Abstract
Since the onset of the pandemic in Wuhan city, China, forecasting and projections of the pandemic are the areas of interest for the investigators, and the basic reproduction rate R0 always stayed the favorite tool. The basic reproduction number (R0) is either ratio or rate or the basic reproductive rate. This dimensionless number was calculated in the past to describe the contagiousness or transmissibility of infectious agents for many communicable diseases. Its importance in the context of COVID-19 is not less, it tells us about the public health measures to be undertaken for disease prevention, and how the transmission of COVID-19 will be affected or eliminated. R0 is affected by several biological, sociobehavioral, and environmental factors which decide agent transmission. R0 is estimated by using complex mathematical models, the results of which are easily distorted, misjudged, and misused. R0 is not a biological constant for an agent or pathogen, it is a rate over time. It can measure the disease severity and also gives an estimate about the herd immunity required for the reversal of epidemic. R0 cannot be altered through vaccination campaigns though it can tell us about the relationship between the population's immune status and epidemic curve. Modeled R0 values are dependent on the model structures and assumptions made. Some R0 values reported in the scientific literature are likely outdated as assumptions are frequently changing in the current pandemic. R0 must be predicted and applied with great caution as this basic metric is far from simple.
Keywords: Basic reproduction number, epidemic models, herd immunity, R0, vaccination
INTRODUCTION
COVID-19 has renewed the interest in infectious disease epidemiology. There is an information overload among health-care workers of COVID-19.[1] With these few words have got prominent, the one such word is R0 (R naught). R0 is the basic reproduction number for an infectious disease. For any infectious disease, understanding of three epidemiological parameters is of importance, namely incubation period (the time taken by an infectious agent for appearance of the first symptom), serial interval (duration of time between the onset of the index case and the secondary case), and reproductive number (number of secondary cases reproduced by a typical case in a susceptible population).[2] It is also a well-known fact that for any novel infectious diseases, estimation of these parameters during pandemics varies widely, and different estimation methods might be used to estimate these parameters.[3]
In this article, we will be focusing on the reproductive number. We would try to understand the reproductive number in the context of the present pandemic of COVID-19. Graphs have been made for illustration with the R version 4.0.3.
ORIGIN OF R0
Interestingly, R0 has its origin in demography. In 1886, Richard Böckh working in Registrar's Office in Berlin, for the first time, added a fertility table in the demographic data for the year 1879. He calculated the total reproduction rate (number of females born from one female during her entire reproductive life), the first modern estimate of R0. Alfred Lotka and Kuczynski developed the theory behind the calculation.[4] Sir Ronald Ross, while giving his famous Mosquito Theorem, used the term “Critical Density” of mosquitoes, below which there could be no transmission of malaria. This was the first rudimentary description of R0 in epidemiology. However, it was a seminal paper by Kermack et al. in 1927, which lead to the development of R0 in epidemiology.[5] Anderson and May give the symbol for basic reproduction rate R0 in Nature in 1979.[6]
MEANING OF R0
The basic reproduction number, R0, is defined as the expected number of secondary cases produced by a single (typical) infection in a completely susceptible population. In other terms, it is the ability to transmit the disease, high R0 leading to a higher rate of transmission and a thus higher number of cases in a susceptible population. For example, if R0 of a disease introduced in a susceptible population is 5, it implies that, on average, the disease is likely to be transmitted by an infectious case to five susceptible hosts. With an R0 value of more than 1, the transmission is likely to continue in a population, and in case the R0 is below 1, the transmission will probably wane off because one infectious case will infect less than one person on average.
Another related term is the effective reproductive number (Rt). It is based on the concept that after introducing the agent into the naïve population, not all persons remain susceptible as the epidemic goes on. Few develop immunity due to infection or due to vaccination. In such circumstances, the term used is an effective reproductive number.
It is important to note that R0 is a dimensionless number and not a rate, which would have units per time. It can be calculated for various communicable diseases irrespective of their route of spread. The calculation of R0 is based on three factors.
R0 = Infection/contact (transmissibility) × contact/time (average rate of contact) × time/infection (duration of infectiousness)
Transmissibility
Transmissibility is probability of infection given contact with a susceptible. Any easily transmissible infection will have a higher R0. For example, an airborne infection like measles, flu has a higher R0 than others requiring direct contacts like HIV or Ebola.
