Lactobacillus spp. are credited with providing the primary defense against gynecological conditions, including BV, most notably through the acidification of the vaginal microenvironment, which results from their production of lactic acid. The microbial production of BAs has been hypothesized to play a mechanistic role in diminishing Lactobacillus species-mediated protection, enabling the colonization and outgrowth of diverse anaerobic bacterial species associated with BV.
KEYWORDS: growth response, bacterial vaginosis, biogenic amines, Lactobacillus, vaginal microbiome
ABSTRACT
Bacterial vaginosis (BV) is the most common vaginal disorder of reproductive-aged women, yet its etiology remains enigmatic. One clinical symptom of BV, malodor, is linked to the microbial production of biogenic amines (BA). Using targeted liquid chromatography mass spectrometry, we analyzed 149 longitudinally collected vaginal samples to determine the in vivo concentrations of the most common BAs and then assessed their relationship to BV and effect upon the growth kinetics of axenically cultured vaginal Lactobacillus species. Increases in cadaverine, putrescine, and tyramine were associated with greater odds of women transitioning from L. crispatus-dominated vaginal microbiota to microbiota that have a paucity of Lactobacillus spp. and from Nugent scores of 0 to 3 to Nugent scores of 7 to 10, consistent with BV. Exposure to putrescine lengthened the lag time and/or slowed the growth of all vaginal Lactobacillus spp. except L. jensenii 62G. L. iners AB107’s lag time was lengthened by cadaverine but reduced in the presence of spermidine and spermine. The growth rate of L. crispatus VPI 3199 was slowed by cadaverine and tyramine, and strain-specific responses to spermine and spermidine were observed. BAs were associated with reduced production of d- and l-lactic acid by vaginal Lactobacillus spp., and this effect was independent of their effect upon Lactobacillus species growth. The exceptions were higher levels of d- and l-lactic acid by two strains of L. crispatus when grown in the presence of spermine. Results of this study provide evidence of a direct impact of common biogenic amines on vaginal Lactobacillus spp.
IMPORTANCE Lactobacillus spp. are credited with providing the primary defense against gynecological conditions, including BV, most notably through the acidification of the vaginal microenvironment, which results from their production of lactic acid. The microbial production of BAs has been hypothesized to play a mechanistic role in diminishing Lactobacillus species-mediated protection, enabling the colonization and outgrowth of diverse anaerobic bacterial species associated with BV. Here, we demonstrate that in vivo increases in the most commonly observed BAs are associated with a loss of Lactobacillus spp. and the development of BV, measured by Nugent score. Further, we show that BAs formed by amino acid decarboxylase enzymes negatively affect the growth of type strains of the most common vaginal Lactobacillus spp. and separately alter their production of lactic acid. These results suggest that BAs destabilize vaginal Lactobacillus spp. and play an important and direct role in diminishing their protection of the vaginal microenvironment.
INTRODUCTION
The vaginal microenvironment comprises microbiota and metabolites that significantly influence gynecological health (1–8). Using molecular approaches, the vaginal microbiota of reproductive-aged women has been broadly classified into five community state types (CSTs), four of which are dominated by Lactobacillus species, namely, L. crispatus (CST I), L. gasseri (CST II), L. iners (CST III), and L. jensenii (CST V) (9). Lactobacillus species dominance is generally associated with positive obstetric and gynecological outcomes (7, 8, 10). Lactobacillus spp. are believed to prevent the colonization of potential pathogens through the production of lactic acid (11), bacteriocins (12, 13), and biosurfactants (14) and through competitive exclusion by adherence to the vaginal mucosa (15, 16). In approximately 30% of women (17, 18), Lactobacillus spp. are depauperate, and they are instead colonized by a diverse assortment of strict and facultative anaerobic bacteria, including Gardnerella vaginalis, Prevotella spp., and Atopobium vaginae (CST IV) (9). These features are also attributes of the vaginal disorder bacterial vaginosis (BV), leading to the recent proposal to describe CST IV as molecular-BV (19).
BV is the most common vaginal condition among reproductive-aged women (17, 18) and is associated with adverse health outcomes, including increased risks of acquiring sexually transmitted infections (STIs) (20–22), such as HIV (22), as well as urinary tract infections (UTIs) (23), the development of pelvic inflammatory disease (24), and infertility (25). Furthermore, in pregnant women, BV is associated with an increased risk for preterm birth (26–28) and miscarriage (29). BV is commonly assessed in research studies using the Nugent score, which applies a scoring metric ranging from 0 to 10 to bacterial morphotypes observed in a Gram stain of vaginal smears, wherein a score of ≥7 is considered BV (30). In a clinical setting, signs and symptoms of BV include an increase in vaginal pH (>4.5), a thin, homogenous gray or white vaginal discharge, the presence of superficial squamous cells with large numbers of adherent bacteria (clue cells), and an amine or “fishy” odor (31). The simultaneous manifestation of three of these four signs is a positive indication of BV, as clinically assessed by the Amsel criteria (31).
Bacterial vaginosis is also reflected in the composition of the vaginal metabolome (2, 4). This relationship is at least partially driven by the associated microbiological changes, with ∼31% of metabolomic variation being explained by CST (6, 32–35). Among metabolomic differences, the vaginal concentrations of cadaverine and putrescine are typically found to be higher in women afflicted with BV, and both are associated with clinical signs of malodor (2, 4, 34, 36–40). Higher concentrations of cadaverine, putrescine, and another biogenic amine (BA), tyramine, have also been observed in women with CST IV vaginal microbiota compared to CSTs dominated by Lactobacillus species (1).
BAs are small cationic molecules that are primarily produced via specific amino acid decarboxylation (AAD) reactions, although the BAs spermine and spermidine are instead produced through modifications of putrescine. AAD is a well-described acid stress resistance mechanism that involves the consumption of intracellular protons with a concomitant increase in extracellular pH (41, 42). BAs have additionally been shown to increase pathogen virulence by mediating resistance to multiple antibiotics (43, 44), protecting against toxicity from oxygen, superoxide, and hydrogen peroxide (45), and altering susceptibility to host innate defenses (42, 46). They have also been shown to generate a proton motive force, thereby providing metabolic energy (47). These functions suggest that BAs have a causal role in the transition from microbiota dominated by Lactobacillus spp. to ones with a paucity of lactobacilli that are associated with bacterial vaginosis (1, 2, 4, 19, 32, 40, 48) as opposed to simply being biomarkers of the condition, as has previously been hypothesized (1).
Beyond reducing the barriers to microbial colonization of the vaginal microenvironment, biogenic amines may directly influence bacterial growth and competitiveness. A few studies have assessed the effect of biogenic amines on the specific growth rates of various nonvaginal bacterial taxa. Cunningham-Rundles and Maas demonstrated that even small concentrations of putrescine led to a reduced growth rate of Escherichia coli (49), while Tabor et al. reported an 87% reduction in the growth rate of E. coli in the absence of spermidine (50). Only one study, conducted in 1964 by Guirard and Snell, examined the effect of BAs on the growth properties of Lactobacillus spp., although this was limited to several species involved in food production (51). Guirard and Snell reported that while spermidine and spermine stimulated the growth rate of several lactobacilli, putrescine and cadaverine significantly reduced growth (51). Thus far, no one has studied the effect of biogenic amines on the growth kinetics of vaginal Lactobacillus spp.
Here, we used targeted liquid chromatography-mass spectrometry (LC-MS) to quantify vaginal biogenic amines in an observational longitudinal study. Using these data, we explored the association between vaginal biogenic amines, vaginal CSTs, and Nugent scores. We also evaluated the effect of physiological concentrations of the most common biogenic amines found in the vagina, namely, cadaverine, putrescine, spermine, spermidine, and tyramine, on the lag time, growth rate, and lactic acid production of vaginal Lactobacillus spp.
RESULTS
Community state type and Nugent dynamics of participants.
Participant characteristics are reported in Data Set S1 in the supplemental material. Of the 32 participants, 25 (78%) were on their menstrual periods for either the selected transition or control event, representing 69 (46%) samples. While menstruating, women were predominantly typified by CST IV (69%) or CST III (23%) microbiota. Each participant had their own baseline in the subsequent analysis, yet among sample control periods and events, there were similar characteristics: samples that were characterized as stable (Nugent 0 to 3; current and previous Nugent score was 0 to 3) were primarily characterized as CST I (36%) or CST III (46%) throughout. Samples that had a stable Nugent score of 7 to 10 were primarily characterized as CST IV (98%) throughout. For the samples that transitioned into a Nugent score of 7 to 10, their vaginal microbiota following transition was largely characterized as CST IV (83%). The vaginal microbiota for samples after transitioning out of a Nugent score of 7 to 10 were characterized as CST I (22%), CST III (22%), and CST IV (56%).
