Abstract
When transplacental therapy is conducted, the maternal serum concentrations of digoxin and flecainide may fluctuate throughout third trimester. Therefore, TDM may be effective in improving the efficacy and safety of treatment.
Keywords: fetus, flecainide, hydrops, supraventricular tachycardia, therapeutic drug monitoring, third trimester, transplacental treatment
When transplacental therapy is conducted, the maternal serum concentrations of digoxin and flecainide may fluctuate throughout third trimester. Therefore, TDM may be effective in improving the efficacy and safety of treatment.

1. INTRODUCTION
We report the case of a fetal supraventricular tachycardia with hydrops treated by transplacental administration of digoxin and flecainide. The maternal serum concentration of digoxin and flecainide fluctuated throughout the third trimester. Therefore, monitoring the maternal concentrations of these drugs may improve efficacy and tolerability.
2. WHAT IS KNOWN AND OBJECTIVE
Fetal tachyarrhythmia occurs in less than 0.1% of pregnancies. The most common type of fetal tachycardia is supraventricular tachycardia (SVT), which may cause fetal hydrops.1 Fetal hydrops is associated with a mortality rate as high as 35% compared with 0%‐4% in non‐hydropic fetuses.2 Transplacental treatment with antiarrhythmic drugs such as digoxin and flecainide has been reported.2, 3 However, no reports have monitored the course of drug concentration and fetal heart rate in combination therapy with digoxin and flecainide for fetal SVT with hydrops during the third trimester. Here, we report a case of fetal SVT with hydrops in which fetal heart rate was successfully controlled with digoxin and flecainide and we discuss the necessity of therapeutic drug monitoring during the third trimester.
3. CASE SUMMARY
The patient was a 36‐year‐old primigravida woman. Her medical history included uterine fibroid, endometriosis, and diabetes. At 29 weeks of gestation, she was admitted to our hospital because of fetal tachycardia and fetal ascites. Fetal M‐mode echocardiography revealed a fetal heart rate of 270 bpm and a 1:1 relationship between atrial and ventricular activity, so we diagnosed fetal SVT. In addition, short ventriculoatrial (VA) interval SVT (short VA SVT) was diagnosed by superior vena cava/aorta doppler recording. Fetal ultrasonography indicated moderate fetal ascites and subcutaneous edema.
Figure 1 shows the treatment course. Digoxin concentrations were measured by chemiluminescent immunoassay, and flecainide concentrations were measured by high‐performance liquid chromatography using the method of Nakagawa et al4 with minor modifications. Flecainide concentrations were measured retrospectively. From the evening of 29 weeks and 0 days of gestation, we intravenously administered digoxin 0.5 mg three times, and following that, orally administered digoxin 0.25 mg three times daily with a target maternal serum concentration of 1.5‐2.0 ng/mL. However, the fetal heart rate did not improve, and fetal ultrasonography revealed that the fetal hydrops had worsened. Therefore, we increased the digoxin dosage to 0.375 mg three times daily and started sotalol 80 mg twice daily from 29 weeks and 3 days of gestation. At 29 weeks and 5 days of gestation, the patient became fatigued, which might have been caused by the sotalol. We decreased sotalol to 40 mg twice daily. At 30 weeks and 2 days of gestation, sotalol was discontinued because the fatigue did not improve, and flecainide 100 mg twice daily was started with digoxin. In our case, flecainide was administered at a fixed‐dose until cesarean delivery, and flecainide concentrations were measured retrospectively. The maternal serum concentration of flecainide at 30 weeks and 5 days was 450.1 ng/mL. At 30 weeks and 5 days of gestation, she felt nausea, and flecainide was discontinued. The fetal heart rate decreased to 130 bpm at 30 weeks and 6 days of gestation but increased again at 31 weeks, when the flecainide maternal serum concentration was 131.6 ng/mL. From the evening of 31 weeks and 2 days of gestation, flecainide was resumed at 100 mg twice daily. From the day after resumption, the fetal heart rate immediately decreased and remained stable until cesarean delivery 24 days later. The maternal serum concentration of flecainide at 4 days (31 weeks 5 days) and 5 days (31 weeks 6 days) after resuming flecainide was 392.4 and 407.2 ng/mL, respectively. After 32 weeks and 4 days, the maternal serum concentration of flecainide remained in the range 600‐650 ng/mL and increased to 788.2 ng/mL at 34 weeks and 4 days. At 32 and 33 weeks and 1 day of gestation, amniocentesis was performed because of abdominal pressure resulting from excessive amniotic fluid; in the amniotic fluid, the concentration of digoxin was 1.2 and 1.8 ng/mL and that of flecainide was 817.1 and 1010.2 ng/mL, respectively. Because she had severe nausea and was considered to have reached the limit of transplacental treatment, a cesarean section was performed at 34 weeks and 5 days of gestation. Table 1 shows the maternal serum concentration, umbilical cord serum concentration, and amniotic fluid concentration of digoxin and flecainide at birth.
