Abstract
Pancreatic ductal adenocarcinoma (PDAC) is projected to emerge as the second leading cause of cancer-related death after 2030. Extreme treatment resistance is perhaps the most significant factor that underlies the poor prognosis of PDAC. To date, combination chemotherapy remains the mainstay of treatment for most PDAC patients. Compared to other cancer types, treatment response of PDAC tumors to similar chemotherapy regimens is clearly much lower and shorter-lived. Aside from typically harboring genetic alterations that to date remain un-druggable and are drivers of treatment resistance, PDAC tumors are uniquely characterized by a densely fibrotic stroma that has well-established roles in promoting cancer progression and treatment resistance. However, emerging evidence also suggests that indiscriminate targeting and near complete depletion of stroma may promote PDAC aggressiveness and lead to detrimental outcomes. These conflicting results undoubtedly warrant the need for a more in-depth understanding of the heterogeneity of tumor stroma in order to develop modulatory strategies in favor of tumor suppression. The advent of novel techniques including single cell RNA sequencing and multiplex immunohistochemistry have further illuminated the complex heterogeneity of tumor cells, stromal fibroblasts, and immune cells. This new knowledge is instrumental for development of more refined therapeutic strategies that can ultimately defeat this disease. Here, we provide a concise review on lessons learned from past stroma-targeting strategies, new challenges revealed from recent preclinical and clinical studies, as well as new prospects in the treatment of PDAC.
Keywords: Stroma, Pancreatic cancer, Treatment resistance, Cancer-associated fibroblasts, Clinical trials
Core Tip: Stromal desmoplasia is not only a prominent histological hallmark of pancreatic cancer, but also a biological barrier to therapies. Various strategies aimed at targeting the stroma to improve therapeutic outcomes have been largely unsuccessful. Here we comprehensively reviewed the rationales and lessons learned from various stromal-targeting strategies and provide prospects on improving these approaches in future clinical trials.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is currently the seventh leading cause of cancer related death in the industrialized world[1]. In the United States, PDAC is projected to be the second leading cause of cancer death by 2030[2]. The current 5-year survival rate of PDAC is 9%, making it one of the deadliest cancers[3].
There are several factors that contribute to the poor outcomes of PDAC. First, non-specific symptoms and lack of PDAC specific markers and screening lead to late detection[4]. Less than 10% of PDAC is resectable at the time of diagnosis. Second, PDAC cells are highly metastatic, evidenced by the fact that most patients develop local or distal recurrences even after seemingly successful surgical resection. Third, PDAC is extremely resistant to chemotherapy and radiation. For example, the triple therapy of folinic acid, 5-fluorouracil (5-FU), irinotecan, oxaliplatin (as FOLFRINOX or FOLFOXIRI) has been used as a first line regimen in the treatment of various gastrointestinal malignancies. A phase III trial conducted by The Groupo Oncoligico Nord Ovest used FOLFOXIRI as a first line treatment in patients with metastatic colorectal cancer. The objective response rate (ORR) to FOLFOXIRI in these patients was 60%[5]. A phase II trial of FOLFIRINOX in patients with advanced gastroesophageal cancer showed an ORR of 61%[6]. This is in contrast to a response rate of only 31.6% in patients with metastatic PDAC receiving FOLFIRINOX[7]. For patients with localized PDAC who have undergone R0 or R1 surgical resection upfront and have postoperative CA19-9 of less than 180 U/mL, treatment with FOLFIRINOX resulted in a 3-year disease free survival rate of 39.7% as opposed to 21.4% with gemcitabine[8]. While these results demonstrate that strong combination chemotherapy can potentially cure additional patients, it is also worth noting that a significant subset (approximately 60%) of patients will still succumb to disease relapse despite having received adjuvant FOLFIRINOX, clearly demonstrating that PDAC tumors are highly chemo-resistant even at the micro-metastatic stage. Another regimen that is commonly used in patients with advanced inoperable PDAC is gemcitabine plus nab-paclitaxel (GnP). A phase III trial including 842 patients with metastatic PDAC (MPACT trial) in 2013 showed the benefit of GnP over gemcitabine alone [median overall survival (OS) 8.5 vs 6.7 mo; 95% confidence interval (CI) 0.62-0.83; P < 0.001], and response rate to GnP was 23%[9]. For patients with locally advanced PDAC, the response rate to FOLFIRINOX was 19% and to GnP was as low as 6%[10]. However, these two regimens are associated with significant toxicities, therefore escalation of these regimens by adding additional cytotoxic agents is expected to be clinically challenging and prohibitive.
PDAC is driven by mutations of multiple genes including KRAS, TP53, CDKN2A and SMAD4[11], which are also present in other cancer types such as non-small cell lung and colorectal cancers. However, PDAC tumors are characterized by a profound desmoplastic tumor microenvironment (TME), which accounts for 80%-90% of the tumor architecture[12]. Major components of the TME include a dense fibrotic matrix deposited by cancer-associated fibroblasts (CAFs), and significant infiltration of various subsets of immunosuppressive myeloid cells, vascular cells, and nerve cells[13-15]. The dense stroma plays an important role in tumor growth, proliferation, epithelial-mesenchymal transition (EMT), immune evasion and resistance to various therapies[16]. The low vascularity combined with elevated interstitial pressure dramatically limits vascular delivery and diffusion of therapeutic agents to tumor cells[17-19]. Therefore, targeting the stroma to improve therapeutic response has been fervently pursued in recent years, albeit with limited success. Importantly, the role of stroma in PDAC progression and treatment resistance has become increasingly controversial. Preclinical mouse models suggest that depletion of stromal fibroblasts alone carries a risk of reverting PDAC cells to a more progenitor-like and aggressive state, with corresponding inferior outcomes[20,21]. These observations underscore the critical need to delineate the diverse interplay between different components of the tumor stroma in order to develop therapies that can modify the tumor stroma in favor of tumor suppression[22]. Herein, we focus specifically on stroma-targeting clinical trials, discuss the outcomes and provide future prospects of this strategy.
COMPONENTS OF THE PANCREATIC TME
Pancreatic stellate cells (PSCs) are star-shaped cells that resemble their hepatic counterparts and were first discovered in 1998[23]. PSCs are found in the exocrine component of healthy pancreas, which when in their quiescent state are characterized by the presence of desmin intermediary filaments, vitamin A and fat droplets. During neoplastic progression, PSCs become activated, acquire a myofibroblast-like phenotype, express α-SMA and secrete extracellular matrix (ECM) proteins (collagen I, collagen III, collagen IV and fibronectin)[24]. These activated PSC are termed CAFs. The ECM proteins secreted/deposited by CAF form a three-dimensional stiff mesh which, along with high molecular weight glycosaminoglycans such as hyaluronan (HA), raises the interstitial pressure that leads to vascular collapse, forming a hypoxic and nutrient poor TME[25,26].