Physiological concentrations of biogenic amines (cross-sectional).
The concentrations of cadaverine, putrescine, spermidine, spermine, and tyramine in swab samples were measured by quantitative targeted liquid chromatography-based metabolomics (Table 1; Data Set S1). Cadaverine, putrescine, and tyramine were the most frequently detected amines (detected in 97% to 98% of the samples). The average concentrations of these amines were highest in samples assigned to CST IV compared to CST I or III samples (P < 0.0001; Table 1). Spermidine and spermine were the least detected amines both among all samples (detected within 55% and 18% of all samples, respectively) and in CST IV women (detected in 48.9% and 8.5% of CST IV samples, respectively). In marked contrast to AAD-synthesized BAs, the average concentrations of spermidine and spermine were higher in samples dominated by L. crispatus (CST I) than those dominated by L. iners (P values of 0.01 and 0.001, respectively) or those characterized as CST IV (P values of 0.0004 and 0.0008, respectively), with their average concentrations being lowest in CST IV samples compared to all others.
TABLE 1.
Physiological concentrations of biogenic amines measured by LC-MS
| Sample type (n) | No. (%) detected in samples |
Mean concn, μM (range) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cadaverine | Putrescine | Spermidine | Spermine | Tyramine | Cadaverine | Putrescine | Spermidine | Spermine | Tyramine | Total AAD-BA | |
| All (149) | 147 (98.7) | 146 (98) | 82 (55) | 28 (18) | 147 (98.7) | 401.5 (2.86–4,990.5) | 332.2 (2.59–3,179.1) | 14.7 (0.31–59.7) | 47.5 (1.18–186.6) | 68.8 (0.14–813.2) | 789.5 (2.79–6,754.6) |
| CST I (17) | 15 (88.2) | 16 (94.1) | 12 (70.6) | 8 (47.1) | 16 (94.1) | 142.5a,d (3.11–833.6) | 144.5d (3.01–796.6) | 29.9b,c,d (3.11–50.2) | 98.9b,c,d (41.03–186.6) | 22.3a,d (0.14–110.3) | 282.9a,d (2.8–1,541.3) |
| CST III (38) | 38 (100) | 38 (100) | 24 (63.2) | 12 (31.6) | 38 (100) | 101.0b (3.1–670.95) | 153.2b (2.6–1,450.96) | 15.8 (0.3–42.7) | 30.6 (1.5–66.7) | 27.0a (0.2–164.1) | 276.3b (6.4–2,229.9) |
| CST IV (94) | 94 (100) | 92 (97.9) | 46 (48.9) | 8 (8.5) | 93 (98.9) | 564.3b,c (2.9–4,990.5) | 438.8b,c (3.1–3,179.2) | 10.1a (0.45–59.7) | 21.3a (1.2–134.2) | 93.8b,c (0.2–813.3) | 1,086.6b,c (4.22–6,754.6) |
P < 0.05 versus concentration in all other combined groups.
P < 0.0009 versus concentration in all other groups combined.
P < 0.05 compared to L. iners-dominated microbiota.
P < 0.006 compared to CST IV.
Increases in cadaverine, putrescine, and tyramine, but not spermidine or spermine, are associated with increased odds of being, or transitioning into, a Nugent score of 7 to 10.
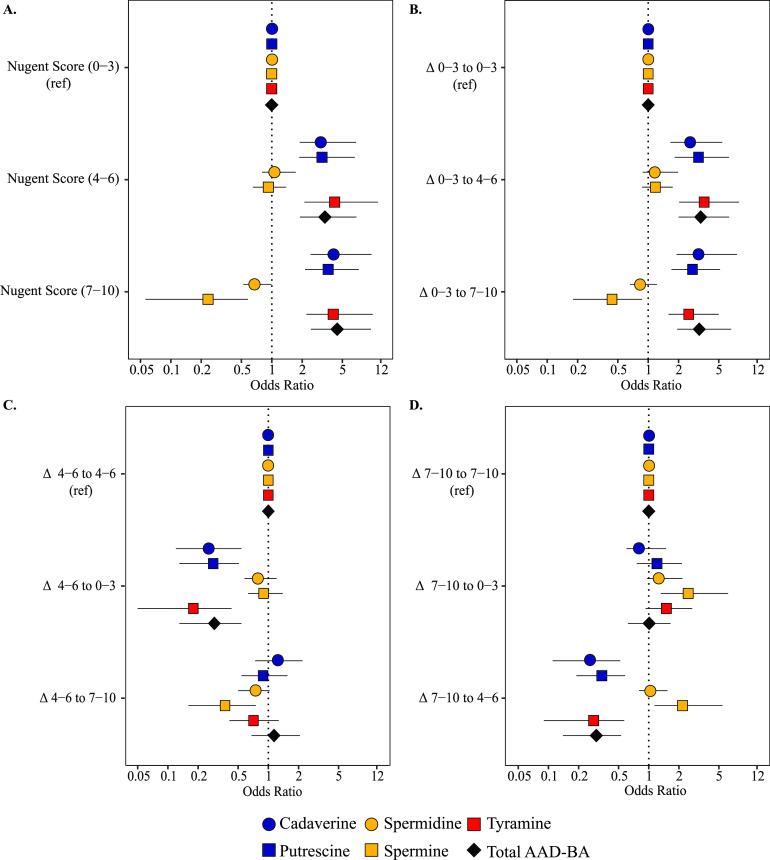
The associations between BAs, Nugent scores, and CSTs were evaluated using Bayesian multinomial logistic mixed-effects regression, with a random intercept for participants and time, and adjusted for menstrual status (Fig. 1 and 2). Increases of cadaverine, putrescine, and tyramine were associated with higher odds of having a Nugent score of 7 to 10 compared to having a low Nugent score of 0 to 3 (no BV) (cadaverine adjusted odds ratio [aOR], 4.5; 95% credible interval [CrI], 2.4 to 9.7; putrescine aOR, 3.7; 95% CrI, 2.1 to 7.3; tyramine aOR, 4.4; 95% CrI, 2.4 to 9.5). Increases in the concentrations of these same metabolites were also associated with higher odds of having a Nugent score of 4 to 6 (intermediate BV) compared to having a Nugent score of 0 to 3 (Fig. 1A). Conversely, the odds of having a Nugent score of 7 to 10 were 62% less likely as vaginal concentrations of spermine increased.
FIG 1.
Odds ratio for Nugent Score (A) and Nugent score transitions by biogenic amine adjusted for menstruation, time, and individual variation (B, C, and D).
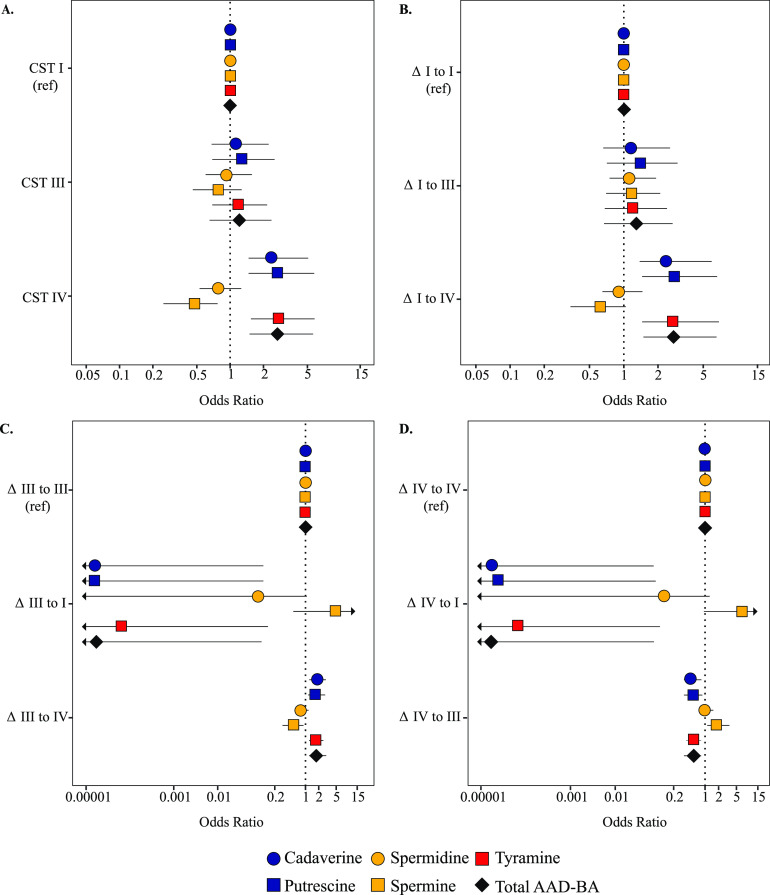
FIG 2.
