Abstract
Background
Patient‐relevant health outcomes for persons with hemophilia should be identified and prioritized to optimize and individualize care for persons with hemophilia. Therefore, an international group of persons with hemophilia and multidisciplinary health care providers set out to identify a globally applicable standard set of health outcomes relevant to all individuals with hemophilia.
Methods
A systematic literature search was performed to identify possible health outcomes and risk adjustment variables. Persons with hemophilia and multidisciplinary health care providers were involved in an iterative nominal consensus process to select the most important health outcomes and risk adjustment variables for persons with hemophilia. Recommendations were made for outcome measurement instruments.
Results
Persons with hemophilia were defined as all men and women with an X‐linked inherited bleeding disorder caused by a deficiency of coagulation factor VIII or IX with plasma activity levels <40 IU/dL. We recommend collecting the following 10 health outcomes at least annually, if applicable: (i) cure, (ii) impact of disease on life expectancy, (iii) ability to engage in normal daily activities, (iv) severe bleeding episodes, (v) number of days lost from school or work, (vi) chronic pain, (vii) disease and treatment complications, (viii) sustainability of physical functioning, (ix) social functioning, and (x) mental health. Validated clinical as well as patient‐reported outcome measurement instruments were endorsed. Demographic factors, baseline clinical factors, and treatment factors were identified as risk‐adjustment variables.
Conclusion
A consensus‐based international set of health outcomes relevant to all persons with hemophilia, and corresponding measurement instruments, was identified for use in clinical care to facilitate harmonized longitudinal monitoring and comparison of outcomes.
Keywords: delivery of health care, health care, hemophilia A, hemophilia B, outcome assessment, patient‐reported outcome measures
Essentials.
A standard set of relevant health outcomes for hemophilia is needed to improve care.
International experts and patients participated in a consensus process to identify such a set.
Ten patient‐relevant health outcomes were selected with recommended outcome measurement instruments.
The standard set can be used in different care systems to track and compare outcomes over time.
1. INTRODUCTION
Hemophilia is an X‐linked inherited bleeding disorder caused by a congenital deficiency of either coagulation factor VIII (hemophilia A) or coagulation factor IX (hemophilia B), which affects 24.6 and 5.0 per 100,000 male live births, respectively. 1 The lack of functional coagulation factor VIII or IX causes spontaneous bleeding in persons with severe hemophilia, especially affecting joints and muscles. 2 Recurrent bleeding into joints causes arthropathy and pain. 3 Persons with a milder form of hemophilia suffer from bleeds after (minor) trauma or surgery. Female carriers of hemophilia may have varying factor levels. Symptomatic carriers experience symptoms usually consistent with mild hemophilia but with a predominance of reproductive tract bleeding. Overall, treatment for severe cases consists of intravenous coagulation factor replacement therapy to treat bleeds (on‐demand treatment) or regular infusions to prevent bleeds (prophylaxis). 4 Additional treatment options such as non–factor‐based replacement therapies have been marketed in recent years, and gene therapy will become available in the near future. 4 , 5 , 6
In recent decades, advances in hemophilia treatment have resulted in a near‐normal life expectancy and lower burden of bleeding in high‐income countries. However, significant disease and treatment burden still exist and availability of treatment varies across the world. Globally, 70% of persons with hemophilia have no access to adequate treatment. 7 , 8
Health care systems should deliver value by achieving health outcomes that matter to patients within available budgets for any given medical condition. 9 Value is measured at the medical condition level and is viewed as a ratio of patient‐relevant health outcomes achieved and the cost of achieving these outcomes over the full cycle of care. 9 , 10 , 11 Selection of a standardized set of well‐defined patient‐relevant health outcomes for a medical condition such as hemophilia is an essential step toward delivering value by enabling monitoring of health outcomes of each individual over time. Concurrent collection of individual patient and treatment characteristics is required for risk‐adjusted comparisons of outcomes between populations. 12
The value‐based health care framework according to Porter 11 distinguishes three hierarchically ordered tiers of outcomes, with outcomes in the lower tiers dependent on the outcomes in the higher tiers. Tier 1 outcomes are generally the most important and reflect the health status achieved or retained, including survival and the degree of health or recovery. Tier 2 outcomes typically include dimensions of time to recovery and disutility of care (discomfort or complications), and tier 3 outcomes relate to long‐term consequences of the disease or treatment. 11 Health care in low‐resource settings (eg, lower‐income countries, more remote areas or hospitals without a hemophilia treatment center) may prioritize assessment and improvement of outcomes in the higher tiers, while care providers in more resource‐rich settings may aim to improve outcomes in all tiers.
Outcome sets evolve over time and build on earlier outcome sets. 13 , 14 , 15 , 16 The recently published patient‐relevant outcomes framework for hemophilia care 13 required broader validation by input from representatives and hemophilia care providers of persons with hemophilia . 17 Therefore, we assembled stakeholders including persons with hemophilia and their representatives, hemophilia care providers with expertise in various disciplines, and experts in value‐based health care to identify a globally applicable standard set of patient‐relevant health outcomes for all persons living with hemophilia.
2. METHODS
Detailed descriptions of participants, literature search, consensus process, panel meetings, and outcome measurement recommendations are documented in the Supporting Information.
2.1. Project overview
A nominal consensus process was applied according to the value‐based health care methodology, 18 as endorsed by the International Consortium for Health Outcomes Measurements (ICHOM) and the National Health Service. 19 In a parallel multistep process, including multiple web‐based meetings, consensus was sought on the elements of the standard set: (i) definition of the patient group for whom the standard outcomes set is intended, that is, the medical condition; (ii) health outcomes; and (iii) risk‐adjustment variables.