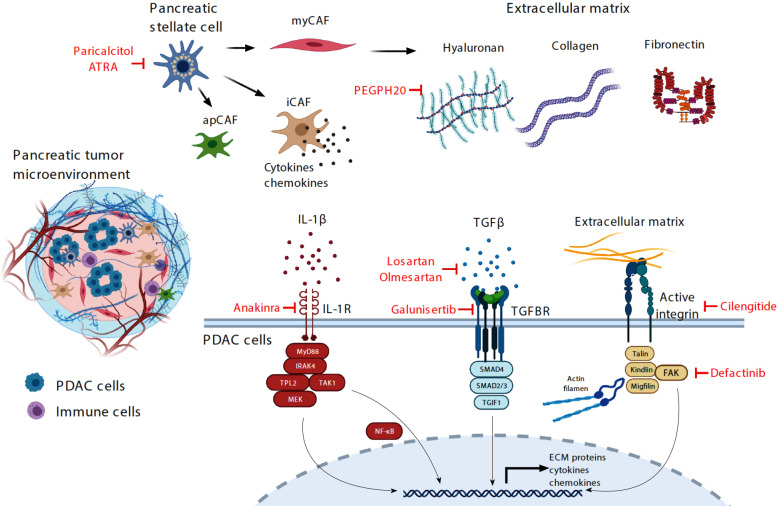
Transcriptomic profiling shows that CAFs consist of at least three distinct subpopulations: Myofibroblast-like (myCAF), inflammatory (iCAF) and antigen-presenting (apCAF, Figure 1)[27,28]. The myCAFs are the most abundant subtype, characterized by high α-SMA expression and hypothesized to have a favorable tumor-restrictive role, potentially explaining paradoxical PDAC progression in genetically engineered mouse models (GEMM) when CAFs are globally depleted[29]. The iCAFs are rich in expression of multiple inflammatory chemokines including interleukin (IL)-6 and proposed to be pro-tumorigenic. The least abundant apCAFs are characterized by abundant expression of MHC class II and therefore may be able to bind and present antigens to CD4+ cytotoxic T-cells. However, due to a lack of co-stimulatory molecules on apCAFs, apCAFs actually dampen, instead of activating the interacting CD4+ T cells[30]. Importantly, it appears that these subtypes are interchangeable, providing an avenue to reprogram these CAFs for therapeutic purposes.
Figure 1.
Illustration of the complex pancreatic ductal adenocarcinoma tumor microenvironment that consists of various cell types and acellular extracellular matrix proteins and proteoglycans. Therapeutic strategies that have recently been tested or are being tested in clinical trials are highlighted in red. PDAC: Pancreatic ductal adenocarcinoma; ATRA: All trans retinoic acid; apCAF: Antigen-presenting cancer-associated fibroblasts; iCAF: Inflammatory cancer-associated fibroblasts; myCAF: Myofibroblast-like cancer-associated fibroblasts; IL: Interleukin; TGF: Transforming growth factor; TGFBR: Transforming growth factor β receptor; PEGPH20: Pegylated PH20; NF-κB: Nuclear factor-κappa β; ECM: Extracellular matrix; FAK: Focal adhesion kinase.
The other predominant cellular components of the pancreatic tumor stroma include endothelial cells, inflammatory cells and nerve cells which have complex interactions with CAFs. Angiogenesis in PDAC is regulated by CAFs, PDAC cells, inflammatory cells and endothelial cells. The hypoxic microenvironment of PDAC acts as a trigger for the transcription factor hypoxia induced factor 1 expression which leads to increased vascular endothelial growth factor (VEGF) production and angiogenesis[31]. VEGF has been identified as an important negative prognostic marker in PDAC which has led to multiple preclinical and clinical studies targeting this pathway[32]. In addition, PDAC stroma is rife with various types of immune cells of myeloid lineages, T cells, B cells that are collectively rendered pro-tumorigenic by various environmental clues[33,34]. Targeting the immune compartment of PDAC, especially the suppressive myeloid cells including macrophages and monocytes, is a rapidly emerging therapeutic strategy, and is outside the scope of this review.
The acellular ECM proteins include collagen I, II, IV, XI-A, fibronectin, laminin, tenascin C and HA. While the dense ECM may physically restrict PDAC progression, ECM proteins have been shown to be protective to PDAC cells. PDAC cells detached from ECM proteins, especially laminin and fibronectin, are prone to apoptosis and necrosis as a result of mitochondrial depolarization and release of cytochrome c and Smac/DIABLO[35]. Various ECM proteins are also capable of promoting EMT and hence therapeutic resistance of PDAC cells[16]. These studies provide solid rationale for targeting these acellular ECM proteins as a therapeutic strategy. The ECM also contains a group of enzymes known as matrix metalloproteinases (MMP), which play a role in degradation of ECM components. The turnover of ECM is a dynamic process and is regulated by tissue MMP inhibitors secreted by stromal cellular structures[36].
Therapies targeting PDAC cells such as chemotherapy, radiation and surgery when used alone or in combination have only provided limited benefit in patients with advanced/metastatic PDAC, rarely improving survival beyond one year. This shifts the focus to the TME which plays a crucial role in inhibiting the effectiveness of cytotoxic therapies, making them less effective by providing a drug free sanctuary enabling them to thrive and proliferate[37]. A number of preclinical studies involving GEMM targeted the tumor stroma showing a beneficial effect toward better drug delivery and overall disease progression. A number of novel therapeutic strategies targeting the complex interaction of various cellular and acellular components of the PDAC microenvironment were first studied in the pre-clinical setting. The promising results of these in-vitro and in-vivo experiments resulted in clinical translation in an effort to alter the tumor micro-environment to improve outcomes of PDAC patients. Herein we review the effectiveness of these therapies and lessons learned from them, which provides guidance towards future treatments.
TARGETING STROMA-PROMOTING PATHWAYS IN PDAC CELLS
Sonic hedgehog pathway
Cancer stem cells (CSCs) are a small population of cancer cells responsible for tumor initiation, recurrence, growth and metastasis. Like normal stem cells, pancreatic CSCs are regulated by several common signaling pathways, one of which is the Sonic hedgehog (SHH) pathway. The SHH signaling pathway is regulated by a few critical nodes: the HH ligand, the Patched (PTCH) transmembrane receptor, the integral smoothened (SMO) protein and the Glioma Associated Oncogene (GLI) transcription factors. In the absence of SHH, PTCH binds and destabilizes SMO, leading to its degradation. Binding of SHH ligand to PTCH results in internalization and lysosomal degradation of both SHH and PTCH, thereby relieving SMO and allowing it to contribute to the activation of the GLI transcription factors. The GLI factors control several genes that participate in developmental patterning and also production of more SHH[38]. Compared to normal pancreatic stem cells, expression of SHH is upregulated in PDAC CSCs[39]. Olive et al[39] showed that targeting SMO with sarigedib (or IPI-926) depletes the stromal tissue and transiently improves the delivery and therapeutic effect of gemcitabine in an orthotopic transplantable mouse model, suggesting that the SHH pathway may contribute to stromal fibrosis and hindrance of therapeutics in PDAC. In January 2012, a phase II trial involving the SHH pathway inhibitor saridegib (IPI-926) was halted due to detrimental outcomes of patients in the gemcitabine plus saridegib arm compared to the gemcitabine plus placebo arm (NCT01130142). This also resulted in early closure of another trial evaluating the efficacy of saridegib with modified FOLFIRINOX (NCT01383538)[40]. Vismodegib (or GDC-0449) is a competitive SMO inhibitor that is FDA-approved for treatment of advanced or metastatic basal cell carcinoma, in which loss-of-function mutations of PTCH1 gene are common. A pilot study involving 25 patients showed downregulation of GLI and PTCH1 expression in patients receiving combination of gemcitabine and vismodegib (NCT01195415)[41]. However, a larger phase IB/II trial evaluating 106 patients showed no improvement in response rate, OS or progression free survival (PFS). Importantly, contrary to the results reported by Olive et al[39] (NCT01064622), vismodegib did not improve intratumoral delivery of gemcitabine, and when combined with gemcitabine also did not statistically improve the survival of autochthonous PDAC mice[42]. A later Phase II trial (NCT01088815) evaluating 67 patients with untreated metastatic PDAC showed that addition of vismodegib to GnP did not improve PFS or OS compared to historical data of chemotherapy alone[43]. Sonidegib is another orally bioavailable SMO receptor antagonist approved for the treatment of recurrent locally advanced basal cell carcinoma[44]. This drug was studied in patients with PDAC in combination with both gemcitabine and GnP. Two small phase I trials showed good tolerance but no improvement in OS or PFS compared to standard chemotherapy (NCT01487785), (NCT02358161)[45,46]. These clinical studies show that targeting the SHH pathway in combination with standard chemotherapy is ineffective in PDAC. Furthermore, these studies underscore the need to obtain on-treatment correlative data to allow investigation into mechanisms of resistance and treatment failure.
