Extracellular electron transfer (EET) is an important biological process in microbial physiology as found in dissimilatory metal oxidation/reduction and interspecies electron transfer in syntrophy in natural environments. EET also plays a critical role in microorganisms relevant to environmental biotechnology in metal-contaminated areas, metal corrosion, bioelectrochemical systems, and anaerobic digesters. Geobacter species exist in a diversity of natural and artificial environments.
KEYWORDS: Geobacter, cytochrome, extracellular electron transfer
ABSTRACT
Extracellular electron transfer (EET) is an important biological process in microbial physiology as found in dissimilatory metal oxidation/reduction and interspecies electron transfer in syntrophy in natural environments. EET also plays a critical role in microorganisms relevant to environmental biotechnology in metal-contaminated areas, metal corrosion, bioelectrochemical systems, and anaerobic digesters. Geobacter species exist in a diversity of natural and artificial environments. One of the outstanding features of Geobacter species is the capability of direct EET with solid electron donors and acceptors, including metals, electrodes, and other cells. Therefore, Geobacter species are pivotal in environmental biogeochemical cycles and biotechnology applications. Geobacter sulfurreducens, a representative Geobacter species, has been studied for direct EET as a model microorganism. G. sulfurreducens employs electrically conductive pili (e-pili) and c-type cytochromes for the direct EET. The biological function and electronics applications of the e-pili have been reviewed recently, and this review focuses on the cytochromes. Geobacter species have an unusually large number of cytochromes encoded in their genomes. Unlike most other microorganisms, Geobacter species localize multiple cytochromes in each subcellular fraction, outer membrane, periplasm, and inner membrane, as well as in the extracellular space, and differentially utilize these cytochromes for EET with various electron donors and acceptors. Some of the cytochromes are functionally redundant. Thus, the EET in Geobacter is complicated. Geobacter coordinates the cytochromes with other cellular components in the elaborate EET system to flourish in the environment.
INTRODUCTION
Extracellular electron transfer (EET) is a biological activity found in various microorganisms in a diversity of environments (1, 2). Recently it was shown that EET occurs in the mammalian gut (3). In EET, cells export electrons generated in the cytoplasm through the cellular membrane onto an extracellular electron acceptor or import electrons drawn from an extracellular electron donor through the cellular membrane into the cytoplasm. Microorganisms can gain energy for growth by drawing electrons from (oxidizing) and donating electrons to (reducing) extracellular metals (4, 5). Microorganisms can grow in syntrophy, in which different microorganisms exchange electrons with each other (6, 7). EET is crucial for environmental biotechnology, such as bioremediation, metal corrosion, bioelectrochemical systems, and anaerobic digesters (8–11). These EET processes can be direct or indirect. In the indirect way, microorganisms synthesize and secrete redox-active compounds or make use of active redox compounds present in their surrounding environments as electron carriers between the microorganisms and electron donors/acceptors (12, 13). In the direct way, microorganisms physically interact with solid electron donors/acceptors (4–7). The direct transfer is considered to be more advantageous because the synthesis and secretion of redox-active compounds may be costly, and these compounds diffuse and may not be available when needed.
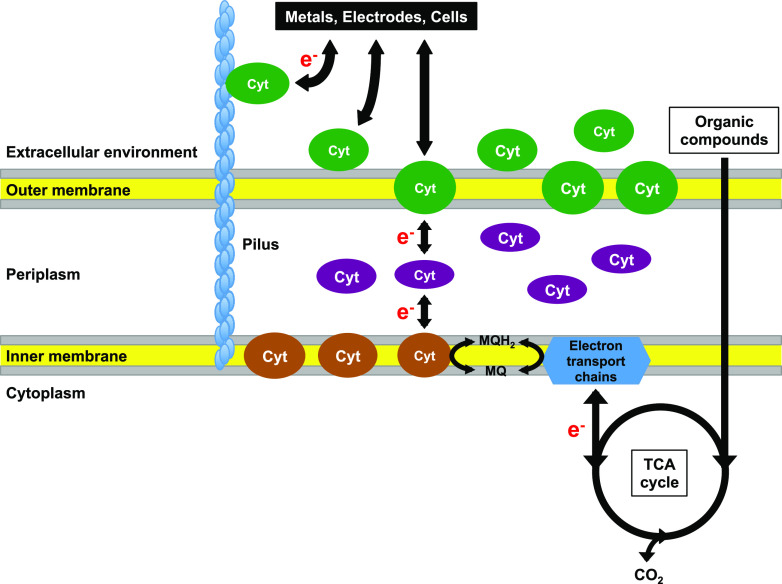
Geobacter species are present in a variety of environments, such as the subsurface where metal reduction is an important biogeochemical process (14) and methanogenic soils and marine sediments where their syntrophic growth occurs with methanogens (6, 7). They also play roles in environmental biotechnology for bioremediation, current production, electrosynthesis, iron corrosion, and anaerobic digesters (6, 7, 14–17). Therefore, they are of ecological significance in the environment and of great value in biotechnology. One of the superb characteristics of Geobacter species is the capability of direct EET with solid metals, electrodes, and other cells (Fig. 1) (18). Geobacter species oxidize organic compounds via the tricarboxylic acid (TCA) cycle (19, 20), which generates electrons that are transported through inner and outer membranes to extracellular electron acceptors, or take up electrons from extracellular electron donors that are transported inside cells. Geobacter sulfurreducens, which possesses the hallmarks of Geobacter species, has been investigated for EET as a model microorganism. G. sulfurreducens reduces metal oxides via direct electron transfer (21); consumes and produces currents via direct electron uptake from the cathode and direct electron donation to the anode, respectively (22, 23); directly accepts electrons from Fe(0) (17); and performs direct interspecies electron transfer (DIET) with other cells in syntrophic cocultures as the electron accepting (24) or donating partner (25).
FIG 1.
Extracellular electron transfer in Geobacter. Cyt, cytochrome; MQ, menaquinone; MQH2, menaquinol.
Electrically conductive pili (e-pili) and c-type cytochromes play critical roles in the direct EET in G. sulfurreducens (Fig. 1) (14, 18, 21). The e-pili are particularly important for long-range direct EET (26–28). The biological function and biotechnology applications of the e-pili have been reviewed recently (26–30), and this review focuses on c-type cytochromes. Genomes of Geobacter species encode an exceptionally large number of c-type cytochromes, but in contrast to high conservation of other energy metabolism proteins, very few cytochromes are well conserved in Geobacter species (31). The genome of G. sulfurreducens (3.81 Mbp) encodes 111 c-type cytochromes (32). Among them, 78 cytochromes have multihemes with an average of 7.5 hemes per cytochrome and are distributed in all subcellular locations (inner membrane, periplasm, outer membrane) (31). The genome of Shewanella oneidensis (4.97 Mbp), another model microorganism for EET study (33), codes for 39 c-type cytochromes (34). S. oneidensis employs indirect EET via flavin as the electron shuttle (35). Substantial redundancy is recognized in each step or subcellular location in the EET in G. sulfurreducens. Cytochromes discussed in this review are listed in Table S1 in the supplemental material. EET in S. oneidensis has been reviewed recently (33, 36), and the research on the molecular mechanism of EET has been most intensively conducted with G. sulfurreducens among Geobacter species. The cytochromes for the EET in G. sulfurreducens are summarized below.
INNER MEMBRANE
Inner membrane cytochromes connect the electron flow between intracellular electron transport chains and periplasmic cytochromes.
Cbc complex.
The G. sulfurreducens genome encodes four putative cytochrome bc (Cbc) complexes (Cbc3 to Cbc6) and the single protein Cbc1 (CbcY, CbcL) containing b-type and c-type cytochrome domains. The Cbc complexes are considered to provide the electron transfer between the menaquinone/menaquinol pool in the inner membrane and periplasmic cytochromes and generate the proton gradient across the inner membrane that influences ATP synthesis (31). CbcC, a c-type cytochrome for Cbc5, was important for reduction of Fe(III) oxide, and CbcV, an iron-sulfur cluster-binding protein for Cbc3, was important for reduction of Fe(III) citrate and Fe(III) oxide (37). Despite downregulation of transcript abundance of cbc1 during growth on Fe(III) oxide in comparison with Fe(III) citrate (37), deletion of cbc1 resulted in decreased rates of Fe(III) oxide reduction (38). The deletion also caused slower current production than the parent strain at the poised potential of −100 mV (38).
The Cbc complexes are well conserved in Geobacter species (31). Because of the electrical link between the inner membrane and periplasm and the potential influence on ATP synthesis, it is likely that the Cbc complexes take critical part in the respiration of Geobacter species in the environment.
ImcH.
ImcH was identified by transposon mutagenesis, and insertion mutations in its gene caused an inability to grow on Fe(III) citrate as the electron acceptor (39). Disruption of imcH eliminated the ability to reduce Fe(III) citrate, Fe(III)-EDTA, and Mn(IV) oxides (39). The gene disruption also inhibited capability to produce currents at poised potentials greater than +100 mV.
It was suggested that two different electron transfer pathways exist in the inner membrane, one containing Cbc1, which is required for reduction of electron acceptors with reduction potentials at or below −100 mV, and the other including ImcH, which is necessary for reduction of electron acceptors with reduction potentials above −100 mV (38–40). Thus, it was proposed that G. sulfurreducens cells could sense the redox potential of extracellular electron acceptors (40). It remains to be seen whether or not disruption of both Cbc1 and ImcH can eliminate the ability to reduce all of these electron acceptors and produce currents at various poised potentials. These suggest that the electron transport between the inner membrane and periplasm may be more elaborate than proposed (31). The Cbc complexes and ImcH have not been purified and biochemically characterized.