Four panels were involved: the coordinating core team, a steering group, the Patients and Health Care Professionals Panel, and the International Academic Council (Table 1). The coordinating core team extracted lists of the definitions, health outcomes, and risk‐adjustment variables from the literature search; earlier outcomes initiatives 13 , 14 , 16 , 20 ; ICHOM standard sets 19 ; and clinical practice. The steering group and Patients and Health Care Professionals Panel members individually voted for the most relevant health outcomes and risk adjustment variables before each web‐based meeting. Voting results were discussed during the web‐based meetings until consensus was reached. Consensus was considered reached when no new topics or questions were raised. The independent International Academic Council reviewed the process and selection of results (Table 1). Finally, the core and steering group assessed and selected available outcome measurement instruments.
TABLE 1.
Overview of the process of standard set development
| Meeting dates | Working group | Meeting objectives |
|---|---|---|
| Jul 19, 2018 | Core and steering group a |
|
| (1) Oct 15, 2018 | Core and steering group |
|
| (2) Dec 20, 2018 | Core and steering group |
|
| (3) Jan 21, 2019 | Patients and Health Care Professionals Panel b |
|
| (4) Feb 12, 2019 | Core and steering group |
|
| (5) Mar 11, 2019 | Patients and Health Care Professionals Panel |
|
| (6) May 6, 2019 | Patients and Health Care Professionals Panel |
|
| (7) May 20, 2019 | Core and steering group |
|
| (8) May 27, 2019 | International Academic Council c |
|
| (9) Jun 17, 2019 | Patients and Health Care Professionals Panel |
|
The core group consisted of four epidemiologists and hematologists and two patient representatives; the steering group consisted the core group and an additional eight hematologists, a nursing specialist, a representative from the World Federation of Hemophilia, and two patient representatives.
The Patients and Health Care Professionals Panel consisted of 17 hemophilia care professionals of eight different disciplines and 15 patient representatives, including persons with hemophilia, parents of children with hemophilia, and female carriers of hemophilia.
The International Academic Council consisted of two hematologists, a gynecologist, a nursing specialist, a physiotherapist, a public health expert, and a value‐based health care expert.
2.2. Identification of health outcomes set
Definition of the medical condition. People included in the medical condition definition have similar medical needs, and the same set of health outcomes is relevant to them. Consensus was sought on the definition of the medical condition, including patient inclusion and exclusion criteria, identification of potential relevant subgroups for whom distinct additional outcomes are needed, establishment of first and last time points of treatment by hemophilia care teams, and available treatment types. 2 , 9 , 21
Selection of health outcomes. The core team defined health outcomes as outcomes that: (i) represent patient value as a result of receiving care; (ii) can be acted upon and improved by the health care team; and (iii) can be reported by persons with hemophilia or documented by health care professionals. 10 Outcome selection was based on the degree to which health care activities affect individual health outcomes, the magnitude of impact on persons with hemophilia, and patient numbers for whom health outcomes were relevant.
Selection of risk‐adjustment variables. Risk‐adjustment variables are patient and treatment characteristics that affect the absolute value of health outcomes. When outcomes are compared between patient populations with different backgrounds, adjustment for such characteristics is required.
2.3. Recommendations for outcome measurement
For outcomes that can be measured directly from clinical or laboratory data, measurement instructions were described. For other outcomes, hemophilia‐specific instruments and item banks from the Patient‐Reported Outcomes Measurement Information System (PROMIS) 22 , 23 were identified. Selection was primarily based on the fit of the instrument’s contents with the health outcome to properly measure the outcome. Then, selection was based on the (i) instrument’s psychometric quality (extracted from systematic reviews and recent literature) 15 , 31 ; (ii) number of available validated translations; and (iii) instrument’s availability and accessibility.
3. RESULTS
3.1. Literature search
The literature search, based on an earlier search strategy and long list with health outcomes by CoreHEM, 14 yielded 382 references; 183 were excluded (Figure S1). From the remaining 199 studies, 3023 potential health outcomes were extracted. After removing duplicates, process indicators, structural indicators, and cost indicators (Supporting Information, p. 13), 136 health outcomes were included in the long list used for round 1 of voting (Supporting Information and Table S5). In total, 57 unique potential risk‐adjustment variables were identified (Table S7).
3.2. Definition of the medical condition hemophilia
Consensus was reached on the medical condition definition for persons with hemophilia: “All people (male or female) with an X‐linked congenital bleeding disorder caused by a deficiency of coagulation factor VIII (hemophilia A) or IX (hemophilia B) with plasma activity levels of factor VIII/IX activity <40 IU/dL.” The deficiency is the result of mutations in the respective coagulation factor genes.
No subgroups were defined, as they were not considered distinctive enough to require additional, specific health outcomes not relevant to the other subgroups. Yet it was acknowledged that there are large differences between individuals (eg, resulting from differences in treatment availability, disease severity, and sex).
The first and last time points of treatment by the hemophilia care team were from time of diagnosis (prenatal or after birth) to death. End‐of‐life care was explicitly included, care delivered before diagnosis, care related to comorbidities, and secondary disease excluded. The four potential treatment modalities were (i) continuous prophylaxis, (2) intermittent periodic prophylaxis (if available), (3) episodic “on‐demand” treatment, and (4) “curative” treatment. 2
3.3. Health outcomes
Steering group members voted on the long list of 136 health outcomes (Table S5). Sixty health outcomes were selected in the first voting round. Ten additional health outcomes were added based on discussions during the steering group meeting and their importance from patients’ and health care perspectives. An additional outcome specific for women (heavy menstrual bleeding) was identified from the literature. In total, 71 outcomes were reviewed in the second voting round (Table S5), after which 45 health outcomes were selected. Collapsing of similar outcomes resulted in an initial short list of 33 outcomes. In parallel, the Patients and Health Care Professionals Panel reviewed and ranked 15 of the 45 health outcomes as the most important. Cross checking of these 15 highest‐ranked health outcomes with the short list of 33 health outcomes from the steering group resulted in 35 outcomes for which the core team drafted preliminary definitions (Table S6). After combining similar outcomes, 27 outcomes remained on the final short list.
