein Kinases Are Activated in Placental Injury in Rat Model of Acute Pancreatitis in Pregnancy. Pancreas. 2016;45:850–857. doi: 10.1097/MPA.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 44.Wan YD, Zhu RX, Bian ZZ, Pan XT. Improvement of Gut Microbiota by Inhibition of P38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in Rats with Severe Acute Pancreatitis. Med Sci Monit. 2019;25:4609–4616. doi: 10.12659/MSM.914538. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Wang F, Lu F, Huang H, Huang M, Luo T. Ultrastructural changes in the pulmonary mechanical barriers in a rat model of severe acute pancreatitis-associated acute lung injury. Ultrastruct Pathol. 2016;40:33–42. doi: 10.3109/01913123.2015.1088907. [DOI] [PubMed] [Google Scholar]
- 46.Han X, Wang Y, Chen H, Zhang J, Xu C, Li J, Li M. Enhancement of ICAM-1 via the JAK2/STAT3 signaling pathway in a rat model of severe acute pancreatitis-associated lung injury. Exp Ther Med. 2016;11:788–796. doi: 10.3892/etm.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoefen RJ, Berk BC. The role of MAP kinases in endothelial activation. Vascul Pharmacol. 2002;38:271–273. doi: 10.1016/s1537-1891(02)00251-3. [DOI] [PubMed] [Google Scholar]
- 48.Naidu S, Vijayan V, Santoso S, Kietzmann T, Immenschuh S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol. 2009;182:7048–7057. doi: 10.4049/jimmunol.0900006. [DOI] [PubMed] [Google Scholar]
- 49.Zhou MM, Zhang WY, Li RJ, Guo C, Wei SS, Tian XM, Luo J, Kong LY. Anti-inflammatory activity of Khayandirobilide A from Khaya senegalensis via NF-κB, AP-1 and p38 MAPK/Nrf2/HO-1 signaling pathways in lipopolysaccharide-stimulated RAW 264.7 and BV-2 cells. Phytomedicine. 2018;42:152–163. doi: 10.1016/j.phymed.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Reboll MR, Schweda AT, Bartels M, Franke R, Frank R, Nourbakhsh M. Mapping of NRF binding motifs of NF-kappaB p65 subunit. J Biochem. 2011;150:553–562. doi: 10.1093/jb/mvr099. [DOI] [PubMed] [Google Scholar]
- 51.Niedick I, Froese N, Oumard A, Mueller PP, Nourbakhsh M, Hauser H, Köster M. Nucleolar localization and mobility analysis of the NF-kappaB repressing factor NRF. J Cell Sci. 2004;117:3447–3458. doi: 10.1242/jcs.01129. [DOI] [PubMed] [Google Scholar]
- 52.Ha YM, Kim MY, Park MK, Lee YS, Kim YM, Kim HJ, Lee JH, Chang KC. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis. 2012;17:463–474. doi: 10.1007/s10495-011-0688-8. [DOI] [PubMed] [Google Scholar]
- 53.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23:177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 54.Ng DC, Long CS, Bogoyevitch MA. A role for the extracellular signal-regulated kinase and p38 mitogen-activated protein kinases in interleukin-1 beta-stimulated delayed signal tranducer and activator of transcription 3 activation, atrial natriuretic factor expression, and cardiac myocyte morphology. J Biol Chem. 2001;276:29490–29498. doi: 10.1074/jbc.M100699200. [DOI] [PubMed] [Google Scholar]
- 55.Shih JH, Tsai YF, Li IH, Chen MH, Huang YS. Hp-s1 Ganglioside Suppresses Proinflammatory Responses by Inhibiting MyD88-Dependent NF-κB and JNK/p38 MAPK Pathways in Lipopolysaccharide-Stimulated Microglial Cells. Mar Drugs. 2020;18 doi: 10.3390/md18100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.