Multidrug-resistant (MDR) strains of bacteria are a major clinical problem, and several reports have linked outbreaks of MDR bacteria with bacterial populations in hospital sinks. Biocides such as octenidine are used clinically in body washes and other products, such as wound dressings for infection control.
KEYWORDS: octenidine, Citrobacter, Enterobacter, Pseudomonas aeruginosa, sink waste trap, smvR
ABSTRACT
Octenidine-based disinfection products are becoming increasingly popular for infection control of multidrug-resistant (MDR) Gram-negative isolates. When a waste trap was removed from a hospital and allowed to acclimatize in a standard tap rig in our laboratory, it was shown that Klebsiella pneumoniae, Pseudomonas aeruginosa, and Citrobacter and Enterobacter spp. were readily isolated. This study aimed to understand the potential impact of prolonged exposure to low doses of a commercial product containing octenidine on these bacteria. Phenotypic and genotypic analyses showed that P. aeruginosa strains had increased tolerance to octenidine, which was characterized by mutations in the Tet repressor SmvR. Enterobacter species demonstrated increased tolerance to many other cationic biocides, although not octenidine, as well as the antibiotics ciprofloxacin, chloramphenicol, and ceftazidime, through mutations in another Tet repressor, RamR. Citrobacter species with mutations in RamR and MarR were identified following octenidine exposure, and this is linked to development of resistance to ampicillin, piperacillin, and chloramphenicol, as well as an increased MIC for ciprofloxacin. Isolates were able to retain fitness, as characterized by growth, biofilm formation, and virulence in Galleria mellonella, after prolonged contact with octenidine, although there were strain-to-strain differences. These results demonstrate that continued low-level octenidine exposure in a simulated sink trap environment selects for mutations that affect smvR. It may also promote microbial adaptation to other cationic biocides and cross-resistance to antibiotics, while not incurring a fitness cost. This suggests that hospital sink traps may act as a reservoir for more biocide-tolerant organisms.
IMPORTANCE Multidrug-resistant (MDR) strains of bacteria are a major clinical problem, and several reports have linked outbreaks of MDR bacteria with bacterial populations in hospital sinks. Biocides such as octenidine are used clinically in body washes and other products, such as wound dressings for infection control. Therefore, increased tolerance to these biocides would be detrimental to infection control processes. Here, we exposed bacterial populations originally from hospital sink traps to repeated dosing with an octenidine-containing product over several weeks and observed how particular species adapted. We found mutations in genes related to biocide and antibiotic susceptibility, which resulted in increased tolerance, although this was species dependent. Bacteria that became more tolerant to octenidine also showed no loss of fitness. This shows that prolonged octenidine exposure has the potential to promote microbial adaptation in the environment and that hospital sink traps may act as a reservoir for increased biocide- and antibiotic-tolerant organisms.
INTRODUCTION
The rise in bacterial infections that are resistant to one or more frontline antibiotics has led to a greater reliance on prevention through effective infection control practices (IPCs). This is essential for reducing the burden of nosocomial infections and includes the use of antiseptics and disinfectants—e.g., the use of body washes to help reduce nosocomial infections in high-risk areas such as intensive care units (ICUs) (1, 2). The antiseptic octenidine has showed promising efficacy against a wide variety of multidrug-resistant (MDR) pathogens (3, 4), as well as reducing the rate of infection with Gram-negative agents through daily body washing (5). Octenidine is a cationic biocide in which the two cationic centers will readily bind to the negatively charged bacterial cell membrane. This leads to disassociation of cations and neutralization of the bacterial cell surface charge (6), which at high concentrations, causes a decrease in membrane fluidity with eventual membrane fracture (7–10). Previous research has shown that Staphylococcus aureus isolates have increased their tolerance to octenidine through repeated exposure (11), and Klebsiella pneumoniae strains isolated in the 1920s and 1930s (i.e., before the introduction of octenidine) have lower octenidine MIC values than those isolated more recently (12). Isolates of Pseudomonas aeruginosa can become more tolerant to octenidine (13), with mutations shown in the TetR efflux pump regulator SmvR and PssA/PgsA, which are enzymes involved in phospholipid biosynthesis (L. J. Bock, P. M. Ferguson, M. Clarke, V. Pumpitakkul, M. E. Wand, P. E. Fady, L. Allison, R. A. Fleck, M. J. Shepherd, A. J. Mason, and J. M. Sutton, unpublished data). K. pneumoniae is also able to survive increased exposure through mutations in the major facilitator superfamily (MFS) efflux pump SmvA, which increased the strength of interactions between the pump and octenidine (12).
Hospital drains and sink waste traps have been well documented as harboring MDR bacteria, with them acting either as a source for potential outbreaks through droplet dispersal (14) or as a reservoir between outbreaks (15–21). MDR bacteria from sinks can reenter the clinical environment during flushing and contribute to onward transmission (22). Upon washing of hands and when biocidal products are used as body washes for decolonization purposes, biocides are regularly discharged into the hospital waste pipes. Therefore, bacteria existing in these waste traps will come into regular long-term exposure to many biocides included in hand and body washes. Constant exposure to the active components of these body washes may either select for “more intrinsically tolerant” Gram-negative bacterial populations and/or force the species present to develop increased tolerance. The aim of this study was to evaluate changes in the culturable Gram-negative bacterial population in sink trap water from traps taken from a hospital upon long-term exposure to octenidine. We looked at whether select bacterial species from this mixed-species environment can adapt phenotypically, leading to decreased susceptibility to octenidine as well as potential cross-resistance to other antimicrobial agents. The genomes of several isolates were also sequenced to understand if mutations following exposure to octenidine were similar to those already seen in monospecies adaptation experiments described previously (12, 13).
RESULTS
Characterization of trap water isolates before treatment with octenidine.
Hospital traps were removed from working hospital wards as part of routine or preventative maintenance and installed in a standard tap rig at Public Health England-Porton Down (PHE-Porton) as described previously (22). As far as the authors were aware, the sink traps had not come into contact with any octenidine-containing products prior to removal. Since we were interested in Gram-negative bacterial populations that could be most readily transmitted by splashing within the hospital environment, we sampled bacterial isolates from the trap water and cultured them on selective agar, as detailed in the Materials and Methods section; this was designated “time original” (Toriginal). Colonies that displayed different morphologies were selected, and individual organisms were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) to gain an understanding of the bacterial species present. There was a mixture of Enterobacteriaceae, including Enterobacter cloacae complex, Kluyvera ascorbata, Klebsiella pneumoniae, and Raoultella and Hafnia spp. present. Several isolates were identified as Pseudomonas nitroreducens or could not be reliably identified (MALDI-TOF MS result of “no ID”). A subset of isolates was sent for whole-genome sequencing (WGS) to confirm/identify species. Many of the no ID isolates were found to be closely related to either Phytobacter spp. (48%) or Kluyvera intestini (70%), with one isolate identified as having high homology to Azospira oryzae (94%). ResFinder identified antibiotic resistance genes, including act-3 and qnrE in Enterobacter asburiae and blaCTX-M-115 in K. intestini. Antimicrobial MIC testing revealed ampicillin and piperacillin resistance (MICs of >512 mg/liter) in many isolates, as well as resistance to colistin in Enterobacter asburiae (MICs of >64 mg/liter) and Klebsiella pneumoniae (MICs of 8 to 16 mg/liter) isolates. Subsequent sequence analysis on these K. pneumoniae isolates showed a unique variation (E57G) in PmrA, although its role in colistin resistance was not confirmed. However, all identified isolates were susceptible to several antibiotics tested, including carbapenems and cephalosporins, and there was no evidence for carriage of MDR plasmids (see Table S1 in the supplemental material). The isolates also had MIC values of between 2 and 32 mg/liter and 1 to 4 mg/liter for the antiseptics chlorhexidine and octenidine, respectively. K. intestini-related isolates showed a degree of lysis when grown in tryptic soy broth (TSB), and we were unable to grow A. oryzae in TSB: therefore, these were left off the MIC analysis.