Odds ratio for community state type (CST) (A) and CST transitions by biogenic amine adjusted for menstruation, time, and individual variation (B, C, and D). Arrows indicate that the 95% credible interval extended past the labeled odds ratio.
Increases in cadaverine, putrescine, and tyramine or the summed concentrations of putrescine, cadaverine, and tyramine (AAD-derived BAs) were associated with higher odds of transitioning to a Nugent score of 7 to 10 when the previous Nugent score was 0 to 3 compared to having a persistent Nugent score of 0 to 3 (cadaverine aOR, 3.4; 95% CrI, 1.9 to 7.8; putrescine aOR, 2.8; 95% CrI, 1.7 to 5.5; tyramine aOR, 2.6; 95% CrI, 1.6 to 5.5; total BA aOR, 3.3; 95% CrI, 1.9 to 6.6). Increases in these same amines were associated with increased odds of transitioning to a Nugent score of 4 to 6 from a score of 0 to 3 (Fig. 1B). Relative to having a stable Nugent score of 4 to 6 or 7 to 10, increases in cadaverine, putrescine, tyramine, or total AAD-derived BAs were associated with decreased odds of transitioning to a Nugent score of 0 to 3 (Fig. 1C and D). Relative to having a stable Nugent score of 0 to 3, increases in spermine were associated with reduced odds of transitioning to a Nugent score of 7 to 10 (aOR, 0.45; 95% CrI, 0.18 to 0.87). This was also true relative to having a stable Nugent score of 4 to 6 (aOR, 0.39; 95% CrI, 0.16 to 0.76). Conversely, relative to maintaining a Nugent score of 7 to 10, increases in spermine were associated with increased odds of transitioning into a Nugent score of 4 to 6 (aOR, 2.5; 95% CrI, 1.3 to 6.2) or 0 to 3 (aOR, 2.2; 95% CrI, 1.1 to 5.14) (Fig. 1D).
Increases in cadaverine, putrescine, and tyramine, but not spermidine or spermine, are associated with increased odds of maintaining or transitioning into CST IV.
The odds of having a CST IV microbiota increased as vaginal concentrations of cadaverine, putrescine, tyramine, or the total AAD-derived BAs increased compared to CST I microbiota (cadaverine aOR, 2.5; 95% CrI, 1.4 to 5.1; putrescine aOR, 2.7; 95% CrI, 1.5 to 5.8; tyramine aOR, 2.7; 95% CrI, 1.5 to 5.8; total AAD-BA aOR, 2.7; 95% CrI, 1.5 to 5.6). Conversely, samples were less likely to have been classified as having CST IV microbiota as the concentrations of the non-AAD-derived BA, spermine, increased (aOR, 0.47; 95% CrI, 0.25 to 0.8) (Fig. 2A).
Increases in cadaverine, putrescine, or tyramine and total AAD-derived BAs were associated with higher odds of transitioning to a CST IV microbiota when the previous CST was CST I compared to maintaining a stable CST I microbiota (cadaverine aOR, 2.5; 95% CrI, 1.3 to 5.9; putrescine aOR, 2.8; 95% CrI, 1.45 to 6.6; tyramine aOR, 2.7; 95% CrI, 1.45 to 6.87; total AAD-BA aOR, 2.8; 95% CrI, 1.49 to 6.6) (Fig. 2B). Increases in these same amines were also associated with higher odds (although to a lesser extent) of transitioning to CST IV from CST III microbiota compared to maintaining a stable CST III microbiota (cadaverine aOR, 1.8; 95% CrI, 1.2 to 2.9; putrescine aOR, 1.7; 95% CrI, 1.1 to 2.8; tyramine aOR, 2.4; 95% CrI, 1.7 to 2.6; total AAD-BA aOR, 1.8; 95% CrI, 1.2 to 2.96) (Fig. 2C). Conversely, increases in spermine were marginally associated with lower odds of transitioning to CST IV from either CST I or III compared to the remaining CST I (aOR, 0.62; 95% CrI, 0.34 to 1.04) or remaining CST III (aOR, 0.55; 95% CrI, 0.3 to 0.9) (Fig. 2).
The effect of biogenic amines on growth properties of vaginal Lactobacillus species.
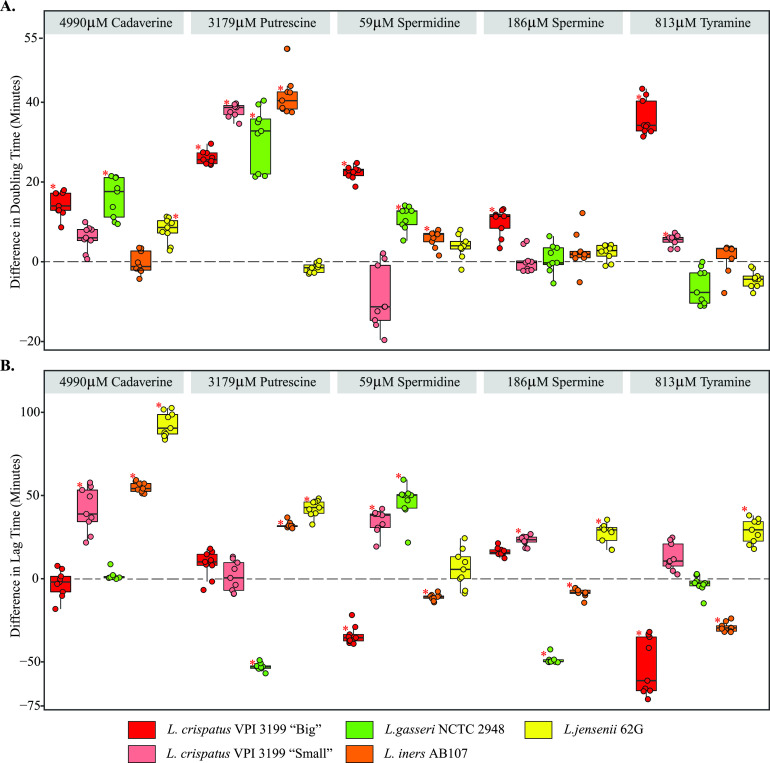
We next assessed the minimum concentration of each biogenic amine necessary to inhibit the in vitro growth of vaginal Lactobacillus species. As these MICs were greater than the measured physiological concentrations (Table S1), we next conducted growth assays using the maximum observed physiological concentration of each biogenic amine measured from vaginal samples (Data Set S1). The maximum concentrations of cadaverine (4,990 μM), putrescine (3,179 μM), spermidine (59 μM), and tyramine (813 μM) were observed in CST IV samples, while the highest concentration of spermine (186 μM) was observed in CST I samples. Growth of pure cultures of several Lactobacillus spp. under in vitro conditions allowed us to evaluate the effect of the biogenic amines upon growth rate (as assessed by doubling times) and lag times (Fig. 3). The average times taken for the positive controls (untreated) of L. crispatus VPI 3199 morphotype big, L. crispatus VPI 3199 morphotype small, L. gasseri NCTC 2948, L. iners AB107, and L. jensenii 62G to double in cell density (doubling time) were 114 (standard deviation [SD], 10), 119 (SD, 14), 134 (SD, 11), 118 (SD, 9), and 98 (SD, 15) min, respectively.
FIG 3.
Effect of biogenic amines on the growth of vaginal Lactobacillus species. (A and B) Differences in doubling time (A) and lag time (B) between treatments and controls for each biogenic amine. Dots represent differences between each treatment and the control. Asterisks indicate significant differences after correcting for multiple comparisons (q value of <0.05).
