Abstract
Statin treatment has been associated with necrotizing autoimmune myopathy and has been linked to myasthenia gravis. We present an unprecedented clinical challenge with both disorders occurring in a patient treated with statins few months earlier.
Keywords: autoimmune disease, myasthenia gravis, necrotizing autoimmune myopathy, statins
Statin treatment has been associated with necrotizing autoimmune myopathy and has been linked to myasthenia gravis. We present an unprecedented clinical challenge with both disorders occurring in a patient treated with statins few months earlier.
1. INTRODUCTION
Statins are commonly prescribed for atherosclerotic disease, and although generally safe, their use has been associated with serious muscular involvement.1 The spectrum of statin‐associated neuromuscular involvement ranges from myalgia with increase of creatine kinase (CK), to severe conditions including necrotizing autoimmune myopathy (NAM), and less commonly, myasthenia gravis (MG).1, 2, 3
Serum myositis‐specific autoantibodies associated with NAM include anti‐3‐hydroxy‐3‐methylglutarylcoenzyme‐A reductase (HMGCR) and antisignal recognition particle (SRP).4 Anti‐HMGCR myopathy is characterized by severe, rapidly progressive proximal muscle weakness along with dysphagia in 16%‐30% of the patients, and elevated serum CK levels.5, 6 Muscle biopsy shows necrosis with scarce or no inflammation. In most patients with a history of statin exposure, symptoms do not resolve after withdrawing the drug, and only a prompt immunosuppressive treatment leads to clinical improvement.4, 7
While lines of evidences suggest a causal relationship between NAM and statin intake, MG is not currently included among adverse drug reactions to statins and in a disproportionality analysis of nearly 4,000 reports mentioning MG, a weak potential signal was found linking statins and MG.8 On the other hand, there have been a number of literature and postmarketing pharmacovigilance reports suggesting that statins may induce, unmask or exacerbate ocular or generalized MG, either seropositive or seronegative for antibodies against acetylcholine receptors (AchR).3, 9, 10, 11, 12, 13, 14
We report on a patient treated with statin and ezetimibe who developed NAM and generalized MG which responded favorably to immunosuppressive therapy.
2. CASE REPORT
A 65‐year‐old male complained of progressive lower limb weakness and calf muscle pain. No autoimmune or hereditary neuromuscular diseases were referred. His medical history was remarkable for arterial hypertension, mild carotid atherosclerosis, subclinical hypothyroidism, and hyperlipidemia. Eight months before admission the patient had an acute myocardial infarct for which percutaneous transluminal coronary angioplasty with placement of two stents in main coronary arteries was performed. Medical therapy at discharge included ramipril, bisoprolol, ticagrelor, aspirin, and high‐intensity statin treatment with 80 mg atorvastatin. Six months later atorvastatin was switched to rosuvastatin/ezetimibe 20 mg/10 mg combination therapy because the patient reported mild weakness and fatigue. The treatment with rosuvastatin/ezetimibe was discontinued 2 weeks prior to admission because of worsening weakness and increased serum CK level (12 309 IU/L; reference range <223 IU/L).
On admission, 3 months after the onset of symptoms, the patient complained of easy fatigability and difficulties in getting up from a chair and walking; his muscle strength was symmetrically reduced at proximal (3/5 by MRC) and distal (4/5 by MRC) segments of all limbs; arm and thigh muscles were hypotrophic. The patient had mild facial and palatal weakness, severe dysphonia, and dysphagia. Sensation and deep tendon reflexes were normal. Severity of weakness fluctuated during the day being worse at evening. During hospitalization, the patient developed dyspnea at rest with decreased oxygen saturation values of 90% in room air.
Serum CK was 32 000 IU/L, AST 950 IU/L (reference range: 5‐38 IU/L), ALT 655 IU/L (reference range 5‐41 IU/L), LDH 1100 (reference: <248 IU/L). Anti‐AchR turned out to be increased to 8.9 nmol/L (NV < 0.5), and anti‐HMGCR antibody titer was 255.2 IU/mL (reference range, <19 IU/mL). ANA, anti‐ds‐DNA, anti‐SSA, and anti‐SSB were negative. Among myositis‐associated autoantibodies, a moderate positivity for anti‐Ro‐52 was detected by immunoblotting. Extensive electrodiagnostic investigations, including bilateral median, ulnar, radial, peroneal, and tibial motor‐conduction studies and median, ulnar, radial, and sural sensory‐conduction studies, were normal. Needle electromyography (EMG) studies showed a myopathic pattern on voluntary recruitment with abnormal spontaneous activity in proximal limb muscles, including trapezius, deltoid, biceps, and vastus lateralis. Single‐fiber EMG (SF‐EMG) performed during voluntary contraction of the extensor digitorum communis at 20 recording sites showed a mean jitter of 65.5 ± 40.2 µs (range 39.7‐196 µs), in the presence of blockings.