Transforming growth factor β pathway
Enhanced intratumoral transforming growth factor β (TGF-β) signaling promotes tumor fibrosis, progression and poor patient survival in PDAC[47,48]. Importantly, more than half of PDAC cases carry inactivating mutations of SMAD4, which encodes a growth-inhibitory transcription factor downstream of the TGF receptor. Unlike other cellular components in the PDAC stroma, pancreatic cancer cells are often deficient in SMAD4. This leads to upregulation of TGF-β ligand and has far reaching effects on other cellular components of PDAC that remain SMAD4 proficient in the stroma. In these cells, TGF-β acts as a driver for desmoplasia. Galunisertib is a small molecule serine/threonine kinase inhibitor of the TGF-β receptor. A phase Ib/II study comparing galunisertib plus gemcitabine vs placebo plus gemcitabine was carried out in patients with advanced PDAC including 104 patients in the treatment arm and 52 patients in the placebo arm. There was a modest survival benefit in the treatment group. The median OS was 8.9 mo (95%CI: 7.3-11.1) for the galunisertib group and 7.1 mo (95%CI: 5.8-9.0) for the placebo group. The overall response rate was numerically superior in the galunisertib group, however, the differences were not statistically significant. No significant difference in PFS was observed in the two groups. Patients in the galunisertib group did not experience increased toxicity (NCT01373164)[49]. This modest but encouraging benefit of TGF-β receptor inhibition has led to further clinical trials evaluating the effect of galunisertib with the anti PD-L1 (programmed death ligand 1) monoclonal antibody durvalumab (NCT02734160). To date, the results of this trial are pending.
The angiotensin II receptor blockers losartan and olmesartan have been evaluated in animal models based on their TGF-β pathway inhibitor effects[50,51]. A retrospective study was carried out at Massachusetts General Hospital evaluating PDAC patients taking or not taking angiotensin pathway inhibitors. These investigators found that patients chronically taking angiotensin pathway inhibitors with non-metastatic PDAC had superior OS independent of chemotherapy[52]. A single arm phase II trial enrolled patients with locally advanced pancreatic cancer (LAPC) and treated with total neoadjuvant therapy of FOLFIRINOX and losartan followed by individualized chemoradiotherapy (NCT01821729). This study showed a high rate of R0 resection (69%) as well as prolonged OS among all patients (31.4 mo) including those who underwent resection (33 mo)[53]. This led to a large multicenter trial using losartan with FOLFIRINOX followed by SBRT and nivolumab in LAPC (NCT03563248). To date, the results of this clinical trial are pending.
Focal adhesion kinase
Enhanced focal adhesion kinase (FAK) activity is commonly found in PDAC cells and CAFs and is a determinant of stromal fibrosis and immune evasion[54,55]. Targeting FAK using small molecule inhibitors dramatically attenuated stromal fibrosis, greatly potentiated chemotherapy and immune checkpoint blockade in several preclinical mouse models including the highly aggressive autochthonous KPC (p48-Cre/p53f/f/LSL-KRASG12D) mouse model[54]. These findings provided a solid rationale for combining a FAK inhibitor (defactinib) with gemcitabine and anti-PD-1 (programmed death 1) in a clinical trial of patients with PDAC after progression on frontline 5FU-based chemotherapy (NCT02546531). Results of this study are pending.
IL-1 receptor pathway
Constitutive activation of the canonical nuclear factor-κappa β (NF-kB) pathway is a major mechanism that contributes to stromal fibrosis, chemoresistance and poor prognosis in PDAC[56]. In PDAC, activation of the canonical NF-kB cascade is driven both by KRAS-MAPK cascades and reciprocal IL-1β signaling, which drives IRAK4-TPL2 and IKK kinases[56-58]. Targeting IKK kinases has proven to be clinically challenging due to lack of safe and effective agents, but other strategies are being developed to target this pathway. For instance, targeting IRAK4 using small molecule kinase inhibitors was shown to reduce stromal fibrosis and potentiate the efficacy of chemotherapy in preclinical mouse models[56,58]. Currently, the IL-1 receptor antagonist Anakinra is being tested in combination with nab-paclitaxel, gemcitabine and cisplatin in patients with resectable or potentially resectable PDAC (NCT02550327). To date, the results for this clinical trial are pending.
Connective tissue growth factor
The PDAC TME is rife with a myriad of pro-tumorigenic and pro-fibrotic growth factors. One of these is connective tissue growth factor (CTGF). Interestingly, in preclinical models, treatment with pamrevlumab (or FG-3019), a humanized monoclonal antibody targeting CTGF, potentiates the effect of gemcitabine by downregulating X-linked inhibitor of apoptosis protein, rather than promoting delivery of gemcitabine[59]. In other PDAC pre-clinical studies pamrevlumab was shown to attenuate tumor growth, metastasis and angiogenesis[60]. These studies collectively suggest that the therapeutic effect of pamrevlumab is predominantly through targeting tumor cells. A phase I/II study evaluated pamrevlumab with gemcitabine and nab-paclitaxel in patients with LAPC. After 6 cycles/months of therapy, more patients treated with pamrevlumab and chemotherapy underwent resection compared to patients receiving chemotherapy only (33.3% vs 7.7%). The higher resection rate translated into improved OS (non-estimable vs 18.56 mo, P = 0.0141). However, it was unclear whether the higher resectability among patients treated with pamrevlumab and chemotherapy was due to higher response rates or lower incidence of disease progression during the six cycles of treatment. Importantly, addition of pamrevlumab did not increase perioperative adverse events or delay in surgical wound healing[61]. A phase III trial is currently underway evaluating the safety and efficacy of pamrevlumab in combination with gemcitabine and nab-paclitaxel for patients with locally advanced PDAC (NCT03941093).