MacA.
The inner membrane-associated diheme c-type cytochrome MacA is important for the reduction of Fe(III) citrate (41), Fe(III) oxide (37), and U(VI) (42). It is proposed that MacA is a regulator for expression of another cytochrome rather than an electron carrier in the EET pathway. Expression of OmcB, an outer membrane c-type cytochrome important for Fe(III) reduction, was not detected in a macA deletion strain, and in trans expression of OmcB in the macA deletion strain restored the ability to reduce Fe(III) (43). Recombinant MacA purified from Escherichia coli contained two low-spin heme groups with reduction potentials of −237 mV and −138 mV (44). The recombinant MacA transferred electrons to PpcA (44, 45), but physiological relevance of this activity is not clear. Fe(III) reductase activity was not observed with the recombinant MacA (44). The sequence of MacA belongs to the family of the diheme cytochrome c peroxidase (46). The recombinant MacA displayed similar electrochemical properties to other bacterial diheme peroxidases and hydrogen peroxide reductase activity (44). However, the deletion of macA had no impact on response to oxidative stress (41).
PERIPLASM
Periplasmic cytochromes link the electron transfer between inner and outer membranes.
PpcA homologs.
G. sulfurreducens has five homologs of small periplasmic c-type cytochromes named PpcA, PpcB, PpcC, PpcD, and PpcE. All of the homologs have three c-type heme binding motifs, their protein sizes are 70 to 75 amino acids in length, and their protein sequences share 45 to 77% identities (47, 48). PpcA has been most intensively studied among periplasmic cytochromes, as it has been considered to be a key periplasmic cytochrome in the EET in G. sulfurreducens (14, 48). Transcript abundance was highest for ppcA, and PpcA was the most abundant protein under several growth conditions among these homologs (49–51).
PpcA was isolated from periplasmic (52) or soluble (53) fractions of G. sulfurreducens and purified to homogeneity. PpcA has a signal sequence, and N-terminal sequence of the purified protein indicated cleavage of the signal sequence (53). The purified PpcA contained three hemes, consistent with the predicted three heme binding motifs (53). The midpoint reduction potentials of PpcA purified from G. sulfurreducens were −167 mV (53) and −169.5 mV (52) (Table 1). Recombinant PpcA purified from E. coli showed a higher midpoint reduction potential of −117 mV (54) than those of PpcA from G. sulfurreducens. Reduction potentials of the three hemes, termed hemes I, III, and IV, in the recombinant PpcA were −147, −104, and −111 mV, respectively (54). The region near heme I was identified as the most dynamic segment, which may be involved in interaction with other molecules (55, 56). In addition to the heme-heme redox interactions, the heme-reduction potentials were modulated by the solution pH (55, 56). This was designated a redox-Bohr effect by analogy with the Bohr effect in hemoglobin (55, 56). The redox-Bohr center was located in the vicinity of heme IV (55, 56). The redox-Bohr effect is considered to be a crucial property, which allows PpcA to perform a concerted electron/proton transfer and to contribute to the proton gradient across the cytoplasmic membrane that drives ATP synthesis (55–57). Recombinant PpcA homologs showed similar reduction potentials (48, 55, 56). Structures of the PpcA homologs have the highest degree of conservation around heme IV, and the protein surface around this heme is positively charged in all homologs, suggesting that all PpcA homologs may interact with similar molecules involving this region (47, 55, 56). The structures and surface characteristics near the other two hemes in the homologs differ, and thus each of the homologs may interact with a unique partner via an interface involving the regions of these hemes (47, 55, 56).
TABLE 1.
Key cytochromes in extracellular electron transfer in G. sulfurreducens
| Name | Location | Reduction potentialb |
Substrateb |
||
|---|---|---|---|---|---|
| MP | W | In vitro | In vivo | ||
| Cbca | Inner membrane | ND | ND | ND | An, FC, FO |
| ImcH | Inner membrane | ND | ND | ND | An, FC, FE, Mn |
| PpcAc | Periplasm | −167 | 210 | Aq, FC, FO, FN, H, Mn, U, Cr, S | Aq, FC, U |
| OmcB | Outer membrane, porin complex | −190 | ND | FC, FN, FO | FC, FO |
| OmcS | Outer membrane, e-pili | −212 | 320 | Aq, Au, Cr, FC, FO, H, Mn, U | An, D, Fe(0), FO, Mn |
| OmcZ | Anode-biofilm interface | −220 | 360 | Aq, Au, Cr, FC, U, Mn | An, Fe(0), U |
Cbc includes Cbc1, Cbc3, and Cbc5 complexes.
ND, not determined; MP, midpoint reduction potential (mV); W, reduction potential window (mV); In vitro, reduction by purified cytochrome; In vivo, negative impact on growth by gene deletion; An, anode; Aq, AQDS; Au, Au(III); Cr, Cr(VI); D, DIET; FC, Fe(III) citrate; FE, Fe(III)-EDTA; FN, Fe(III)-nitrilotriacetic acid; FO, Fe(III) oxide; H, humic acid; Mn, Mn(IV) oxide; S, elemental sulfur; U, U(VI).
Reduction potentials for PpcA are from reference 52.
The purified PpcA could reduce a variety of substrates (Table 1) (52, 53). Cell suspensions of a G. sulfurreducens ppcA deletion strain reduced Fe(III) citrate at a rate 60% of that of the wild-type, and the deletion strain extended a lag period before adapting to growth on Fe(III) citrate as the electron acceptor (53). The cell suspension assays also demonstrated decreased reduction rates for the humic substance analogue anthraquinone-2,6-disulfonate (AQDS) and U(VI) (53). These suggest that PpcA is an important, but not essential, component for the EET.
Potential substitutes for PpcA for facilitating the electron transfer across the periplasm are the PpcA homologs, PpcB to PpcE, because the homologs may interact with common redox partners as described above (47, 48). A G. sulfurreducens strain possessing PpcA but lacking PpcB to PpcE could reduce Fe(III) citrate, but a G. sulfurreducens strain lacking all of the PpcA homologs could not (58). The strain lacking all of the PpcA homologs did not adapt to growth on Fe(III) citrate (58), unlike the strain lacking only PpcA (53), suggesting that one or more of PpcB to PpcE played a role in the adaptation of the strain lacking only PpcA to growth on Fe(III) citrate. It remains to be seen if one of PpcB to PpcE is engineered to be expressed in a level as high as PpcA in the strain lacking all of the PpcA homologs and can confer the ability to reduce Fe(III) citrate similar to the strain possessing only PpcA but lacking PpcB to PpcE.
As the PpcA homologs are highly conserved in Geobacter species (31), it is likely that they play an essential role in the respiration of Geobacter species in the environment. The presence of the multiple homologs may ensure that a route for the electron transfer between the inner and outer membranes is always established in Geobacter cells.
PccH.
PccH was identified as a highly upregulated gene by comparing the transcriptomes of G. sulfurreducens cells grown under current consuming conditions versus current producing conditions (59). Deletion of pccH from G. sulfurreducens resulted in failure of the current consumption but not production (59). The deletion also caused impaired reduction of Fe(III) oxide (37). PccH is a monoheme c-type cytochrome that is predicted to be localized in periplasm, but its actual location has not been verified yet. Recombinant PccH was purified from the periplasmic fraction of E. coli (60). PccH presents a unique structure, different from all known c-type cytochromes (60–62). PccH has the lowest reduction potential observed to date for a monoheme cytochrome, −24 mV at pH 7, which is about 300 mV more negative than the measurement of horse heart cytochrome c (60, 62). In the case of monoheme cytochrome, its reduction potential corresponds to the heme group’s reduction potential. These distinctive properties, along with phylogenetic analysis, set PccH apart from the other class I cytochromes and monoheme cytochromes from G. sulfurreducens, suggesting that it belongs to a new subfamily (60–62). An electron transfer partner for PccH was proposed to be the cytochrome GSU2515 (63). However, GSU2515 has not been studied previously, and this proposal has not been investigated yet.
OUTER MEMBRANE
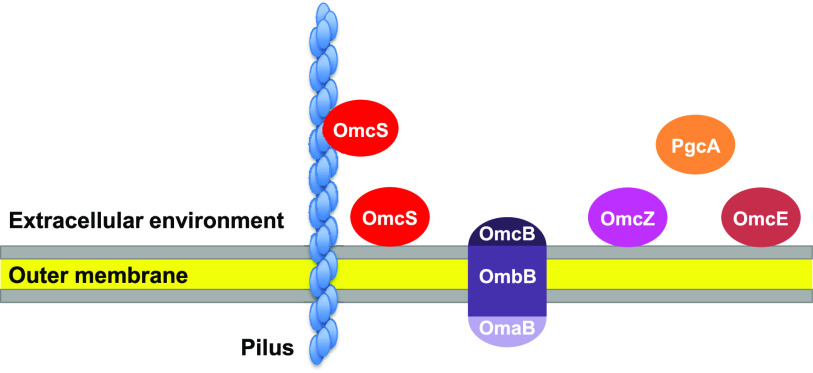
Outer membrane cytochromes are the first or final step in the direct EET. Although the term “outer membrane” is used, the actual location of these cytochromes varies significantly (Fig. 2). This variety appears to greatly contribute to the ability of direct EET with diverse extracellular electron donors and acceptors.