The final voting rounds by the steering group and the Patients and Health Care Professionals Panel’s subsequent discussions during web‐based meetings resulted in a final set of 10 health outcomes. The final set was discussed in the final meetings of all panels. The health outcome “life‐threatening bleeding episodes” was initially included in the final set because it consistently scored higher than the more broadly defined “bleeding outcomes (frequency of bleeding episodes/frequency of bleeding episodes requiring treatment).” However, several participants felt that a more broadly defined bleeding outcome should be included. It was proposed to replace the outcome “life‐threatening bleeding episodes” with the modified outcome “severe bleeding episodes,” which also covered bleeding outcomes for women. After discussions in writing, full consensus was reached in the steering group on including the modified outcome “severe bleeding episodes” and its definition.
The final international set consisted of the following 10 health outcomes (Figure 1): (i) cure; (ii) impact of disease on life expectancy; (iii) ability to engage in normal daily activities; (iv) severe bleeding episodes; (v) number of days lost from school or work; (vi) chronic pain; (vii) complications of hemophilia and its treatment; (viii) sustainability of physical functioning; (ix) social functioning; and (x) mental health. Consensus‐based definitions of each of the health outcomes are listed in Table 2, including the type of reporting (clinician‐reported or patient‐reported) and the corresponding domain of the International Classification of Functioning model. 32
FIGURE 1.
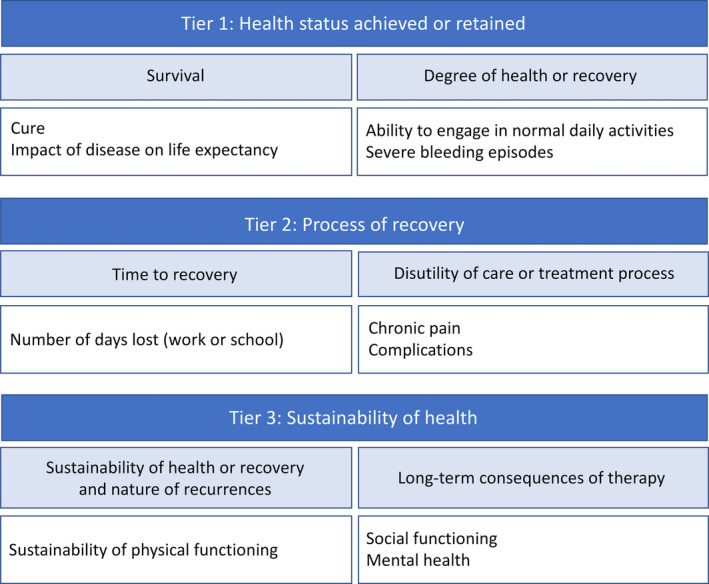
International set of health outcomes for hemophilia. Health outcomes are listed as a hierarchy, with the most important health outcomes in tier 1
TABLE 2.
Health outcomes and definitions
| Health outcome | Definition | Type of data | ICF domain |
|---|---|---|---|
| Tier 1: Health status achieved or retained | |||
| 1. Cure | Complete correction of previous bleeding tendency with normalized clotting factor levels 5 years after curative treatment, requiring no further treatment (with coagulation factor or other treatments), not even for surgery or bleeding. Cure is phenotypically intended and does not include: eliminating transmission of hemophilia to children or fully reverting established damage. | Clinician‐reported | Body function and structures |
| 2. Impact of disease on life expectancy | Decrease in number of years a person is expected to live due to hemophilia compared to an age‐ and sex‐matched reference population. | Clinician‐reported | Body function and structures |
| 3. Ability to engage in normal daily activities | Actual or potential ability of individuals with hemophilia to perform activities of daily living, including self‐care and looking after the household or children, and going to work or school, without support from others. | Patient‐reported | Activities and participation |
| 4. Severe bleeding episodes | Number of severe bleeding episodes or recurrent bleeding as perceived by persons with hemophilia, including but not limited to causing acute severe pain, substantial loss of range of motion, and the need for an extended treatment course. This includes any serious or life‐threatening bleed requiring hospitalization, transfusion of blood products, or emergency surgery (ie, decompression or compartment release) | Patient‐reported | Body function and structures |
| Tier 2: Process of recovery | |||
| 5. Number of days lost (work or school) | Absence from work or school due to hemophilia (because of bleeding, hospital admission, outpatient visit, picking up medication), as a proportion of the regular number of days worked or in school. | Patient‐reported | Activities and participation |
| 6. Chronic pain | Chronic pain is patient‐reported pain that is present for more than 3 months. Pain is multidimensional (including emotional affect and effect on persons with hemophilia), may be intermittent or continuous, and may be of variable intensity over this time. Chronic pain is not due to an acute bleeding episode and may have different causes. | Patient‐reported | Body function and structures |
| 7. Complications | Any clinician‐reported health complication, caused by the condition or by administration of treatment: inhibitor development and treatment‐related infections, other infection‐related complications, thromboembolic complications of medication, difficult venous access, infections, thrombosis or obstruction of central venous access devices, postpartum hemorrhage, and iron deficiency. Complications also include complications that result from other treatment, such as orthopedic interventions and physiotherapy. | Clinician‐reported | Body function and structures |
| Tier 3: Sustainability of health | |||
| 8. Sustainability of physical function | Functional status over time. Functional status is defined as endurance, strength, and mobility of the body and body structures. | Clinician‐reported or patient‐reported | Body function and structures |
| 9. Social functioning | The degree to which a person is able to maintain and manage interactions with other people in a contextually and socially appropriate manner and to contribute to society. | Patient‐reported | Activities and participation |
| 10. Mental health | Degree of overall well‐being, satisfaction with life, and anxiety and depression. | Patient‐reported | Body function and structures |
Definitions of each health outcome are listed along with the type of health outcome (clinician‐reported or patient‐reported) and the corresponding domain of the International Classification of Functioning (ICF). 32
3.4. Risk‐adjustment variables
Of the 57 risk‐adjustment variables extracted from the literature search, the steering group removed two and added six others, resulting in a long list of 61 risk‐adjustment variables (Table S7). Steering group voting resulted in a top‐15 list of risk‐adjustment variables. Six nonselected risk‐adjustment variables were added again during the discussions in the steering group, as they were considered relevant in affecting health outcomes. Consecutive voting rounds of the steering group resulted in a short list of 19 risk‐adjustment variables (Table S7). After final voting and steering group discussion to reach consensus, the 11 risk‐adjustment variables selected were: age, sex, individual socioeconomic status, availability of and access to treatment, comorbidities, severity of hemophilia, degree of joint damage, psychological well‐being, inhibitor status, health literacy, and which hemophilia care professionals are involved in the management of hemophilia (Table S8).