TARGETING STROMA-PROMOTING PATHWAYS IN CAFs
Vitamin D receptor
The role of vitamin D in the risk of developing pancreatic cancer is highly controversial, with studies showing high serum vitamin D levels to be protective, detrimental or have no impact on pancreatic cancer development[62-64]. However, the role of vitamin D repletion in PDAC patients after initial diagnosis and during treatment is actively being pursued in clinical studies. This is based on observational studies showing that higher pre-diagnostic serum vitamin D levels were shown to be associated with better survival in PDAC patients[65], and overwhelming preclinical studies demonstrating protective effects of vitamin D. Specifically, in mouse models ligation of vitamin D receptor with the vitamin D receptor ligand calcipotriol markedly impeded PSC activation, leading to stromal remodeling that augmented intratumoral gemcitabine, reduced tumor volume and prolonged the survival of KPC mice by 57% compared to mice treated with gemcitabine alone[66]. However, it is critical to emphasize that although calcipotriol impedes activation of PSCs, it fails to block a-SMA expression or collagen I production of fully activated PSCs[67], raising the concern for the modest efficacy of stromal effect of vitamin D receptor ligands. However, vitamin D receptor ligands could have tumor-intrinsic effects. In PDAC cells, calcipotriol lowered the expression of low-density lipoprotein receptor-related protein 6 and inhibits autocrine Wnt signaling[68]. Paricalcitol was also shown to impede PDAC cell proliferation by upregulating cell cycle inhibitors p21 (Waf1/CIP1) and p27 (Kip1)[69]. On these premises, multiple phase I or II studies testing the impact of paricalcitol are currently opened. These include in combination with cisplatin, gemcitabine and nab-paclitaxel for patients with treatment-naïve metastatic PDAC (NCT04054362); in combination with hydroxychloroquine, gemcitabine and nab-paclitaxel for patients with treatment-naïve metastatic PDAC (NCT04524702); in combination with 5-FU/Liposomal irinotecan for patients who have progressed through frontline gemcitabine-based therapies (NCT03883919); and in combination with anti-PD-1 (pembrolizumab) as maintenance treatment for patients who have achieved partial response or stable disease for at least two months on chemotherapy (NCT03331562).
All-trans retinoic acid
There is an association with lower levels of fat-soluble vitamin A and risk of pancreatic cancer, theorized to be due to impaired absorption of fat-soluble vitamins[70]. Preclinical data has shown that vitamin A deficiency leads to activation of PSC, while repletion of vitamin A in culture media converts the PSCs from an activated to a quiescent state. Using the KPC model of human PDAC, treatment with all-trans retinoic acid (ATRA) induced quiescence of PSC, reduced proliferation of PDAC cells and led to increased apoptosis of PDAC cells in part by down-regulating Wnt signaling[71]. Based on this data, a phase I clinical trial was conducted enrolling 27 patients with unresectable PDAC, treated with GnP in combination with ATRA using the established dose for acute promyelocytic leukemia. This study demonstrated the safety and tolerability of the regimen, and diffusion weighted-magnetic resonance imaging identified signals of stromal modulation. This has led to the possibility of repurposing ATRA as a stromal targeting agent in PDAC. Based on these data, the combination of ATRA along with GnP is currently being studied in the Phase II randomized STAR_PAC trial (NCT03307148) enrolling patients with locally advanced or metastatic disease[72].
Other agents targeting PSCs
Pirfenidone is an anti-inflammatory and anti-fibrotic agent that is clinically used for treatment of idiopathic pulmonary fibrosis, however with an unknown mechanism of action. In primary human lung fibroblasts, pirfenidone inhibits proliferation, TGF-β-induced myofibroblast differentiation and pro-collagen expression[73]. Pirfenidone also blocks proliferation, production of collagen, fibronectin and periostin by PSCs in vitro and in vivo, and potentiates the anti-tumor effect of gemcitabine by reducing stromal fibrosis[74].
Halofunginone (HF) is another anti-fibrotic drug that is of interest in pre-clinical studies. The exact mechanism of action of this agent is unclear however it has shown to cause resolution of pathologic liver fibrosis. In animal models, HF works by inhibiting the activation of PSC’s which in-turn decreases the deposition of ECM proteins such as collagen and HA. HF inhibits the downstream signaling of TGF-β by inhibiting SMAD2 and SMAD3 receptors. HF not only improves drug delivery by decreasing fibrosis, but it also fosters favorable immune response by augmenting cytotoxic T-cells and stimulatory myeloid cells in the tumor stroma[75]. To date, no clinical trials are opened yet incorporating these two agents for PDAC patients.
TARGETING ECM
HA
HA is a high molecular weight glycosaminoglycan that is synthesized by HA synthases (HAS1, HAS2, and HAS3) in PDAC cells and CAFs and deposited into the ECM framework[76]. Intratumoral HA undergoes constant turnover via degradation by hyaluronidases (HYAL1-4, HYALP1, and PH20). Excessive HA deposition, which is common in PDAC tumors, causes elevated water retention and consequently high interstitial pressure that collapses tumor vasculature and limits delivery of therapeutics[19]. High expression of HA in tumors has been associated with shorter survival post-surgical resection in patients with PDAC[77]. Importantly, aside from being an important structural component of the ECM, HA has an active signaling function. CD44 is a well-established cellular receptor for HA. Engagement of CD44 on PDAC cells enhances invasion, metastasis, angiogenesis and survival[78]. In PDAC mouse models, addition of hyaluronidase such as pegylated PH20 (PEGPH20) reduces intratumoral HA content and interstitial pressure, thereby permitting re-expansion of the microvasculature. In mouse models, this results in improved delivery of chemotherapy into the PDAC TME and prolongation of survival[19,79].
The above promising preclinical data led to incorporation of PEGPH20 with gemcitabine in a phase Ib clinical trial. Patients with high intratumoral HA content had improved PFS (7.2 mo vs 3.5 mo) and OS (13 mo vs 5.7 mo) compared to patients with low HA[80]. This encouraging data led to a larger randomized placebo-controlled phase II study (HALO 202) comparing gemcitabine/nab-paclitaxel plus either PEGPH20 (PAG arm) or placebo (AG arm). Again, patients with high HA level (defined as HA staining of > 50% of tumor surface at any intensity) had improved PFS [9.2 mo vs 5.2 mo, hazard ratio (HR), 0.51; 95%CI: 0.26-1.00; P = 0.048] and OS (11.5 mo vs 8.5 mo, HR, 0.96; 95%CI: 0.57-1.61) compared to patients with low HA tumors[81]. Unfortunately, these promising results failed to be recapitulated in a subsequent larger randomized phase III study (HALO 109-301). In this study, only patients with high HA tumors were enrolled, and 492 patients were included in intention-to-treat analysis. Median OS for PAG vs AG was 11.2 mo vs 11.5 mo (HR, 1.00, 95%CI: 0.80-1.27; P = 0.97); median PFS was 7.1 vs 7.1 mo (HR, 0.97, 95%CI: 0.75-1.26); confirmed ORR was 34% vs 27% (NCT02715804)[82]. In this study, all patients treated with PEGH20 were anticoagulated with low molecular weight heparin to prevent venous thromboembolism. In another phase Ib/II study (SWOG S1313), PEGPH20 was tested in combination with modified FOLFIRINOX vs modified FOLFIRINOX alone for treatment naïve PDAC patients. This study had to be halted after interim futility analysis showing inferior outcomes in the PEGPH20 group. Median OS and PFS was strikingly inferior in the PEGPH20 group with a HR of 2.07 (7.7 mo vs 14.4 mo, 95%CI: 1.28-3.34, P < 0.01) and 1.74 (4.3 mo vs 6.2 mo, 95%CI: 1.14-2.66, P = 0.01). ORR in PEGPH20 group was also lower, although this difference did not reach statistical significance (33% vs 45%, P = 0.2) (NCT01959139)[83]. A critical factor leading to the poor outcome of PEGPH20 group was the unexpectedly increased gastrointestinal toxicity and thromboembolic events, which likely resulted in dose reduction of chemotherapy or treatment interruptions[84]. These setbacks led to the cessation of further development of PEGPH20 in cancer clinical trials.