FIG 2.
Location of major outer membrane cytochromes. OmcB forms a complex with the porin-like outer membrane protein OmbB and the periplasmic c-type cytochrome OmaB.
Porin-cytochrome complex.
Porin-cytochrome (Pcc) complex is important for EET (64, 65). The complex consists of a porin-like outer membrane protein, a periplasmic c-type cytochrome, and an outer membrane c-type cytochrome (Fig. 2). Four gene clusters for Pcc complexes—GSU2739-2737 (ombB-omaB-omcB), GSU2733-2731 (ombC-omaC-omcC), GSU2642-2645 (extABCD), and GSU2726-2724 (extEFG)—were identified in the G. sulfurreducens genome (66).
The Pcc complex containing the outer membrane c-type cytochrome OmcB is important for Fe(III) reduction in G. sulfurreducens (67–69). This complex consists of the porin-like protein OmbB, the periplasmic cytochrome OmaB, and the outer membrane cytochrome OmcB (Fig. 2). The OmaB/OmbB/OmcB complex and MtrABC, a Pcc complex from S. oneidensis, appear to be similar in overall structure, subcellular localization, and physiological function, but they share little protein sequence similarity (68). OmcB has a signal sequence similar to those of lipoproteins (70). Electrochemical studies of purified OmcB estimated a midpoint reduction potential of −190 mV (70) (Table 1). For large cytochromes with multiple hemes, the assignment of the reduction potentials to the individual redox centers is not possible with the currently available methodologies, and thus the individual redox properties of the hemes cannot be determined for all cytochromes (48). Purified OmcB reduced Fe(III)-nitrilotriacetic acid and synthetic ferrihydrite (70) (Table 1). The OmaB/OmbB/OmcB complex was isolated, and the average heme content of the isolated complex was 20, corresponding to the sum of c-type heme binding motifs in OmcB (12) and OmaB (8) (68). The isolated complex reconstituted in proteoliposomes transferred electrons from reduced methyl viologen across the lipid bilayer of the liposomes to Fe(III) citrate and ferrihydrite (68) (Table 1). Studies by proteinase K treatment and immunogold labeling revealed that OmcB was embedded within the outer membrane, but a portion of OmcB was exposed on the outer surface (71).
Deletion of omcB greatly impaired the ability to reduce Fe(III) citrate and Fe(III) oxide (67–69). The deletion slightly decreased the rate of AQDS reduction (72). G. sulfurreducens has an OmcB homolog, OmcC (67). In contrast to OmcB, OmcC was dispensable for Fe(III) reduction (67) but might compensate for the lack of OmcB for Fe(III) reduction, as omcC transcript was upregulated in the OmcB deletion strain that was adapted to growth on Fe(III) citrate (73). In addition to omcC, other genes for outer membrane c-type cytochromes were upregulated in the adapted OmcB deletion strain (73). In contrast to Fe(III) citrate, the OmcB deletion strain never adapted to growth on Fe(III) oxide (73). The abundance of omcB transcript and OmcB protein was higher during growth on Fe(III) than on fumarate (49, 51). Expression of OmcB appears to be controlled by other outer membrane c-type cytochromes, such as OmcF, OmcG, and OmcH, and the inner membrane cytochrome MacA as described above. Deletions of these cytochromes caused defective ability to reduce Fe(III) citrate, but the function of these cytochromes might be regulation of OmcB expression rather than an electron carrier to Fe(III), as OmcB was not expressed in these deletion mutants (41, 43, 74, 75). The regulatory mechanism of these cytochromes in OmcB expression is not elucidated.
GSU2724 (omcV, extG) encoding an outer membrane c-type cytochrome was identified as a gene whose transcript abundance increased during growth on Fe(III) oxide or Mn(IV) oxide in comparison with growth on Fe(III) citrate, and deletion of this gene impaired growth on Fe(III) oxide (37). OmcV could contribute to Fe(III) citrate reduction when genes for other outer membrane cytochromes, omcBCEST, were deleted (58).
GSU2642 (omcW, extD) encoding an outer membrane c-type cytochrome was not essential for Fe(III) citrate reduction but could play a role in Fe(III) citrate reduction when genes for other outer membrane cytochromes, omcBCESTV, were deleted (58). The GSU2642-2645 (extABCD) cluster was also identified by transposon analysis for defective growth on an anode (76). The cluster seems to be required for optimal current production, as a deletion strain of the extABCD cluster exhibited delayed initiation and decreased rates of current production at −100 mV (76).
OmcB alone may be sufficient for Fe(III) reduction as the outer membrane c-type cytochrome in the Pcc complex homologs in G. sulfurreducens. Reintroduction of omcB in the septuple deletion strain, which lacked genes for outer membrane cytochromes (omcBCESTVW) and could not reduce Fe(III), restored the ability to reduce Fe(III) (58). The OmcB complex appears to be the major route in the electron transfer connecting periplasmic cytochromes and extracellular electron acceptors. Fe(III) reduction is considered to be the vital mode of respiration for Geobacter species in the natural environment. Gene clusters for Pcc complexes were identified in all sequenced Geobacter species (66). Therefore, OmcB or its counterpart may play a crucial role in growth of Geobacter species in the natural environment.
OmcS.
OmcS was identified as one of the most abundant proteins that were easily sheared from the outer surface of G. sulfurreducens cells grown on Mn(IV) oxide as the electron acceptor (77). Immunogold study showed that OmcS was localized on the outer surface and along the e-pili (78). Isolated OmcS was shown to form conductive filaments, indicating new possibilities for long-range EET (79, 80). The filaments may increase the cell surface area that is coated with redox active components and provide a route for the electron transfer between outer surface cytochromes and the e-pili for the long-range EET. However, OmcS filaments emanating from G. sulfurreducens cells were not observed (78). Further investigation is expected to better understand OmcS filaments. OmcS was predicted to have a signal sequence, and the molecular mass of purified OmcS was closer to the calculated molecular mass without the signal sequence than with the signal sequence (81). The purified OmcS had six hemes, the same as the number of predicted c-type heme binding motifs, showed a low midpoint potential (−212 mV) with a redox curve that spans over a broad range of reduction potentials (−360 to −40 mV), and could reduce a diverse range of substrates (81) (Table 1). The broad range of its reduction potential may allow OmcS to accept electrons from and donate electrons to a variety of electron carrier compounds and proteins, consistent with the physiological function.
OmcS was essential for EET to insoluble Mn(IV) oxide and Fe(III) oxide (77). OmcS was also involved in reduction of AQDS and soil humic acid (72). OmcS might also play a role in Fe(III) citrate reduction when OmcB was absent because the OmcB deletion strain adapted to growth on Fe(III) citrate expressed more omcS transcripts than the wild type (73). Deletion of OmcS inhibited current production when grown on an anode in an H-cell (see Fig. S1 in the supplemental material) that was not being operated in the flowthrough mode and when the biofilm was very thin (82). However, the OmcS deletion did not have a negative effect on transferring electrons to an anode in the flowthrough mode when the biofilm was thick (83) or on drawing electrons from a cathode (59). OmcS was essential for G. sulfurreducens as the electron-accepting partner in syntrophic coculture with Geobacter metallireducens via DIET (24). It has been hypothesized that OmcS serves as an electrical contact to promote electron transfer to and from the e-pili (26). Magnetite could serve as a substitute for OmcS (84). Magnetite was associated with the e-pili, and the expression of omcS was downregulated in the presence of magnetite (84). The OmcS deletion strain was effective in DIET and Fe(III) oxide reduction in the presence of magnetite (84). OmcS could also play a critical role in drawing electrons from Fe(0), as the omcS transcript was upregulated during growth on Fe(0) as the electron donor and deletion of omcS resulted in failure to grow on Fe(0) (17). Therefore, OmcS facilitates direct contact with the solid extracellular electron donors and acceptors. However, it was shown that G. sulfurreducens strain KN400 did not require OmcS for DIET and Fe(III) oxide reduction, indicating that the requirement of OmcS for EET is strain specific (85).
In the natural environment, insoluble forms of Fe(III) are more prevalent than soluble forms and are likely the source of the electron acceptor for the respiration of Geobacter species. OmcS is not conserved in Geobacter species (31). However, a cytochrome that was identified by the proteomic analysis of Geobacter species in the environment and had no sequence similarity to OmcS could substitute for OmcS in the reduction of Fe(III) oxide (86). Thus, an equivalent of OmcS may be involved in the reduction of insoluble Fe(III) by Geobacter species in the environment.
OmcZ.