FIGURE 1.
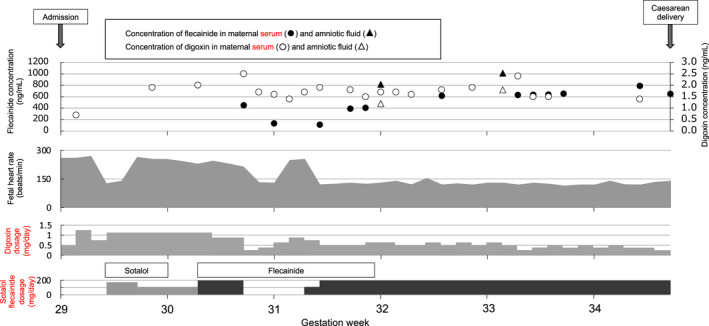
Changes in doses of digoxin, sotalol, and flecainide, concentrations of digoxin and flecainide, and fetal heart rate. The patient was hospitalized at our hospital from 29 wk of gestation and had a cesarean delivery at 34 wk and 5 d of gestation
TABLE 1.
Concentrations of flecainide and digoxin at birth
| Digoxin (ng/mL) | Flecainide (ng/mL) | |
|---|---|---|
| Maternal serum | 1.3 | 650.1 |
| Umbilical vein | 0.5 | 451.6 |
| Umbilical artery | 0.5 | 484.7 |
| Amniotic fluid | 1.6 | 1051.2 |
The baby was bornt by cesarean delivery. At that time, maternal serum, umbilical cord serum, and amniotic fluid were collected and the concentrations of digoxin and flecainide were measured.
At birth, the baby's length was 45 cm, weight was 2418 g, and the Apgar scores were 8 at both 1 and 5 minutes. His pulse rate was 100‐140 bpm, ascites and subcutaneous edema were mild, and there was no cardiac hypertrophy or pleural effusion. We administered digoxin and flecainide to the baby from the day after birth.
In previous reports, no significant difference was noted in maternal serum concentration of flecainide between flecainide responders and nonresponders, but no fetus achieved cardioversion with a maternal flecainide concentration <250 ng/mL; the median time to cardioversion was 3 days.5 In our case, fetal heart rate decreased 4 days after the start of flecainide, and the maternal serum concentration of flecainide was 450.1 ng/mL at 3 days after the start of flecainide. Fetal heart rate increased when flecainide was discontinued, but after resumption of flecainide, the maternal serum concentration of flecainide remained above 400 ng/mL after 31 weeks and 6 days, and the fetal heart rate remained stable until cesarean delivery. These results suggest that the maternal serum concentration of flecainide should be measured and assessed if the transplacental effect of flecainide is deemed insufficient.
Miyoshi et al6 reported the safety and efficacy of transplacental treatment based on an original protocol for fetal SVT and atrial flutter. According to that protocol, the combination of digoxin and sotalol must be administered for short VA SVT with fetal hydrops because digoxin alone has a low success rate for fetal hydrops.7, 8 Flecainide continues to cross the placenta readily even when hydrops is present in the fetus.2 In our case, the transplacental rates of digoxin and flecainide calculated from the maternal serum concentration and umbilical cord venous serum at delivery were 38.5% and 69.5%, respectively (Table 1). The transplacental rate of flecainide was higher than that of digoxin, which was consistent with previous reports.5, 9, 10 In our case, the fetal heart rate did not decrease under co‐administration of digoxin and sotalol. Some reports have indicated that flecainide should be selected as the first‐line treatment for fetal SVT instead of sotalol, particularly in the presence of fetal hydrops.7, 8, 11 The first choice of antiarrhythmic drug for SVT with fetal hydrops remains controversial.