Upon installation of the trap at PHE-Porton, the Gram-negative bacterial population was allowed to stabilize for 28 days before challenge with octenidine. This was because water conditions such as water hardness are different from those of the hospital from which the traps were acquired. After 28 days, the population was reanalyzed in the same manner as Toriginal (designated time 0 [T0]). Citrobacter spp. and Pseudomonas aeruginosa were now identified, and K. pneumoniae and P. nitroreducens were present at counts of over 1 × 106 CFU/ml. There was also infrequent identification of several species, such as Klebsiella michigenensis, Stenotrophomonas spp., Aeromonas caviae, and Delftia tsuruhatensis. We did not detect any Kluyvera spp., and there were only sporadic isolates of Phytobacter spp.
Challenge with a variety of antimicrobial agents showed again that the majority of isolates studied were susceptible, with the exception of ampicillin and piperacillin (MICs of 256 to >512 mg/liter), and that all isolates showed low levels of tolerance to octenidine (MICs of 0.5 to 8 mg/liter) (see Table S2 in the supplemental material).
Change in specific Gram-negative organisms present over the time course of challenge with Octenisan.
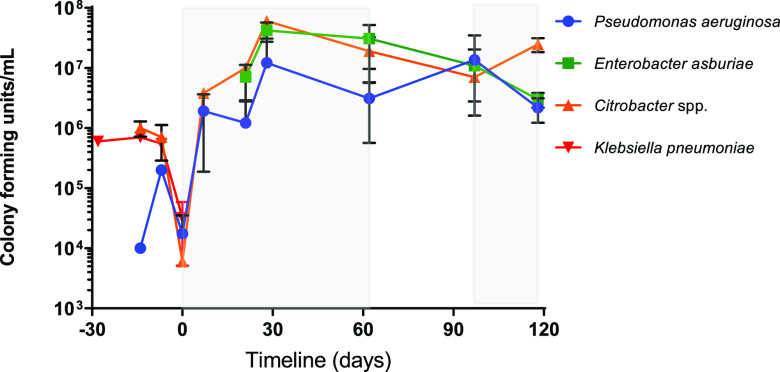
Following the stabilization period, at T0 dosing of the sink trap with Octenisan (Schülke & Mayr, Sheffield, United Kingdom) was initiated (see the Materials and Methods section for the dosing regimen). Since it was reasoned that K. pneumoniae, Citrobacter spp., E. asburiae, and P. aeruginosa were of the greatest clinical significance, analysis was concentrated on these organisms. Samples of trap water were taken at various time points over the course of the experiment and plated on selective agar for identification of these organisms. We sporadically identified other organisms, including D. tsuruhatensis, Hafnia paralvei, and Klebsiella michigenensis at various time points (see Fig. S1 in the supplemental material), but P. aeruginosa, E. asburiae, and Citrobacter spp. were consistently isolated across all time points (Fig. 1). K. pneumoniae was only identified when the Octenisan challenge was removed later in the experiment (time points 76 and 84 days [T76 and T84, respectively]), indicating that it might be present in the biofilm but was not present in the trap water in large numbers.
FIG 1.
Analysis of population changes of P. aeruginosa, Citrobacter spp., Enterobacter spp., and K. pneumoniae in a drain trap after installation in an automated sink drainage system with prolonged exposure to Octenisan. Drain trap water was sampled at regular intervals across the experimental timeline and selectively cultured for Gram-negative organisms. The number of CFU was calculated, and an average of all isolates at each time point sampled is shown. Error bars indicate standard deviation (SD). Shaded areas indicate the time frame when the sink was dosed with Octenisan, and nonshaded areas indicate when there was no dosing with Octenisan. T0 (where the dosing with Octenisan begins) is indicated as 0 days. Time points prior to this represent the establishment of the sink trap in the laboratory.
Decreased susceptibility to octenidine in P. aeruginosa linked to mutations in the TetR family repressor smvR.
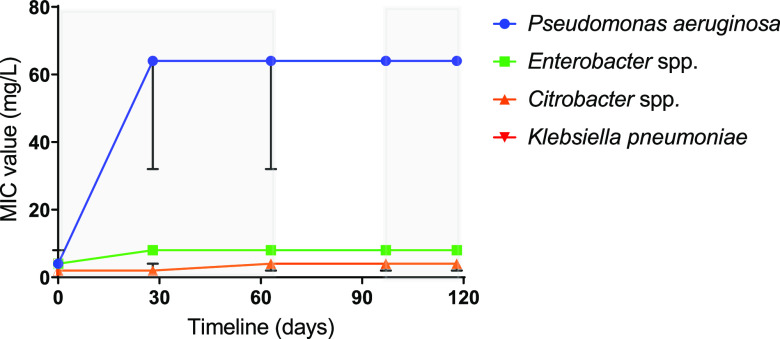
At time points T0, T28, T62, T97, and T118, at least two isolates that were identified as either P. aeruginosa, Enterobacter spp., or Citrobacter spp. were cultured, and the MIC/minimum bactericidal concentration (MBC) was tested against octenidine to determine if there has been an increase in tolerance (Fig. 2 and Table 1). For P. aeruginosa isolates, there was an increase in octenidine tolerance following challenge, with MIC and MBC values increasing around 16-fold (from 4 mg/liter to >64 mg/liter) after 28 days. This level of tolerance was maintained in all isolates tested throughout the remaining time points, even when the Octenisan selection was removed (between time points T62 and T97). For Citrobacter spp., there was a consistent 2-fold increase in the modal MIC and MBC values at the later time points (T62 onwards) compared to T0. For Enterobacter spp., from T28 onwards, a different sequence type was isolated (ST997), which was identified as Enterobacter roggenkampii rather than E. asburiae. The E. roggenkampii isolates had comparable octenidine tolerance to E. asburiae (MICs of 4 to 8 mg/liter compared to 4 mg/liter), and these MIC/MBC values did not change during repeated challenge with octenidine. Since Octenisan contains other components that have antibacterial properties namely, lactic acid, MIC/MBC analyses were performed against this. Throughout the time course, there was little change in the levels of tolerance to lactic acid in all three species tested (Table 1).
FIG 2.
Octenidine susceptibility over time for organisms isolated from sink trap water. The MIC value (mg/liter) for octenidine was tested for a range of P. aeruginosa, Enterobacter, Citrobacter, and K. pneumoniae isolates at the time points indicated. The values shown are the mode at each time point. Error bars represent the upper and lower limits. Shaded areas indicate the time frame when the sink was dosed with Octenisan, and nonshaded areas indicate when there was no dosing with Octenisan.
TABLE 1.