3.5. Recommendations for outcome measurement
Measurement instructions were summarized for each outcome. The outcomes cure, impact of disease on life expectancy, severe bleeding episodes, number of days lost from work or school, and complications can be assessed directly from clinical or laboratory data. Recommended clinical instruments, hemophilia‐specific instruments and generic PROMIS item banks are presented for the other outcomes (ability to engage in daily activities, chronic pain, sustainability of physical functioning, social functioning, and mental health) (Table 3).
TABLE 3.
Measurement instructions and instruments for the health outcomes set
| Health outcome | What to measure | Recommended measurement instruments | |
|---|---|---|---|
| Hemophilia‐specific | PROMIS item bank | ||
| Tier 1: Health status achieved or retained | |||
| Cure |
|
NA | NA |
| Impact of disease on life expectancy a |
|
NA | NA |
| Ability to engage in normal daily activities |
|
Adults For high‐income societies For lower‐income societies
Children For high‐income societies For lower‐income societies
|
Adults
Children
|
| Severe bleeding episodes |
|
NA | NA |
| Tier 2: Process of recovery | |||
| Number of days lost (work or school) |
|
NA | NA |
| Chronic pain |
|
Adults
Children
|
Adults Children |
| Complications |
|
||
| Tier 3: Sustainability of health | |||
| Sustainability of physical functioning |
|
Adults
Children |
Adults
Children
|
| Social functioning |
|
Adults
Children
|
Adults
Children
|
| Mental health |
|
Adults
Children
|
Adults
Children
|
For the measurement of the standard set of outcomes we recommend to use the listed hemophilia specific outcome measurement instruments or PROMIS item banks. In resource‐limited settings, health care providers may start measuring outcomes (in tier 1). Based on feasibility and preference instruments may be selected for clinical practice from either category.
The most recent versions of the measurement instruments are recommended for use.
The health outcomes Cure, Impact of disease on life expectancy, Severe bleeding episodes, Number of days lost from work or school, and Complications may be measured at baseline and annually, when possible.
Abbreviations: ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate; CHO‐KLAT, Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool; FISH, Functional Independence Score in Hemophilia; HAL, Hemophilia Activities List; HJHS, Hemophilia Joint Health Score; NA, not applicable; PedHAL, Pediatric Hemophilia Activities List; PROBE, Patient‐Reported Outcomes Burdens and Experiences; PROMIS, Patient‐Reported Outcomes Measurement Information System.
Impact on life expectancy can be measured by collecting data on the number of deaths and the age at death.
The HAL (adults) and PedHAL (children) measure self‐perceived functional abilities due to hemophilia in seven domains in the previous month.
The FISH is a performance‐based tool to assess an individual's functional ability. Eight activities of daily living are assessed: eating, grooming, dressing, chair transfer, squatting, walking, step climbing, and running. For children, the e‐FISH is currently under development (A. Srivastava, personal communication).
PROBE measures general health issues, use of mobility aids or assistive devices, pain, daily activities, current work or student status, surgeries or procedures and comorbid diseases.
PROMIS has pain‐related item banks (pain intensity and pain interference). However, the fit with the HaemoValue outcome “chronic pain” lasting >3 months is limited, as pain in the previous 7 days is assessed and the emotional impact of pain is lacking.
The HJHS assesses functional impairment in the six main joints commonly affected by hemophilia.
Haemo‐QoL‐A is a hemophilia‐specific instrument that measures health‐related quality of life in adults in six domains.
CHO‐KLAT is a hemophilia‐specific instrument that measures several aspects of quality of life in children.CHO‐KLAT 3.0 is currently under development. 48
Initially, a total of 25 potential outcome measurement instruments were identified for adults (six hemophilia‐specific instruments, 11 PROMIS item banks, and eight clinical instruments) and 26 instruments for children (six hemophilia‐specific instruments, 12 PROMIS item banks, and eight clinical instruments). Scoring of instruments led to the selection of the recommended outcome measurement instruments (Tables S3 and S9).
Hemophilia‐specific instruments generally measure several domains of health‐related quality of life (eg, physical functioning, social functioning, mental health, and others). The most appropriate subscales were selected if subscale scoring was available. Life satisfaction, which is part of the outcome mental health, is not measured in any hemophilia‐specific instrument. It is therefore recommended to use the PROMIS item bank Life Satisfaction. We recommend choosing the instrument that is most feasible in each situation, for example, depending on language and availability of clinical or research staff. Where possible, measurement of outcomes should be embedded into routine clinical care.
4. DISCUSSION
We present a standard set of health outcomes for all persons with hemophilia that can be used by hemophilia treatment centers and health systems to assess the value provided for persons with hemophilia in different geographic and health care settings. The standard set was developed by persons with hemophilia and their representatives and international panels of health care professionals with expertise across various disciplines. We propose appropriate measurement instruments with the best content fit and the best reported psychometric properties.