Putrescine significantly reduced the growth rate of L. crispatus VPI 3199 morphotype big, L. crispatus VPI morphotype small, L. gasseri NCTC 2948, and L. iners AB107, increasing their doubling times by an average of 26.1 (+22% change; 95% confidence interval [CI], 21 to 31), 37.9 (+33% change; 95% CI, 31 to 44), 31.2 (+25% change; 95% CI, 20 to 41), and 41.7 (+32.3% change; 95% CI, 36 to 47) min, respectively. Cadaverine also significantly increased the doubling times of L. crispatus VPI 3199 morphotype big, L. gasseri NCTC 2948, and L. jensenii 62G by 14.5 (+12% change; 95% CI, 7.6 to 21), 16 (+12.8% change; 95% CI, 9.2 to 22.8), and 8 (+7.5%; 95% CI, 2.5 to 13.5) min, respectively, and was associated with longer lag times of L. crispatus VPI 3199 morphotype small (+22%), L. iners AB107 (+44%), and L. jensenii 62G (+78.6%) (Fig. 3B). Conversely, spermidine decreased the lag times of L. crispatus VPI 3199 morphotype big (−14.7%) and L. iners AB107 (−8.1%), and spermine decreased lag times of L. gasseri NCTC 2948 (−27.8%) and L. iners AB107 (−5%).
The effect of biogenic amines upon lactic acid production of vaginal Lactobacillus species.
Finally, we assessed whether biogenic amines affected the production of d- and l-lactic acid isomers by vaginal Lactobacillus spp. when controlling for cell density (Table 2). In general, when grown in the presence of biogenic amines, vaginal Lactobacillus spp. produced fewer d- and l-lactic acid isomers. The notable exceptions were higher levels of d-lactic acid by L. crispatus VPI 3199 morphotype big and l-lactic acid by L. crispatus VPI 3199 morphotype small when grown in the presence of spermine (P < 0.05). Cadaverine negatively affected the production of d-lactic acid by L. crispatus VPI 3199 morphotype big and l-lactic acid by L. iners AB107 (P < 0.05). Both L. crispatus VPI 3199 morphotypes and L. jensenii 62G were associated with decreased production of d-lactic acid when grown in the presence of putrescine, although these relationships were no longer significant after correction for multiple comparisons. Growth in tyramine was associated with decreased production of d-lactic acid in all vaginal Lactobacillus spp. except for L. iners AB107, which does not have a known genetic capacity to produce d-lactic acid. However, tyramine did significantly reduce the concentration of l-lactic acid that L. iners AB107 produced (q value of <0.05). After correction for multiple comparisons, only the associations between tyramine and L. iners AB107 and tyramine and L. gasseri NCTC 2948 remained significant. It is also worth noting, as previously shown, that both d- and l-lactic acid production varied by species, even among the positive controls (52).
TABLE 2.
Effect of BAs upon vaginal Lactobacillus species d- and l-lactic acid production
| Biogenic amine | Lactic acid | Value(s) (mM) for: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
L. crispatus VPI 3199 big |
L. crispatus VPI 3199 small |
L. gasseri NCTC 2948 |
L. iners AB107 |
L. jensenii 62G |
|||||||
| Positive control (n = 3) |
Treatment (n = 3) |
Positive control (n = 3) |
Treatment (n = 3) |
Positive control (n = 3) |
Treatment (n = 3) |
Positive control (n = 3) |
Treatment (n = 3) |
Positive control (n = 3) |
Treatment (n = 3) |
||
| 4,990 μM cadaverine | d-Lactic acid (avg) | 70.09 | 55.77 | 70.9 | 65.25 | 22.88 | 21.67 | NAc | NA | 126.29 | 123.56 |
| d-Lactic acid (SD) | 0.29 | 1.14 | 0.85 | 1.43 | 1.43 | 1.43 | NA | NA | 0 | 1.14 | |
| l-Lactic acid (avg) | 55.12a | 45.94a | 51.94 | 50.88 | 16.65 | 13.3 | 52.65a | 39.24a | 4.13 | 3.78 | |
| l-Lactic acid (SD) | 1.75 | 0.25 | 0.25 | 0.25 | 0.25 | 1.5 | 0.75 | 1.24 | 0 | 0 | |
| 3179 μM P,utrescine | d-Lactic acid (avg) | 58.19a | 50.92a | 63.23a | 52.56a | 67.88 | 56.98 | NA | NA | 60.05a | 58.99a |
| d-Lactic acid (SD) | 0.56 | 1.14 | 1.42 | 0.6 | 0 | 2.28 | NA | NA | 0.57 | 0.57 | |
| l-Lactic acid (avg) | 45.41 | 44 | 56 | 46.82 | 48.94 | 47.705 | 47.88 | 46.46 | 3.77 | 3.77 | |
| l-Lactic acid (SD) | 0.5 | 2.5 | 1.99 | 1 | 0.5 | 0.25 | 0.5 | 0.25 | 1 | 0.5 | |
| 59 μM spermidine | d-Lactic acid (avg) | 61.02 | 50.52 | 32.23 | 27.12 | 13.26 | 14.2 | NA | NA | 97.47 | 81.06 |
| d-Lactic acid (SD) | 4.58 | 0.4 | 1.42 | 4.55 | 0.46 | 0.81 | NA | NA | 11.66 | 7.49 | |
| l-Lactic acid (avg) | 40.43 | 37.77 | 30.47 | 25.65 | 8.95 | 11.19 | 40.59 | 39.06 | 4.48 | 4.01 | |
| l-Lactic acid (SD) | 8.82 | 1.78 | 2.04 | 3.58 | 0.41 | 1.41 | 1.24 | 2.2 | 0.35 | 0.2 | |
| 186 μM spermine | d-Lactic acid (avg) | 47.7a | 51.57a | 49.99 | 49.31 | 53.52 | 51.48 | NA | NA | 65.72a | 54.69a |
| d-Lactic acid (SD) | 1.06 | 0.93 | 3.24 | 0.4 | 2.94 | 1.53 | NA | NA | 3.75 | 3.43 | |
| l-Lactic acid (avg) | 42 | 38.83 | 44.94a | 50.12a | 33.53 | 33.89 | 42 | 39.41 | 2.25 | 2.36 | |
| l-Lactic acid (SD) | 1.08 | 4.48 | 0.89 | 1.13 | 1.74 | 2.07 | 4.79 | 0 | 0.2 | 0.35 | |
| 813 μM tyramine | d-Lactic acid (avg) | 103.79a | 91.01a | 91.95a | 29.54a | 29.81a | 16.09a | NA | NA | 139.03a | 162.34a |
| d-Lactic acid (SD) | 4.96 | 2.22 | 1.82 | 12.94 | 2.22 | 2.03 | NA | NA | 10.84 | 6.69 | |
| l-Lactic acid (avg) | 85.17 | 78.82 | 70.11 | 38.33 | 23.3b | 17.65b | 59.06b | 43.53b | 7.89 | 10.6 | |
| l-Lactic acid (SD) | 5.47 | 2.7 | 4.07 | 11.8 | 1.81 | 1.02 | 1.94 | 1.81 | 0.2 | 1.81 | |
Treatment and control were significantly different; P < 0.05.
Treatment and control were significantly different; q value, <0.05.
NA, not applicable (L. iners does not produce d-lactic acid).
DISCUSSION
We have previously hypothesized that the biosynthesis of BAs within the vagina reduces Lactobacillus species-mediated protection and alters the microenvironment sufficiently to enable colonization by a diverse set of anaerobic bacterial species, as observed in molecular-BV (1, 3, 5, 32, 53). We examined this hypothesis in the context of both an observational cohort study and in vitro experimentation to examine the direct influence of BAs on Lactobacillus species growth properties and lactic acid production.
Measured BA concentrations were consistent with previously reported literature (1, 2, 4, 32, 40) and were found to be associated with both CST and Nugent score. Also consistent with previously reported findings (3–5, 34), we observed higher concentrations of cadaverine, putrescine, and tyramine in CST IV samples than Lactobacillus species-dominant CSTs (I and III); conversely, the non-AAD-derived BAs, spermine, and spermidine were minimally detected in CST IV samples but observed in higher concentrations among Lactobacillus species-dominated CSTs (1). Further, we show that increases in concentrations of putrescine, cadaverine, and tyramine are associated with greater odds of transitioning into CST IV compared to CST I women, after adjustment for menstrual status, as well as with increased odds of transitioning into a Nugent score of 4 to 6 (intermediate BV) or a Nugent score of 7 to 10 (indicative of BV) compared to having a stable Nugent score of 0 to 3. These data support the hypothesis that BAs are important precursors, rather than simply biomarkers, of BV (1, 2, 4). Certainly, genetic analyses indicate that several vaginal taxa are capable of producing BAs (1); however, it is also important to note that putrescine, spermine, and spermidine can be produced endogenously by human cells (54).