Contact rate
It is the average rate of contact between susceptible and infected individuals. The higher the contact rate, the higher would be R0. This factor is not specific to the disease; instead, it depends on the population characteristic and is amenable to be modified by public health measures like quarantine and travel ban.
Duration of infectiousness
It is also known as the infectious period. It is the duration in which the person infected can transmit the infection to susceptible. The longer the duration of infectiousness, the higher is the R0. The duration of infectiousness is the agent characteristic and depends on the underlying condition of the host. The younger population and diseased people may have a higher infectious period.
The values estimated for the above three factors vary with agent characteristic, individuals' factors and socioenvironmental influences. Hence, at best, the estimates are known. The calculated R0 from these estimates itself remains an estimate.
For the direct method of calculating R0, every new confirmed case should be linked with the previous one, which is seldom possible in the real world. The other ways are the calculation of R0 based on reported incident cases. The various programs for calculation of the same are available with various software including R.[7] However, this article would limit itself to the understanding of the concept of R0.
UNDERSTANDING R0
R0 may be utilized to understand various characteristics of the pandemic. Some of them are illustrated below
Establishment of a pathogen in the population
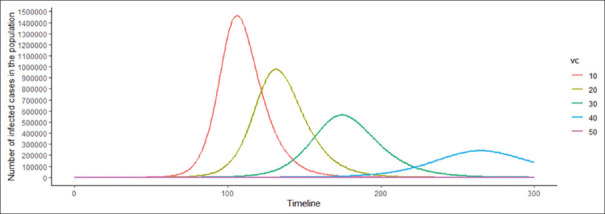
This is the easiest concept to understand. When R0 is >1, an agent would be able to spread through the population. When it is smaller than one, which means one case would be able to infect <1 case, the chain of transmission would break.[8] The R0 would also give the likely time and numbers of infection in a population. As an illustrative example, let us consider a population of 10,000,000 (10 million), which is susceptible to a novel pathogen. Figure 1 illustrates the likely establishment trajectory or the natural course of infections in the population depending on the R0. Two aspects of pathogen epidemiology can be understood from Figure 1. First, on a temporal scale, the higher the R0, the quicker the pathogen will likely infect the population, resulting in an epidemic. Second, in terms of the proportion of the population infected at the peak, the higher the R0, the higher is the proportion of the population infected at the peak of the epidemic. The figure depicts the public health importance of the R0, as with higher R0 cases being concentrated in a shorter period of time; thus, the health system may get overwhelmed easily. Herein, public health measures to decrease contact rate may be helpful to reduce the number of peak cases. In the end, the equilibrium between agent, host, and environment would be established, and the disease may or may not become endemic.
Figure 1.
The path for the establishment of pathogen in a susceptible population based on R0 of the pathogen
Herd immunity
R0 gives an estimate about the herd immunity required for the epidemic to reverse. If a sufficient number of individuals are immunized (either naturally or through vaccination), the effective reproductive ratio will be below one, and the number of infected cases would be reduced in future. The threshold is given by equation = 1 − 1/R0. Thus, measles with an R0 of up to 20 requires around 95% immune persons in the population, and chickenpox with an R0 of 5 would require 80% immune persons for the epidemic to reverse. For the present pandemic, the pooled estimate of R0 using the random effect model on 23 studies was found to be 3.32 (95% confidence interval: 2.81–3.82).[9] Hence, the percentage of the population required to be immune before the epidemic may be reversed is 69.9%. From an acquired immunization point of view, vaccination would be required for a minimum of 69.9% of the population if it is 100% effective.
To understand, let us do an illustration on R0. If a susceptible population of 10,000,000, a novel virus with an R0 of 3.32 enters, an epidemic is the most likely outcome. However, assuming that the population under consideration develops a vaccine against the novel virus and immunizes 10% of its population, will the epidemic be averted? Will it be averted at 30% vaccination coverage before an infectious person transmitting the novel virus enters this population? The answer to the dilemma illustrated above can be answered by the critical vaccination threshold or the herd immunity threshold, which is defined as the level or percentage or proportion of the total population which needs to be immune to the disease under consideration so that the R0 remains below one. The higher the R0, the higher is the critical vaccination threshold and the higher is the herd immunity threshold. The same is illustrated in Figure 2, wherein the effect of increasing vaccination coverage (assumed vaccine effectiveness of 100% and vaccination carried out before the introduction of novel disease agent) in a susceptible population of 100,000,000 on epidemic trajectories is represented. It can be seen from the trajectories that even 50% if effectively covered by natural or acquired immunity can blunt the epidemic to a great extent. This has been projected by modeling the COVID-19 pandemic by various authors.[10] If 70% of the population is immune, a newer agent with R0 3.2 would not establish itself in that population.