This work was performed in close collaboration with earlier working groups: the CoreHEM core outcomes set for hemophilia gene therapy trials 14 ; the Patient Reported Outcomes, Burdens, and Experiences (PROBE) study 20 ; the Cost of Hemophilia in Europe: a Socioeconomic Survey 33 ; the Value Framework 13 ; core outcomes set for clinical research in hemophilia 34 ; the Scientific and Standardization Committee/International Society on Thrombosis and Haemostasis definitions in hemophilia project group, 2 an expert review on tools for outcome measurement 15 , 16 and systematic reviews on the psychometric properties of hemophilia‐specific instruments for joint health, activities and participation, and health‐related quality of life. 24 , 25 , 26
Improving value for persons with hemophilia should be the overarching goal of health care delivery. 9 Without focus on value, limited health care resources may be wasted on activities that do not improve outcomes. In many health care systems or clinics, outcomes that matter to persons with hemophilia are not measured, or efforts are aimed at measuring process indicators (ie, volume of patient visits or units coagulation factor consumption) or at outcomes that are irrelevant to persons with hemophilia in their daily lives. 11 Moreover, lack of focus on value fails to provide insight into the level of patient‐relevant outcomes achieved and sustained through individualized tailoring of treatment. For most conditions treated through a value‐based system, a focus on achieving outcomes will eventually reduce costs because health care activities that do not contribute to better outcomes are eliminated. 11
In high‐income countries, up to 99% of measured total health care costs for severe hemophilia are currently attributed to coagulation factor replacement therapy. 33 As a result, decision makers tend to focus on a per‐unit or per‐patient cost for product. There is no tabulation of the overall cost to the health care budget or to society long term (ie, surgeries, hospital admissions, unemployment) of achieving the current outcomes. In spite of this, over the past 20 years most payers have agreed to increased and widespread use of coagulation factor prophylaxis in all age groups through recognition of its long‐term beneficial outcomes. These benefits include reducing bleeding complications with the prevention or slowing of disability and enhancing labor market participation. Still, the relative system cost saved by avoiding poor health outcomes remains unmeasured. Measuring the relative value of therapies by comparing outcomes relevant to persons with hemophilia, rather than relative costs through consumption of products, is urgently needed in the light of recently developed non–factor‐based therapies and gene therapy, which will affect coagulation factor use and be priced similarly high or higher.
4.1. Strengths and limitations
A strength of this study is the representation of persons with hemophilia. A large representation of persons with hemophilia, carriers, and parents in the steering group and the Patients and Health Care Professionals Panel (26% and 47%) ensured that the standard set of outcomes is relevant for persons with hemophilia. Care was taken to include a variety of persons with hemophilia in the Patients and Health Care Professionals Panel, including individuals with hemophilia A and B, different severities, symptomatic carriers of hemophilia, and parents of children with hemophilia from various geographic backgrounds. Furthermore, since the standard outcomes set needs to be applicable in health care settings with varying resources, participants represented high‐income countries, upper‐middle‐income countries, and one lower‐middle‐income country. Since we aimed to identify health outcomes relevant to persons with hemophilia, we did not involve policy makers and payers to avoid bias in the selection of the outcomes.
A limitation of this work is that some (sub)groups of persons with hemophilia may be underrepresented. We attempted to reach out to stakeholders from low‐income countries but did not succeed, in part due to language barriers. Therefore, the applicability of the standard health outcomes set in such resource‐constrained settings remains to be assessed. Furthermore, outcomes specific to women, such as menorrhagia and pregnancy issues, may have received less attention, as women and children with hemophilia were underrepresented in the panels. To overcome this, patient representatives were asked to also represent women and children with hemophilia. In addition, during the final review step, a gynecologist with extensive expertise in the area of women with bleeding disorders reviewed the standard set. Finally, persons with mild hemophilia were not included but represented by others in the working groups. Some outcomes, notably those in tiers 1 and 2, may be less relevant for persons with mild hemophilia than outcomes in tier 3. In future revisions of the outcomes set, we will aim for a more extensive representation of women, children, and individuals with mild hemophilia in order to ensure relevance of the outcomes. The majority of persons with hemophilia and their representatives in the steering group and Patients and Health Care Professionals Panel were active members of regional, national, and global patient organizations. Their expertise may have led to different opinions than expressed by an “average” person with hemophilia. Even though participants were instructed to represent all persons with hemophilia, we cannot rule out that this affected the selection of health outcomes. Furthermore, participants were required to be proficient in English, which is not typical for persons with hemophilia around the world. However, this was necessary for participation in assignments and discussions during web‐based meetings. For these reasons, relevance of the set to all persons with hemophilia around the world will need to be further evaluated in practice.
It is also acknowledged that some of the recommended hemophilia‐specific outcome measurement instruments still need further validation, particularly in the areas of structural validity (ie, the degree to which the scores of an instrument are an adequate reflection of the dimensionality of the construct 35 ), responsiveness (ie, the ability of an instrument to detect change over time 35 ), and cross‐cultural validity. 24 , 25 , 26 , 36 , 37 It is important to note that the use of patient‐reported outcomes measures (PROMs) may have some limitations. First, PROMs (including digital PROMs) may be less feasible in settings with high functional illiteracy rates. Second, PROMs that have been developed in high‐income countries may not be culturally appropriate for lower‐income countries and vice‐versa. Cross‐cultural adaptation is essential to safeguard performance. Several items in the Hemophilia Activities List (HAL), for example, are not applicable in India and Jamaica, while the Functional Independence Score in Hemophilia (FISH), an instrument developed in India, performs well in these countries. 38 , 39 Similarly, the FISH shows ceiling effects and fails to detect early changes in joint health in high‐income countries with early prophylaxis. 40 Health care organizations may choose the tool that is most appropriate and feasible in their situation. Third, PROMs are subjective by definition and may demonstrate response shift if used to assess changes over time. 41 , 42 Therefore, assessment of health outcomes with clinical tools will be needed to supplement PROMs when possible. Finally, a PROM that measures all outcomes in the standard set is currently unavailable, and several instruments are needed to measure all outcomes. Having to complete multiple instruments that may be partially overlapping may pose a burden on persons with hemophilia, or for parents or guardians completing an instrument for children with hemophilia. The length and unknown responsiveness of current hemophilia‐specific outcome assessment instruments may hamper their usefulness in clinical practice.
