Abstract
Background
Adenosine stress T1-mapping on cardiovascular magnetic resonance (CMR) can differentiate between normal, ischemic, infarcted, and remote myocardial tissue classes without the need for contrast agents. Regadenoson, a selective coronary vasodilator, is often used in stress perfusion imaging when adenosine is contra-indicated, and has advantages in ease of administration, safety profile, and clinical workflow. We aimed to characterize the regadenoson stress T1-mapping response in healthy individuals, and to investigate its ability to differentiate between myocardial tissue classes in patients with coronary artery disease (CAD).
Methods
Eleven healthy controls and 25 patients with CAD underwent regadenoson stress perfusion CMR, as well as rest and stress ShMOLLI T1-mapping. Native T1 values and stress T1 reactivity were derived for normal myocardium in healthy controls and for different myocardial tissue classes in patients with CAD.
Results
Healthy controls had normal myocardial native T1 values at rest (931 ± 22 ms) with significant global regadenoson stress T1 reactivity (δT1 = 8.2 ± 0.8% relative to baseline; p < 0.0001). Infarcted myocardium had significantly higher resting T1 (1215 ± 115 ms) than ischemic, remote, and normal myocardium (all p < 0.0001) with an abolished stress T1 response (δT1 = −0.8% [IQR: −1.9–0.5]). Ischemic myocardium had elevated resting T1 compared to normal (964 ± 57 ms; p < 0.01) with an abolished stress T1 response (δT1 = 0.5 ± 1.6%). Remote myocardium in patients had comparable resting T1 to normal (949 ms [IQR: 915–973]; p = 0.06) with blunted stress reactivity (δT1 = 4.3% [IQR: 3.1–6.3]; p < 0.0001).
Conclusions
Healthy controls demonstrate significant stress T1 reactivity during regadenoson stress. Regadenoson stress and rest T1-mapping is a viable alternative to adenosine and exercise for the assessment of CAD and can distinguish between normal, ischemic, infarcted, and remote myocardium.
Keywords: Coronary artery disease, Cardiovascular magnetic resonance, T1-mapping, ShMOLLI, Regadenoson
Highlights
-
•
Regadenoson has advantages over adenosine in terms of administration, safety profile, and clinical workflow.
-
•
There are distinct tissue characteristics for normal, ischemic, infarcted, and remote myocardium.
-
•
Healthy controls demonstrate significant stress T1 reactivity during vasodilator stress.
-
•
Regadenoson stress T1-mapping can distinguish between different myocardial tissue classes.
-
•
Regadenoson stress T1-mapping is a viable alternative to adenosine and exercise for the assessment of coronary artery disease.
1. Introduction
Cardiovascular magnetic resonance (CMR) has demonstrable utility in the assessment of suspected coronary artery disease (CAD) and the selection of patients for invasive coronary catheterization. First-pass myocardial perfusion CMR using gadolinium contrast directly assesses global and focal reductions in myocardial blood flow (MBF) at rest and during vasodilator stress, and has high diagnostic accuracy in detecting significant coronary disease [[1], [2], [3], [4]]. However, myocardial blood volume (MBV), which represents both coronary macro- and microcirculations, may constitute a more comprehensive global marker of ischemia than MBF [5,6]. Satisfactory augmentation of MBF and MBV requires adequate coronary vasodilatory reserve, which may be interrogated by pharmacological vasodilatory stress [7,8].
Application of this principle for the assessment of CAD is based on the fact that myocardial territories downstream of a significant coronary stenosis have increased resting coronary vasodilation and capillary recruitment [9] and thus increased MBV with significantly impaired stress coronary vasoreactivity. These changes in MBV may be interrogated by stress T1-mapping, based on the principle that native T1 values are sensitive to tissue free water content and may thus detect changes in MBV during vasodilatory stress [10]. Previous studies have shown that adenosine stress T1-mapping on CMR can distinguish between normal, ischemic, infarcted, and remote myocardium, without the need for gadolinium-based contrast agents (GBCAs) [9,[11], [12], [13]].