The dose required to adjust the maternal serum concentration of digoxin to 1.5‐2.0 ng/mL decreased with each gestational week, and the average daily dose at 30 and 34 weeks was 0.821 and 0.375 mg/d, respectively. In a previous report, the maternal serum concentration of digoxin in the third trimester was found to be higher than that in the postpartum period, even though digoxin renal clearance was higher.12 In the third trimester, decreased bowel mobility leads to increased transit time in the small bowel.13, 14 As a result, digoxin absorption may increase. In addition, increased cardiac output associated with greater perfusion of intestinal blood flow13 may also increase drug absorption. In our case, the digoxin dosage to maintain the maternal serum concentration in the target range changed over time from 29 weeks of gestation. This phenomenon suggests that continuous therapeutic drug monitoring of digoxin is necessary in the third trimester. In contrast, flecainide was administered at a fixed dose. The retrospectively measured maternal serum concentration of flecainide increased over time, reaching 788.2 ng/mL before cesarean delivery. In general, pharmacokinetics change during pregnancy.15 The increase in serum levels of flecainide may be due to increased absorption for the same reasons as digoxin, but it is not certain. In our case, close monitoring of drug concentrations throughout the third trimester revealed that the ratios of maternal serum concentrations to dosage of digoxin and flecainide increased over time. Furthermore, it has been reported that a higher serum concentration of flecainide increases the frequency of side effects such as supraventricular arrhythmias, central nervous system symptoms, and liver dysfunction.16 In our case, there was intermittent nausea from 30 weeks and 5 days of gestation, and it persisted even while flecainide was discontinued. Because of serious nausea, cesarean delivery was performed. Maternal serum concentration of flecainide 2 days before cesarean section was highest all over the course. Nausea may be caused by dilation of the uterus in addition to the drug, but we cannot deny the possibility that the nausea may have been caused by high serum levels of flecainide. On the other hand, the fetal adverse event such as arrhythmia was not observed in the serum concentration range of our case. Therefore, it is possible to avoid side effects and achieve efficacy by monitoring the maternal serum concentration and adjusting the flecainide dose as needed. Flecainide monotherapy for fetal SVT has been previously reported.3, 7, 8, 11 It has also been reported that combination therapy has a higher risk of adverse maternal and fetal effects compared with monotherapy.17 Similarly, it is possible that flecainide monotherapy could have been continued by controlling the serum concentration of flecainide.
The concentrations of digoxin and flecainide were nearly unchanged in the umbilical vein and artery (Table 1). The amniotic fluid concentrations of digoxin and flecainide were 1.23 and 1.62 times the maternal serum concentrations. However, a previous report found that the concentration of flecainide in the amniotic fluid was 26.6 times the maternal serum concentration.10 In addition, there are large individual differences. Assessment of drug concentrations in the fetus and amniotic fluid during the course of pregnancy is invasive and not easily performed. Therefore, it is considered that the target serum concentrations of digoxin and flecainide are 1.5‐2.0 and >400 ng/mL, respectively. And it is recommended to adjust dosage in response to the fetal heart rate as a clinical index. On the other hand, we must monitor adverse effects in both of patient and fetus. In this case, it may be important to reduce the dose so that it does not fall below 400 ng/mL. Based on the above, when treating SVT with fetal hydrops, monitoring the maternal serum concentration of flecainide and digoxin may contribute to improved efficacy and tolerability.