ECM-remodeling enzyme lysyl oxidase-like 2
The ECM-remodeling enzyme lysyl oxidase-like 2 (LOXL2) is a secreted enzyme that maintains the stromal microenvironment in PDAC. Preclinical data identified LOXL2 to be upregulated in cell lines with high invasive potential, and furthermore in human tissue, elevated LOXL2 expression correlates with greater depth of tumor invasion, lymph node involvement, and inferior OS[85]. Simtuzumab, an immunoglobulin G4 monoclonal antibody against LOXL2, was studied in a phase II randomized double-blind placebo-controlled study in combination with gemcitabine in metastatic PDAC patients (NCT01472198). The 240 patients were divided into three groups and received simtuzumab 700 mg, simtuzumab 200 mg or placebo along with gemcitabine. Unfortunately, despite encouraging preclinical and correlative clinical data, when prospectively evaluated, the addition of simtuzumab to gemcitabine did not prolong OS in either cohort of patients with metastatic PDAC compared to gemcitabine alone[86].
Integrins
Integrins are cell surface receptors that mediate surface adhesion of various components of ECM including fibronectin, laminin, collagen and fibrinogen[87]. They play an important role in tumor angiogenesis and lymphangiogenesis, and hence are attractive therapeutic targets[88]. Cilengitide is a low molecular weight anti-angiogenic molecule that acts by inhibiting the integrin surface receptors of endothelial cells[89]. A randomized multi-center phase II trial enrolled patients with unresectable PDAC and treated them with cilengitide and gemcitabine vs gemcitabine alone. There were no signs of efficacy, including a median OS of 6.7 mo for those receiving cilengitide and gemcitabine vs 7.7 mo for those receiving gemcitabine alone (ISRCTN13413322)[90].
Drugs targeting MMPs
The MMPs are proteolytic enzymes that play an important role in remodeling of ECM proteins and modifying the tumor stroma in favor of tumor proliferation, invasion and metastasis. MMP inhibitors have been evaluated in multiple solid tumors based on their fundamental role in modulating tumor stroma, with encouraging preclinical data[91]. However, clinical outcomes have been disappointing in PDAC. Specifically, the MMP inhibitors marimastat and BAY12-9566 were studied in comparison to gemcitabine as first line therapy in advanced PDAC patients. These studies both demonstrated superior outcomes with gemcitabine compared to the MMP inhibitors[92,93].
LESSONS AND PROSPECTS
The histologic predominance and tumor-modulating role of stroma continues to propel preclinical and clinical research into stromal-targeting strategies for PDAC (Figure 1). However, clinical success remains limited (Table 1). Until the present time, clinical progress in PDAC has been the result of increasingly aggressive combination chemotherapies, demonstrating that targeting PDAC cells with cytotoxic agents remains a viable strategy, although not ideal, as it is no longer clinically feasible to add further cytotoxic agents to current regimens considering treatment-limiting toxicity. For this reason, stroma-targeting approaches are gaining significant attention. The excitement surrounding stromal targeting is well deserved, given that in preclinical models these approaches permit effective immunotherapy and durable tumor control. As described above, these mechanisms include re-activating T cell and myeloid compartments with or without directly altering the physical immunosuppressive stroma. In contrast to many other solid tumors, clinical efficacy has not yet been realized in PDAC. Studies to date have taught us that PDAC is a very unique tumor type, such that findings in other solid tumors cannot be extrapolated to PDAC. Thus, a more in depth understanding of the different cellular and acellular components of the pancreatic stroma is needed, in order for subsets of cellular or acellular components to be targeted. We have learned that oversimplified preclinical models, especially the widely used GEMM such as KPC mice, which consists of merely two mutations (KRAS and TP53), are clearly inadequate in PDAC, with findings that do not faithfully translate to patients.
Table 1.
Summary of stromal-targeting clinical trials in pancreatic cancer
| Drug | Mechanism of action | Backbone therapy | Outcome |
| Targeting stroma-promoting pathways in PDAC cells | |||
| Saridegib (IPI-926) | SHH pathway inhibitor | Gemcitabine; mFOLFIRINOX | No improvement in OS or PFS (NCT01130142); No improvement in OS or PFS (NCT01383538) |
| Vismodegib | SHH pathway inhibitor | Gemcitabine; Gemcitabine + nab-paclitaxel | No improvement in OS or PFS (NCT01064622); No improvement in OS or PFS (NCT01088815) |
| Sonidegib | SHH pathway inhibitor (SMO receptor antagonist) | Gemcitabine; Gemcitabine + nab-Paclitaxel | No improvement in OS or PFS (NCT01487785); No improvement in OS or PFS (NCT02358161) |
| Galunisertib | TGF-β receptor inhibitor | Gemcitabine; Gemcitabine + Durvalumab | Ongoing trial (NCT01373164); Ongoing trial (NCT02734160) |
| Losartan | TGF-β ligand inhibitor | FOLFRINOX; Nivolumab + FOLFRINOX | Ongoing trial (NCT01821729); Ongoing trial (NCT03563248) |
| Defactinib | Focal Adhesion Kinase inhibitor | Gemcitabine + Pembrolizumab | Ongoing trial (NCT02546531) |
| Anakinra | IL-1 receptor inhibitor | GnP + Cisplatin | Ongoing trial (NCT02550327) |
| Targeting stroma-promoting pathways in CAFs | |||
| Pamrevlumab | Antibody against CTGF | Gemcitabine + nab-paclitaxel | Ongoing trial (NCT03941093) |
| Paricalcitol | Vitamin D agonist; Inactivation of PSC | GnP + Cisplatin; GnP + Hydroxychloroquine; 5FU/Leucovorin/liposomal Irinotecan; Pembrolizumab (2nd line) | Ongoing trial (NCT04054362); Ongoing trial (NCT04524702); Ongoing trial (NCT03883919); Ongoing trial (NCT03331562) |
| ATRA | Inactivation of PSC | Gemcitabine + nab-paclitaxel | Ongoing trial (NCT03307148) |
| Targeting ECM | |||
| PEGPH20 | Hyaluronan degradation | Gemcitabine + nab-paclitaxel; mFOLFIRINOX | No improvement in OS or PFS (NCT02715804); Poor OS due to poor tolerance (NCT01959139) |
| Simtuzumab | Antibody against LOXL2 | Gemcitabine | No improvement in OS (NCT01472198) |
| Cilengitide | Integrin inhibitor | Gemcitabine | No improvement in OS (ISRCTN13413322) |
| Marimastat | MMP inhibitor | Gemcitabine | No improvement in OS |
| Bay-12-9566 | MMP inhibitor | Gemcitabine | No improvement in OS |
| Others | |||
| Halofunginone | PSC/CAF and SMAD2,3 inhibitor | - | Positive pre-clinical outcomes |
| Pirfenidone | Cell cycle inhibitor of CAF | - | Positive pre-clinical outcomes |
SHH: Sonic hedgehog; OS: Overall survival; PFS: Progression free survival; SMO: Smoothened; TGF: Transforming growth factor; IL: Interleukin; GnP: Gemcitabine plus nab-paclitaxel; CTGF: Connective tissue growth factor; CAF: Cancer-associated fibroblasts; PSC: Pancreatic stellate cell; ECM: Extracellular matrix; LOXL2: Lysyl oxidase-like 2; MMP: Matrix metalloproteinases.