Biocide MIC/MBC values during time course
| Time point (days) | MIC/MBC (mg/liter) value or % of active ingredient fora: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Octenisan | OCT | CET | CPC | HDPCM | BAC | CTAB | TRC | CHX | L acid | |
| P. aeruginosa | ||||||||||
| 0 | 25 (25) | 4 (8–16) | 6.25–12.5 (25) | 512 (>512) | 512 (>512) | 32–64 (64–128) | 512 (>512) | >64 (>64) | 16–32 (32) | 0.16 (0.16) |
| 28 | 25–50 (50) | 32–64 (64–>64) | 6.25–12.5 (12.5–25) | 512 (>512) | 512 (>512) | 64 (128) | 512 (>512) | >64 (>64) | 32–64 (>128) | 0.16 (0.16) |
| 62 | 25–50 (50) | 32–64 (64) | 6.25–25 (12.5–25) | 512 (>512) | 512 (>512) | 64 (128–256) | 512 (>512) | >64 (>64) | 32–64 (128–>128) | 0.16 (0.16) |
| 97 | 25–50 (50) | 32–64 (64–>64) | 6.25–12.5 (12.5–50) | 512 (>512) | 512 (>512) | 64 (128–256) | 512 (>512) | >64 (>64) | 32 (>128) | 0.16 (0.16) |
| 118 | 25–50 (50) | 32–64 (64–>64) | 6.25–12.5 (25) | 512 (>512) | 512 (>512) | 64 (128–256) | 512 (>512) | >64 (>64) | 32 (128–>128) | 0.16 (0.16) |
| E. asburiae/E. roggenkampii | ||||||||||
| 0 | 12.5 (12.5–25) | 4 (4–8) | 0.19–0.39 (0.39) | 8–16 (32) | 16 (16–32) | 32 (32) | 16–32 (32–64) | 1 (1–2) | 16–32 (32) | 0.16–0.33 (0.33) |
| 28 | 12.5–25 (25) | 4 (4–8) | 0.78 (0.78–1.56) | 16–32 (64) | 16–32 (16–32) | 32–64 (64) | 32–128 (128) | 2 (4–8) | 32 (32) | 0.33 (0.33) |
| 62 | 12.5–25 (25) | 4–8 (8–16) | 3.125–6.25 (6.25) | 128 (128–256) | 128 (128–256) | 32–64 (64) | 128–256 (256) | 4–8 (8) | 64–128 (64–128) | 0.16–0.33 (0.33) |
| 97 | 25 (25) | 4 (4–16) | 1.56–3.125 (3.125–6.25) | 64–256 (128–256) | 128–256 (256) | 32–64 (64) | 128–256 (128–256) | 4–8 (8) | 128–256 (256–512) | 0.16–0.33 (0.33) |
| 118 | 25 (25) | 4 (8) | 1.56–3.125 (3.125) | 128–256 (128–256) | 128–256 (256) | 32–64 (64) | 128–256 (128–256) | 4–8 (8) | 128–256 (128–256) | 0.16–0.33 (0.33) |
| Citrobacter spp. | ||||||||||
| 0 | 1.56–3.125 (3.125) | 2 (2) | 0.09–0.19 (0.19–0.39) | 8 (8–16) | 4–8 (4–8) | 8–16 (16–32) | 8–16 (16–32) | 0.25 (0.25) | 8–16 (16) | 0.16–0.33 (0.33) |
| 28 | 3.125 (3.125) | 2 (2) | 0.09–0.39 (0.19–0.39) | 8–16 (16) | 8–16 (8–16) | 8–16 (16) | 16–32 (16–32) | 0.125–0.25 (1) | 8–16 (32) | 0.16–0.33 (0.33) |
| 62 | 1.56–3.125 (3.125–6.25) | 2 (2) | 0.19–0.39 (0.19–0.39) | 8–16 (16) | 8–16 (8–16) | 8–16 (16–32) | 16–32 (16–32) | 0.25–0.5 (>4) | 8–16 (16–32) | 0.16–0.33 (0.33) |
| 97 | 3.125–6.25 (12.5) | 2 (2-4) | 0.09–0.39 (0.09–0.39) | 8–16 (16) | 8–16 (16) | 16–32 (32) | 16–32 (16–32) | 0.25–0.5 (>4) | 16 (16–32) | 0.16–0.33 (0.33) |
| 118 | 3.125–6.25 (6.25–12.5) | 2 (2-4) | 0.09–0.19 (0.19–0.39) | 8–16 (16) | 8–16 (8–16) | 16–32 (32) | 16–32 (16–32) | 0.25–0.5 (>4) | 16–32 (16–32) | 0.16–0.33 (0.33) |
MIC/MBC values are shown for the biocides cetylpyridinium chloride (CPC), hexadecylpyridinium chloride monohydrate (HDPCM), benzalkonium chloride (BAC), cetyltrimethylammonium bromide (CTAB), triclosan (TRC), and chlorhexidine digluconate (CHX), as well as the octenidine-containing body wash Octenisan. Cetrimide (CET) and lactic acid (L acid) values are expressed as a percentage of the active ingredient. MBC values are shown in parentheses.
At least two isolates for P. aeruginosa, Citrobacter spp., and Enterobacter spp. from each time point were sent for whole-genome sequencing (WGS) to understand genetic changes following exposure to Octenisan (see Table S3 in the supplemental material). All P. aeruginosa isolates from T28 onwards contained changes in the TetR family regulator, which we have termed SmvR, which functions to regulate the divergently transcribed MFS pump SmvA. The appearance of mutations in smvR coincides with the increase in tolerance levels to octenidine. Subsequent analysis of transposon mutants of this gene and smvA in strain PAO1 indicated that there was little change in the MIC values between PAO1 and PAO1ΔsmvR (2 mg/liter for both), but there was an increase in the MBC value from 2 mg/liter to 4 to 8 mg/liter in PAO1ΔsmvR. For PAO1ΔsmvA, the MIC and MBC values for octenidine were 1 mg/liter. Since SmvA is an efflux pump, the effect of the efflux pump inhibitors CCCP (carbonyl cyanide m-chlorophenylhydrazone) and PaβN (phenylalanine-arginine β-naphthylamide) was tested. The addition of PaβN reduced the MIC/MBC values slightly (2- to 4-fold), while CCCP increased the MIC/MBC values of T0 isolates 8- to 16-fold (see Table S4 in the supplemental material). Mutations were also observed in the catalase KatA, the motility protein MorA, and the promoter regions of cheZ and arcD (Table S3).
For E. roggenkampii, when isolates were mapped to MPT87 (from time point T28), there were two distinct lineages identified: those that contained deletions or deleterious single nucleotide polymorphisms (SNPs) in the regulator of the maltose regulon, malT, and the anaerobically synthesized molybdoenzyme torA, and those isolates that contained a conservative change (D21E) in SmvA (see Table S5 in the supplemental material). Citrobacter strains isolated at later time points showed mutations in the transcriptional regulator marR and the gene coding for the two-component sensor kinase involved in porin regulation, envZ (see Table S6 in the supplemental material).
Enterobacter spp. acquired increased tolerance to several other cationic biocides, while Citrobacter spp. developed increased resistance to β-lactam and fluoroquinolone antibiotics.
To understand if adaptation to octenidine led to cross-tolerance to other biocides and antibiotics, susceptibility of isolates to several antimicrobial agents was tested at all time points. There was little change in MIC/MBC levels against biocides for P. aeruginosa, except for chlorhexidine, for which the MBC values increased from 32 mg/liter to >128 mg/liter (Table 1). For Citrobacter, there was a 2-fold increase for most biocides tested: up to 4-fold for Octenisan and triclosan (Table 1). In Enterobacter, there was an increase in MIC values (>4-fold) for the following biocides tested: cetrimide (CET), cetylpyridinium chloride (CPC), hexadecylpyridinium chloride monohydrate (HDPCM), cetyltrimethylammonium bromide (CTAB), triclosan (TRC), and chlorhexidine digluconate (CHX). There was not an increase in MIC values for Octenisan and benzalkonium chloride (BAC). The increase was stable and was not lost when the octenidine challenge was removed (between time points T62 and T97).