One mechanism through which BAs have been hypothesized to affect the incidence and maintenance of molecular-BV is by directly affecting the growth properties of vaginal lactobacilli (1). In general, vaginal microbiota dominated by Lactobacillus spp. is thought to resist the colonization and outgrowth of BV-associated bacteria as well as other reproductive tract infections. This is largely attributed to the production of lactic acid by vaginal Lactobacillus spp., which acidifies the vaginal microenvironment to a pH of <4.5 and limits the growth of potential pathogens (55), although lactobacilli contribute other known anticompetitive activities, including the lactic acid-driven rendering of epithelial cells resistant to infection, as was recently shown with Chlamydia trachomatis (56). Consistent with this hypothesis, we show in this study that physiological concentrations of cadaverine, putrescine, and tyramine decrease the in vitro growth rate and/or increase the lag times of representative strains of each of the major vaginal Lactobacillus spp. as well as affect production of lactic acid of L. iners AB107 and L. gasseri NCTC 2948.
Given findings that women with L. iners (CST III)-dominant microbiota are more likely to transition to CST IV than women with L. crispatus-dominant microbiota (CST I) and are reportedly more susceptible to vaginal disorders, including BV and STIs (56–60), we were interested in comparing the growth responses of L. iners and L. crispatus relative to the concentrations of BAs reported for CST IV and BV. While the growth rate of L. iners appeared less impacted by exogenous BAs than other Lactobacillus spp., the lag time was comparatively longer in the presence of cadaverine and putrescine. It is not entirely clear if growth rate or lag time would be more important in vivo; however, we hypothesize that under more acidic conditions there is reduced growth of all vaginal Lactobacillus spp. based on our own assessment of these Lactobacillus spp. in the laboratory and assessment of Lactobacillus species growth that has been reported elsewhere to be inhibited at pH ∼3.6 to 4.0, dependent upon species and strain (61). Therefore, Lactobacillus spp. may only grow as pH increases above these limits, such as is hypothesized to occur as AAD-derived BAs are produced (1). In this scenario, lag time, which would represent the time to respond to this change in environmental acidity, would be the most important factor, and the more greatly impacted lag times of L. iners could help to explain why CST III vaginal microbiota more frequently transition to CST IV than other CSTs (62). Similarly, when spontaneously clearing molecular BV without antibiotics, wherein levels of putrescine and cadaverine are maximal and the pH is >4.5, a growth rate that is more resistant to these BAs would be favorable, again potentially explaining why L. iners more commonly dominates immediately following a temporary transition to CST IV (62) or BV (63). The ability for L. crispatus to dominate in other scenarios may then simply relate to its higher growth rate under conditions where BA concentrations have returned to normal and/or with other competitive strategies not assessed in this study.
There are, of course, several limitations to our study. First, this was a relatively small sample size, and we could not assess behavioral and confounding variables, such as age, time-varying sexual activity, antibiotic use, partner concurrency, and douching in the models. This limited power is reflected in the large credible intervals associated with transitioning from a CST IV microbiota to CST I. Additionally, our results were limited to the type strains of the four major vaginal Lactobacillus species; thus, it remains to be determined if these observations are generalizable to other strains and clinical isolates of Lactobacillus species. Similarly, it is important to determine if and how these biogenic amines affect the anaerobic taxa associated with BV. Future experiments should assess the effect of specific BA producers on Lactobacillus spp. and whether there is evidence of pH-dependent competition for nutrients. We also evaluated the effect of biogenic amines upon specific growth properties of vaginal Lactobacillus species grown in vitro, utilizing media that may not sufficiently recapitulate the nutrient resources available in vivo; however, it is noteworthy that these in vitro growth responses reflect the changes in relative abundances of Lactobacillus species determined in vivo when biogenic amine concentrations differ. It is also important to consider that biogenic amines do not occur in the vaginal microenvironment in isolation; thus, the synergistic and/or antagonistic effects of the biogenic amines upon one another and in the presence of other metabolites within the vaginal environment needs to be explored before the full impact of biogenic amines on the growth of vaginal Lactobacillus spp. can be fully understood. Finally, putrescine, spermine, and spermidine each are microbially and host produced, and this study cannot tease apart host from microbial contributions.
Conclusions.
Here, we quantified the physiological concentration of biogenic amines from a longitudinal cohort of participants. We observed that increases in the biogenic amines putrescine, cadaverine, and tyramine were associated with increased odds of having molecular-BV, having a Nugent score of 7 to 10, transitioning into a Nugent score of 7 to 10, and transitioning to molecular-BV. We then interrogated the effect of exogenous biogenic amines upon the growth properties of four vaginal Lactobacillus spp. representing the major vaginal CSTs in vitro. We observed the biogenic amines commonly associated with the malodor characteristic of BV, putrescine and cadaverine, to have adverse effects on the growth rate, lag time, and lactic acid production of vaginal lactobacilli. Biogenic amines are important in reducing vaginal acidity, overcoming acid stress resistance, and providing a source of energy to bacteria. Several biogenic amines have recently been associated with STIs and increased pathogen resistance and the vaginal disorder bacterial vaginosis. The results here provide pertinent information on the potential role that biogenic amines play in affecting vaginal reproductive and sexual health.
MATERIALS AND METHODS
Study population and sample collection.
This study utilized samples from a repository collected in prior studies (62, 63). Briefly, between September 2009 and July 2010, 135 nonpregnant, reproductive-aged women were enrolled in a longitudinal study at the University of Alabama at Birmingham and provided daily, self-collected mid-vaginal swabs for 10 weeks. Vaginal secretions were collected using Copan ESwabs (Copan Diagnostics, Murrieta, CA) placed in Amies liquid transport medium and frozen at −80°C until use. An additional sample for metabolomics was collected using a Starplex double-headed Dacron swab at the same visit (Starplex Scientific, Cleveland, TN) and stored dry in a tube at −80°C until later use.
Taxonomic assignment and CST profiling.
For a subset of the samples (n = 38) (see Data Set S1 in the supplemental material), DNA extraction, PCR amplification, and Roche 454 pyrosequencing of 16S rRNA gene amplicons (V1-V3) using primers 27F-YM +3 and 543R were previously described (63). For the rest of the samples (n = 111), DNA extraction, PCR amplification, and sequencing of the 16S rRNA gene V3-V4 and bioinformatics processing using the dada2 pipeline (64) followed the methods described by Holm et al. (65). Taxonomic assignments were performed using the RDP Classifier trained with SILVA (version 128) and species of specific vaginal bacteria determined with SpeciateIT (ravel-lab.org/speciateit). For each sample, CST classifications were assigned using VALENCA, as described by France et al. (66).
Sample selection and targeted liquid chromatography-mass spectrometry.
We were interested in evaluating the associations between vaginal biogenic amines and Nugent scores as participants transitioned into or from a Nugent score of 7 to 10 as well as into or from a vaginal microbiota of CST IV. This is a secondary study based on samples from two previously published studies (62, 63) in which 135 women self-collected daily vaginal samples for 10 weeks. For the present study, vaginal swabs from 32 participants were selected based on transitions into or from a Nugent score of 7 to 10 and their corresponding CSTs characterized (Fig. S1 and Data Set S1). The median contribution was 4 samples per participant (range, 2 to 8 samples), for a total of 149 samples, with the median number of days between samples being 2 (interquartile range [IQR], 1 day). There were 6 samples for which we did not have a Nugent score but we did have CST characterization. Of those 149 samples, vaginal microbiota types were of CST I (L. crispatus, n = 17), CST III (L. iners, n = 38), and CST IV (n = 94). Of the samples for which we had current Nugent scores, 39 were of Nugent 0 to 3, 33 were of Nugent 4 to 6, and 71 were of Nugent 7 to 10.