We selected relevant PROMIS item banks 22 because they may in part solve these issues. PROMIS item banks have been developed for many patient‐relevant outcomes and have been validated in diseased and healthy populations. PROMIS item banks are available in many languages and offer greater precision of outcome assessment than other generic instruments. Since item banks were developed based on modern Item Response Theory, they allow for selecting any number of items from the bank to produce a short form whose scores can be compared to any other selection of items from the same item bank. This increases flexibility and reduces response burden, especially if administered as computerized adaptive test. 36 However, PROMIS item banks have yet to be formally validated for use in hemophilia populations.
4.2. Implications for clinical practice
We recommend that health care providers start measuring the outcomes from the international standard set in clinical practice. This is relevant for patients because it allows individualized adjustment of treatment. Still, feasibility and applicability in different care settings and patient groups should be evaluated in annual meetings in which health care providers exchange their experiences with using the standard set.
It may not be feasible or necessary to measure the complete outcomes set at once. When time or resources are limited, we encourage users of the outcomes set to start with regular assessments of the outcomes in tier 1. Data collection may be expanded to the health outcomes in tiers 2 and 3 at a later stage.
Feasibility of implementation in different healthcare systems was our foremost priority. Therefore, we recommend the use of widely accepted measurement instruments that are publicly available in multiple languages and can be administered during routine clinical practice. We have summarized the length of each instrument, availability and validity in multiple languages, and accessibility (Table S9) to assist with implementation.
National registries already collect outcome data on a regular basis. The World Bleeding Disorders Registry of the World Federation of Hemophilia promotes standardized patient data collection from treatment centers around the world 43 and may be used to start measuring health outcomes and risk‐adjustment variables. An acceptable burden of outcome assessments for both persons with hemophilia and health care providers is crucial for a broad acceptability and use of any standard set. We expect that e‐health developments such as a PROMs mobile app or routine data collection from electronic medical records will greatly reduce the burden for both persons with hemophilia and health care providers.
4.3. Future directions
Hemophilia care is in transition. Novel and potentially curative treatments will be increasingly available for persons with hemophilia in the near future. This may have implications for the definition of hemophilia (eg, cutoff points for baseline coagulation factor levels 44 ) as well as for which health outcomes are the most relevant. Therefore, the currently presented definitions of the medical condition and health outcomes may need to be adapted in the future.
In addition, outcome measurement instruments must be continuously improved and adapted to health care developments. An enhanced version of the FISH (A. Srivastava, personal communication) is aimed at reducing the ceiling effects that have been found for children and individuals with mild hemophilia. 24 Similarly, the Hemophilia Joint Health Score is currently under revision to enhance efficacy 45 and to further improve convergent and discriminant validity in adults. 46 PROBE is expanding country and language availability, implementing longitudinal data collection to improve detection of change over time and testing performance in new hemophilia populations. 47 It may be more contemporary than existing instruments, potentially replacing older instruments over time. Finally, PROMs such as the Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool questionnaire are currently updated to increase sensitivity for detecting improvements in treatment burden of novel treatments and to include outcomes that may become relevant in the future, such as caregiver and family burden. 48
For all these reasons, we emphasize that the recommendations regarding the standard set of health outcomes and measurement instruments are dynamic entities and that revisions should be scheduled biennially.
5. CONCLUSION
The presented international standard set of health outcomes that matter to persons with hemophilia will form the basis for harmonized longitudinal monitoring and comparison of health outcomes. Broad implementation will enable a more personalized approach in hemophilia care within a framework of continuous improvement of treatment with increasing value for persons with hemophilia.
RELATIONSHIP DISCLOSURE
EvB, BO, GD, DG, JO, BK, DN, DC, and CS declare no conflicts of interest. MC has received grants from governmental research institutes, such as the Dutch Research Institute (NWO), ZonMW, Innovation fund, NWO‐NWA, and unrestricted investigator initiated research grants, as well as educational and travel funding from various companies (Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, and Nordic Pharma), and has served as a member on steering boards of Roche and Bayer. All grants, awards, and fees go to the Erasmus MC. VB’s institution holds the copyright to CHO‐KLAT. KF’s institution holds the copyright to HAL and PedHAL. AI’s institution has received project‐based funding via research or service agreements with Bayer, CSL, Grifols, NovoNordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, and Takeda. Shannon Jackson has received research funding from Bioverativ, Sanofi, Bayer, and Spark Therapeutics. MS’s institution received independent investigator‐initiated research support for the PROBE study from Bayer, CSL, Novo Nordisk, Roche, and Sanofi, and his institution holds the copyright to PROBE. AS’s institution holds the copyright to the Functional Independence Score in Hemophilia (FISH). FvE’s firm received financial compensation for facilitation of the process. JvdB reports personal fees from Bayer (teaching activities), personal fees from NovoNordisk (consulting), outside the submitted work. SCG reports SOBI medical research grants, outside the submitted work.
AUTHOR CONTRIBUTIONS
SCG, JGvdB, BO, MHC conceived the project. ECvB conducted the literature search and prepared the web‐based meetings. ECvB, JGvdB, MHC and SCG wrote the manuscript. FvE chaired web‐based meetings and provided practical and methodological support. All authors actively participated in the assignments and web‐based steering group meetings. All authors approved the final manuscript and provided relevant feedback.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Vincent Wiersma and Matthijs van der Linde (The Decision Group) for assistance with project management, as well as the Patients and Health Care Professionals Panel for their participation and the International Academic Council for their valuable feedback: Rezan Abdul‐Kadir, Sulochana Badagabettu, Marlène Beijlevelt, Petra Buckova, Frederica Cassis, Randall Curtis, Susan Cutter, Judy Ann David, Mariette Driessens, Brian Feldman, Leonard Friedman, Cesar Garrido, Khalid Habaybeh, Lotte Haverman, Mathieu Jackson, Pedro Jardim, Radek Kaczmarek, Kate Khair, Piet de Kleijn, Ilmar Kruis Ed Kuebler, Annamma Kurien, Johnny Mahlangu, Mike Makris, Paul McLaughlin, Pamela Narayan, Rungrote Natesirinilkul, Yasu Nishida, Declan Noone, David Page, Glenn Pierce, Suzie Peterson, Pia Petrini, Suely Rezende, R. Sathyanarayanan, Sheldon Simson, Roberto Solinis Nuño, Fendi Valdez, Pamela Wilton, and Deon York. Affiliations of participants are included in the Supporting Information. We would like to thank Elizabeth Clearfield for sharing the literature search of the CoreHEM project, medical librarian Jan Schoones for his assistance in the literature search update, and Nancy Young for critical feedback on the first draft of the manuscript.
van Balen EC, O'Mahony B, Cnossen MH, et al. Patient‐relevant health outcomes for hemophilia care: Development of an international standard outcomes set. Res Pract Thromb Haemost. 2021;5::e12488. 10.1002/rth2.12488
Handling Editor: Cihan Ay
Contributor Information
Marjon H. Cnossen, @CnossenM.