However, although adenosine is widely used in CMR units as a coronary vasodilator stress agent, it has a potentially increased side-effect profile due to non-selective activation of all adenosine receptors (A1, A2A, A2B, and A3) and requires an intravenous (IV) infusion (3–4 min) before images may be acquired. Regadenoson, a selective A2A-receptor agonist coronary vasodilator, has similar cardiac efficacy as adenosine whilst minimizing adverse effects, is often used in stress perfusion imaging when adenosine is contra-indicated, and has advantages in ease of administration (IV bolus), safety profile, and clinical workflow [14]. The normal stress T1-mapping response to regadenoson vasodilatory stress has not been previously studied for its potential clinical applicability. We aimed to characterize the normal stress T1-mapping response to regadenoson in healthy controls, and to then investigate the ability of regadenoson stress T1-mapping to differentiate between normal, ischemic, infarcted, and remote myocardium as an additional method for contrast-free assessments of CAD in the future.
2. Methods
2.1. Study population
Eleven healthy controls with no history of cardiovascular disease or cardiac symptoms, and twenty-five patients with known chronic CAD undergoing clinically indicated regadenoson stress perfusion CMR, were prospectively included. Participants with contraindications to stress perfusion CMR scanning (e.g. claustrophobia, implantable cardiac devices or other metallic implants, significant renal impairment with eGFR < 30 ml/min/1.73m2, allergy to gadolinium contrast or regadenoson) or other significant comorbidities (e.g. severe valvular heart disease or cardiomyopathy, acute myocardial infarction, concurrent malignancy) or pregnancy were excluded. All participants were advised to avoid caffeine for ≥24 h before the scan. Ethics approval was obtained from the local ethics committee for study procedures, and all participants provided written informed consent.
2.2. Image acquisition
CMR imaging was performed using a 1.5 T MRI scanner (MAGNETOM Avanto Fit, Siemens Healthcare, Erlangen, Germany) using an 18-channel phased-array coil. After standard planning, cine images were acquired in three long-axis views (HLA, VLA, LVOT) and in short-axis slices covering the whole left ventricle (LV) using balanced steady-state free precession cine imaging [8,15]. T1-mapping was performed using the Shortened Modified Look-Locker Inversion recovery (ShMOLLI) prototype sequence as previously described [16]. In-line quality assessment of ShMOLLI T1-maps was performed using parametric goodness-of-fit (R2) maps at time of acquisition [[16], [17], [18], [19]]. In healthy controls, native T1-maps were acquired at rest and during peak (first acquisition within 30 s) vasodilator stress (regadenoson 400μg intravenous (IV) bolus injection over 10 s followed by a 10 ml 0.9% sodium chloride saline flush over 10 s) in 3 short-axis slices (basal, mid-ventricular, and apical).
CAD patients underwent rest and regadenoson stress T1-mapping as described above. Stress first-pass perfusion imaging was performed on matching short-axis slices to the T1-maps during peak stress, with a bolus of gadolinium (0.05 mmol/kg IV, gadoterate meglumine, Dotarem, Guerbet SA, Paris, France) followed by a 15-20 ml saline flush, both administered at 4-6 ml/s [9]. Regadenoson stress was reversed using aminophylline (100 mg IV over 10 s followed by a 10 ml saline flush also over 10 s). Rest first-pass perfusion imaging was then performed as described above, typically at least 5–10 min after aminophylline reversal. Late gadolinium enhancement (LGE) imaging was performed ~8 to 10 min after an additional bolus of gadolinium (0.1 mmol/kg) in long and short-axis slices covering the LV, as well as matched to the T1-maps and perfusion slices.