For each sample, one head of a Starplex vaginal swab was eluted using 70% methanol and analyzed with the 5500 QTRAP LC-MS/MS system (Sciex, Framingham, MA) in the Metabolomics Laboratory of the Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign. Software Analyst 1.6.2 was used for data acquisition and analysis. The 1200 series high-performance liquid chromatography (HPLC) system (Agilent Technologies, Santa Clara, CA) includes a degasser, an autosampler, and a binary pump. The LC separation was performed on an Agilent Eclipse XDB-C18 (4.6 by 150 mm, 5 μm) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The flow rate was 0.4 ml/min. The linear gradient was the following: 0 to 3 min, 95% A; 9 to 13 min, 5% A; and 13.5 to 18 min, 95% A. The autosampler was set to 10°C. The injection volume was 5 μl. Mass spectra were acquired under positive electrospray ionization (ESI) with an ion spray voltage of +5,000 V. The source temperature was 500°C. The curtain gas, ion source gas 1, and ion source gas 2 were 33, 65, and 55 lb/in2, respectively. Multiple reaction monitoring (MRM) was used for quantitation: cadaverine (m/z 103.0→m/z 69.0), putrescine (m/z 189.0→m/z 30.0), spermine (m/z 199.9→m/z 91.1), spermidine (m/z 146.0→m/z 30.0), and tyramine (m/z 138.1→m/z 77.1). The levels of detection for each metabolite were cadaverine (100 nM), putrescine (100 nM), spermidine (20 nM), spermine (20 nM), and tyramine (5 nM). Quantitative measurements are reported in Table 1 and Data Set S1 in the supplemental material. For the purpose of plotting, missing values were imputed with one-half the minimum value obtained for a given metabolite (67–71). Boxplots of the range of amines were constructed in R Statistical Software.
Association between CSTs and biogenic amines.
Bayesian multinomial logistic mixed-effect regression with a random intercept for individuals was utilized for modeling the association between the log2-transformed vaginal BAs, vaginal CSTs, and Nugent score categories. We evaluated the following associations: model 1, BAs and Nugent score (outcome); model 2, BAs and Nugent transitions (outcome); model 3, BAs and CST (outcome); and model 4, BAs and CST transitions (outcome). All models were adjusted for a binary variable on current menstruation status and included random intercepts for individual-specific variation and time.
For model 1, the reference was Nugent score 0 to 3 at the present visit. To compare the transition into a Nugent score of 7 to 10 from a previous Nugent score of 0 to 3, we set the reference to a current and previous Nugent score of 0 to 3. We adjusted the reference for model 2 to allow for comparisons between transitioning into Nugent scores compared to having a stable Nugent score of 4 to 6 (present and previous visit having a Nugent score of 4 to 6) and 7 to 10 (present and previous visit having a Nugent score of 7 to 10). There were 9 observations for which we did not have either current or prior Nugent score data, so these events were excluded from models 1 and 2. For model 3, the reference was CST I for current CST. To model transition to a current CST IV from a previous CST I, the reference was set to current and previous CST I. To model transition to the current CST IV from a previous CST III, the reference was adjusted to the current and previous CST III. To model transition to the current CST of I or III from a previous CST IV, the reference was adjusted to the current and previous CST IV. In all instances, an increase refers to a doubling of a given metabolite. Finally, we evaluated the concerted effect of total BAs using the four models listed above. We created a variable wherein we summed the concentrations of cadaverine, putrescine, and tyramine (AAD-BA).
For all models, estimation was carried out using Hamiltonian Monte Carlo (72–74) and its extension, the No-U-Turn (NUTS) sampler (75). We used weakly informative priors for the fixed and random effects (i.e., we did not impose any prior information on the estimates, instead using default priors; fixed-effect parameters have an improper flat prior over the real parameters, and random-effect parameters are restricted to be nonnegative, have a half student t-prior with three degrees of freedom, and have a minimal scale parameter of at least 10 [76]). Bayesian multinomial logistic regression was performed in the brms package (76) in R, which runs RStan (Stan Development Team, 2015) in the background. Statistical significance was defined as Bayesian credible intervals for odds ratios excluding 1. All reported results represent between-participant associations.
Bacterial strains.
The following bacteria were obtained from the ATCC (American Type Culture Collection) and used to evaluate the effect of biogenic amines upon bacterial growth in axenic cultures: L. crispatus VPI 3199 (ATCC 33820), L. iners AB107 (ATCC 55195), L. gasseri NCTC 2948 (ATCC 9857), and L. jensenii 62G (ATCC 25258). When grown in isolation on MRS-NYC III agar (MNC; 10 g/liter proteose peptone, 10 g/liter beef extract, 5 g/liter yeast extract, 5 g/liter NaCl, 0.1 g/liter MgSO4, 0.05 g/liter MnSO4, 2 g/liter K2HPO4, 20 g/liter glucose, 100 ml/liter fetal bovine serum), L. crispatus VPI 3199 had two distinct colony morphologies. One colony type was small, irregular undulate, opaque, buff in color, and flat; we termed this L. crispatus small. The other colony type was larger and opaque, with a distinct, dot-like center that became translucent closer to the perimeter, and irregular undulate; we termed this L. crispatus big. Colony PCR was conducted on both morphotypes using 16S rRNA gene primers 42F and 1023R, as described by Fredricks et al. (77), and amplicon sequencing confirmed the identity of the two morphologies as L. crispatus. Both colony morphologies had distinct growth properties, and, as such, we conducted all growth assays on both morphotypes.
Growth conditions and biogenic amines.
Lactobacillus spp. were grown in MNC as described by Witkin et al. (52). For all experiments, all cultures were incubated at 37°C in an atmosphere with 5% CO2 and grown in sterile, 15-ml Pyrex round-bottom, screwcap, glass test tubes with no shaking. The approximate bacterial cell density was calculated using a modified most probable number enumeration wherein 2-fold dilutions were carried out in 96-well plates beginning with an OD600 of ∼0.5 for each bacterium until the maximum dilution was apparent by no visible growth after 24 h. Plates were incubated for 24 h at 37°C, and the cell density was approximated and associated with the corresponding optical density (OD) (Table S2). Cadaverine, spermidine, spermine, and tyramine were obtained from ACROS Organics. Putrescine was obtained from MPBiomedicals. Biogenic amine treatments were prepared within 30 min of use by diluting premade 1 M stocks (putrescine and cadaverine) and 0.1 M stocks (spermidine and spermine) or a powder form (tyramine) into the appropriate concentration with MNC that had been prewarmed in an incubator at 37°C.
MICs of biogenic amines.
The MIC was defined as the minimal biogenic amine concentration needed to inhibit visible bacterial growth and was determined with a standard microdilution procedure according to Clinical and Laboratory Standards Institute guidelines (78) (Table S1). Biogenic amine concentrations ranged from 900 mM to 0.78 mM in a 2-fold dilution series. To each dilution of the amine, bacteria were added 1:100, vortexed, and then plated in a 96-well microtiter plate. For each bacterium and amine, the test was carried out in triplicate in MNC and grown for 24 h at 37°C and evaluated in both anaerobic (grown in a vinyl anaerobic chamber [Coy Laboratories Products, Inc.]) and aerobic environments.
Effect of biogenic amines on the growth characteristics of Lactobacillus spp.
Growth curves were conducted to evaluate the direct effect of the biogenic amines upon the growth of the Lactobacillus species. Bacteria were inoculated into 15 ml of MNC and grown overnight at 37°C in an atmosphere of 5% CO2 with no shaking until they reached an OD600 of 1.4. Saturated overnight cultures were diluted 1:100 into 15 ml of prewarmed, fresh MNC. Once the day cultures reached an OD600 of ≈0.5 (Table S2), they were inoculated with a 1:100 dilution into 15 ml of the respective treatment and control. For each bacterium, the amine treatment and positive controls were run in triplicate. The pH of the initial medium was 6.5 as tested by the MilliporeSigma MColorPhast pH strips, and there was no observed immediate effect upon the addition of the amines or upon the addition of the Lactobacillus species seed cultures. Biogenic amines are volatile compounds; to ensure that the observed effects were due to the amine of interest, for each bacterium, treatments were completed in isolation over three separate days, yielding nine replicates per positive control and biogenic amine treatment. Samples (200 μl) were obtained from each replicate, and the OD was measured using the BioTek Epoch 2 at least every 30 min until stationary phase. Generation and lag times were calculated using the package GrowthRates in R statistical software (79). Differences in the generation and lag time were calculated using the t.test function, and P values were adjusted using the false discovery rate method in the p.adjust function in R statistical software.