A range of antibiotics were tested, which included frontline therapies for treatment of P. aeruginosa, Citrobacter, and Enterobacter infections. Little cross-resistance was seen, except for Citrobacter, for which there was an increase (4-fold) in resistance to ampicillin, piperacillin, and ceftazidime, with a 2- to 4-fold increase in resistance to ciprofloxacin and meropenem (Table 2).
TABLE 2.
Antibiotic MICs during the time course of this study
| Time point (days) | MIC (mg/liter) fora: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AMK | GEN | AMP | PIP | CAZ | MEM | CIP | CHL | CST | |
| P. aeruginosa | |||||||||
| 0 | 4–8 | 2–4 | >512 | 8 | 1–2 | 2–4 | 0.125–0.25 | 128 | 0.5–1 |
| 28 | 4–8 | 2–4 | >512 | 8 | 2 | 2–4 | 0.06–0.25 | NT | 0.5–1 |
| 62 | 4–8 | 2–8 | >512 | 8 | 2 | 2–4 | 0.125–1 | NT | 0.5–1 |
| 97 | 4–8 | 4–8 | >512 | 8 | 2 | 1–4 | 0.06–1 | NT | 0.5–2 |
| 118 | 4–8 | 4–8 | >512 | 8–16 | 2 | 1–2 | 0.06–0.125 | NT | 0.5–1 |
| E. asburiae/E. roggenkampii | |||||||||
| 0 | 2–4 | 2–4 | 512–>512 | 2–4 | 1–2 | 0.15–0.25 | 0.06–0.125 | 4–8 | >64 |
| 28 | 16–32 | 8 | 4–16 | 4–8 | 0.5–1 | ≤0.06 | ≤0.06 | 8–16 | 16–32 |
| 62 | 8–16 | 4–8 | 16–32 | 4–16 | 1–2 | ≤0.06 | 0.125 | 8–16 | 16–32 |
| 97 | 16–32 | 8–16 | 8–16 | 8–16 | 1–2 | ≤0.06 | 0.125–0.5 | 16 | 16–64 |
| 118 | 16–32 | 8–16 | 8–16 | 8 | 1–2 | ≤0.06 | 0.125–0.5 | 8–16 | 32–64 |
| Citrobacter spp. | |||||||||
| 0 | 2–4 | 4 | 4–8 | 2–4 | 0.5–1 | 0.015 | 0.015 | 4–8 | 0.06–0.125 |
| 28 | 2–4 | 4–8 | 8–16 | 2–4 | 0.5–1 | 0.015 | 0.015 | 4–8 | 0.125–0.25 |
| 62 | 2–8 | 4–8 | 8–16 | 8–16 | 1–2 (16–32)b | 0.015–0.03 | 0.03 | 8 | 0.125–0.25 |
| 97 | 4–8 | 2–4 | 16–32 | 8 | 2–4 | 0.03 | 0.03 | 8–16 | 0.125–0.25 |
| 118 | 4–8 | 2–4 | 16–32 | 8–16 | 1–4 | 0.03–0.06 | 0.03 | 16–32 | 0.125 |
MIC values are shown for the antibiotics amikacin (AMK), gentamicin (GEN), ampicillin (AMP), piperacillin (PIP), ceftazidime (CAZ), meropenem (MEM), ciprofloxacin (CIP), chloramphenicol (CHL), and colistin (CST). NT, not tested.
Value in parentheses represents one isolate which had consistently higher CAZ MIC values.
In P. aeruginosa, acquisition of increased octenidine tolerance leads to retention of preexposure fitness levels.
To understand if continued exposure and adaption to low levels of octenidine had affected the growth rate, P. aeruginosa, Enterobacter, and Citrobacter isolates from all time points were examined in both R2A broth, to stimulate low-nutrient conditions encountered in the sink trap, but also in a rich medium, TSB. There was no significant difference in the growth rates of all P. aeruginosa isolates in both TSB and R2A (Fig. 3). For Enterobacter, there was no difference in the growth rates of E. roggenkampii isolates from time points T28 through to T118. There was, however, a difference in growth rates between the E. asburiae isolates from T0 and the E. roggenkampii isolates, with the latter isolates being slower growing in both TSB and, more extremely, in R2A broth, although this is likely to reflect differences between E. asburiae and E. roggenkampii rather than differences caused by exposure to Octenisan. Citrobacter isolates from the later time points had a growth defect compared to isolates from the T0 and T28 time points; this effect was more pronounced in TSB, suggesting that prolonged exposure to Octenisan resulted in growth retardation.
FIG 3.
Growth rates of P. aeruginosa (a and b), Enterobacter spp. (c and d), and Citrobacter spp. (e and f) in R2A medium (a, c, and e) and TSB (b, d, and f) at different time points during the time course. Time points are indicated by the number of days into the time course. T28, T62, and T118 are the time points (days) after a prolonged period of Octenisan dosing, whereas T97 is the day after a period of 35 days when Octenisan dosing was stopped.
Since biofilms are often the source of bacterial contamination in wastewater/sink water, the ability of the isolates to form biofilms was examined in both TSB and R2A broth. There was no statistical significance between any of the time points within the same species for biofilm formation in both TSB and R2A broth. Typically, P. aeruginosa was able to form increased biofilm in TSB, but surprisingly, Enterobacter appeared to be a better biofilm former in R2A medium rather than TSB (see Fig. S2 in the supplemental material).
To further understand the effect on fitness, virulence of isolates was examined in the invertebrate model Galleria mellonella. Isolates from all time points were injected into Galleria at 105 and 106 CFU for Enterobacter and Citrobacter spp., respectively, and 102 CFU for P. aeruginosa. The results showed that for Enterobacter and P. aeruginosa, there was no difference in any of the time points investigated. Citrobacter isolates for later time points showed a significant loss of virulence when compared to T0 (P = 0.0005) (Fig. 4).
FIG 4.
Virulence in Galleria of P. aeruginosa, Enterobacter spp., and Citrobacter spp. following prolonged exposure to octenidine. Groups of 30 larvae were challenged with 1 × 106 CFU of Citrobacter, 1 × 105 CFU of Enterobacter, and 1 × 102 CFU of P. aeruginosa. The number of live versus dead larvae was determined every 24 h postinfection up to 120 h. Significance as determined by the Mantel-Cox test is shown next to the species name. ***, P = 0.0004. Time points are indicated by the number of days into the time course. T28, T62, and T118 are time points (days) after a prolonged period of Octenisan dosing, whereas T97 is after a period of 35 days when Octenisan dosing was stopped.
A second independent sink trap replicates the study’s findings.
A second sink trap, which had been left to stabilize at PHE-Porton for several weeks, was used to understand if the findings from the first sink were reproduceable. Strains of P. aeruginosa, Enterobacter spp. (a mixture of E. roggenkampii and strains more closely related to E. asburiae), Citrobacter spp. (C. freundii), and K. pneumoniae were readily isolated from the initial sink trap population (see Fig. S3 in the supplemental material). These were different sequence types from those found in the previous sink waste trap and included strains of Enterobacter spp. and K. pneumoniae that were carbapenem resistant due to the presence of plasmids containing blaKPC-2. Following the same dosing and sampling regimen, isolates of P. aeruginosa, Enterobacter spp., and C. freundii were obtained at all time points. Again, K. pneumoniae was only sporadically isolated, and at the later time points, a different sequence type (ST915) was identified from those of isolates at T0 (ST11). The sink population also contained multiple sequence types of Enterobacter spp. and P. aeruginosa, which made comparison of the susceptibility profiles of isolates between time points problematic. However, regardless of sequence type, all P. aeruginosa isolates at the later time points after exposure to Octenisan contained changes in smvR, and again the appearance of these changes corresponded with an increase in tolerance to octenidine (see Table S7 in the supplemental material). Other interesting changes observed in P. aeruginosa include changes in an marR-type regulator (PAO1 equivalent PA3458) and a divergently transcribed gene (PA3459) encoding an N-acetylglutaminylglutamine amidotransferase. Changes in PA3458 were observed in multiple isolates, but were not universal, and similar mutations were also found in isolates from the previous sink trap analysis. For Enterobacter spp., mutations were seen in the TetR family regulator RamR and the chemotaxis protein CheA as well as select isolates showing a conservative mutation in SmvA, D31E (see Table S8 in the supplemental material). C. freundii isolates from the later time points also contained mutations in RamR, as well as SNPs in the trimethylamine N-oxide (TMAO) reductase system sensor kinase TorS. Specific isolates also had mutations in the promoter region of smvAR (see Table S9 in the supplemental material).