A lower limb MRI revealed asymmetric edema and atrophy of proximal and distal thigh muscles (Figure 1). A left vastus lateralis muscle biopsy showed slight variation in fiber caliber, with few atrophic fibers, randomly distributed necrotic myofibers, either hyalinized or myophagocytic, and rare basophilic fibers, but not inflammatory infiltrates. Echocardiogram and total body CT scan were normal.
FIGURE 1.
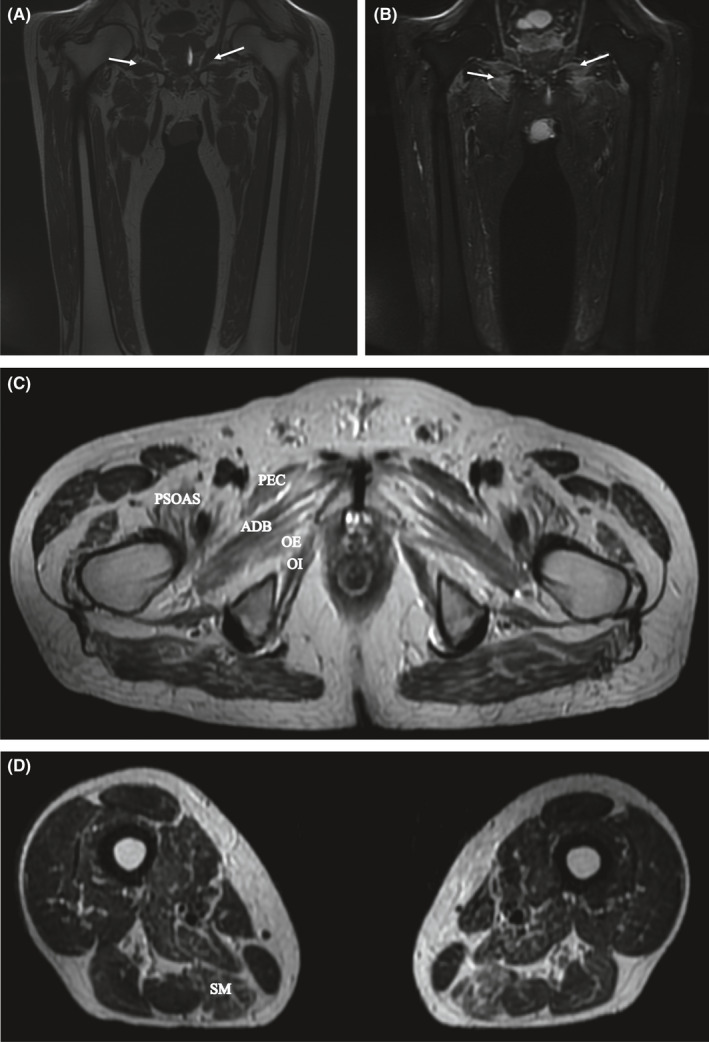
Muscle MRI of lower limbs. Coronal T1‐weighted images (A) reveal muscle atrophy and hyperintensity reflecting connective tissue and fatty replacement (arrows). Coronal short‐tau inversion recovery (STIR) sequences (B) show edema and asymmetrically increased signals in muscle compartments (arrows). Axial T2‐weighted sequences show atrophy in proximal (C) and in the distal (D) thigh muscles. (semimembranosus = SM). PEC, denotes pectineus, PSOAS, ileopsoas, ADB, adductor brevis, OE, obturator externus, and OI, obturator internus
The patient was treated with IVIg (0.4 g/kg die) for 5 days, prednisone 1 mg/kg, and azathioprine 100 mg/die. After 8 days, azathioprine was discontinued because the patient had a diffuse rash, and intramuscular injections of methotrexate 15 mg/week were given; in addition, piridostigmine at a dose of 30 mg three times a day was started.