Lactic acid assays.
We evaluated whether the biogenic amines influenced d- and l-lactic acid production by vaginal Lactobacillus species. Axenic cultures of each bacterium in pure MNC and in MNC supplemented with appropriate concentrations of biogenic amines were grown in triplicate. As biogenic amines are volatile, the effect of each amine was tested on independent days. Once each culture had reached stationary phase, it was spun down at 4,500 × g for 10 min. For each sample, 1 ml of supernatant was collected, transferred to clean microcentrifuge tubes, and stored at −20°C. Samples were thawed in batches and d- and l-lactate measured colorimetrically by using EnzyChrom l-lactate and EnzyChrom d-lactate kits from BioAssay Systems (Hayward, CA). Values were converted to nanograms per milliliter or millimolar by reference to a standard curve that was generated in parallel to the test samples. Differences in concentrations of d- and l-lactic acid were analyzed using the Student's t test, as it has been shown this method is appropriate even with sample sizes of N = 2 if the effect size is large (80). P values were adjusted for multiple comparisons using the false discovery rate method and the p.adjust function in R Statistical Software.
Ethics.
All participants provided written informed consent. Ethical approval was obtained from the Institutional Review Boards of the University of Alabama at Birmingham (UAB), the University of Maryland School of Medicine, and Montana State University (MSU), and all research was conducted in compliance with relevant guidelines and regulations.
Availability of data and materials.
The metabolite data set and participant characteristics used and/or analyzed during the current study are included as a supplemental data set (Data Set S1). The doubling and lag times for type strains are available upon reasonable request. The sequence data are available in SRA under BioProject accession numbers PRJNA208535 (samples beginning with UAB) and PRJNA575586 (samples beginning with AYAC and EM).
Supplementary Material
ACKNOWLEDGMENTS
We thank Deanna DeSon, Herlin Kadriu, and Trent Jones for their efforts in bacterial culturing and Elizabeth O’Hanlon for her advice about MPNs.
J.R. is the cofounder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health. All remaining authors have no disclosures to declare.
The research reported in this publication was supported by the National Institutes of Allergy and Infectious Diseases (NIAID), General Medical Sciences (NIGMS), and Nursing Research (NINR) of the National Institutes of Health under award numbers R21AI111145 (C.J.Y.), U54GM115371 (C.J.Y.), UH2AI083264 (J.R.), and R01NR015495 (J.R.) and by the Montana Agricultural Experiment Station.
C.J.Y., T.M.N., and J.-L.C.B. conceived of the study. J.R., L.F., and R.M.B. implemented the parent study. A.U. and Z.L. performed metabolomics. J.-L.C.B., E.K.S., B.A., and S.G.G. performed growth curves and lactic acid assays. B.A. performed PCR validation on all vaginal taxa. J.-L.C.B. and M.D.S. performed statistical analysis. J.-L.C.B., M.D.S., R.M.B., J.R., and C.J.Y. advised on statistical analysis and interpretation of the data. J.-L.C.B. and C.J.Y. wrote the main manuscript text. All authors reviewed and contributed to the final version of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nelson TM, Borgogna JL, Brotman RM, Ravel J, Walk ST, Yeoman CJ. 2015. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol 6:253. 10.3389/fphys.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. 2015. Metabolic signatures of bacterial vaginosis. mBio 6:e00204-15. 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgogna JC, Shardell MD, Santori EK, Nelson TM, Rath JM, Glover ED, Ravel J, Gravitt PE, Yeoman CJ, Brotman RM. 2020. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis. BJOG 127:182–192. 10.1111/1471-0528.15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeoman CJ, Thomas SM, Miller MEB, Ulanov AV, Torralba M, Lucas S, Gillis M, Cregger M, Gomez A, Ho M, Leigh SR, Stumpf R, Creedon DJ, Smith MA, Weisbaum JS, Nelson KE, Wilson BA, White BA. 2013. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One 8:e56111. 10.1371/journal.pone.0056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgogna J-LC, Shardell MD, Yeoman CJ, Ghanem KG, Kadriu H, Ulanov AV, Gaydos CA, Hardick J, Robinson CK, Bavoil PM, Ravel J, Brotman RM, Tuddenham S. 2020. The association of Chlamydia trachomatis and Mycoplasma genitalium infection with the vaginal metabolome. Sci Rep 10:3420. 10.1038/s41598-020-60179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillier S, Holmes K. et al. 1999. Bacterial vaginosis, p 563–586. Holmes K, Sparling P, Mardh P (ed), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, NY. [Google Scholar]
- 7.Marrazzo JM, Martin DH, Watts DH, Schulte J, Sobel JD, Hillier SL, Deal C, Fredricks DN. 2010. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sexually Transm Dis 37:732–744. 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DH. 2011. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci 343:2–9. 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin DH, Zozaya M, Lillis R, Miller J, Ferris MJ. 2012. The microbiota of the human genitourinary tract: trying to see the forest through the trees. Trans Am Clin Climatol Assoc 123:242–256. [PMC free article] [PubMed] [Google Scholar]
- 11.Boskey ER, Cone RA, Whaley KJ, Moench TR. 2001. Origins of vaginal acidity: high d/l lactate ratio is consistent with bacteria being the primary source. Hum Reprod 16:1809–1813. 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 12.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. 2001. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379. 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 13.Ocana VS, Pesce De Ruiz Holgado AA, Nader-Macias ME. 1999. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl Environ Microbiol 65:5631–5635. 10.1128/AEM.65.12.5631-5635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid G, Heinemann C, Velraeds M, van der Mei HC, Busscher HJ. 1999. Biosurfactants produced by Lactobacillus. Methods Enzymol 310:426–433. 10.1016/s0076-6879(99)10033-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaewsrichan J, Peeyananjarassri K, Kongprasertkit J. 2006. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunol Med Microbiol 48:75–83. 10.1111/j.1574-695X.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 16.Boris S, Barbés C. 2000. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2:543–546. 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 17.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. 2019. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 46:304–311. 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 18.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE. 2007. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 34:864–869. 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 19.McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, Jaspan HB, Kaul R, Kaushic C, Klatt N, Kwon DS, Marrazzo JM, Masson L, McClelland RS, Ravel J, van de Wijgert J, Vodstrcil LA, Tachedjian G. 2019. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 35:219–228. 10.1089/AID.2018.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. 2003. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 36:663–668. 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 21.Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, Schwebke JR. 2010. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 202:1907–1915. 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180:1863–1868. 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 23.Sumati AH, Saritha NK. 2009. Association of urinary tract infection in women with bacterial vaginosis. J Glob Infect Dis 1:151–152. 10.4103/0974-777X.56254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson PG, Bergman B, Forsum U, Platz-Christensen JJ, Pahlson C. 1989. Mobiluncus and clue cells as predictors of PID after first-trimester abortion. Acta Obstet Gynecol Scand 68:217–220. 10.3109/00016348909020992. [DOI] [PubMed] [Google Scholar]
- 25.van Oostrum N, De Sutter P, Meys J, Verstraelen H. 2013. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod 28:1809–1815. 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 26.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, Stevenson DK, Holmes SP, Relman DA. 2015. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 112:11060–11065. 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. 2003. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 189:139–147. 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 28.Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, Ravel J. 2019. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun 10:1305. 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Memar M, Bobdiwala S, Fourie H, Mannino R, Lee YS, Smith A, Marchesi JR, Timmerman D, Bourne T, Bennett PR, MacIntyre DA. 2020. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG 127:264–274. 10.1111/1471-0528.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301. 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 32.Nelson TM, Borgogna JC, Michalek RD, Roberts DW, Rath JM, Glover ED, Ravel J, Shardell MD, Yeoman CJ, Brotman RM. 2018. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci Rep 8:852. 10.1038/s41598-017-14943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Koppolu S, Chappell C, Moncla BJ, Hillier SL, Mahal LK. 2015. Studying the effects of reproductive hormones and bacterial vaginosis on the glycome of lavage samples from the cervicovaginal cavity. PLoS One 10:e0127021. 10.1371/journal.pone.0127021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobel JD, Karpas Z, Lorber A. 2012. Diagnosing vaginal infections through measurement of biogenic amines by ion mobility spectrometry. Eur J Obstet Gynecol Reprod Biol 163:81–84. 10.1016/j.ejogrb.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Witkin SS, Ledger WJ. 2012. Complexities of the uniquely human vagina. Sci Transl Med 4:132fs11. 10.1126/scitranslmed.3003944. [DOI] [PubMed] [Google Scholar]