2.3. Image analysis
Image analysis for biventricular indices was performed offline in accordance with SCMR guidelines [8], using cmr42 post-processing software (version 5.10.1, Circle Cardiovascular Imaging Inc., Calgary, Canada). First-pass myocardial perfusion and LGE images were analyzed as previously described [9,20]. Offline post-processing of ShMOLLI T1-maps was performed using MC-ROI (dedicated in-house software developed by SKP in Interactive Data Language v6.1, Exelis Visual Information Solutions, Boulder, Colorado, USA). Endocardial and epicardial contours were placed using dedicated automated software and manually checked for errors. Quality assessment of parametric goodness-of-fit (R2) maps resulted in exclusion of 6.6% of myocardial segments. For healthy controls, mean myocardial T1 values were derived from rest and stress T1-maps on a per-slice and per-segment basis according to the American Heart Association (AHA) 16-segment model [21]. Stress T1 reactivity was calculated as:
In CAD patients, mean T1 values were derived from regions of interest (ROI) carefully placed within ischemic, infarcted, and remote myocardium, and LV blood pool for reference, based on first-pass perfusion and LGE findings as previously described (Fig. 1) [9]. Briefly, ischemic myocardium was defined as an area corresponding to a reversible perfusion defect on first-pass stress perfusion imaging in the absence of LGE. Infarcted myocardium was defined as an area of infarction on LGE images; infarct ROIs were placed in the core of the infarcts to avoid partial volume contamination from the LV blood pool, carefully referenced against corresponding cine images [9]. Remote myocardium was defined as areas contralateral to ischemic myocardium and without evidence of first-pass perfusion defects, regional wall motion abnormalities, or LGE. ROIs were carefully matched between rest and stress images.
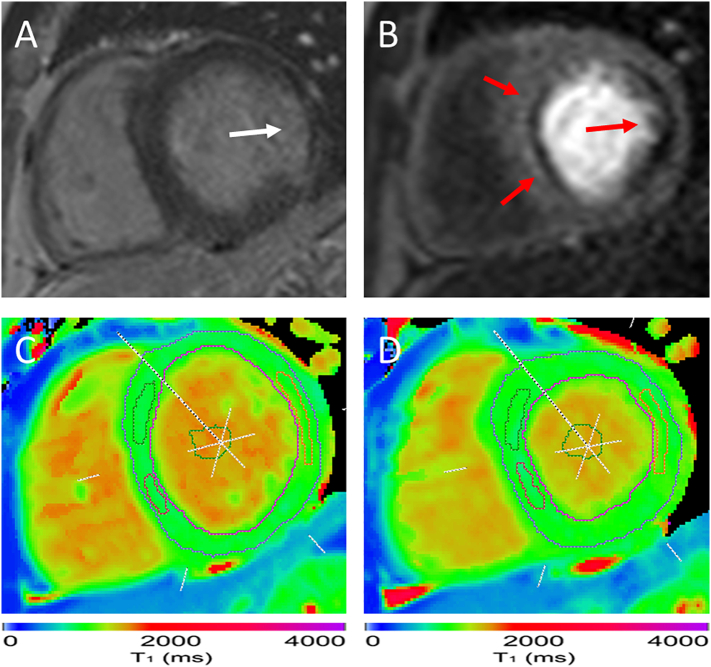
Fig. 1.
Example image of a patient with coronary artery disease. Late gadolinium enhancement image (A) shows evidence of a lateral myocardial infarction (white arrow). On first-pass stress perfusion (B) there is a fixed hypoperfusion defect in the lateral wall corresponding to the area of infarction, as well as inducible hypoperfusion in the septum (red arrows). Regions of interest are placed in the areas of ischemia and infarction and LV blood pool on T1 maps at rest (C) and stress (D). δT1 was significantly abolished in the anteroseptum (1.3%), inferoseptum (−0.8%), and in the lateral infarction (−0.4%). For comparison, the remote myocardium in this case had a δT1 of 3.7%. This participant had severe left anterior descending artery disease on invasive angiography, as well as a chronic total occlusion of the left circumflex artery. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.4. Statistical analysis
Data are presented as mean ± SD for all parametric data, and median with interquartile ranges (IQR) for non-parametric data, based on Shapiro-Wilks tests for normality. For parametric data, paired t-tests are used whenever possible to assess differences between rest and stress in the same individuals; unpaired t-tests are used to assess differences between groups for selected myocardial tissue types. For non-parametric data, Wilcoxon signed rank test and Mann Whitney U test are used. Repeated measures within subjects were accounted for using a linear mixed effects model. P < 0.05 is considered statistically significant. Statistical analysis and data modelling were performed using R Studio (RStudio Team (2018). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA).