OmcZ was identified by transcriptome analysis comparing G. sulfurreducens biofilms grown on an anode as the electron acceptor and fumarate as the electron acceptor and was essential for high-density current production with thick biofilms in the flowthrough H-cell system (83). In contrast, OmcZ was not important for current production with thin biofilms in the H-cell system that was not being operated in the flowthrough mode (82). It was predicted that OmcZ has eight heme binding motifs and a signal sequence, and its signal-sequence-cleaved form does not have transmembrane signatures (87). OmcZ exists as two forms, 50-kDa and 30-kDa proteins, termed OmcZL and OmcZS, respectively, and OmcZS is a proteolytic product of OmcZL and contains eight hemes (87). Conductive filaments comprising OmcZ were observed, and this observation may lead to better understanding of the EET via OmcZ (88). Contribution of OmcZ filaments to the conductivity of G. sulfurreducens biofilms was reported (88), but earlier studies showed that inactivating cytochromes had no impact on the biofilm conductivity, and the biofilm conductivity was attributed to networks of the e-pili (89). OmcZ filaments associating with G. sulfurreducens cells were not found (90). Redox titration analysis revealed that the midpoint reduction potential of OmcZS was −220 mV with a large reduction potential range (−420 to −60 mV) (87) (Table 1). Its large reduction potential range was considered to be important for the ability to reduce various extracellular electron acceptors (Table 1) except Fe(III) oxide (87). This is consistent with the phenotype that the G. sulfurreducens strain lacking omcZ retains the ability to reduce Fe(III) oxide (37). Recombinant OmcZS was shown to bind riboflavin (91). Riboflavin was proposed to function as a redox cofactor bound to the outer membrane c-type cytochromes, not as an electron shuttle, for current production (92), reduction of Mn(IV) mineral birnessite (93), and syntrophic cocultures (94). It is unlikely that flavin or other small soluble molecules serve as the electron shuttle for G. sulfurreducens (95).
Although the deletion of omcZ alone did not inhibit reduction of AQDS and soil humic acid, OmcZ could contribute to the reduction of these extracellular electron acceptors when genes for other outer membrane cytochromes, omcBSTE, were deleted (72). OmcZ was also necessary for optimal reduction of U(VI) (96). As described above, G. sulfurreducens could adapt to the loss of outer membrane cytochromes in order to reduce soluble electron acceptors (58, 72, 73). However, the OmcZ deletion strain has never adapted to regain the ability of high-density current production with biofilms (83). Immunogold study demonstrated that OmcZ was highly concentrated at the biofilm-anode interface when G. sulfurreducens was grown on an anode as the electron acceptor (90). This specific localization was not observed when G. sulfurreducens was grown on an anode but fumarate was the electron acceptor. Electrochemical analyses suggested higher resistance of electron transfer between the biofilm and the anode in the OmcZ deletion cells than in the wild type (97). OmcZ may serve as an electrochemical gate facilitating electron transfer from G. sulfurreducens biofilms to the anode surface. In contrast, deletion of omcZ had no negative impact on drawing electrons from a cathode (59). However, OmcZ could play an important role in accepting electrons from Fe(0) because omcZ expression was upregulated during growth on Fe(0) as the electron donor and deletion of omcZ inhibited growth on Fe(0) (17).
Other outer membrane c-type cytochromes.
In the G. sulfurreducens genome, downstream of omcS, there is a gene termed omcT that is cotranscribed with omcS, and OmcT is a homolog of OmcS with 62% protein sequence identity (77). The transcript of omcT was highly upregulated during growth on Fe(III) or Mn(IV) oxide as the electron acceptor (37) and on Fe(0) as the electron donor (17). However, the OmcT protein was not detected among the sheared proteins (77), and proteome analysis did not identify OmcT as an abundant protein during growth on Fe(III) oxide (50). Posttranscriptional regulation may exist for its expression. OmcT was not required for reduction of Fe(III) oxide, Mn(IV) oxide, Fe(III) citrate, and fumarate (77).
OmcE was another abundant c-type cytochrome among the sheared proteins (77). It was important for the reduction of Fe(III) oxide but with less impact than OmcS, as an omcE deletion strain was still capable of reducing Fe(III) oxide with a long lag period and at a lower reduction rate than the wild type (77). Reduction of Mn(IV) oxide was also affected with a lag period in contrast to the wild type. The ability to reduce AQDS and soil humic acid was slightly impaired by the deletion of OmcE (72). U(VI) reduction was influenced by the OmcE deletion (42). One or more of OmcB, OmcE, OmcS, OmcT, and OmcZ may be involved in reduction of Hg(II), as the deletion strain of these cytochromes decreased Hg(II) reduction (98). OmcE has not been characterized by biochemical methods.
PgcA is an extracellular c-type cytochrome. It was identified by an adaptive evolution study for faster reduction of Fe(III) oxide by the wild-type G. sulfurreducens strain (99). The adaptive evolution generated mutations in a GEMM (genes related to the environment, membranes, and motility) riboswitch sequence for expression of pgcA that resulted in its increased expression and enhanced ability to reduce Fe(III) oxide. Another adaptive evolution study with G. sulfurreducens strain KN400 lacking the gene for the pilin PilA of the e-pili also identified PgcA (95). The PilA deletion strain was severely impaired for Fe(III) oxide reduction, but an adapted PilA deletion strain gained capability to reduce Fe(III) oxide by producing PgcA as the electron shuttle. Addition of purified PgcA to cell suspension assays for Fe(III) oxide reduction increased the reduction rates (100). However, the adapted PilA deletion strain remained unable to produce high levels of currents (95). Producing electron shuttles is costly, and diffusion of electron shuttles is problematic as they may be lost when required. Accordingly, the strain expressing the e-pili capable of direct EET to Fe(III) oxide outcompeted the adapted strain lacking the e-pili and producing PgcA when they were grown on Fe(III) oxide (95). This adapted evolution of the PilA deletion strain also created a mutation in the GEMM riboswitch sequence for pgcA, and increased pgcA expression was observed (95).
OmcF is important for reduction of Fe(III) citrate (74), Fe(III) oxide (37), and U(VI) (42) and for current production (101). As described above, OmcF appears to influence the expression of OmcB and is considered as a regulator rather than an electron transfer carrier (74). A soluble part of OmcF was purified from E. coli (102). Unlike the other outer membrane cytochromes (OmcB, OmcS, OmcZ), the reduction potential of the recombinant OmcF was +180 mV (102), which is much higher than those of OmcB, OmcS, and OmcZ and may be related to the consideration that OmcF is a regulator and not an electron transfer component. Structure of the recombinant OmcF was similar to that of cytochrome c6 from the green alga Monoraphidium braunii (102). The cytochrome c6 in algae and cyanobacteria mediates electron transfer from cytochrome b6f to photosystem I (103).
SPECIFICITY OF CYTOCHROMES
It has been thought that the outer membrane cytochromes for the reduction of the soluble extracellular electron acceptors such as Fe(III) citrate and humic substances are less specific while OmcS and OmcZ for the EET with insoluble electron donors and acceptors are highly specific. OmcS and OmcZ are not highly conserved in Geobacter species (31). Locations of these cytochromes are considered to be important for their EET ability, as access to the insoluble electron donors/acceptors is more restricted than the soluble ones. When the gscA gene encoding a c-type cytochrome that is homologous to the Geobacter cytochrome identified in the in situ uranium bioremediation site was expressed in the OmcS deletion strain, the capacity for Fe(III) oxide reduction was restored (86). Atomic force microscopy showed that GscA was associated with the e-pili like OmcS (86). OmcS and GscA are 432 and 1,027 amino acids in length with 6 and 10 c-type heme binding motifs, respectively, and are not similar to each other, with only 24% identity in protein sequence. These support that location of a cytochrome is critical for the Fe(III) oxide reduction. Similar study for OmcZ has not been conducted yet.
MODEL FOR EET
It is likely that the Cbc complexes and ImcH in the inner membrane are involved in redox reactions with the menaquinone/menaquinol pool in G. sulfurreducens, but this remains to be verified. The predominant electron transfer bridge between the inner and outer membranes is the periplasmic cytochrome PpcA (Fig. 3). The major electron transfer pathway from the outer membrane to extracellular Fe(III) is mediated by the OmaB/OmbB/OmcB complex (Fig. 3). It is considered that the electron transfer from PpcA to OmcB occurs via the periplasmic cytochrome OmaB in the complex. Although the reduction potential of OmaB is not known, the reduction potential window of PpcA overlaps the midpoint reduction potential of OmcB (Table 1). In the case of soluble Fe(III) citrate, OmcB is likely the terminal Fe(III) reductase, and the electron transfer from OmcB to Fe(III) citrate (reduction potential, +372 mV) (104) appears to go downhill. For insoluble Fe(III) oxide, the OmaB/OmbB/OmcB complex transfers electrons to OmcS, which is associated with the e-pili and acts as the terminal reductase (Fig. 3). The reduction potential window of OmcS overlaps the midpoint reduction potential of OmcB (Table 1) and reduction potentials of various Fe(III) oxides (104). In addition to the cytochromes and the e-pili, putative multicopper oxidases are known to be important for the EET to Fe(III) oxide in G. sulfurreducens (105, 106). One of the putative multicopper oxidases, OmpB, was very loosely associated with the cell surface, and its majority was present in the culture supernatant (71). The function of these putative multicopper oxidases is not known. Flagella could enhance the ability of G. sulfurreducens to reduce Fe(III) oxide (107). Resurrecting the gene for the master regulator of flagella and motility gene expression conferred flagella production and motility in the otherwise nonmotile G. sulfurreducens. The motility is considered to be important for growth of Geobacter in the environment in order to locate Fe(III) oxide (14).
FIG 3.
Model for minimal route in extracellular electron transfer to Fe(III) in G. sulfurreducens.
For the anode as the electron acceptor, OmcZ localized at the biofilm-anode interface functions as the terminal electron carrier from the cell to the anode. The reduction potential window of OmcZ (Table 1) covers various anode potentials poised in G. sulfurreducens fuel cells, suggesting that OmcZ is able to directly transfer electrons to the anode. Unlike OmcS, interaction of OmcZ with the e-pili was not observed (90), and it is not clear how electron transfer takes place far from the anode surface within biofilms. G. sulfurreducens appears to differentially utilize the Cbc complex and ImcH in the inner membrane with respect to the potential of the anode (40). Periplasmic cytochromes essential for the EET to the anode have not been identified yet.