Alfonso Iorio, @AlfonsoIorio.
Mark W. Skinner, @MSkinnerDC.
Samantha C. Gouw, Email: s.c.gouw@lumc.nl.
REFERENCES
- 1. Iorio A, Stonebraker JS, Chambost H, Makris M, Coffin D, Herr C, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta‐analytic approach using national registries. Ann Intern Med. 2019;171(8):540. 10.7326/m19-1208 [DOI] [PubMed] [Google Scholar]
- 2. Blanchette VS, Key NS, Ljung LR, Manco‐Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–9. 10.1111/jth.12672 [DOI] [PubMed] [Google Scholar]
- 3. van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: pathophysiology, pain and imaging. Haemophilia. 2018;24(Suppl 6):44–9. 10.1111/hae.13449 [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1–158. 10.1111/hae.14046 [DOI] [PubMed] [Google Scholar]
- 5. Balkaransingh P, Young G. Novel therapies and current clinical progress in hemophilia A. Therapeutic Adv Hematol. 2018;9:49–61. 10.1177/2040620717746312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nossair F, Thornburg CD. The role of patient and healthcare professionals in the era of new hemophilia treatments in developed and developing countries. Therapeutic Adv Hematol. 2018;9:239–49. 10.1177/2040620718784830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Kleijn P, Odent T, Berntorp E, Hilliard P, Pasta G, Srivastava A, et al. Differences between developed and developing countries in paediatric care in haemophilia. Haemophilia. 2012;18(Suppl 4):94–100. 10.1111/j.1365-2516.2012.02875.x [DOI] [PubMed] [Google Scholar]
- 8. WFH . Report on the annual global survey. Montreal; 2019. [Google Scholar]
- 9. Porter ME. What is value in health care? New Engl J Med. 2010;363:2477–81. 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 10. Porter ME, Larsson S, Lee TH. Standardizing patient outcomes measurement. New Engl J Med. 2016;374:504–6. 10.1056/NEJMp1511701 [DOI] [PubMed] [Google Scholar]
- 11. Porter ME. The strategy that will fix health care. Harvard Bus Rev. 2013;50–70. [Google Scholar]
- 12. Feldman BM. The outcomes of haemophilia and its treatment: why we need a core set. Haemophilia. 2017;23:485–7. 10.1111/hae.13234 [DOI] [PubMed] [Google Scholar]
- 13. O'Mahony B, Dolan G, Nugent D, Goodman C. Patient‐centred value framework for haemophilia. Haemophilia. 2018;24(6):873–9. 10.1111/hae.13456 [DOI] [PubMed] [Google Scholar]
- 14. Iorio A, Skinner MW, Clearfield E, Messner D, Pierce GF, Witkop M, et al. Core outcome set for gene therapy in haemophilia: results of the coreHEM multistakeholder project. Haemophilia. 2018;24:e167–e172. 10.1111/hae.13504 [DOI] [PubMed] [Google Scholar]
- 15. Fischer K, Poonnoose P, Dunn AL, Babyn P, Manco‐Johnson MJ, David JA, et al. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia. 2017;23:11–24. 10.1111/hae.13088 [DOI] [PubMed] [Google Scholar]
- 16. Dover S, Blanchette VS, Srivastava A, Fischer K, Abad A, Feldman BM. Clinical outcomes in hemophilia: towards development of a core set of standardized outcome measures for research. Res Pract Thrombosis Haemostasis. 2020;4(4):652–8. 10.1002/rth2.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nugent DJ, O'Mahony B, Dolan G. Value of prophylaxis vs on‐demand treatment: application of a value framework in hemophilia. Haemophilia. 2018;24(5):755–65. 10.1111/hae.13589 [DOI] [PubMed] [Google Scholar]
- 18. Porter ME, Teisberg EO. Redefining health care. Boston: Harvard Business Review Press; 2006. [PubMed] [Google Scholar]
- 19. ICHOM . International Consortium for Health Outcomes Measurements Standard sets. Boston; 2019. [Google Scholar]
- 20. Skinner MW, Chai‐Adisaksopha C, Curtis R, Frick N, Nichol M, Noone D, et al. The Patient Reported Outcomes, Burdens and Experiences (PROBE) Project: development and evaluation of a questionnaire assessing patient reported outcomes in people with haemophilia. Pilot Feasib Stud. 2018;4:58. 10.1186/s40814-018-0253-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porter ME, Lee TH. Why strategy matters now. New Engl J Med. 2015;372:1681–4. 10.1056/NEJMp1502419 [DOI] [PubMed] [Google Scholar]
- 22. Ader DN. Developing the patient‐reported outcomes measurement information system (PROMIS). Med Care. 2007;45:S1–S2. 10.1097/01.mlr.0000260537.45076.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prinsen CAC, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. Guideline for selecting outcome measurement instruments for outcomes included in a Core Outcome Set. Amsterdam; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timmer MA, Gouw SC, Feldman BM, Zwagemaker A, de Kleijn P, Pisters MF, et al. Measuring activities and participation in persons with haemophilia: a systematic review of commonly used instruments. Haemophilia. 2017;24(2):e33–49. 10.1111/hae.13367 [DOI] [PubMed] [Google Scholar]
- 25. Limperg PF, Terwee CB, Young NL. Health‐related quality of life questionnaires in individuals with haemophilia: a systematic review of their measurement properties. Haemophilia. 2017;23(4):497–510. 10.1111/hae.13197 [DOI] [PubMed] [Google Scholar]