3. Results
3.1. Myocardial stress T1 reactivity to regadenoson in healthy controls
All controls completed the CMR protocol without complications. One participant was excluded after incidental detection of a large myocardial infarction on LGE imaging, leaving a total of ten healthy controls (50% male; 32 [IQR: 29–37] years). All remaining healthy controls had structurally normal hearts with normal resting cardiac volumes (LVEDVi 79 [IQR: 67–88] ml/m2) and systolic function (LVEF 59 [IQR: 57–63] %), no inducible perfusion defects, and no LGE (Table 1). All participants had a significant heart-rate response to regadenoson (healthy controls 67 ± 12 to 114 ± 12 bpm; CAD patients 65 ± 11 to 93 ± 17 bpm; all p < 0.0001).
Table 1.
Baseline characteristics of study participants. Values are n (%) or median [IQR].
| Healthy controls |
CAD patients |
|
|---|---|---|
| (n = 10) | (n = 25) | |
| Male | 5 (50) | 23 (92) |
| Age (years) | 32 [29–37] | 69 [55–74] |
| Body mass index (kg/m2) | 25 [24–26] | 27 [24–29] |
| Clinical risk factors | ||
| Smoker | – | 7 (28) |
| Hypertension | – | 11 (44) |
| Hypercholesterolemia | – | 4 (16) |
| Diabetes mellitus | – | 14 (56) |
| CMR clinical indices | ||
| Left ventricular ejection fraction (%) | 59 [57–63] | 52 [43–64] |
| Left ventricular end diastolic volume index (ml/m2) | 79 [67–88] | 86 [78–108] |
| Number of remote myocardial segments (no ischemia or infarction) | – | 54 (13) |
| Number of myocardial segments with ischemia (first-pass perfusion) | – | 58 (15) |
| Number of myocardial segments with infarction (LGE) | – | 56 (14) |
| Angiographic data (n = 10) | ||
| ≥1 lesion (>50% visual diameter stenosis) | – | 10 (100) |
| Left anterior descending artery | – | 10 |
| Left circumflex artery | – | 6 |
| Right coronary artery | – | 8 |
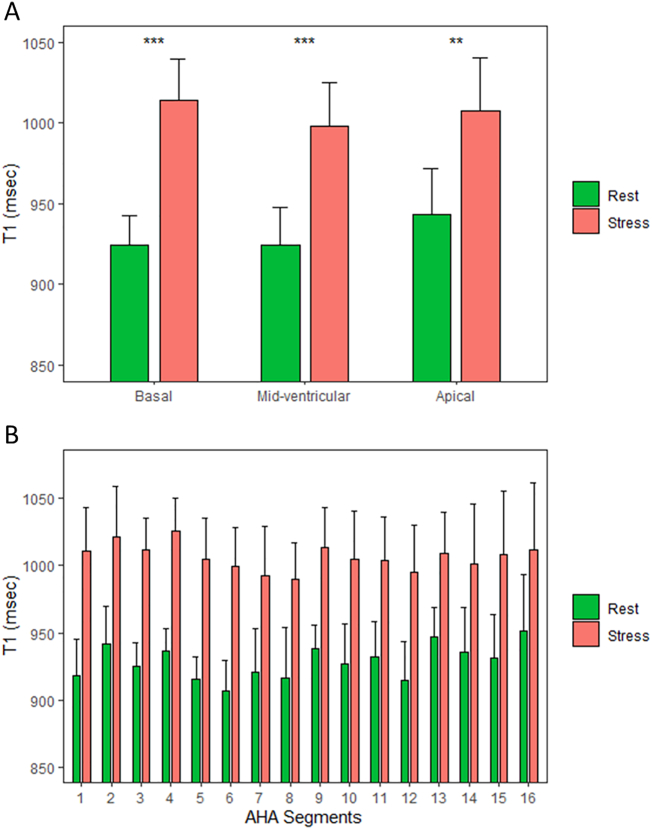
Healthy controls had normal global myocardial native T1 values at rest (Table 2) in accordance with previously published normal ranges [22]. Compared to rest, there was a significant rise in myocardial T1 values with regadenoson stress (p < 0.0001). On per slice analysis, there was a significant rise in myocardial T1 values with regadenoson for the basal (rest: 924 ± 18 ms; stress: 1014 ± 25 ms; δT1 = 9.7 ± 1.6%), mid-ventricular (rest: 926 ± 24 ms; stress 999 ± 24 ms; δT1 = 7.7 ± 1.2%), and apical (rest: 943 ± 29 ms; stress: 1008 ± 33 ms; δT1 = 7.1 ± 1.4%) slices (p < 0.0001 for all comparisons; Fig. 2A), as well as for individual myocardial segments (Fig. 2B). There were no significant differences in stress T1 reactivity between the basal, mid-ventricular, and apical slices (all p > 0.05).