No cross-tolerance to biocides was observed in P. aeruginosa, but again, Enterobacter isolates showed a significant decrease in susceptibility to several cationic biocides (>4-fold), but not for octenidine (see Table S10 in the supplemental material). C. freundii isolates did not show any biocidal cross-tolerance, but there was a decrease in susceptibility to ampicillin, piperacillin, and ciprofloxacin (4-fold) and development of resistance to chloramphenicol. Enterobacter isolates at the later time points also showed a decrease in susceptibility to ciprofloxacin and chloramphenicol. These isolates are already resistant to ampicillin, piperacillin, and ceftazidime (see Table S11 in the supplemental material).
DISCUSSION
This study focused on the impact of disposal of the cationic antiseptic octenidine on Gram-negative populations of K. pneumoniae, Citrobacter, Enterobacter, and P. aeruginosa in a mixed-species environment. The study showed that the latter three organisms were able to survive continuous exposure to Octenisan, and for P. aeruginosa, this led to the culture of isolates that had become increasingly tolerant to octenidine. Why K. pneumoniae numbers were greatly reduced in the water samples is unknown. K. pneumoniae is typically not more susceptible to octenidine than the other species, and its growth is R2A broth is similar to that of Citrobacter and Enterobacter. We have evidence of maintenance of K. pneumoniae in non-octenidine-treated sink traps installed in the same tap rig (data not shown), which suggests that the observed reduction in K. pneumoniae in the sink trap water is a result of the Octenisan treatment. A summary of the results for P. aeruginosa and Citrobacter and Enterobacter spp. from both sinks analyzed is shown in Table 3.
TABLE 3.
Summary of findings from both sink traps for P. aeruginosa, Citrobacter spp., and Enterobacter spp.
| Organism | Decrease in octenidine susceptibility | Biocide cross-tolerance | Antibiotic cross-resistance | Growth | Virulence | Important genes |
|---|---|---|---|---|---|---|
| P. aeruginosa | Yes (>4-fold) | No | No | WTa | WT | smvA, PA3458–PA3461 |
| Enterobacter spp. | Slight (2-fold) | Yes (multiple biocides) | Yes (ciprofloxacin) | WT | WT | smvA, malT, ramR, torA |
| Citrobacter spp. | Slight (2-fold) | No | Yes (select β-lactams, ciprofloxacin) | Retardation | Loss of virulence | marR, ramR, torS, envZ |
WT, wild type.
Octenisan selection in multiple sequence types of P. aeruginosa, including the international MDR clone readily isolated from wastewater (ST235) (23), resulted in deletion of a functional smvR, which in Klebsiella leads to derepression of the smvA promoter and increased expression of the SmvA efflux pump (40). Laboratory-adapted P. aeruginosa to octenidine also showed deletions in smvR (Bock et al., unpublished), some of which are replicated here (notably a 12-bp deletion of bases 318 to 329 in smvR). Therefore, it is likely that in P. aeruginosa SmvA acts as an efflux pump that ameliorates the effects of octenidine, probably through direct efflux of the biocide. Mutations in SmvA and the smvAR promoter region were also observed in Enterobacter and Citrobacter spp., respectively, including identification of Enterobacter isolates with the D21E mutation from both sinks. This amino acid is present in a highly conserved predicted transmembrane region of Enterobacteriaceae SmvA. Increased efflux is a recognized mechanism of increased tolerance to biocides (24–26).
The transcriptional regulators MarR and RamR are important in the regulation of the MDR efflux pump AcrAB-TolC and function to repress the activators MarA and RamA, respectively (27). Mutations in MarR lead to increased expression of the marRAB operon, increased expression of oxidative stress genes either directly or through SoxRS (28, 29), and increased antibiotic resistance through overexpression of the efflux pump AcrAB-TolC, which is also activated through mutations in RamR (30). Mutations were seen in both marR and ramR in Citrobacter spp. and in ramR in Enterobacter spp., and these are linked to the decrease in susceptibility to antibiotics such as ampicillin, ciprofloxacin, piperacillin, ceftazidime, and chloramphenicol, all antibiotics known to be substrates for AcrAB-TolC (31). Mutations in RamR have also been described before in Salmonella enterica following exposure to specific biocides (32) as well as being implicated in increased tolerance to the biocides benzalkonium chloride and triclosan (33, 34). Although there have been limited studies on the role of RamR and MarR in Citrobacter and Enterobacter, it is predicted that they act in a similar manner to other members of the Enterobacteriaceae. Therefore, the potential for continuous exposure to octenidine to repeatedly select for mutations leading to increased antibiotic resistance, through mutations in important regulators of efflux pumps, is a cause for concern. Discussion of other mutations is continued in the supplemental material.
A limitation of the current study is that the sink trap biofilm was not studied, and a detailed analysis of the sink trap population was not undertaken. While we have generated evidence that there was a shift in the Gram-negative population following challenge with Octenisan (i.e., the P. aeruginosa and Citrobacter and Enterobacter spp. appear to be more prevalent), the study did not look at changes in the Gram-positive population and those viable but nonculturable organisms. It is plausible that by only sampling the trap water, there could be species such as K. pneumoniae, which are underrepresented in the water samples but are present within the deeper layers of the biofilm, at least during Octenisan challenge. This multispecies biofilm-mediated tolerance to biofilms is something we have observed previously (35), but it would require a different study protocol to evidence clearly in the sink trap biofilm model. We equally recognize that the biofilm populations of different sink traps are not likely to be entirely consistent with each other, as evidenced by a recent publication (36), especially given different experimental conditions, but we found that there was consistent isolation in the trap water of P. aeruginosa and Enterobacter and Citrobacter spp. that allowed a degree of comparison between both sinks analyzed.
As well as selecting for specific mutations or species, particular phenotypic characteristics were also selected for such as decreased biocide susceptibility in Enterobacter spp. that were not necessarily defined by individual mutations: e.g., isolates with SmvA mutations did not appear to be more biocide tolerant than those with mutations in MalT. In both sinks, E. roggenkampii became the dominant Enterobacter sp. at the later time points, with E. asburiae only cultured from isolates at the earlier time points. This suggests that E. roggenkampii either is intrinsically more tolerant to octenidine, is more genetically adaptable, or is better able to exist synergistically with other bacteria such as P. aeruginosa. There is also little evidence to suggest that particular P. aeruginosa sequence types are more able to adapt to octenidine since we isolated multiple sequence types (ST27, ST282, and ST319) with high MIC values against octenidine; what was common were mutations in SmvR. In previous studies, isolates from multiple sequence types were able to adapt to octenidine (13), again suggesting that, at least for P. aeruginosa, octenidine does not select for particular sequence types. P. aeruginosa and Enterobacter isolates that had adapted to octenidine also displayed no loss of fitness or virulence compared to preexposure strains. There is precedence for strains of Enterobacteriaceae with mutations, particularly in smvR, to persist in the environment with no obvious selective disadvantage (37).