Numerous challenges remain ahead in pancreatic cancer. In the design of future clinical trials, several factors should be taken into consideration. First, the robustness of preclinical data needs to be evaluated carefully before proceeding into the clinical setting. The most pertinent questions to ask are, what type of models were used (patient-derived xenograft, organoid, GEMM)? How many different models were studied to confirm results? How predictive is the current model, in light of prior investigations? What endpoints were considered as significant and meaningful anti-tumor activities (survival, tumor shrinkage)? How was suppressed tumor growth or tumor shrinkage in the animal models defined? Was any synergistic effect seen between the agent of interest and cytotoxic agents? How dramatic is the effect that was seen preclinically? Given that the effect seen in patients is nearly always more modest than that seen in preclinical studies, only those combinations with profound preclinical efficacy, rather than those that meet statistically significant P values, should be advanced into the clinic. Despite all of these considerations, the apparent discrepancies between preclinical and clinical success in PDAC research should remind us of the fact that none of the current experimental models are by themselves adequate. Multiple models, both human and mouse-based, must be tested, and ultimately better preclinical models need to be developed.
The phase I trial is a great opportunity for pharmacokinetic and pharmacodynamic (PD) investigation; however, a recent trend has arisen that shifts the focus of the phase I trial to identifying an early efficacy signal, such that subsequent investigations can directly move into the phase III setting. Tissue biopsy collection is essential for PDAC trials for two important reasons. First, rigorous collection of tissues or surrogate biospecimens for the purpose of in-human verification of PD target effects is critical. This will verify in vivo on-target activity of the agent being evaluated, which is necessary to confirm relative dose sufficiency and appropriate frame treatment failures. Second, tissue collection will allow an initial assessment of in vivo mechanisms of resistance and correlative analyses. Finally, every effort should be made to enroll PDAC patients in appropriate clinical trials to allow patient access to the most advanced therapeutics, optimizing their outcome to the greatest extent possible. Recent failed clinical trials based on impressive preclinical data have provided us with an undesired but valuable opportunity to reexamine the challenges of PDAC and re-iterate the importance of more rigorously designed and scientifically-based clinical investigations. In summary, much progress has been made in recent years understanding the pancreatic tumor stroma, however, lack of subsequent clinical success is evidence that much work remains to be done.
CONCLUSION
The poor response of PDAC to standard cytotoxic regimens has directed attention towards the dense fibrous stroma. The stroma is thought to be a fortress that protects PDAC cells from immune invasion, leads to chemotherapeutic resistance, and thereby provides a sanctuary for these cells to proliferate[16]. There are multiple pre-clinical and in vitro studies wherein stroma depletion led to decreased progression and improved survival among animal models. Based on these a variety of novel approaches have been adapted in human trials for clinical translation[35,37,79]. Some of these approaches were initially promising, however, most if not all have led to negative outcomes, financial waste and frustration in the clinic. This has made stromal targeting in PDAC a much controversial subject. It has been established time and again that indiscriminate targeting and near complete depletion of tumor stroma can cause more harm than good[40,94]. However, these attempts have enhanced our understanding of the tumor micro-environment. There is increased need for caution when targeting these matrix components as we have discovered cellular and acellular components of the stroma that in fact restrain tumor growth and progression[95].
A better understanding of plasticity of the stroma will lead to development of therapeutics that can accurately modulate the tumor micro-environment in favor of tumor suppression. The discovery of heterogeneity among CAF and their paradoxical role in tumor growth further delineates the importance of development of targeted therapies that downregulate subsets of CAF (such as iCAF or possibly apCAF) by selectively modifying the stroma[27,30]. Furthermore, vigorous evaluation of pre-clinical data, comparison of its effectiveness in multiple models and assessment of synergistic response of these novel therapeutics with existing cytotoxic therapy in both human and mouse models is vital to avoid detrimental clinical outcomes. There is a need for development of more standardized pre-clinical models and critical analysis of the data in relation to tumor response in these models before we can translate preclinical findings into clinical success. Such agents once developed may synergistically improve the efficacy of currently available cytotoxic and immune modulating therapies.
ACKNOWLEDGEMENTS
We thank the researchers whose related work was not cited in this review.
Footnotes
Conflict-of-interest statement: The authors have declared no conflict of interests.
Manuscript source: Invited manuscript
Peer-review started: January 8, 2021
First decision: February 23, 2021
Article in press: April 21, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen I, Yamada T S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
Contributor Information
Faran Polani, Division of Oncology, Department of Internal Medicine, Barnes-Jewish Hospital and The Alvin J. Siteman Comprehensive Cancer Center, Washington University School of Medicine, Saint Louis, MO 63110, United States.
Patrick M Grierson, Division of Oncology, Department of Internal Medicine, Barnes-Jewish Hospital and The Alvin J. Siteman Comprehensive Cancer Center, Washington University School of Medicine, Saint Louis, MO 63110, United States.