Table 2.
Rest and regadenoson stress native T1-mapping myocardial tissue profiles. Values are mean ± SD or median [IQR].
| Regadenoson | Healthy controls (n = 10) | CAD patients (n = 25) |
|||
|---|---|---|---|---|---|
| Remote | Ischemic | Infarct | LV blood pool | ||
| Rest T1 (ms) | 931 ± 22 | 949 [915–973] | 964 ± 57 | 1214 ± 115 | 1506 ± 78 |
| Stress T1 (ms) | 1008 ± 24 | 988 [955–1013] | 969 ± 55 | 1213 ± 108 | 1503 ± 75 |
| δT1 (%) | 8.2 ± 0.8 | 4.3 [3.1–6.3] | 0.5 ± 1.6 | −0.8 [−1.9–0.5] | −0.01 ± 1.1 |
Fig. 2.
Normal regadenoson stress T1 responses in healthy controls. Comparison of native myocardial T1 values at rest and during regadenoson stress in healthy controls in standard short-axis slice positions (A) and in the American Heart Association (AHA) 16-segments (B). There are significant increases in T1 values during stress for all slices and myocardial segments. Data presented as mean and standard deviation (error bars). **p < 0.01; ***p < 0.001; ****p < 0.0001; ns = not significant.
3.2. Myocardial stress T1 reactivity to regadenoson in patients with CAD
Twenty-five patients (92% male; 69 [IQR: 55–74] years) with known CAD underwent clinically indicated stress perfusion CMR at 1.5 T, including LV function, regadenoson stress/rest T1-mapping, first-pass perfusion, and LGE. Patients with CAD had normal LV volumes (LVEDVi 86 [IQR: 78–108] ml/m2) with mildly reduced systolic function (LVEF 52 [IQR: 43–64] %) (Table 1). All patients had evidence of myocardial ischemia on first-pass perfusion imaging (referenced to ≥1 angiographically significant coronary stenosis in n = 10 patients) or infarction on LGE with n = 13 (52%) having imaging evidence of both ischemia and infarction. No patients with acute myocardial infarction were included in the study.
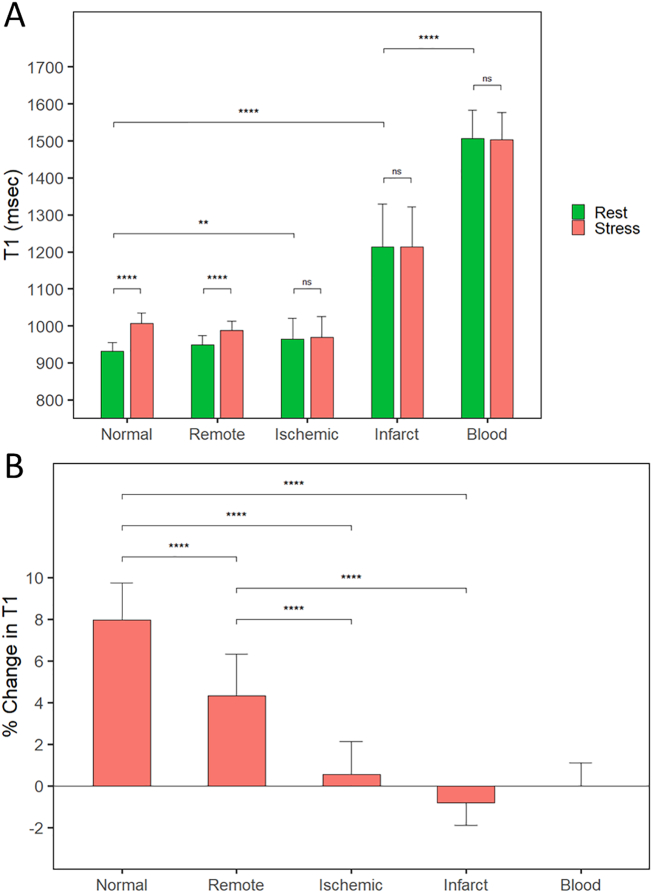
Rest and stress T1 values and stress reactivity (δT1) for patients with CAD are presented in Table 2. At rest, remote myocardial T1 in CAD patients was similar to healthy controls (p = 0.06). Ischemic myocardial T1 was significantly higher than normal myocardium (p < 0.01), but not remote myocardium (p = 0.15). Infarcted myocardium had significantly higher resting T1 than ischemic, remote, and normal myocardium (all p < 0.0001) (Fig. 3A) but shorter than that of LV blood pool (p < 0.0001). There were no significant correlations between infarcted myocardial T1 and ischemic (r = 0.18, p = 0.19) or remote (r = 0.1, p = 0.46) myocardium or with LV blood pool (r = 0.01, p = 0.94).