It appears to be possible that reverse flow of electrons from other cells to G. sulfurreducens cells in DIET takes place via OmcS and the e-pili. OmcS as well as OmcZ may also be the interaction point with Fe(0) as the electron donor. However, the electron transfer route from the outer membrane into the cell has not been determined for the EET from the other cell and Fe(0) yet. The periplasmic cytochrome PccH may be an electron carrier from the outer membrane to the inner membrane in a similar manner proposed for the cathode as the electron donor. However, the function of PccH has not been evaluated for DIET and the electron uptake from Fe(0). Cytochromes in the outer and inner membranes that are important for the direct EET from the cathode have not been identified yet.
The majority of the cytochromes in Geobacter species have multiple hemes (31). The broad reduction potential windows of the multiheme cytochromes appear to allow direct EET with a variety of electron donors/acceptors in different reduction potentials, permitting Geobacter species to grow in a diversity of environments (108, 109).
PERSPECTIVES
The EET from electron donors is less understood. The wild-type G. sulfurreducens can consume currents. However, its current consumption rate is low and its current-consuming biofilms are much thinner than its current-producing biofilms. Consequently, investigation of the electron uptake with the cathode-grown cells has not progressed much. In contrast, G. sulfurreducens strain ACL consumed currents at a 10-fold higher rate and formed much thicker biofilms on the cathode than the wild type (15). Thus, the ACL strain may guide future investigation of electron uptake. With the thick biofilms grown on the cathode, transcriptome and proteome analyses as conducted with the biofilms grown on the anode will be readily feasible, and genes and proteins that are differentially expressed will be identified. The function of the identified genes and proteins in the EET from the cathode may be elucidated by genetic methods. The proteins can be characterized by biochemical and biophysical methods as employed for those involved in other EET pathways. Although Geobacter species have not been isolated as an iron-corroding agent from environments, G. sulfurreducens may become a model microorganism for iron corrosion research. G. sulfurreducens is the only genetically tractable microorganism that is able to directly draw electrons from iron (17).
Structural study is required for the OmaB/OmbB/OmcB complex. Recently, the atomic structure of the S. oneidensis MtrABC complex was reported (110). Similar approaches should be applicable to the structural study in G. sulfurreducens. Although structures of isolated OmcS and OmcZ filaments were reported, physiological function of these filaments is elusive. These filaments were polymerization of the cytochromes (79, 80, 88). Structural investigation with biochemically active OmcS (81) and OmcZ (87) as purified previously may provide insights into the structure-function relationship. Mutation analysis as conducted for the e-pili may reveal the mechanism of the conductivity of the cytochrome filaments. Unlike the e-pili, OmcS and OmcZ are not conserved in Geobacter species (31). Further research is expected to see if other Geobacter species produce filaments of cytochromes. Protein nanowires in G. sulfurreducens are under debate (26, 111). Magnetite could substitute for OmcS in the direct EET, but the e-pili were still required (84). The G. sulfurreducens pilA deletion strain could not carry out the EET in the presence of OmcS (21, 24). The addition of magnetite did not enable the pilA deletion strain to perform the EET (84). In addition, the e-pili were essential for the EET to the anode in thick biofilms in the presence of OmcZ (83, 112). Therefore, OmcS and OmcZ are unable to fulfill the function of the e-pili in the EET and vice versa. The filaments of OmcS (78) and OmcZ (90) emerging from G. sulfurreducens cells were not observed while the e-pili emanating from G. sulfurreducens cells were shown (113). G. sulfurreducens pili assembled from PilA pilin monomers without cytochromes were demonstrated to be electrically conductive (114). Evaluation of electron transfer between the cytochromes and between OmcS and the e-pili is crucial. Structural analysis of the e-pili associated with OmcS will provide new insight for the EET mechanism.
The minimal route for the EET to Fe(III) is proposed (Fig. 3), but to evaluate this, it is necessary to construct G. sulfurreducens strains in which the potential routes for EET are clearly defined and controlled at each subcellular location. This construction would be labor-intensive and time-consuming since G. sulfurreducens has the substantial redundancy. Alternatively, genetically tractable microorganisms with no or few cytochromes, such as E. coli, can be used for engineering heterologous electron transport chains (115). Recently, an E. coli strain was constructed in which G. sulfurreducens e-pili were heterologously assembled (116). Thus, E. coli may serve as a chassis for building direct EET chains from G. sulfurreducens.
There are a number of putative cytochromes that have not been characterized in G. sulfurreducens. G. sulfurreducens can reduce various metals (14). Uncharacterized cytochromes appear to be involved in reduction of Pd(II) (117). There may be other cytochromes that are indirectly involved in the electron transfer, for example, as a regulator for expression of other cytochromes as described above and electron sink or capacitor to monitor and regulate the redox status of cells and redox proteins in the electron transport chains (118, 119). G. sulfurreducens appears to have homeostasis for electron flux by changing Fe(II)/Fe(III) ratios in the multiheme cytochromes (120).
Better understanding of EET is beneficial for advancing environmental biotechnology practices. EET is important for bioremediation of contaminated environments, metal corrosion, microbial current production, microbial electrosynthesis/electrofermentation, and anaerobic digesters. Gaining fundamental knowledge about the molecular mechanism in EET will enable the engineering of EET systems to develop more efficient strategies for these biotechnology practices. The cytochrome filaments would provide a new resource in electronics applications, as the e-pili are utilized as electrically conductive nanowires in various electronics applications, such as electricity generation, biocompatible artificial neurons, and sensors (30). More knowledge of the cytochrome filaments and techniques for engineering the cytochrome filaments are needed for electronics applications.
CONCLUDING REMARKS
The mechanism of the EET in G. sulfurreducens is very elaborate. With the myriad genes for cytochromes in its genome, a variety of cytochromes are differentially expressed for the EET with different electron donors and acceptors and are carefully arranged in the inner membrane, periplasm, outer membrane, and extracellular space. Geobacter harmonizes the cytochromes with other cellular components to optimize the EET system. This flexibility may make Geobacter suitable for growth in diverse environments where EET is advantageous. However, despite intensive studies, a complete picture of the EET mechanism has not yet been achieved. Further research for the EET mechanism is warranted.
Supplementary Material
ACKNOWLEDGMENTS
I thank Derek R. Lovley for his support. I am grateful to Trevor L. Woodard for critical reading of the manuscript.
Biography

Toshiyuki Ueki is a Research Assistant Professor in the Department of Microbiology at University of Massachusetts Amherst. He received his M.S. and Ph.D. from Tokyo Institute of Technology in Japan. His research interests are understanding fundamental knowledge of molecular mechanisms of sensing and responding to a myriad of conditions that microorganisms face in the environment and engineering their biological programs for biotechnological applications. He is motivated to develop a synthetic biology toolbox for these interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Chabert N, Amin Ali O, Achouak W. 2015. All ecosystems potentially host electrogenic bacteria. Bioelectrochemistry 106:88–96. 10.1016/j.bioelechem.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Koch C, Harnisch F. 2016. Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem 3:1282–1295. 10.1002/celc.201600079. [DOI] [Google Scholar]
- 3.Wang W, Du Y, Yang S, Du X, Li M, Lin B, Zhou J, Lin L, Song Y, Li J, Zuo X, Yang C. 2019. Bacterial extracellular electron transfer occurs in mammalian gut. Anal Chem 91:12138–12141. 10.1021/acs.analchem.9b03176. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Shi M, Shi L. 2019. Molecular underpinnings for microbial extracellular electron transfer during biogeochemical cycling of earth elements. Sci China Life Sci 62:1275–1286. 10.1007/s11427-018-9464-3. [DOI] [PubMed] [Google Scholar]