This study has shown that repeated exposure to low concentrations of octenidine, in a simulated clinical environment, enhanced selection of individual P. aeruginosa mutants with elevated tolerance (≥8-fold) to octenidine. These tolerance levels are still below the in-use concentrations of octenidine, and therefore octenidine remains an effective product for decontamination. However, the SmvA efflux pump-mediated increased-tolerance phenotype in P. aeruginosa could be an intermediate step toward development of resistance, through secondary mutations such as membrane modification. This is evidenced by a stepwise development of fluoroquinolone resistance, with efflux-mediated resistance first, followed by mutations in grlA (38) or elevation of expression of the NorA expression pump potentiating a fitness benefit for DNA topoisomerase mutations in S. aureus (39). Our own research showed that smvR mutations predated PhoPQ mutations in the development of increased chlorhexidine tolerance in K. pneumoniae (40), and smvR mutations were first observed before secondary high-level tolerance to octenidine in P. aeruginosa (13; Bock et al., unpublished). Mutations in regulators of efflux pumps (e.g., MexZ in P. aeruginosa), while not actually increasing the tolerance to antibiotics, may enable increased fitness in the presence of antibiotic (41). Taken together with the evidence of long-lasting colonization of hospital wastewater systems with MDR pathogens, notably carbapenem-resistant Enterobacteriaceae (CRE) (42–44), it is perhaps timely to reevaluate whether it is sensible to discharge unneutralized cationic biocides into an environmental reservoir that might be colonized with complex biofilms of MDR Gram-negative pathogens and that could pose an elevated infection risk. Residues of antimicrobial substances have been detected in the drainpipes from sinks (45) and in wastewater (46), and therefore it is plausible that octenidine discharged down the sink would remain at very low concentrations. Exposure to low concentrations of biocides remains a poorly understand risk factor for the development of decreased antibiotic and biocide susceptibility. Previous studies have shown that long-term exposure to biocides in drains resulted in little alteration in antimicrobial susceptibility (47–49), but other experiments have indicated that low-level biocide exposure leads to cross-tolerance to other disinfectants or antibiotics (20, 40, 50). Therefore, more studies are required to fully understand the role of environmental biocide exposure on bacterial adaptation and potential progression toward tolerance. However, biocides remain an effective weapon against the spread of MDR pathogens, and their continued use is essential for infection control.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The P. aeruginosa, K. pneumoniae, Citrobacter, and Enterobacter strains in this study are described in Table S12 (original sink waste trap) and Table S13 (second sink waste trap) in the supplemental material. All strains were grown in tryptic soy broth (TSB) with aeration or on tryptic soy agar (TSA) plates at 37°C unless otherwise stated.
Automated model sink drain system.
Water from a United Kingdom hospital sink waste trap known to be contaminated with a mixed-species population biofilm was sampled on site for analysis. The sink waste trap was transported at room temperature and attached to an automated model sink drain system at PHE-Porton within 48 h, where over the duration of the time course, the water temperature was kept constant at 41°C. Details of the sink drain system have been described previously (22). After installation, the waste trap was allowed to “acclimatize” for 21 days, during which it was dosed daily with 5 ml of TSB to maintain the original levels of Enterobacteriaceae. Throughout the time course, the associated tap was operated four times a day for 40 s at a flow rate of 4 liters/min. Ten seconds into the flush, the sink was dosed with 2 ml of a commercial octenidine formulation body wash (Octenisan; Schülke & Mayr) containing 0.3% octenidine. The Octenisan dosing was sustained for 62 days, paused for 35 days, and then resumed for another 21 days. This is shown diagrammatically in Fig. S4 in the supplemental material. Water from the waste trap was sampled throughout the acclimatization period, immediately and again prior to commencement of challenge with octenidine (T0), as well as at various time points across the experimental timeline. Again, this process is demonstrated diagrammatically in Fig. S5 in the supplemental material. Each sample was serially diluted (10-fold) and selectively cultured for Gram-negative organisms by plating out 100 μl of select dilutions on ChromID CARBA agar (bioMérieux, Basingstoke, United Kingdom), Brilliance E. coli/coliform selective agar, MacConkey no. 3 agar, cetrimide agar, and, to obtain a heterotrophic plate count, R2A agar (all from Oxoid, Ltd., Basingstoke, United Kingdom). Before plating out, Octenisan was neutralized using Dey-Engley (D/E) neutralizing broth. Different Gram-negative morphotypes were subcultured and identified via MALDI-TOF MS (MALDI Biotyper; Bruker Daltonics, Germany) and stored at −80°C. Up to three isolates that had been identified as either P. aeruginosa, Citrobacter spp., or Enterobacter spp. were phenotypically analyzed and sent for whole-genome sequencing.
Determination of MIC/MBC and evaluation of growth and biofilm formation.
The MICs for antibiotics and biocides were determined by a standard broth microdilution method at a starting inoculum of 5 × 105 CFU/ml following guidelines detailed by EUCAST, except that 96-well polypropylene plates (Griener Bio-One, Ltd., Stonehouse, United Kingdom) were used instead of polystyrene plates to test colistin (51). Antibiotic susceptibility was measured utilizing EUCAST breakpoints for Pseudomonas spp. and Enterobacterales. MBCs were measured by plating out onto TSA plates 10 μl of MIC dilutions from and including the MIC level and the subsequent three further higher biocide concentrations (where applicable). The efflux pump inhibitors carbonyl cyanide m-chlorophenylhydrazone (CCCP) and phenyl-arginine β-naphthylamide (PaβN) were added at concentrations of 10 and 25 mg/liter, respectively. Bacterial growth in both TSB and R2A broth was measured by taking an optical density at 600 nm (OD600) reading every hour for 20 h using a FLUOstar Omega plate reader (BMG Labtech, GmbH, Ortenberg, Germany). Biofilm formation was carried out in both TSB and R2A broth using a modification of the Calgary method already described (52).
Virulence in Galleria mellonella.
Wax moth (G. mellonella) larvae were purchased from Livefood UK, Ltd. (Rooks Bridge, Somerset, United Kingdom), and were maintained on wood chips in the dark at 14°C. They were stored for not longer than 2 weeks. Bacterial infection of G. mellonella was performed as essentially described previously (53). Briefly, three groups of 10 larvae were challenged with isolates from all time points at starting inocula in phosphate-buffered saline (PBS) of 1 × 105 and 1 × 106 CFU for Enterobacter and Citrobacter, respectively, and 1 × 102 CFU for P. aeruginosa. The number of live versus dead larvae was determined every 24 h postinfection up to 120 h. Galleria larvae injected with sterile PBS were used as a control, and results were only validated if there were no deaths in the control group. Data were analyzed by the Mantel-Cox method using Prism Software version 8 (GraphPad, San Diego, CA, USA).
Whole-genome sequencing.