The patient gradually improved and after 4 months of treatment, he could walk and feed unaided. CK was 241 IU/L, anti‐HMGCR 25 IU/mL, and anti‐AchR 9.3 nmol/L. A repeated EMG recorded a myopathic pattern without denervation. Six months later, prednisone was tapered to 15 mg/die and methotrexate to 10 mg/week because of persistent improvement of symptoms.
3. DISCUSSION
To our knowledge, this is the first reported patient with statin‐associated NAM with anti‐HMGCR IgG occurring concomitantly with anti‐AchR seropositive MG.
As reported in previous cases, the duration of statin exposure before the onset of NAM ranges from few months to 2 years, and the course of the disease is remarkably varied, usually within months and years.15 In addition, while the majority of patients with NAM require immunosuppressive and/or immunomodulatory medications, subjects with mild weakness may have spontaneous remission. The latter evolution is at variance with the disappearance of minor symptoms, such as myalgias and cramps, which cease a few weeks after discontinuation of statins.15
The variability in clinical presentation and outcome suggests that immune‐mediated muscle injury and reversible statin myotoxicity might contribute to varying degrees of perceived and objective weakness in a given patient, and also provides an explanation for the persistence of muscular involvement months after statin withdrawal. In the present case, all clinical and paraclinical features observed at disease onset and evolution are consistent with statin‐associated NAM, but not myotoxicity.
However, in our patient distinctive clinical features, such as fatigable and fluctuating muscle weakness, dysphonia, facial and palatal weakness, dysphagia, and respiratory distress, suggested a coexistent dysfunction of the neuromuscular junction. Indeed, beside the occurrence of dysphagia occurring in 16%‐30% of patients with NAM,4 only one patient with facial weakness has been reported in revised case series, while there is no consistent evidence of respiratory involvement.16 The clinical suspicion of MG was further supported by neurophysiological and serological findings. In our case, the possibility of a subclinical form of MG exacerbated by statin treatment is not consistent with previous observations reporting MG worsening within 1‐16 weeks of statin treatment.17 On the contrary, in statin‐associated MG the time interval from the initiation of statins to the onset of MG ranges from 6 months to 6 years.13 The most likely hypothesis to account for the coexistence of both NAM and MG in our patient is based on the immunomodulatory effects of statins,5, 18 which may trigger an increased immunogenicity to neuromuscular peptides in subjects with distinctive HLA haplotypes. These drugs are recognized to induce other autoimmune disorders such as a lupus‐like syndrome, myositis, and immune hepatitis.19 The mechanisms underlying the production of antibodies to HMGCR and AchR, in addition to anti‐Ro‐52, are unknown, although autoimmunity could be driven by drug‐induced loss of immune tolerance.
Timely immunosuppressive treatment in statin‐exposed patients usually leads to muscle strength improvement and decrease in anti‐HMGCR and CK levels, as we observed in our patient.1 This is keeping with previous observations showing a strict correlation among anti‐HMGCR titer, CK level, and muscle weakness.4 Notably, in refractory cases or in patients receiving delayed treatment, accelerated muscle atrophy represents the major contributor to long‐term disability.7
In conclusion, clinicians should be alerted to the possible association between statin exposure and incident NAM/MG in order to discontinue the drug and promptly begin an immunosuppressive treatment.
CONFLICT OF INTEREST
None of the authors has potential conflicts of interest to be disclosed.
AUTHOR CONTRIBUTIONS
EF: collected the information and drafted the manuscript. MS: contributed in writing the paper and preparing images. LB: contributed in designing the paper. GC: was in charge of the patient's treatment plan. CV, GR, AP, and MGP: involved in responsible for the treatment of the patient and reviewed the case. SM: contributed in writing and designing the paper and approved the final version of the manuscript.
ETHICAL APPROVAL
We all authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Patient's written informed consent to publication was obtained.
ACKNOWLEDGMENTS
The authors thank Dr Paola Tonin for helpful suggestions.
Frasson E, Simonetto M, Bertolasi L, et al. Statin‐associated necrotizing autoimmune myopathy with concurrent myasthenia gravis. Clin Case Rep. 2021;9:e03925. 10.1002/ccr3.3925
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the patient.