Fig. 3.
Regadenoson stress T1-mapping distinguishes between different myocardial tissue classes. Myocardial tissue T1 profiles in healthy controls and CAD patients are based on absolute T1 values (A) and the percentage change in T1 between rest and stress (B). Normal and remote myocardium demonstrate significant stress T1 reactivity, whereas areas of myocardial ischemia and infarction have a near-abolished stress T1 response. Data presented as mean and standard deviation or median and IQR (error bars). **p < 0.01; ***p < 0.001; ****p < 0.0001; ns = not significant.
Ischemic and infarcted myocardium both had an abolished stress T1 response compared with normal controls (both p < 0.0001). Remote myocardial stress T1 reactivity was significantly blunted compared to normal controls (p < 0.0001), although still significantly greater than ischemic and infarcted myocardium (both p < 0.0001) (Fig. 3B). For comparison, LV blood pool had little stress T1 reactivity.
4. Discussion
To our knowledge, this is the first study to demonstrate the normal regadenoson stress T1-mapping response, and its ability to distinguish between normal, ischemic, infarcted and remote myocardium, further supporting the findings that regadenoson is a viable alternative to adenosine stress T1-mapping in the assessment of CAD [23].
4.1. Regadenoson stress T1 reactivity in healthy controls
In healthy controls we saw an overall global regadenoson stress T1 response of 8.2 ± 0.8%, with reactivities of 9.7 ± 1.6%, 7.7 ± 1.2%, and 7.1 ± 1.4% for the basal, mid-ventricular, and apical slices respectively. Although not statistically significant, there seemed to be a numerically greater stress T1 response in the basal slice, which is worthy of discussion.
Regadenoson is a more potent in vitro vasodilator than adenosine, with greater selectivity for the coronary circulation in animals [24]. Interestingly, this has been seen to translate to significantly higher stress MBF (ml/min/g) and myocardial perfusion reserve (MPR) with regadenoson compared with adenosine and dipyridamole [25]. However, these effects on MBF and MPR seemed to be driven by the higher heart rate responses achieved with regadenoson rather than by more potent vasodilation [25]. It is possible that the acquisition timings for the basal slice in our study (acquired early within 30 s of regadenoson administration) could have coincided with peak heart rate effects of the drug. This could have contributed to a numerically higher stress T1 response, especially given the known physiological myocardial changes that occur during elevated heart rates and their additional biological effects on myocardial T1 values in vivo (6 ms increase in ShMOLLI T1 for every 10 bpm rise in heart rate) [22]. Other possible confounds may be due to partial-volume effects during stress, as the basal slice may be subject to changes in the extent of longitudinal atrioventricular plane descent with regadenoson through heart rate and ventricular filling. Further work is required to fully ascertain the true relationship of these effects over time, particularly given the known rapid rise and slow decay pharmacokinetic profile of regadenoson [26], as opposed to the more steady-state adenosine infusion.
4.2. Regadenoson stress T1 reactivity for the assessment of CAD
Our findings are consistent with previous adenosine and exercise stress T1-mapping studies in patients with CAD [9,[11], [12], [13],27]. We also saw similar results to Bohnen et al. [23], who used regadenoson stress T1-mapping to assess for the presence of inducible ischemia, although notably their study did not include healthy controls for reference nor investigated the normal regadenoson stress T1 response in healthy individuals. The lack of stress reactivity seen in ischemic myocardium may reflect depletion of coronary vasodilatory reserve and suggests that myocardial blood volume at rest may already be maximally elevated in areas of significant coronary artery stenosis. This is further supported by the observed elevation in native T1 values at rest in these segments. Areas of infarction, which have significantly elevated T1 values at rest due to the presence of fibrosis and myocardial scar, are not expected to display much T1 reactivity, as observed previously [9,[11], [12], [13]].