Whole-genome sequencing (WGS) was carried out as previously described (40), and PHE Galaxy was used to analyze genetic changes (54). Species identification from the genome sequences was carried out using ribosomal multilocus sequence typing (rMLST) (55), and antibiotic resistome analysis was carried out using ResFinder (56).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Public Health England Grant in Aid 111743. The views expressed are those of the authors and not necessarily those of the funding body.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Harris PN, Le BD, Tambyah P, Hsu LY, Pada S, Archuleta S, Salmon S, Mukhopadhyay A, Dillon J, Ware R, Fisher DA. 2015. Antiseptic body washes for reducing the transmission of methicillin-resistant Staphylococcus aureus: a cluster crossover study. Open Forum Infect Dis 22:ofv051. 10.1093/ofid/ofv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viray MA, Morley JC, Coopersmith CM, Kollef MH, Fraser VJ, Warren DK. 2014. Daily bathing with chlorhexidine-based soap and the prevention of Staphylococcus aureus transmission and infection. Infect Control Hosp Epidemiol 35:243–250. 10.1086/675292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conceição T, de Lencastre H, Aires-de-Sousa M. 2016. Efficacy of octenidine against antibiotic-resistant Staphylococcus aureus epidemic clones. J Antimicrob Chemother 71:2991–2994. 10.1093/jac/dkw241. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Marin R, Aires-de-Sousa M, Nordmann P, Kieffer N, Poirel L. 2017. Antimicrobial activity of octenidine against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36:2379–2383. 10.1007/s10096-017-3070-0. [DOI] [PubMed] [Google Scholar]
- 5.Meißner A, Hasenclever D, Brosteanu O, Chaberny IF. 2017. Effect of daily antiseptic body wash with octenidine on nosocomial primary bacteraemia and nosocomial multidrug-resistant organisms in intensive care units: design of a multicentre, cluster-randomised, double-blind, cross-over study. BMJ Open 7:e016251. 10.1136/bmjopen-2017-016251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malanovic N, Ön A, Pabst G, Zellner A, Lohner K. 2020. Octenidine: novel insights into the detailed killing mechanism of Gram-negative bacteria at a cellular and molecular level. Int J Antimicrob Agents 56:106146. 10.1016/j.ijantimicag.2020.106146. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert P, Moore LE. 2005. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 99:703–715. 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 8.Davies A. 1973. The mode of action of chlorhexidine. J Periodontal Res Suppl 12:68–75. 10.1111/j.1600-0765.1973.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 9.Hugo WB, Longworth AR. 1966. The effect of chlorhexidine on the electrophoretic mobility, cytoplasmic constituents, dehydrogenase activity and cell walls of Escherichia coli and Staphylococcus aureus. J Pharm Pharmacol 18:569–578. 10.1111/j.2042-7158.1966.tb07935.x. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy K, Sunnucks K, Gil H, Shabir S, Trampari E, Hawkey P, Webber M. 2018. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of Staphylococcus aureus. mBio 9:e00894-18. 10.1128/mBio.00894-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wand ME, Jamshidi S, Bock LJ, Rahman KM, Sutton JM. 2019. SmvA is an important afflux pump for cationic biocides in Klebsiella pneumoniae and other Enterobacteriaceae. Sci Rep 9:1344. 10.1038/s41598-018-37730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd MJ, Moore G, Wand ME, Sutton JM, Bock LJ. 2018. Pseudomonas aeruginosa adapts to octenidine in the laboratory and a simulated clinical setting, leading to increased tolerance to chlorhexidine and other biocides. J Hosp Infect 100:e23–e29. 10.1016/j.jhin.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Hajar H, Mana T, Cadnum JL, Donskey CJ. 2019. Dispersal of Gram-negative bacilli from contaminated sink drains to cover gowns and hands during hand washing. Infect Control Hosp Epidemiol 40:460–462. 10.1017/ice.2019.25. [DOI] [PubMed] [Google Scholar]
- 15.Regev-Yochay G, Smollan G, Tal I, Pinas Zade N, Haviv Y, Nudelman V, Gal-Mor O, Jaber H, Zimlichman E, Keller N, Rahav G. 2018. Sink traps as the source of transmission of OXA-48 producing Serratia marcescens in an intensive care unit. Infect Control Hosp Epidemiol 39:1307–1315. 10.1017/ice.2018.235. [DOI] [PubMed] [Google Scholar]
- 16.Decker BK, Palmore TN. 2013. The role of water in healthcare-associated infections. Curr Opin Infect Dis 26:345–351. 10.1097/QCO.0b013e3283630adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H, Covens L, Piérard D, Wybo I. 2017. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control 6:24. 10.1186/s13756-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Högenauer C, Sill H, Krause R, Zollner-Schwetz I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decraene V, Phan HTT, George R, Wyllie DH, Akinremi O, Aiken Z, Cleary P, Dodgson A, Pankhurst L, Crook DW, Lenney C, Walker AS, Woodford N, Sebra R, Fath-Ordoubadi F, Mathers AJ, Seale AC, Guiver M, McEwan A, Watts V, Welfare W, Stoesser N, Cawthorne J, Aiken Z, Akinremi O, Cawthorne J, Cleary P, Crook DW, Decraene V, Dodgson A, Doumith M, Ellington M, Eyre DW, George R, Guiver M, Hill R, Hopkins K, Jones R, Lenney C, Mathers AJ, McEwan A, Moore G, Neilson S, Peto TEA, Phan HTT, Regan M, Seale AC, Stoesser N, Turner-Gardner J, Watts V, TRACE Investigators’ Group . et al. 2018. A large, refractory nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Escherichia coli demonstrates carbapenemase gene outbreaks involving sink sites require novel approaches to infection control. Antimicrob Agents Chemother 62:e01689-18. 10.1128/AAC.01689-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RC, Deming C, Conlan S, Zellmer CJ, Michelin AV, Lee-Lin S, Thomas PJ, Park M, Weingarten RA, Less J, Dekker JP, Frank KM, Musser KA, McQuiston JR, Henderson DK, Lau AF, Palmore TN, Segre JA. 2018. Investigation of a cluster of Sphingomonas koreensis infections. N Engl J Med 379:2529–2539. 10.1056/NEJMoa1803238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weingarten RA, Johnson RC, Conlan S, Ramsburg AM, Dekker JP, Lau AF, Khil P, Odom RT, Deming C, Park M, Thomas PJ, Henderson DK, Palmore TN, Segre JA, Frank KM, NISC Comparative Sequencing Program . 2018. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio 9:e02011-17. 10.1128/mBio.02011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aranega-Bou P, George RP, Verlander NQ, Paton S, Bennett A, Moore G, Aiken Z, Akinremi O, Ali A, Cawthorne J, Cleary P, Crook DW, Decraene V, Dodgson A, Doumith M, Ellington M, Eyre DW, George RP, Grimshaw J, Guiver M, Hill R, Hopkins K, Jones R, Lenney C, Mathers AJ, McEwan A, Moore G, Neilson M, Neilson S, Peto TEA, Phan HTT, Regan M, Seale AC, Stoesser N, Turner-Gardner J, Watts V, Walker J, Sarah Walker A, Wyllie D, Welfare W, Woodford N, TRACE Investigators' Group . 2019. Carbapenem-resistant Enterobacteriaceae dispersal from sinks is linked to drain position and drainage rates in a laboratory model system. J Hosp Infect 102:63–69. 10.1016/j.jhin.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treepong P, Kos VN, Guyeux C, Blanc DS, Bertrand X, Valot B, Hocquet D. 2018. Global emergence of the widespread Pseudomonas aeruginosa ST235 clone. Clin Microbiol Infect 24:258–266. 10.1016/j.cmi.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Hatt JK, Weigand MR, Krishnan R, Pavlostathis SG, Konstantinidis KT. 2018. Genomic and transcriptomic insights into how bacteria withstand high concentrations of benzalkonium chloride biocides. Appl Environ Microbiol 84:e00197-18. 10.1128/AEM.00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slipski CJ, Zhanel GG, Bay DC. 2018. Biocide selective TolC-independent efflux pumps in Enterobacteriaceae. J Membr Biol 251:15–33. 