- 5.Pankratova G, Hederstedt L, Gorton L. 2019. Extracellular electron transfer features of Gram-positive bacteria. Anal Chim Acta 1076:32–47. 10.1016/j.aca.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Lovley DR. 2017. Happy together: microbial communities that hook up to swap electrons. ISME J 11:327–336. 10.1038/ismej.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovley DR. 2017. Syntrophy goes electric: direct interspecies electron transfer. Annu Rev Microbiol 71:643–664. 10.1146/annurev-micro-030117-020420. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Aulenta F, Puig S, Esteve-Núñez A, He Y, Mu Y, Rabaey K. 2020. Microbial electrochemistry for bioremediation. Environ Sci Ecotechnology 1:100013. 10.1016/j.ese.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan BE, Rossi R, Ragab A, Saikaly PE. 2019. Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol 17:307–319. 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- 10.Baek G, Kim J, Kim J, Lee C. 2018. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 11:107. 10.3390/en11010107. [DOI] [Google Scholar]
- 11.Li Y, Xu D, Chen C, Li X, Jia R, Zhang D, Sand W, Wang F, Gu T. 2018. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J Mater Sci Technol 34:1713–1718. 10.1016/j.jmst.2018.02.023. [DOI] [Google Scholar]
- 12.Glasser NR, Saunders SH, Newman DK. 2017. The colorful world of extracellular electron shuttles. Annu Rev Microbiol 71:731–751. 10.1146/annurev-micro-090816-093913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B, Gao S, Xu Z, He H, Pan X. 2018. The functional mechanisms and application of electron shuttles in extracellular electron transfer. Curr Microbiol 75:99–106. 10.1007/s00284-017-1386-8. [DOI] [PubMed] [Google Scholar]
- 14.Lovley DR, Ueki T, Zhang T, Malvankar NS, Shrestha PM, Flanagan KA, Aklujkar M, Butler JE, Giloteaux L, Rotaru AE, Holmes DE, Franks AE, Orellana R, Risso C, Nevin KP. 2011. Geobacter: the microbe electric's physiology, ecology, and practical applications. Adv Microb Physiol 59:1–100. 10.1016/B978-0-12-387661-4.00004-5. [DOI] [PubMed] [Google Scholar]
- 15.Ueki T, Nevin KP, Woodard TL, Aklujkar MA, Holmes DE, Lovley DR. 2018. Construction of a Geobacter strain with exceptional growth on cathodes. Front Microbiol 9:1512. 10.3389/fmicb.2018.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang X, Kalathil S, Divitini G, Wang Q, Reisner E. 2020. A three-dimensional hybrid electrode with electroactive microbes for efficient electrogenesis and chemical synthesis. Proc Natl Acad Sci U S A 117:5074–5080. 10.1073/pnas.1913463117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang HY, Holmes DE, Ueki T, Palacios PA, Lovley DR. 2019. Iron corrosion via direct metal-microbe electron transfer. mBio 10:e00303-19. 10.1128/mBio.00303-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley DR. 2012. Electromicrobiology. Annu Rev Microbiol 66:391–409. 10.1146/annurev-micro-092611-150104. [DOI] [PubMed] [Google Scholar]
- 19.Galushko AS, Schink B. 2000. Oxidation of acetate through reactions of the citric acid cycle by Geobacter sulfurreducens in pure culture and in syntrophic coculture. Arch Microbiol 174:314–321. 10.1007/s002030000208. [DOI] [PubMed] [Google Scholar]
- 20.Segura D, Mahadevan R, Juarez K, Lovley DR. 2008. Computational and experimental analysis of redundancy in the central metabolism of Geobacter sulfurreducens. PLoS Comput Biol 4:e36. 10.1371/journal.pcbi.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 22.Gregory KB, Bond DR, Lovley DR. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604. 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 23.Bond DR, Lovley DR. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 25.Ha PT, Lindemann SR, Shi L, Dohnalkova AC, Fredrickson JK, Madigan MT, Beyenal H. 2017. Syntrophic anaerobic photosynthesis via direct interspecies electron transfer. Nat Commun 8:13924. 10.1038/ncomms13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley DR, Walker DJF. 2019. Geobacter protein nanowires. Front Microbiol 10:2078. 10.3389/fmicb.2019.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley DR. 2017. Electrically conductive pili: biological function and potential applications in electronics. Curr Opin Electrochem 4:190–198. 10.1016/j.coelec.2017.08.015. [DOI] [Google Scholar]
- 28.Lovley DR, Holmes DE. 2020. Protein nanowires: the electrification of the microbial world and maybe our own. J Bacteriol 202:e00331-20. 10.1128/JB.00331-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley DR. 2017. e-Biologics: fabrication of sustainable electronics with “green” biological materials. mBio 8:e00695-17. 10.1128/mBio.00695-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovley DR, Yao J. 5 January 2020. Intrinsically conductive microbial nanowires for ‘green’ electronics with novel functions. Trends Biotechnol 10.1016/j.tibtech.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Butler JE, Young ND, Lovley DR. 2010. Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes. BMC Genomics 11:40. 10.1186/1471-2164-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Methé BA, Nelson KE, Eisen JA, Paulsen IT, Nelson W, Heidelberg JF, Wu D, Wu M, Ward N, Beanan MJ, Dodson RJ, Madupu R, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Gwinn M, Kolonay JF, Sullivan SA, Haft DH, Selengut J, Davidsen TM, Zafar N, White O, Tran B, Romero C, Forberger HA, Weidman J, Khouri H, Feldblyum TV, Utterback TR, Van Aken SE, Lovley DR, Fraser CM. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967–1969. 10.1126/science.1088727. [DOI] [PubMed] [Google Scholar]
- 33.Beblawy S, Bursac T, Paquete C, Louro R, Clarke TA, Gescher J. 2018. Extracellular reduction of solid electron acceptors by Shewanella oneidensis. Mol Microbiol 109:571–583. 10.1111/mmi.14067. [DOI] [PubMed] [Google Scholar]
- 34.Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, Eisen JA, Seshadri R, Ward N, Methe B, Clayton RA, Meyer T, Tsapin A, Scott J, Beanan M, Brinkac L, Daugherty S, DeBoy RT, Dodson RJ, Durkin AS, Haft DH, Kolonay JF, Madupu R, Peterson JD, Umayam LA, White O, Wolf AM, Vamathevan J, Weidman J, Impraim M, Lee K, Berry K, Lee C, Mueller J, Khouri H, Gill J, Utterback TR, McDonald LA, Feldblyum TV, Smith HO, Venter JC, Nealson KH, Fraser CM. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol 20:1118–1123. 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- 35.Kotloski NJ, Gralnick JA. 2013. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. mBio 4:e00553-12. 10.1128/mBio.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirose A, Kouzuma A, Watanabe K. 2019. Towards development of electrogenetics using electrochemically active bacteria. Biotechnol Adv 37:107351. 10.1016/j.biotechadv.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Aklujkar M, Coppi MV, Leang C, Kim BC, Chavan MA, Perpetua LA, Giloteaux L, Liu A, Holmes DE. 2013. Proteins involved in electron transfer to Fe(III) and Mn(IV) oxides by Geobacter sulfurreducens and Geobacter uraniireducens. Microbiology (Reading) 159:515–535. 10.1099/mic.0.064089-0. [DOI] [PubMed] [Google Scholar]
- 38.Zacharoff L, Chan CH, Bond DR. 2016. Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens. Bioelectrochemistry 107:7–13. 10.1016/j.bioelechem.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Levar CE, Chan CH, Mehta-Kolte MG, Bond DR. 2014. An inner membrane cytochrome required only for reduction of high redox potential extracellular electron acceptors. mBio 5:e02034-14. 10.1128/mBio.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levar CE, Hoffman CL, Dunshee AJ, Toner BM, Bond DR. 2017. Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens. ISME J 11:741–752. 10.1038/ismej.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler JE, Kaufmann F, Coppi MV, Nunez C, Lovley DR. 2004. MacA, a diheme c-type cytochrome involved in Fe(III) reduction by Geobacter sulfurreducens. J Bacteriol 186:4042–4045. 10.1128/JB.186.12.4042-4045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelobolina ES, Coppi MV, Korenevsky AA, DiDonato LN, Sullivan SA, Konishi H, Xu H, Leang C, Butler JE, Kim BC, Lovley DR. 2007. Importance of c-type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol 7:16. 10.1186/1471-2180-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BC, Lovley DR. 2008. Investigation of direct vs. indirect involvement of the c-type cytochrome MacA in Fe(III) reduction by Geobacter sulfurreducens. FEMS Microbiol Lett 286:39–44. 10.1111/j.1574-6968.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 44.Seidel J, Hoffmann M, Ellis KE, Seidel A, Spatzal T, Gerhardt S, Elliott SJ, Einsle O. 2012. MacA is a second cytochrome c peroxidase of Geobacter sulfurreducens. Biochemistry 51:2747–2756. 10.1021/bi300249u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dantas JM, Brausemann A, Einsle O, Salgueiro CA. 2017. NMR studies of the interaction between inner membrane-associated and periplasmic cytochromes from Geobacter sulfurreducens. FEBS Lett 591:1657–1666. 10.1002/1873-3468.12695. [DOI] [PubMed] [Google Scholar]