- 26. Gouw SC, Timmer MA, Srivastava A, de Kleijn P, Hilliard P, Peters M, et al. Measurement of joint health in persons with haemophilia: a systematic review of the measurement properties of haemophilia‐specific instruments. Haemophilia. 2019;25(1):e1–e10. 10.1111/hae.13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funk SM, Engelen S, Benjamin K, Moshkovich O, Gentile B, Church N, et al. Validity and reliability of the Colorado Adult Joint Assessment Scale in adults with moderate‐severe hemophilia A. J Thrombosis Haemostasis. 2020;18:285–94. 10.1111/jth.14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chai‐Adisaksopha C, Skinner MW, Curtis R, Frick N, Nichol MB, Noone D, et al. Psychometric properties of the Patient Reported Outcomes, Burdens and Experiences (PROBE) questionnaire. BMJ Open. 2018;8:e021900. 10.1136/bmjopen-2018-021900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chai‐Adisaksopha C, Skinner MW, Curtis R, Frick N, Nichol MB, Noone D, et al. Test‐retest properties of the Patient Reported Outcomes, Burdens and Experiences (PROBE) questionnaire and its constituent domains. Haemophilia. 2019;25:75–83. 10.1111/hae.13649 [DOI] [PubMed] [Google Scholar]
- 30. Ligocki CC, Abadeh A, Wang KC, Adams‐Webber T, Blanchette VS, Doria AS. A systematic review of ultrasound imaging as a tool for evaluating haemophilic arthropathy in children and adults. Haemophilia. 2017;23:598–612. 10.1111/hae.13163 [DOI] [PubMed] [Google Scholar]
- 31. Chai‐Adisaksopha C, Skinner MW, Curtis R, Frick N, Nichol MB, Noone D, et al. Exploring regional variations in the cross‐cultural, international implementation of the Patient Reported Outcomes Burdens and Experience (PROBE) study. Haemophilia. 2019;25:365–72. 10.1111/hae.13703 [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization . International Classification of Functioning, Disability and Health (ICF). ICF core sets. [Google Scholar]
- 33. O'Hara J, Hughes D, Camp C, Burke T, Carroll L, Diego DG. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12:106. 10.1186/s13023-017-0660-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feldman BM, Srivastava A, Fischer K, Dover S, Abad A, Blanchette VS. Towards the development of a core set for standardized assessment of outcomes in persons with hemophilia. In: International Society on Thrombosis and Haemostasis conference. ISTH poster presentation, Berlin; 2017. [Google Scholar]
- 35. Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health‐related patient‐reported outcomes. J Clin Epidemiol. 2010;63:737–45. 10.1016/j.jclinepi.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 36. Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short‐forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133–41. 10.1007/s11136-007-9204-6 [DOI] [PubMed] [Google Scholar]
- 37. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poonnoose PM, Thomas R, Keshava SN, Cherian RS, Padankatti S, Pazani D, et al. Psychometric analysis of the Functional Independence Score in Haemophilia (FISH). Haemophilia. 2007;13:620–6. 10.1111/j.1365-2516.2007.01508.x [DOI] [PubMed] [Google Scholar]
- 39. Wharfe G, Buchner‐Daley L, Gibson T, Hilliard P, Usuba K, Abad A, et al. The Jamaican Haemophilia Registry: describing the burden of disease. Haemophilia. 2018;24:e179–e186. 10.1111/hae.13517 [DOI] [PubMed] [Google Scholar]
- 40. Poonnoose PM, van der Net J. Musculoskeletal outcome in hemophilia: bleeds, joint structure and function, activity, and health‐related fitness. Semin Thromb Hemost. 2015;41:872–9. 10.1055/s-0034-1543997 [DOI] [PubMed] [Google Scholar]
- 41. Barclay‐Goddard R, Epstein JD, Mayo NE. Response shift: a brief overview and proposed research priorities. Qual Life Res. 2009;18:335–46. 10.1007/s11136-009-9450-x [DOI] [PubMed] [Google Scholar]
- 42. Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM. The clinical significance of adaptation to changing health: a meta‐analysis of response shift. Qual Life Res. 2006;15:1533–50. 10.1007/s11136-006-0025-9 [DOI] [PubMed] [Google Scholar]
- 43. WFH . World Bleeding Disorders registry. 2018 data report. Montreal; 2019. [Google Scholar]
- 44. Soucie JM, Monahan PE, Kulkarni R, Konkle BA, Mazepa MA. The frequency of joint hemorrhages and procedures in nonsevere hemophilia A vs B. Blood Adv. 2018;2:2136–44. 10.1182/bloodadvances.2018020552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuijlaars IAR, van der Net J, Feldman BM, Aspdahl M, Bladen M, de Boer W, et al. Evaluating international Haemophilia Joint Health Score (HJHS) results combined with expert opinion: options for a shorter HJHS. Haemophilia. 2020;26(6):1072–80. 10.1111/hae.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feldman B, Zourikian N, Funk S, Tilak M, Lobet S, Manco‐Johnson MJ, et al. Elevated HJHS scores in healthy adult males without hemophilia. Abstracts. Haemophilia. 2018;24:3–196. 10.1111/hae.13478 [DOI] [PubMed] [Google Scholar]
- 47. Clinicaltrials.gov . Patient reported outcomes burdens and experiences ‐ phase 3 (PROBE‐3). [Google Scholar]
- 48. Price VE, Dover S, Blanchette VS, Klaassen RJ, Wakefield C, Belletrutti M, et al. Measuring Health Related Quality of Life (HRQoL) in boys with hemophilia using extended half‐life clotting factor concentrates (CFCs). Melbourne, Australia: International Society for Thrombosis and Hemostasis; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