Kian-Huat Lim, Division of Oncology, Department of Internal Medicine, Barnes-Jewish Hospital and The Alvin J. Siteman Comprehensive Cancer Center, Washington University School of Medicine, Saint Louis, MO 63110, United States. klim@dom.wustl.edu.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Hasan S, Jacob R, Manne U, Paluri R. Advances in pancreatic cancer biomarkers. Oncol Rev. 2019;13:410. doi: 10.4081/oncol.2019.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 6.Park H, Jin RU, Wang-Gillam A, Suresh R, Rigden C, Amin M, Tan BR, Pedersen KS, Lim KH, Trikalinos NA, Acharya A, Copsey ML, Navo KA, Morton AE, Gao F, Lockhart AC. FOLFIRINOX for the Treatment of Advanced Gastroesophageal Cancers: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020;6:1231–1240. doi: 10.1001/jamaoncol.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, Overman M, Pant S, Javle M, Koay EJ, Herman J, Kim M, Ikoma N, Tzeng CW, Lee JE, Katz MHG. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020;155:832–839. doi: 10.1001/jamasurg.2020.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 12.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11:734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulle A, Lim KH. Beyond just a tight fortress: contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct Target Ther. 2020;5:249. doi: 10.1038/s41392-020-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong X, Li L, Li Z, Xie K. Targeted destruction of the orchestration of the pancreatic stroma and tumor cells in pancreatic cancer cases: molecular basis for therapeutic implications. Cytokine Growth Factor Rev. 2012;23:343–356. doi: 10.1016/j.cytogfr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 19.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Li J, Chen X, Duan W, Ma Q, Li X. Disrupting the balance between tumor epithelia and stroma is a possible therapeutic approach for pancreatic cancer. Med Sci Monit. 2014;20:2002–2006. doi: 10.12659/MSM.892523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 25.DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, Thompson CB, Connor RJ, Thanos CD, Scott Brockenbrough J, Provenzano PP, Frost GI, Michael Shepard H, Hingorani SR. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys J. 2016;110:2106–2119. doi: 10.1016/j.bpj.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuFort CC, DelGiorno KE, Hingorani SR. Mounting Pressure in the Microenvironment: Fluids, Solids, and Cells in Pancreatic Ductal Adenocarcinoma. Gastroenterology 2016; 150: 1545-1557. :e2. doi: 10.1053/j.gastro.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res . 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djurec M, Graña O, Lee A, Troulé K, Espinet E, Cabras L, Navas C, Blasco MT, Martín-Díaz L, Burdiel M, Li J, Liu Z, Vallespinós M, Sanchez-Bueno F, Sprick MR, Trumpp A, Sainz B Jr, Al-Shahrour F, Rabadan R, Guerra C, Barbacid M. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci USA . 2018;115:E1147–E1156. doi: 10.1073/pnas.1717802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahab ZA, Metzgar RS. Human cytotoxic lymphocytes reactive with pancreatic adenocarcinoma cells. Pancreas. 1991;6:307–317. doi: 10.1097/00006676-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SK, Lee J, Beatty GL. Paracrine and cell autonomous signalling in pancreatic cancer progression and metastasis. EBioMedicine. 2020;53:102662. doi: 10.1016/j.ebiom.2020.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 36.Stetler-Stevenson WG, Liotta LA, Kleiner DE Jr. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 37.Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: The microenvironment needs attention too! Pancreatology. 2015;15:S32–S38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Sari IN, Phi LTH, Jun N, Wijaya YT, Lee S, Kwon HY. Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells. 2018;7 doi: 10.3390/cells7110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, Venook AP, Kindler HL. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016;45:370–375. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, Zalupski MM, Simeone DM. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Jesus-Acosta A, Sugar EA, O'Dwyer PJ, Ramanathan RK, Von Hoff DD, Rasheed Z, Zheng L, Begum A, Anders R, Maitra A, McAllister F, Rajeshkumar NV, Yabuuchi S, de Wilde RF, Batukbhai B, Sahin I, Laheru DA. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br J Cancer. 2020;122:498–505. doi: 10.1038/s41416-019-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burness CB. Sonidegib: First Global Approval. Drugs. 2015;75:1559–1566. doi: 10.1007/s40265-015-0458-y. [DOI] [PubMed] [Google Scholar]
- 45.Macarulla T, Tabernero J, Palmer DH, Sharma S, Yu KH, Sellami DB, Zhou J, Yi W, Boss H, Kwak EL. A phase Ib dose escalation, safety, and tolerability study of sonidegib in combination with gemcitabine in patients with locally advanced or metastatic pancreatic adenocarcinoma. J Clin Oncol . 2016;34:371–371. [Google Scholar]
- 46.Lee K, Molenaar RJ, Klaassen R, Bijlsma MF, Weterman MJ, Richel DJ, Wymenga M, van Laarhoven HWM, Wilmink JW. A Phase I study of LDE225 in combination with gemcitabine and nab-paclitaxel in patients with metastasized pancreatic cancer. Ann Oncol . 2017;28:v260. [Google Scholar]
- 47.Principe DR, DeCant B, Mascariñas E, Wayne EA, Diaz AM, Akagi N, Hwang R, Pasche B, Dawson DW, Fang D, Bentrem DJ, Munshi HG, Jung B, Grippo PJ. TGFβ Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016;76:2525–2539. doi: 10.1158/0008-5472.CAN-15-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friess H, Yamanaka Y, Büchler M, Ebert M, Beger HG, Gold LI, Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 49.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Smith C, Estrem ST, Gueorguieva I, Lahn MMF, Blunt A, Benhadji KA, Tabernero J. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119:1208–1214. doi: 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masamune A, Hamada S, Kikuta K, Takikawa T, Miura S, Nakano E, Shimosegawa T. The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand J Gastroenterol. 2013;48:602–609. doi: 10.3109/00365521.2013.777776. [DOI] [PubMed] [Google Scholar]
- 51.Arnold SA, Rivera LB, Carbon JG, Toombs JE, Chang CL, Bradshaw AD, Brekken RA. Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation. PLoS One. 2012;7:e31384. doi: 10.1371/journal.pone.0031384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Naxerova K, Pinter M, Incio J, Lee H, Shigeta K, Ho WW, Crain JA, Jacobson A, Michelakos T, Dias-Santos D, Zanconato A, Hong TS, Clark JW, Murphy JE, Ryan DP, Deshpande V, Lillemoe KD, Fernandez-Del Castillo C, Downes M, Evans RM, Michaelson J, Ferrone CR, Boucher Y, Jain RK. Use of Angiotensin System Inhibitors Is Associated with Immune Activation and Longer Survival in Nonmetastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2017;23:5959–5969. doi: 10.1158/1078-0432.CCR-17-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, Ferrone CR, Parikh AR, Weekes CD, Nipp RD, Kwak EL, Allen JN, Corcoran RB, Ting DT, Faris JE, Zhu AX, Goyal L, Berger DL, Qadan M, Lillemoe KD, Talele N, Jain RK, DeLaney TF, Duda DG, Boucher Y, Fernández-Del Castillo C, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaghdoudi S, Decaup E, Belhabib I, Samain R, Cassant-Sourdy S, Rochotte J, Brunel A, Schlaepfer D, Cros J, Neuzillet C, Strehaiano M, Alard A, Tomasini R, Rajeeve V, Perraud A, Mathonnet M, Pearce OM, Martineau Y, Pyronnet S, Bousquet C, Jean C. FAK activity in cancer-associated fibroblasts is a prognostic marker and a druggable key metastatic player in pancreatic cancer. EMBO Mol Med. 2020;12:e12010. doi: 10.15252/emmm.202012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D, Li L, Jiang H, Knolhoff BL, Lockhart AC, Wang-Gillam A, DeNardo DG, Ruzinova MB, Lim KH. Constitutive IRAK4 Activation Underlies Poor Prognosis and Chemoresistance in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2017;23:1748–1759. doi: 10.1158/1078-0432.CCR-16-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodhiawala PB, Khurana N, Zhang D, Cheng Y, Li L, Wei Q, Seehra K, Jiang H, Grierson PM, Wang-Gillam A, Lim KH. TPL2 enforces RAS-induced inflammatory signaling and is activated by point mutations. J Clin Invest. 2020;130:4771–4790. doi: 10.1172/JCI137660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D, Li L, Jiang H, Li Q, Wang-Gillam A, Yu J, Head R, Liu J, Ruzinova MB, Lim KH. Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018;78:1700–1712. doi: 10.1158/0008-5472.CAN-17-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 61.Picozzi VJ, Pishvaian MJ, Mody K, Winter JM, Glaspy JA, Larson T, Matrana MR, Saikali K, Carney M, Porter S, Yu P, Kouchakji E, Carrier E. Effect of anti-CTGF human recombinant monoclonal antibody pamrevlumab on resectability and resection rate when combined with gemcitabine/nab-paclitaxel in phase 1/2 clinical study for the treatment of locally advanced pancreatic cancer patients. J Clin Oncol . 2018;36:4016–4016. [Google Scholar]