Figure 2.
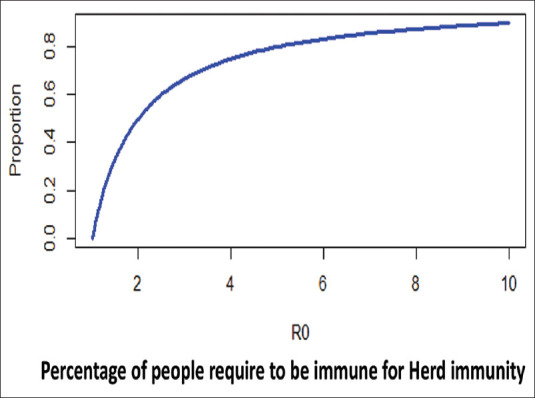
Relationship between the increasing proportion of the population immune to a disease on epidemic trajectories
Final epidemic size
Another question that is often asked how many people would get infected at the end of the epidemic or what is the final epidemic size? according to the approximate relationship of (1 – exp [−R0]). The assumption here is the closed epidemic model. However, the calculation is only an approximation and does hold good for R0 >2.5 but overestimates the final epidemic size for smaller R0 and may not be applicable for R0 <1.[11]
The final epidemic size for various R0 is represented in Figure 3. For an epidemic with R0 of 3.32, the final population which would eventually get infected would be 96.4%. As shown in Figure 3, the proportion of the population infected would be approximately 100% for R0 of 4 or more.
Figure 3.
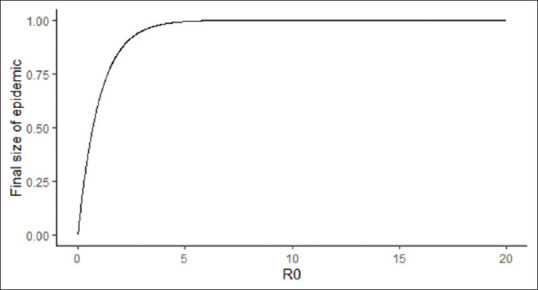
Relationship between the proportion of the population infected at the end of epidemic and R)
Mean age of infection
In a stable host population, the mean age of infection may also be estimated by R0. The mean age of infection is L/(R0 − 1), where L is host life expectancy.[12] Figure 4 represents the relationship between the mean age of infection and the life expectancy in a population when a novel disease agent is introduced in a closed population with an R0 of 3.
Figure 4.
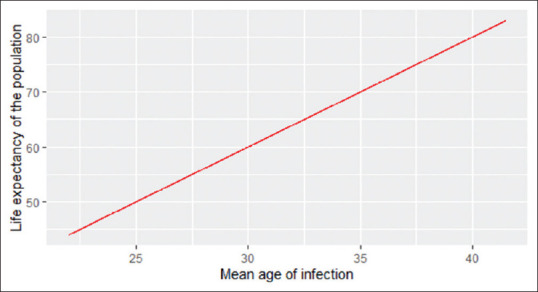
Relationship between the life expectancy and mean age of infection in a closed population for an assumed R0 of 3
Competitive dominance
At any given point in time, multiple organisms are competing for dominance. The susceptible fraction at equilibrium is inversely related to R0. This implying that besides acute invasion, which is highest with increasing R0, the organism with lower R0 would eventually become dominant.[13]
Limitation of R0
Real world is not mathematical. The assumption used in the calculation of R0, such as availability of data, exponential part of curve, homogenous population, similar contact rate in the population, and closed population, is seldom satisfied in the actual world as explained R0 is dependent on a variety of parameters including agent factors such as contagiousness of the pathogen, duration of infectiousness, and survival once excreted by the host. In terms of the classical epidemiological triad, R0 depends on the host and environmental factors as well. The host factors, such as susceptibility, age, sex, and occupation are likely to have a bearing on the values of R0.[14] Similarly, the environmental factors such as seasonality (summer or winter), and living conditions (overcrowding and ventilation) also contribute to the transmission dynamics and thus the Ro. This is the precise reason that there are different R0 values for the same pathogen in different populations, for measles, 20 different values of R0 were reported during different populations and in different study periods.[15] A systemic review of 18 studies reported R0 values of 3.7–203.3 for measles.[16] Thus, the value of R0 is highly variable in different regions and varied population characteristics.[17] Further, the duration of infectiousness and contact rate may vary by implementing control measures such as quarantine, isolation of the infected, personal hygiene, good disinfection practices, wearing masks, and adequate ventilation, thus limiting the use of R0 within the same population. Hence, it is important to look at the empirical data to predict the epidemic's course rather than only R0. Few authors have also advocated the use of other epidemiological parameters for understanding the epidemic.[17]