REFERENCES
- 1.Mammen AL. Statin‐Associated Autoimmune Myopathy. N Engl J Med. 2016;374:664‐669. [DOI] [PubMed] [Google Scholar]
- 2.Du Souich P, Roederer G, Dufour B. Myotoxicity of statins: Mechanism of action. Pharmacol Ther. 2017;175:1‐16. [DOI] [PubMed] [Google Scholar]
- 3.Khalid R, Ibad A, Thompson PD. Statins and myasthenia gravis. Muscle Nerve. 2016;54:509. [DOI] [PubMed] [Google Scholar]
- 4.Mohassel P, Mammen AL. Anti‐HMGCR myopathy. J Neuromuscul Dis. 2018;5:11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yongchairat K, Tanboon J, Waisayarat J, et al. Clinical spectrums and outcomes of necrotizing autoimmune myopathy versus other idiopathic inflammatory myopathies: a multicenter case‐control study. Clin Rheumatol. 2019;38:3459‐3469. [DOI] [PubMed] [Google Scholar]
- 6.Allenbach Y, Mammen AL, Benveniste O, Stenzel W, Immune‐Mediated Necrotizing Myopathies Working Group . 224th ENMC International Workshop: Clinico‐sero‐pathological classification of immune‐mediated necrotizing myopathies. Zandvoort, The Netherlands, 14‐16 October 2016. Neuromuscul Disord. 2018;28:87‐99. [DOI] [PubMed] [Google Scholar]
- 7.Rudski L, Rabinovitch MA, Danoff D. Systemic immune reactions to HMG‐CoA reductase inhibitors. Report of 4 cases and review of the literature. Medicine (Baltimore). 1998;77:378‐383. [DOI] [PubMed] [Google Scholar]
- 8.Gras‐Champel V, Batteux B, Masmoudi K, Liabeuf S. Statin‐induced myasthenia: a disproportionality analysis of the WHO's VigiBase pharmacovigilance database. Muscle Nerve. 2019;60:382‐386. [DOI] [PubMed] [Google Scholar]
- 9.Parmar B, Francis PJ, Ragge NK. Statins, fibrates, and ocular myasthenia. Lancet. 2002;360:717. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright MS, Jeffery DR, Nuss GR, Donofrio PD. Statin‐associated exacerbation of myasthenia gravis. Neurology. 2004;63:2188. [DOI] [PubMed] [Google Scholar]
- 11.Keogh MJ, Findlay JM, Leach S, Bowen J. Statin‐associated weakness in myasthenia gravis: a case report. J Med Case Rep. 2010;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale J, Danesh‐Meyer HV. Statins can induce myasthenia gravis. J Clin Neurosci. 2014;21:195‐197. [DOI] [PubMed] [Google Scholar]
- 13.Purvin V, Kawasaki A, Smith KH, Kesler A. Statin‐associated myasthenia gravis: report of 4 cases and review of the literature. Medicine (Baltimore). 2006;85:82‐85. [DOI] [PubMed] [Google Scholar]
- 14.Negevesky GJ, Kolsky MP, Laureno R, Yau TH. Reversible atorvastatin‐associated external ophthalmoplegia, anti‐acetylcholinereceptor antibodies, and ataxia. Arch Ophthalmol. 2000;118:427‐428. [PubMed] [Google Scholar]
- 15.Ramanathan S, Langguth D, Hardy TA, et al. Clinical course and treatment of anti‐HMGCR antibody‐associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe Y, Uruha A, Suzuki S, et al. Clinical features and prognosis in anti SRP andanti‐HMGCR necrotising myopathy. J Neurol Neurosur Psychiatry. 2016;87:1038‐1044. [DOI] [PubMed] [Google Scholar]
- 17.Oh SJ, Dhall R, Young A, Morgan MB, Lu L, Claussen GC. Statins may aggravate myasthenia gravis. Muscle Nerve. 2008;38:1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loganathan P, Oddis CV, Aggarwal R. Immune‐mediated statin myopathy. Expert Rev Clin Immunol. 2016;12:33‐38. [DOI] [PubMed] [Google Scholar]
- 19.Dehnavi S, Sohrabi N, Sadeghi M, et al. Statins and autoimmunity: State‐of‐the‐art. Pharmacol Ther. 2020;214:1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the patient.