- 62.van Duijnhoven FJB, Jenab M, Hveem K, Siersema PD, Fedirko V, Duell EJ, Kampman E, Halfweeg A, van Kranen HJ, van den Ouweland JMW, Weiderpass E, Murphy N, Langhammer A, Ness-Jensen E, Olsen A, Tjønneland A, Overvad K, Cadeau C, Kvaskoff M, Boutron-Ruault MC, Katzke VA, Kühn T, Boeing H, Trichopoulou A, Kotanidou A, Kritikou M, Palli D, Agnoli C, Tumino R, Panico S, Matullo G, Peeters P, Brustad M, Olsen KS, Lasheras C, Obón-Santacana M, Sánchez MJ, Dorronsoro M, Chirlaque MD, Barricarte A, Manjer J, Almquist M, Renström F, Ye W, Wareham N, Khaw KT, Bradbury KE, Freisling H, Aune D, Norat T, Riboli E, Bueno-de-Mesquita HBA. Circulating concentrations of vitamin D in relation to pancreatic cancer risk in European populations. Int J Cancer. 2018;142:1189–1201. doi: 10.1002/ijc.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterhouse M, Risch HA, Bosetti C, Anderson KE, Petersen GM, Bamlet WR, Cotterchio M, Cleary SP, Ibiebele TI, La Vecchia C, Skinner HG, Strayer L, Bracci PM, Maisonneuve P, Bueno-de-Mesquita HB, Zaton Ski W, Lu L, Yu H, Janik-Koncewicz K, Polesel J, Serraino D, Neale RE Pancreatic Cancer Case–Control Consortium (PanC4) Vitamin D and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2015;26:1776–1783. doi: 10.1093/annonc/mdv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res. 2012;72:1190–1198. doi: 10.1158/0008-5472.CAN-11-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan C, Qian ZR, Babic A, Morales-Oyarvide V, Rubinson DA, Kraft P, Ng K, Bao Y, Giovannucci EL, Ogino S, Stampfer MJ, Gaziano JM, Sesso HD, Buring JE, Cochrane BB, Chlebowski RT, Snetselaar LG, Manson JE, Fuchs CS, Wolpin BM. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J Clin Oncol. 2016;34:2899–2905. doi: 10.1200/JCO.2015.66.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O'Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallbaum P, Rohde S, Ehlers L, Lange F, Hohn A, Bergner C, Schwarzenböck SM, Krause BJ, Jaster R. Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J Gastroenterol. 2018;24:170–178. doi: 10.3748/wjg.v24.i2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arensman MD, Nguyen P, Kershaw KM, Lay AR, Ostertag-Hill CA, Sherman MH, Downes M, Liddle C, Evans RM, Dawson DW. Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer. Mol Cancer Res. 2015;13:1509–1519. doi: 10.1158/1541-7786.MCR-15-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz GG, Eads D, Naczki C, Northrup S, Chen T, Koumenis C. 19-nor-1 alpha,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther. 2008;7:430–436. doi: 10.4161/cbt.7.3.5418. [DOI] [PubMed] [Google Scholar]
- 70.Huang X, Gao Y, Zhi X, Ta N, Jiang H, Zheng J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci Rep. 2016;6:38936. doi: 10.1038/srep38936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011; 141: 1486-1497, 1497.e1-1497. 14 doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 72.Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, deSouza NM, De Paepe KN, Goulart MR, Hughes C, Imrali A, Roberts R, Pawula M, Houghton R, Lawrence C, Yogeswaran Y, Mousa K, Coetzee C, Sasieni P, Prendergast A, Propper DJ. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11:4841. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–2356. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 75.Elahi-Gedwillo KY, Carlson M, Zettervall J, Provenzano PP. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019;79:372–386. doi: 10.1158/0008-5472.CAN-18-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 77.Cheng XB, Sato N, Kohi S, Yamaguchi K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS One. 2013;8:e80765. doi: 10.1371/journal.pone.0080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22:2848–2854. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 82.Tempero MA, Cutsem EV, Sigal D, Oh D-Y, Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, Li C-P, Fouchardiere CDL, Heinemann V, Maraveyas A, Bahary N, Layos L, Sahai V, Zheng L, Lacy J, Bullock AJ, Investigators H- HALO 109-301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20) + nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA) J Clin Oncol . 2020;38:638–638. doi: 10.1200/JCO.20.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, Mehan PT, Gaur R, Seery T, Guthrie KA, Hochster HS. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062–1069. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang-Gillam A. Targeting Stroma: A Tale of Caution. J Clin Oncol. 2019;37:1041–1043. doi: 10.1200/JCO.19.00056. [DOI] [PubMed] [Google Scholar]
- 85.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 86.Benson AB 3rd, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, Kudrik F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist. 2017;22:241–e15. doi: 10.1634/theoncologist.2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brooks PC. Role of integrins in angiogenesis. Eur J Cancer. 1996;32A:2423–2429. doi: 10.1016/s0959-8049(96)00381-4. [DOI] [PubMed] [Google Scholar]
- 88.Garmy-Susini B, Varner JA. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res Biol. 2008;6:155–163. doi: 10.1089/lrb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Böttcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39:917–926. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 90.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, Hossfeld DK, Stöger H, Neyns B, Herzog P, Piedbois P, Dobrowolski F, Scheithauer W, Hawkins R, Katz F, Balcke P, Vermorken J, van Belle S, Davidson N, Esteve AA, Castellano D, Kleeff J, Tempia-Caliera AA, Kovar A, Nippgen J. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther. 2018;17:1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B, Tu D, Ottaway J, Humphrey R, Seymour L National Cancer Institute of Canada Clinical Trials Group. Comparison of gemcitabine vs the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 93.Evans JD, Stark A, Johnson CD, Daniel F, Carmichael J, Buckels J, Imrie CW, Brown P, Neoptolemos JP. A phase II trial of marimastat in advanced pancreatic cancer. Br J Cancer. 2001;85:1865–1870. doi: 10.1054/bjoc.2001.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Mackelenbergh MG, Stroes CI, Spijker R, van Eijck CHJ, Wilmink JW, Bijlsma MF, van Laarhoven HWM. Clinical Trials Targeting the Stroma in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2019;11 doi: 10.3390/cancers11050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang H, Torphy RJ, Steiger K, Hongo H, Ritchie AJ, Kriegsmann M, Horst D, Umetsu SE, Joseph NM, McGregor K, Pishvaian MJ, Blais EM, Lu B, Li M, Hollingsworth M, Stashko C, Volmar K, Yeh JJ, Weaver VM, Wang ZJ, Tempero MA, Weichert W, Collisson EA. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest. 2020;130:4704–4709. doi: 10.1172/JCI136760. [DOI] [PMC free article] [PubMed] [Google Scholar]