CONCLUSION
The present manuscript has been developed to provide an introduction and highlight important aspects of R0 in understanding disease epidemiology. The article aimed to bring forward the salient features of R0 in the context of infectious disease modeling. The R0 is not a mathematical number having precision. Rather, it is a complex number having limited application in the real world.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Datta R, Yadav AK, Singh A, Datta K, Bansal A. The infodemics of COVID-19 amongst healthcare professionals in India. Med J Armed Forces India. 2020;76:276–83. doi: 10.1016/j.mjafi.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Last accessed on 2020 Aug 12]. Available from: https://www.who.int/csr/disease/swineflu/global_pandemic_influenza_surveilance_apr09.pdf .
- 3.Boëlle PY, Ansart S, Cori A, Valleron AJ. Transmission parameters of the A/H1N1 (2009) influenza virus pandemic: A review. Influenza Other Respir Viruses. 2011;5:306–16. doi: 10.1111/j.1750-2659.2011.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heesterbeek J. The concept of Ro in epidemic theory. Stat Neerl. 1996;50:89–110. [Google Scholar]
- 5.Kermack WO, McKendrick AG, Walker GT. A contribution to the mathematical theory of epidemics. Proceedings of the royal society of London series a, containing papers of a mathematical and physical character. 1927;772(115):700–21. [Google Scholar]
- 6.Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature. 1979;280:361–7. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 7.Obadia T, Haneef R, Boëlle PY. The R0 package: A toolbox to estimate reproduction numbers for epidemic outbreaks. BMC Med Inform Decis Mak. 2012;12:147. doi: 10.1186/1472-6947-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JR, Dobson AP, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–7. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alimohamadi Y, Taghdir M, Sepandi M. Estimate of the basic reproduction number for COVID-19: A systematic review and meta-analysis. J Prev Med Public Health. 2020;53:151–7. doi: 10.3961/jpmph.20.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee K, Chatterjee K, Kumar A, Shankar S. Healthcare impact of COVID-19 epidemic in India: A stochastic mathematical model. Med J Armed Forces India. 2020;76:147–55. doi: 10.1016/j.mjafi.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjørnstad ON. Ist edition. Springer International Publishing; 2018. Epidemics. Models and Data Using R. [Google Scholar]
- 12.Dietz K, Schenzle D. Proportionate mixing models for age-dependent infection transmission. J Math Biol. 1985;22:117–20. doi: 10.1007/BF00276550. [DOI] [PubMed] [Google Scholar]
- 13.Shrestha S, Bjørnstad ON, King AA. Evolution of acuteness in pathogen metapopulations: Conflicts between “classical” and invasion-persistence trade-offs. Theor Ecol. 2014;7:299–311. doi: 10.1007/s12080-014-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RM, editor. The Population Dynamics of Infectious Diseases: Theory and Applications. Boston, MA, USA: Springer; 1982. [Last accessed on 2020 Dec 01]. Directly transmitted viral and bacterial infections of man. Available from: https://link.springer.com/book/10.1007/978-1-4899-2901-3 . [Google Scholar]
- 16.Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, et al. The basic reproduction number (R0) of measles: A systematic review. Lancet Infect Dis. 2017;17:e420–8. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 17.Ridenhour B, Kowalik JM, Shay DK. Unraveling R0: Considerations for public health applications. Am J Public Health. 2014;104:e32–41. doi: 10.2105/AJPH.2013.301704. [DOI] [PMC free article] [PubMed] [Google Scholar]