10.1007/s00232-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amsalu A, Sapula SA, Lopes MB, Hart BJ, Nguyen AH, Drigo B, Turnidge J, Ex Leong L, Venter H. 2020. Efflux pump driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated form different ecological niches: a case study in the development of multidrug resistance in environmental hotspots. Microorganisms 8:1647. 10.3390/microorganisms8111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weston N, Sharma P, Ricci V, Piddock LJV. 2018. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res Microbiol 169:425–431. 10.1016/j.resmic.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Amábile-Cuevas CF, Demple B. 1991. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res 19:4479–4484. 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariza RR, Cohen SP, Bachhawat N, Levy SB, Demple B. 1994. Repressor mutations in the marRAB operon that activate stress genes and multiple antibiotic resistance in Escherichia coli. J Bacteriol 176:143–148. 10.1128/jb.176.1.143-148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneiders T, Amyes SG, Levy SB. 2003. Role of AcrR and ramA in fluoroquinolone resistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob Agents Chemother 47:2831–2837. 10.1128/aac.47.9.2831-2837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido H, Pagès JM. 2012. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363. 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webber MA, Whitehead RN, Mount M, Loman NJ, Pallen MJ, Piddock LJ. 2015. Parallel evolutionary pathways to antibiotic resistance selected by biocide exposure. J Antimicrob Chemother 70:2241–2248. 10.1093/jac/dkv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber MA, Randall LP, Cooles S, Woodward MJ, Piddock LJ. 2008. Triclosan resistance in Salmonella enterica serovar Typhimurium. J Antimicrob Chemother 62:83–91. 10.1093/jac/dkn137. [DOI] [PubMed] [Google Scholar]
- 34.Curiao T, Marchi E, Viti C, Oggioni MR, Baquero F, Martinez JL, Coque TM. 2015. Polymorphic variation in susceptibility and metabolism of triclosan-resistant mutants of Escherichia coli and Klebsiella pneumoniae clinical strains obtained after exposure to biocides and antibiotics. Antimicrob Agents Chemother 59:3413–3423. 10.1128/AAC.00187-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touzel RE, Sutton JM, Wand ME. 2016. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J Hosp Infect 92:154–160. 10.1016/j.jhin.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Constantinides B, Chau KK, Quan TP, Rodger G, Andersson M, Jeffery K, Lipworth S, Gweon HS, Peniket A, Pike G, Millo J, Byukusenge M, Holdaway M, Gibbons C, Mathers A, Crook DW, Peto TEA, Walker AS, Stoesser N. 2020. Multi-omic surveillance of Escherichia coli and Klebsiella spp. in hospital sink drains and patients. bioRxiv https://www.biorxiv.org/content/10.1101/2020.02.19.952366v3. [DOI] [PMC free article] [PubMed]
- 37.Pelling H, Bock LJ, Nzakizwanayo J, Wand ME, Denham EL, MacFarlane WM, Sutton JM, Jones BV. 2019. De-repression of the smvA efflux system arises in clinical isolates of Proteus mirabilis and reduces susceptibility to chlorhexidine and other biocides. Antimicrob Agents Chemother 63:e01535-19. 10.1128/AAC.01535-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos Costa S, Viveiros M, Rosato AE, Melo-Cristino J, Couto I. 2015. Impact of efflux in the development of multidrug resistance phenotypes in Staphylococcus aureus. BMC Microbiol 15:232. 10.1186/s12866-015-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papkou A, Hedge J, Kapel N, Young B, MacLean RC. 2020. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat Commun 11:3970. 10.1038/s41467-020-17735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wand ME, Bock LJ, Bonney LC, Sutton JM. 2017. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 61:e01162-16. 10.1128/AAC.01162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frimodt-Møller J, Løbner-Olesen A. 2019. Efflux-pump upregulation: from tolerance to high-level antibiotic resistance? Trends Microbiol 27:291–293. 10.1016/j.tim.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Wilson AP, Livermore DM, Otter JA, Warren RE, Jenks P, Enoch DA, Newsholme W, Oppenheim B, Leanord A, McNulty C, Tanner G, Bennet S, Cann M, Bostock J, Collins E, Peckitt S, Ritchie L, Fry C, Hawkey P. 2016. Prevention and control of multi-drug-resistant Gram-negative bacteria: recommendations from a Joint Working Party. J Hosp Infect 92(Suppl 1):S1–S44. 10.1016/j.jhin.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Kanamori H, Weber DJ, Rutala WA. 2016. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 62:1423–1435. 10.1093/cid/ciw122. [DOI] [PubMed] [Google Scholar]
- 44.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, Peto TEA, Crook DW, Stoesser N. 2017. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections—a systemic review of the literature. Clin Infect Dis 64:1435–1444. 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 45.Voigt AM, Faerber HA, Wilbring G, Skutlarek D, Felder C, Mahn R, Wolf D, Brossart P, Hornung T, Engelhart S, Exner M, Schmithausen RM. 2019. The occurrence of antimicrobial substances in toilet, sink and shower drainpipes of clinical units: a neglected source of antibiotic residues. Int J Hyg Environ Health 222:455–467. 10.1016/j.ijheh.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Grillitsch B, Gans O, Kreuzinger N, Scharf S, Uhl M, Fuerhacker M. 2006. Environmental risk assessment for quaternary ammonium compounds: a case study from Austria. Water Sci Technol 54:111–118. 10.2166/wst.2006.840. [DOI] [PubMed] [Google Scholar]
- 47.McBain AJ, Bartolo RG, Catrenich CE, Charbonneau D, Ledder RG, Price BB, Gilbert P. 2003. Exposure of sink drain microcosms to triclosan: population dynamics and antimicrobial susceptibility. Appl Environ Microbiol 69:5433–5442. 10.1128/aem.69.9.5433-5442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. 2004. Effects of quaternary-ammonium based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol 70:3449–3456. 10.1128/AEM.70.6.3449-3456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall BM, Robleto E, Dumont T, Levy SB. 2012. The frequency of antibiotic-resistant bacteria in homes differing in their use of surface antibacterial agents. Curr Microbiol 65:407–415. 10.1007/s00284-012-0172-x. [DOI] [PubMed] [Google Scholar]
- 50.Capita R, Vicente-Velasco M, Rodríguez-Melcón C, García-Fernández C, Carballo J, Alonso-Calleja C. 2019. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci Rep 9:15905. 10.1038/s41598-019-51907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bock LJ, Hind CK, Sutton JM, Wand ME. 2018. Growth media and assay plate material can impact on the effectiveness of cationic biocides and antibiotics against different bacterial species. Lett Appl Microbiol 66:368–377. 10.1111/lam.12863. [DOI] [PubMed] [Google Scholar]
- 52.Wand ME, Bock LJ, Turton JF, Nugent PG, Sutton JM. 2012. Acinetobacter baumannii virulence is enhanced in Galleria mellonella following biofilm adaptation. J Med Microbiol 61:470–477. 10.1099/jmm.0.037523-0. [DOI] [PubMed] [Google Scholar]
- 53.Wand ME, Müller CM, Titball RW, Michell SL. 2011. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol 11:11. 10.1186/1471-2180-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. 2018. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544. 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jolley KA, Bliss CM, Bennett JS, Bratcher HB, Brehony C, Colles FM, Wimalarathna H, Harrison OB, Sheppard SK, Cody AJ, Maiden MCJ. 2012. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology (Reading) 158:1005–1015. 10.1099/mic.0.055459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.