- 46.Pettigrew GW, Echalier A, Pauleta SR. 2006. Structure and mechanism in the bacterial dihaem cytochrome c peroxidases. J Inorg Biochem 100:551–567. 10.1016/j.jinorgbio.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Pokkuluri PR, Londer YY, Yang X, Duke NE, Erickson J, Orshonsky V, Johnson G, Schiffer M. 2010. Structural characterization of a family of cytochromes c7 involved in Fe(III) respiration by Geobacter sulfurreducens. Biochim Biophys Acta 1797:222–232. 10.1016/j.bbabio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Santos TC, Silva MA, Morgado L, Dantas JM, Salgueiro CA. 2015. Diving into the redox properties of Geobacter sulfurreducens cytochromes: a model for extracellular electron transfer. Dalton Trans 44:9335–9344. 10.1039/c5dt00556f. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Y, Cho BK, Park YS, Lovley D, Palsson BO, Zengler K. 2010. Structural and operational complexity of the Geobacter sulfurreducens genome. Genome Res 20:1304–1311. 10.1101/gr.107540.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding YH, Hixson KK, Aklujkar MA, Lipton MS, Smith RD, Lovley DR, Mester T. 2008. Proteome of Geobacter sulfurreducens grown with Fe(III) oxide or Fe(III) citrate as the electron acceptor. Biochim Biophys Acta 1784:1935–1941. 10.1016/j.bbapap.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Ding YHR, Hixson KK, Giometti CS, Stanley A, Esteve-Nunez A, Khare T, Tollaksen SL, Zhu WH, Adkins JN, Lipton MS, Smith RD, Mester T, Lovley DR. 2006. The proteome of dissimilatory metal-reducing microorganism Geobacter sulfurreducens under various growth conditions. Biochim Biophys Acta 1764:1198–1206. 10.1016/j.bbapap.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Seeliger S, Cord-Ruwisch R, Schink B. 1998. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J Bacteriol 180:3686–3691. 10.1128/JB.180.14.3686-3691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lloyd JR, Leang C, Hodges Myerson AL, Coppi MV, Cuifo S, Methe B, Sandler SJ, Lovley DR. 2003. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem J 369:153–161. 10.1042/BJ20020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgado L, Bruix M, Orshonsky V, Londer YY, Duke NE, Yang X, Pokkuluri PR, Schiffer M, Salgueiro CA. 2008. Structural insights into the modulation of the redox properties of two Geobacter sulfurreducens homologous triheme cytochromes. Biochim Biophys Acta 1777:1157–1165. 10.1016/j.bbabio.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Morgado L, Bruix M, Pessanha M, Londer YY, Salgueiro CA. 2010. Thermodynamic characterization of a triheme cytochrome family from Geobacter sulfurreducens reveals mechanistic and functional diversity. Biophys J 99:293–301. 10.1016/j.bpj.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgado L, Paixao VB, Schiffer M, Pokkuluri PR, Bruix M, Salgueiro CA. 2012. Revealing the structural origin of the redox-Bohr effect: the first solution structure of a cytochrome from Geobacter sulfurreducens. Biochem J 441:179–187. 10.1042/BJ20111103. [DOI] [PubMed] [Google Scholar]
- 57.Pessanha M, Morgado L, Louro RO, Londer YY, Pokkuluri PR, Schiffer M, Salgueiro CA. 2006. Thermodynamic characterization of triheme cytochrome PpcA from Geobacter sulfurreducens: evidence for a role played in e-/H+ energy transduction. Biochemistry 45:13910–13917. 10.1021/bi061394v. [DOI] [PubMed] [Google Scholar]
- 58.Ueki T, DiDonato LN, Lovley DR. 2017. Toward establishing minimum requirements for extracellular electron transfer in Geobacter sulfurreducens. FEMS Microbiol Lett 364:fnx093. 10.1093/femsle/fnx093. [DOI] [PubMed] [Google Scholar]
- 59.Strycharz SM, Glaven RH, Coppi MV, Gannon SM, Perpetua LA, Liu A, Nevin KP, Lovley DR. 2011. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80:142–150. 10.1016/j.bioelechem.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Dantas JM, Tomaz DM, Morgado L, Salgueiro CA. 2013. Functional characterization of PccH, a key cytochrome for electron transfer from electrodes to the bacterium Geobacter sulfurreducens. FEBS Lett 587:2662–2668. 10.1016/j.febslet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Dantas JM, Campelo LM, Duke NE, Salgueiro CA, Pokkuluri PR. 2015. The structure of PccH from Geobacter sulfurreducens - a novel low reduction potential monoheme cytochrome essential for accepting electrons from an electrode. FEBS J 282:2215–2231. 10.1111/febs.13269. [DOI] [PubMed] [Google Scholar]
- 62.Santos TC, de Oliveira AR, Dantas JM, Salgueiro CA, Cordas CM. 2015. Thermodynamic and kinetic characterization of PccH, a key protein in microbial electrosynthesis processes in Geobacter sulfurreducens. Biochim Biophys Acta 1847:1113–1118. 10.1016/j.bbabio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira LR, Portela PC, Morgado L, Pantoja-Uceda D, Bruix M, Salgueiro CA. 2019. Backbone assignment of cytochrome PccH, a crucial protein for microbial electrosynthesis in Geobacter sulfurreducens. Biomol NMR Assign 13:321–326. 10.1007/s12104-019-09899-6. [DOI] [PubMed] [Google Scholar]
- 64.Richardson DJ, Butt JN, Fredrickson JK, Zachara JM, Shi L, Edwards MJ, White G, Baiden N, Gates AJ, Marritt SJ, Clarke TA. 2012. The 'porin-cytochrome' model for microbe-to-mineral electron transfer. Mol Microbiol 85:201–212. 10.1111/j.1365-2958.2012.08088.x. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Shi L, Gu JD. 2018. Microbial electrocatalysis: redox mediators responsible for extracellular electron transfer. Biotechnol Adv 36:1815–1827. 10.1016/j.biotechadv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Shi L, Fredrickson JK, Zachara JM. 2014. Genomic analyses of bacterial porin-cytochrome gene clusters. Front Microbiol 5:657. 10.3389/fmicb.2014.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leang C, Coppi MV, Lovley DR. 2003. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 185:2096–2103. 10.1128/jb.185.7.2096-2103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Wang Z, Liu J, Levar C, Edwards MJ, Babauta JT, Kennedy DW, Shi Z, Beyenal H, Bond DR, Clarke TA, Butt JN, Richardson DJ, Rosso KM, Zachara JM, Fredrickson JK, Shi L. 2014. A trans-outer membrane porin-cytochrome protein complex for extracellular electron transfer by Geobacter sulfurreducens PCA. Environ Microbiol Rep 6:776–785. 10.1111/1758-2229.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Fredrickson JK, Zachara JM, Shi L. 2015. Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe(III) by Geobacter sulfurreducens PCA. Front Microbiol 6:1075. 10.3389/fmicb.2015.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magnuson TS, Isoyama N, Hodges-Myerson AL, Davidson G, Maroney MJ, Geesey GG, Lovley DR. 2001. Isolation, characterization and gene sequence analysis of a membrane-associated 89 kDa Fe(III) reducing cytochrome c from Geobacter sulfurreducens. Biochem J 359:147–152. 10.1042/0264-6021:3590147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian X, Reguera G, Mester T, Lovley DR. 2007. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol Lett 277:21–27. 10.1111/j.1574-6968.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 72.Voordeckers JW, Kim BC, Izallalen M, Lovley DR. 2010. Role of Geobacter sulfurreducens outer surface c-type cytochromes in reduction of soil humic acid and anthraquinone-2,6-disulfonate. Appl Environ Microbiol 76:2371–2375. 10.1128/AEM.02250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leang C, Adams LA, Chin KJ, Nevin KP, Methe BA, Webster J, Sharma ML, Lovley DR. 2005. Adaptation to disruption of the electron transfer pathway for Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 187:5918–5926. 10.1128/JB.187.17.5918-5926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim BC, Leang C, Ding YH, Glaven RH, Coppi MV, Lovley DR. 2005. OmcF, a putative c-type monoheme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J Bacteriol 187:4505–4513. 10.1128/JB.187.13.4505-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim BC, Qian X, Leang C, Coppi MV, Lovley DR. 2006. Two putative c-type multiheme cytochromes required for the expression of OmcB, an outer membrane protein essential for optimal Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 188:3138–3142. 10.1128/JB.188.8.3138-3142.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan CH, Levar CE, Jimenez-Otero F, Bond DR. 2017. Genome scale mutational analysis of Geobacter sulfurreducens reveals distinct molecular mechanisms for respiration and sensing of poised electrodes versus Fe(III) oxides. J Bacteriol 199:e00340-17. 10.1128/JB.00340-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta T, Coppi MV, Childers SE, Lovley DR. 2005. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol 71:8634–8641. 10.1128/AEM.71.12.8634-8641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leang C, Qian X, Mester T, Lovley DR. 2010. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl Environ Microbiol 76:4080–4084. 10.1128/AEM.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Filman DJ, Marino SF, Ward JE, Yang L, Mester Z, Bullitt E, Lovley DR, Strauss M. 2019. Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire. Commun Biol 2:219. 10.1038/s42003-019-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F, Gu Y, O'Brien JP, Yi SM, Yalcin SE, Srikanth V, Shen C, Vu D, Ing NL, Hochbaum AI, Egelman EH, Malvankar NS. 2019. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell 177:361–369. 10.1016/j.cell.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian XL, Mester T, Morgado L, Arakawa T, Sharma ML, Inoue K, Joseph C, Salgueiro CA, Maroney MJ, Lovley DR. 2011. Biochemical characterization of purified OmcS, a c-type cytochrome required for insoluble Fe(III) reduction in Geobacter sulfurreducens. Biochim Biophys Acta 1807:404–412. 10.1016/j.bbabio.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Holmes DE, Chaudhuri SK, Nevin KP, Mehta T, Methe BA, Liu A, Ward JE, Woodard TL, Webster J, Lovley DR. 2006. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ Microbiol 8:1805–1815. 10.1111/j.1462-2920.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- 83.Nevin KP, Kim BC, Glaven RH, Johnson JP, Woodard TL, Methe BA, Didonato RJ, Covalla SF, Franks AE, Liu A, Lovley DR. 2009. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS One 4:e5628. 10.1371/journal.pone.0005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu F, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. 2015. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ Microbiol 17:648–655. 10.1111/1462-2920.12485. [DOI] [PubMed] [Google Scholar]