4. WHAT IS NEW AND CONCLUSION
The maternal serum concentration of digoxin and flecainide may fluctuate throughout the third trimester. Monitoring the maternal concentrations of these drugs and adjusting the dosage may improve efficacy and tolerability. However, this is a single case report, and thus this issue needs to be studied further in the future.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
HT, KW: wrote the manuscript; SY, HT, TS, and II: supervised the writing of the manuscript; HT, SY, HT, AO, and MS: participated in patient's evaluation and treatment. All authors read and approved the final manuscript.
ETHICAL APPROVAL
This report was conducted in compliance with the medical research ethics committee at Chiba University (approval No. 3363).
ACKNOWLEDGMENTS
We thank all the Chiba university hospital staff involved in treating this patient. Published with written consent of the patient.
Takatsuka H, Wakabayashi K, Yamazaki S, et al. Transition of maternal serum concentration of digoxin and flecainide in the third trimester—A case report of fetal supraventricular tachycardia with hydrops. Clin Case Rep. 2021;9:e03992. 10.1002/ccr3.3992
Hirokazu Takatsuka and Kayo Wakabayashi contributed equally to this work.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1.Simpson JM. Fetal arrhythmias. Ultrasound Obstet Gynecol. 2006;27:599‐606. [DOI] [PubMed] [Google Scholar]
- 2.Jaeggi ET, Carvalho JS, De Groot E, et al. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation. 2011;124:1747‐1754. [DOI] [PubMed] [Google Scholar]
- 3.Alsaied T, Baskar S, Fares M, et al. First‐line antiarrhythmic transplacental treatment for fetal tachyarrhythmia: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagawa R, Homma M, Kuga K, et al. High performance liquid chromatography for routine monitoring of serum flecainide. J Pharm Biomed Anal. 2002;30:171‐174. [DOI] [PubMed] [Google Scholar]
- 5.Vigneswaran TV, Callaghan N, Andrews RE, et al. Correlation of maternal flecainide concentrations and therapeutic effect in fetal supraventricular tachycardia. Heart Rhythm. 2014;11:2047‐2053. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi T, Maeno Y, Hamasaki T, et al. Antenatal therapy for fetal supraventricular tachyarrhythmias: multicenter trial. J Am Coll Cardiol. 2019;74:874‐885. [DOI] [PubMed] [Google Scholar]
- 7.Krapp M, Kohl T, Simpson JM, Sharland GK, Katalinic A, Gembruch U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89:913‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill GD, Kovach JR, Saudek DE, Singh AK, Wehrheim K, Frommelt MA. Transplacental treatment of fetal tachycardia: a systematic review and meta‐analysis. Prenat Diagn. 2017;37:1076‐1083. [DOI] [PubMed] [Google Scholar]
- 9.Chan V, Tse TF, Wong V. Transfer of digoxin across the placenta and into breast milk. Br J Obstet Gynaecol. 1978;85:605‐609. [DOI] [PubMed] [Google Scholar]
- 10.Bourget P, Pons JC, Delouis C, Fermont L, Frydman R. Flecainide distribution, transplacental passage, and accumulation in the amniotic fluid during the third trimester of pregnancy. Ann Pharmacother. 1994;28:1031‐1034. [DOI] [PubMed] [Google Scholar]
- 11.Oudijk MA, Michon MM, Kleinman CS, et al. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721‐2726. [DOI] [PubMed] [Google Scholar]
- 12.Luxford AM, Kellaway GS. Pharmacokinetics of digoxin in pregnancy. Eur J Clin Pharmacol. 1983;25:117‐121. [DOI] [PubMed] [Google Scholar]
- 13.Krauer B, Krauer F. Drug kinetics in pregnancy. Clin Pharmacokinet. 1977;2:167‐181. [DOI] [PubMed] [Google Scholar]
- 14.Manninen V, Apajalahti A, Melin J, Karesoja M. Altered absorption of digoxin in patients given propantheline and metoclopramide. Lancet. 1973;1:398‐400. [DOI] [PubMed] [Google Scholar]
- 15.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39:512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salerno DM, Granrud G, Sharkey P, et al. Pharmacodynamics and side effects of flecainide acetate. Clin Pharmacol Ther. 1986;40:101‐107. [DOI] [PubMed] [Google Scholar]
- 17.Donofrio MT, Moon‐Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183‐2242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