- 36.Chen KC, Forsyth PS, Buchanan TM, Holmes KK. 1979. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J Clin Investig 63:828–835. 10.1172/JCI109382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen KC, Amsel R, Eschenbach DA, Holmes KK. 1982. Biochemical diagnosis of vaginitis: determination of diamines in vaginal fluid. J Infect Dis 145:337–345. 10.1093/infdis/145.3.337. [DOI] [PubMed] [Google Scholar]
- 38.Hillier SL, Krohn MA, Nugent RP, Gibbs RS. 1992. Characteristics of three vaginal flora patterns assessed by gram stain among pregnant women. Vaginal infections and pematurity study group. Am J Obstet Gynecol 166:938–944. 10.1016/0002-9378(92)91368-K. [DOI] [PubMed] [Google Scholar]
- 39.Wolrath H, Boren H, Hallen A, Forsum U. 2002. Trimethylamine content in vaginal secretion and its relation to bacterial vaginosis. APMIS 110:819–824. 10.1034/j.1600-0463.2002.1101108.x. [DOI] [PubMed] [Google Scholar]
- 40.Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L. 2015. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur J Clin Microbiol Infect Dis 34:2367–2376. 10.1007/s10096-015-2490-y. [DOI] [PubMed] [Google Scholar]
- 41.Kanjee U, Houry WA. 2013. Mechanisms of acid resistance in Escherichia coli. Annu Rev Microbiol 67:65–81. 10.1146/annurev-micro-092412-155708. [DOI] [PubMed] [Google Scholar]
- 42.Shah P, Swiatlo E. 2008. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol 68:4–16. 10.1111/j.1365-2958.2008.06126.x. [DOI] [PubMed] [Google Scholar]
- 43.Kwon DH, Lu CD. 2007. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob Agents Chemother 51:2070–2077. 10.1128/AAC.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon DH, Lu CD. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother 50:1615–1622. 10.1128/AAC.50.5.1615-1622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chattopadhyay MK, Tabor CW, Tabor H. 2003. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci U S A 100:2261–2265. 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goytia M, Dhulipala VL, Shafer WM. 2013. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 343:64–69. 10.1111/1574-6968.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira CI, Matos D, San Romao MV, Crespo MT. 2009. Dual role for the tyrosine decarboxylation pathway in Enterococcus faecium E17: response to an acid challenge and generation of a proton motive force. Appl Environ Microbiol 75:345–352. 10.1128/AEM.01958-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMillan A, Macklaim JM, Burton JP, Reid G. 2013. Adhesion of Lactobacillus iners AB-1 to human fibronectin: a key mediator for persistence in the vagina? Reprod Sci 20:791–796. 10.1177/1933719112466306. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham-Rundles S, Maas WK. 1975. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol 124:791–799. 10.1128/JB.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabor CW, Tabor H, Hafner EW. 1978. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem 253:3671–3676. 10.1016/S0021-9258(17)34853-6. [DOI] [PubMed] [Google Scholar]
- 51.Guirard BM, Snell EE. 1964. Effect of polyamine structure on growth stimulation and spermine and spermidine content of lactic acid bacteria. J Bacteriol 88:72–80. 10.1128/JB.88.1.72-80.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. 2013. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio 4:e00460-13. 10.1128/mBio.00460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borgogna J-LC, Yeoman CJ. 2017. The application of molecular methods towards an understanding of the role of the vaginal microbiome in health and disease. Methods Microbiol 44:37–91. 10.1016/bs.mim.2017.08.003. [DOI] [Google Scholar]
- 54.Pegg AE. 2009. Mammalian polyamine metabolism and function. IUBMB Life 61:880–894. 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Hanlon DE, Moench TR, Cone RA. 2011. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis 11:200. 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards VL, Smith SB, McComb EJ, Tamarelle J, Ma B, Humphrys MS, Gajer P, Gwilliam K, Schaefer AM, Lai SK, Terplan M, Mark KS, Brotman RM, Forney LJ, Bavoil PM, Ravel J. 2019. The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. mBio 10:e01548-19. 10.1128/mBio.01548-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Houdt R, Ma B, Bruisten SM, Speksnijder A, Ravel J, de Vries HJC. 2018. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: a case-control study. Sex Transm Infect 94:117–123. 10.1136/sextrans-2017-053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borgdorff H, Armstrong SD, Tytgat HL, Xia D, Ndayisaba GF, Wastling JM, van de Wijgert JH. 2016. Unique insights in the cervicovaginal Lactobacillus iners and L. crispatus proteomes and their associations with microbiota dysbiosis. PLoS One 11:e0150767. 10.1371/journal.pone.0150767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, Sung J, Ko G. 2013. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8:e63514. 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, Gravitt PE. 2014. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 210:1723–1733. 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammes WP, Hertel C. 2015. Lactobacillus. In Trujillo ME, Dedysh S, DeVos P, Hedlund B, Kämpfer P, Rainey FA, Whitman WB (ed), Bergey's manual of systematics of Archaea and Bacteria. Springer-Verlag, New York, NY. 10.1002/9781118960608.gbm00604. [DOI] [Google Scholar]
- 62.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra52. 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh D, Sakamoto J, Koenig SS, Fu L, Zhou X, Hickey R, Schwebke J, Forney LJ. 2013. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 1:29. 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holm JB, Humphrys MS, Robinson CK, Settles ML, Ott S, Fu L, Yang H, Gajer P, He X, McComb E, Gravitt PE, Ghanem KG, Brotman RM, Ravel J. 2019. Ultrahigh-throughput multiplexing and sequencing of >500-base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems 4:e00029-19. 10.1128/mSystems.00029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.France MT, Ma B, Gajer P, Brown S, Humphrys MS, Holm JB, Waetjen LE, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dareng EO, Ma B, Famooto AO, Adebamowo SN, Offiong RA, Olaniyan O, Dakum PS, Wheeler CM, Fadrosh D, Yang H, Gajer P, Brotman RM, Ravel J, Adebamowo CA. 2016. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect 144:123–137. 10.1017/S0950268815000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Zhao X, Lu X, Lin X, Xu G. 2015. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front Mol Biosci 2:4. 10.3389/fmolb.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei R, Wang J, Su M, Jia E, Chen S, Chen T, Ni Y. 2018. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep 8:663. 10.1038/s41598-017-19120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smilde AK, van der Werf MJ, Bijlsma S, van der Werff-van der Vat BJ, Jellema RH. 2005. Fusion of mass spectrometry-based metabolomics data. Anal Chem 77:6729–6736. 10.1021/ac051080y. [DOI] [PubMed] [Google Scholar]
- 71.Xia J, Wishart DS. 2011. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc 6:743–760. 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- 72.Duane S, Kennedy AD, Pendleton BJ, Roweth D. 1987. Hybrid Monte Carlo. Phys Lett B 195:216–222. 10.1016/0370-2693(87)91197-X. [DOI] [Google Scholar]
- 73.Brooks S, Gelman A, Jones G, Meng X-L. 2012. Handbook of Markov Chain Monte Carlo. Chapman and Hall/CRC Press, Boca Raton, FL. 10.1080/09332480.2012.668472. [DOI] [Google Scholar]
- 74.Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A. 2017. Stan: a probabilistic programming language. J Stat Softw 76:1–32. 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffman MDGA. 2014. The No-U-Turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. J Machine Learning Res 15:1593–1623. [Google Scholar]
- 76.Bürkner P-C. 2017. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28. 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- 77.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. 2007. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45:3270–3276. 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9. CLSI, Wayne, PA. [Google Scholar]
- 79.Hall BG, Acar H, Nandipati A, Barlow M. 2014. Growth rates made easy. Mol Biol Evol 31:232–238. 10.1093/molbev/mst187. [DOI] [PubMed] [Google Scholar]
- 80.de Winter JCF. 2013. Using the Student's t-test with extremely small sample sizes. Pract Assess Res Eval 18:1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metabolite data set and participant characteristics used and/or analyzed during the current study are included as a supplemental data set (Data Set S1). The doubling and lag times for type strains are available upon reasonable request. The sequence data are available in SRA under BioProject accession numbers PRJNA208535 (samples beginning with UAB) and PRJNA575586 (samples beginning with AYAC and EM).