- 85.Walker DJF, Li Y, Meier D, Pinches S, Holmes DE. 2020. Cytochrome OmcS is not essential for long-range electron transport in Geobacter sulfurreducens strain KN400. bioRxiv 10.1101/2020.07.22.214791. [DOI] [PMC free article] [PubMed]
- 86.Yun J, Malvankar NS, Ueki T, Lovley DR. 2016. Functional environmental proteomics: elucidating the role of a c-type cytochrome abundant during uranium bioremediation. ISME J 10:310–320. 10.1038/ismej.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue K, Qian X, Morgado L, Kim BC, Mester T, Izallalen M, Salgueiro CA, Lovley DR. 2010. Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl Environ Microbiol 76:3999–4007. 10.1128/AEM.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yalcin SE, O’Brien JP, Gu Y, Reiss K, Yi SM, Jain R, Srikanth V, Dahl PJ, Huynh W, Vu D, Acharya A, Chaudhuri S, Varga T, Batista VS, Malvankar NS. 2020. Electric field stimulates production of highly conductive microbial OmcZ nanowires. Nat Chem Biol 16:1136–1142. 10.1038/s41589-020-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malvankar NS, Tuominen MT, Lovley DR. 2012. Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energy Environ Sci 5:8651–8659. 10.1039/c2ee22330a. [DOI] [Google Scholar]
- 90.Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, Lovley DR. 2011. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ Microbiol Rep 3:211–217. 10.1111/j.1758-2229.2010.00210.x. [DOI] [PubMed] [Google Scholar]
- 91.Thirumurthy MA, Jones AK. 2020. Geobacter cytochrome OmcZs binds riboflavin: implications for extracellular electron transfer. Nanotechnology 31:124001. 10.1088/1361-6528/ab5de6. [DOI] [PubMed] [Google Scholar]
- 92.Okamoto A, Saito K, Inoue K, Nealson KH, Hashimoto K, Nakamura R. 2014. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species. Energy Environ Sci 7:1357–1361. 10.1039/C3EE43674H. [DOI] [Google Scholar]
- 93.Michelson K, Sanford RA, Valocchi AJ, Werth CJ. 2017. Nanowires of Geobacter sulfurreducens require redox cofactors to reduce metals in pore spaces too small for cell passage. Environ Sci Technol 51:11660–11668. 10.1021/acs.est.7b02531. [DOI] [PubMed] [Google Scholar]
- 94.Huang L, Liu X, Ye Y, Chen M, Zhou S. 2020. Evidence for the coexistence of direct and riboflavin-mediated interspecies electron transfer in Geobacter co-culture. Environ Microbiol 22:243–254. 10.1111/1462-2920.14842. [DOI] [PubMed] [Google Scholar]
- 95.Smith JA, Tremblay PL, Shrestha PM, Snoeyenbos-West OL, Franks AE, Nevin KP, Lovley DR. 2014. Going wireless: Fe(III) oxide reduction without pili by Geobacter sulfurreducens strain JS-1. Appl Environ Microbiol 80:4331–4340. 10.1128/AEM.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orellana R, Leavitt JJ, Comolli LR, Csencsits R, Janot N, Flanagan KA, Gray AS, Leang C, Izallalen M, Mester T, Lovley DR. 2013. U(VI) reduction by diverse outer surface c-type cytochromes of Geobacter sulfurreducens. Appl Environ Microbiol 79:6369–6374. 10.1128/AEM.02551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richter H, Nevin KP, Jia HF, Lowy DA, Lovley DR, Tender LM. 2009. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ Sci 2:506–516. 10.1039/b816647a. [DOI] [Google Scholar]
- 98.Lin H, Morrell-Falvey JL, Rao B, Liang L, Gu B. 2014. Coupled mercury-cell sorption, reduction, and oxidation on methylmercury production by Geobacter sulfurreducens PCA. Environ Sci Technol 48:11969–11976. 10.1021/es502537a. [DOI] [PubMed] [Google Scholar]
- 99.Tremblay PL, Summers ZM, Glaven RH, Nevin KP, Zengler K, Barrett CL, Qiu Y, Palsson BO, Lovley DR. 2011. A c-type cytochrome and a transcriptional regulator responsible for enhanced extracellular electron transfer in Geobacter sulfurreducens revealed by adaptive evolution. Environ Microbiol 13:13–23. 10.1111/j.1462-2920.2010.02302.x. [DOI] [PubMed] [Google Scholar]
- 100.Zacharoff LA, Morrone DJ, Bond DR. 2017. Geobacter sulfurreducens extracellular multiheme cytochrome PgcA facilitates respiration to Fe(III) oxides but not electrodes. Front Microbiol 8:2481. 10.3389/fmicb.2017.02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim BC, Postier BL, Didonato RJ, Chaudhuri SK, Nevin KP, Lovley DR. 2008. Insights into genes involved in electricity generation in Geobacter sulfurreducens via whole genome microarray analysis of the OmcF-deficient mutant. Bioelectrochemistry 73:70–75. 10.1016/j.bioelechem.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 102.Pokkuluri PR, Londer YY, Wood SJ, Duke NE, Morgado L, Salgueiro CA, Schiffer M. 2009. Outer membrane cytochrome c, OmcF, from Geobacter sulfurreducens: high structural similarity to an algal cytochrome c6. Proteins 74:266–270. 10.1002/prot.22260. [DOI] [PubMed] [Google Scholar]
- 103.Dikiy A, Carpentier W, Vandenberghe I, Borsari M, Safarov N, Dikaya E, Van Beeumen J, Ciurli S. 2002. Structural basis for the molecular properties of cytochrome c6. Biochemistry 41:14689–14699. 10.1021/bi026473v. [DOI] [PubMed] [Google Scholar]
- 104.Bird LJ, Bonnefoy V, Newman DK. 2011. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol 19:330–340. 10.1016/j.tim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Mehta T, Childers SE, Glaven R, Lovley DR, Mester T. 2006. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology (Reading) 152:2257–2264. 10.1099/mic.0.28864-0. [DOI] [PubMed] [Google Scholar]
- 106.Holmes DE, Mester T, O'Neil RA, Perpetua LA, Larrahondo MJ, Glaven R, Sharma ML, Ward JE, Nevin KP, Lovley DR. 2008. Genes for two multicopper proteins required for Fe(III) oxide reduction in Geobacter sulfurreducens have different expression patterns both in the subsurface and on energy-harvesting electrodes. Microbiology (Reading) 154:1422–1435. 10.1099/mic.0.2007/014365-0. [DOI] [PubMed] [Google Scholar]
- 107.Ueki T, Leang C, Inoue K, Lovley DR. 2012. Identification of multicomponent histidine-aspartate phosphorelay system controlling flagella and motility gene expression in Geobacter species. J Biol Chem 287:10958–10966. 10.1074/jbc.M112.345041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chong GW, Karbelkar AA, El-Naggar MY. 2018. Nature's conductors: what can microbial multi-heme cytochromes teach us about electron transport and biological energy conversion? Curr Opin Chem Biol 47:7–17. 10.1016/j.cbpa.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Edwards MJ, Richardson DJ, Paquete CM, Clarke TA. 2020. Role of multiheme cytochromes involved in extracellular anaerobic respiration in bacteria. Protein Sci 29:830–842. 10.1002/pro.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edwards MJ, White GF, Butt JN, Richardson DJ, Clarke TA. 2020. The crystal structure of a biological insulated transmembrane molecular wire. Cell 181:665–673. 10.1016/j.cell.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yalcin SE, Malvankar NS. 2020. The blind men and the filament: understanding structures and functions of microbial nanowires. Curr Opin Chem Biol 59:193–201. 10.1016/j.cbpa.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rotaru AE, Woodard TL, Nevin KP, Lovley DR. 2015. Link between capacity for current production and syntrophic growth in Geobacter species. Front Microbiol 6:744. 10.3389/fmicb.2015.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ueki T, Walker DJF, Tremblay PL, Nevin KP, Ward JE, Woodard TL, Nonnenmann SS, Lovley DR. 2019. Decorating the outer surface of microbially produced protein nanowires with peptides. ACS Synth Biol 8:1809–1817. 10.1021/acssynbio.9b00131. [DOI] [PubMed] [Google Scholar]
- 114.Cologgi DL, Lampa-Pastirk S, Speers AM, Kelly SD, Reguera G. 2011. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc Natl Acad Sci U S A 108:15248–15252. 10.1073/pnas.1108616108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jensen HM, Albers AE, Malley KR, Londer YY, Cohen BE, Helms BA, Weigele P, Groves JT, Ajo-Franklin CM. 2010. Engineering of a synthetic electron conduit in living cells. Proc Natl Acad Sci U S A 107:19213–19218. 10.1073/pnas.1009645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ueki T, Walker DJF, Woodard TL, Nevin KP, Nonnenmann SS, Lovley DR. 2020. An Escherichia coli chassis for production of electrically conductive protein nanowires. ACS Synth Biol 9:647–654. 10.1021/acssynbio.9b00506. [DOI] [PubMed] [Google Scholar]
- 117.Hernandez-Eligio A, Pat-Espadas AM, Vega-Alvarado L, Huerta-Amparan M, Cervantes FJ, Juarez K. 2020. Global transcriptional analysis of Geobacter sulfurreducens under palladium reducing conditions reveals new key cytochromes involved. Appl Microbiol Biotechnol 104:4059–4069. 10.1007/s00253-020-10502-5. [DOI] [PubMed] [Google Scholar]
- 118.Esteve-Nunez A, Sosnik J, Visconti P, Lovley DR. 2008. Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ Microbiol 10:497–505. 10.1111/j.1462-2920.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 119.Malvankar NS, Mester T, Tuominen MT, Lovley DR. 2012. Supercapacitors based on c-type cytochromes using conductive nanostructured networks of living bacteria. ChemPhysChem 13:463–468. 10.1002/cphc.201100865. [DOI] [PubMed] [Google Scholar]
- 120.Chabert V, Babel L, Füeg MP, Karamash M, Madivoli ES, Herault N, Dantas JM, Salgueiro CA, Giese B, Fromm KM. 2020. Kinetics and mechanism of mineral respiration: how iron hemes synchronize electron transfer rates. Angew Chem Int Ed Engl 59:12331–12336. 10.1002/anie.201914873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.