We also confirm significantly blunted stress T1 reactivity in remote myocardium compared to healthy controls, in line with similar findings from other studies [9,12,23]. This may suggest a degree of microvascular dysfunction, possibly in combination with compensatory resting vasodilation or other pathophysiological factors in chronic CAD not yet elucidated. Blunted stress T1 reactivity has also been seen in patients with type 2 diabetes mellitus in the absence of obstructive CAD, possibly reflecting coronary microvascular dysfunction [28]. Other CMR methods have shown similar blunted responses in absolute myocardial perfusion and stress/rest blood oxygen level dependence in remote myocardium, also postulating whether this may be due to microvascular dysfunction [29]. Further work is required to determine the underlying mechanism behind these blunted physiological responses in remote myocardium and in other cardiovascular pathologies.
4.3. Limitations
This is a proof-of-principle study for regadenoson stress T1-mapping in healthy controls and in patients with CAD referred for a clinical CMR. Having a larger cohort of healthy controls would provide greater confidence in the regadenoson stress T1 response and the SD, as smaller sample sizes may be subject to sampling bias. Our healthy controls were not age-matched to CAD patients, which is a possible confounder given the known reductions in hyperemic flow and myocardial perfusion reserve with increasing age [30]. Additionally, there may be potential sex bias given the majority (92%) of our CAD cohort were male, compared with the controls (50%). This is potentially relevant given higher quantitative perfusion and MBV in female healthy volunteers during adenosine stress [31], although no significant sex differences in the diagnostic performance of CMR have been reported [32]. Further work with larger cohorts is required to study the effects of sex on stress T1 reactivity.
We used stress-perfusion CMR as the non-invasive reference for inducible myocardial ischemia, which is known to have high sensitivity and specificity for CAD [2]. Although not all patients underwent invasive coronary angiography, the fact that stress T1-mapping is able to detect CMR signal changes on perfusion and LGE imaging supports the validity of this technique, as done in previous studies [9,[11], [12], [13],27], including the use of SPECT as a reference [27]. However, although this demonstrates the utility of stress T1-mapping to differentiate myocardial tissue classes, areas of ischemia or infarction were identified from myocardial first-pass perfusion and LGE images as reference. Further work is required to develop a completely gadolinium-free approach for identification of pathology in patients with cardiovascular disease based solely on stress T1 mapping.
5. Conclusions
Healthy controls demonstrate significant stress T1 reactivity during regadenoson stress. Regadenoson stress and rest T1-mapping is a viable alternative to adenosine and exercise for the assessment of CAD and can distinguish between normal, ischemic, infarcted, and remote myocardium.
Authors’ note
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding
VMF, SKP, and SN are supported by the British Heart Foundation. MKB is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/19/65/34692). The authors acknowledge support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre at Oxford University Hospitals NHS Foundation Trust, (University of Oxford, UK). SKP, QZ, and VMF acknowledge the John Fell Oxford University Press Research Fund and the British Heart Foundation Centre of Research Excellence in Oxford (RE/18/3/34214).
Disclosures
SKP has patent authorship rights for U.S. patent 9285446 B2 (systems and methods for Shortened Look Locker Inversion Recovery [Sh-MOLLI] cardiac gated mapping of T1), granted March 15, 2016; all rights transferred to Siemens Medical. KC is employed by company Siemens Medical Solutions USA Inc. The authors report no other relationships that could be construed as conflict of interest.
References
- 1.Rieber J., Huber A., Erhard I. Cardiac magnetic resonance perfusion imaging for the functional assessment of coronary artery disease: a comparison with coronary angiography and fractional flow reserve. Eur. Heart J. 2006;27(12):1465–1471. doi: 10.1093/eurheartj/ehl039. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood J.P., Maredia N., Younger J.F. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379(9814):453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel E., Greenwood J.P., McCann G.P. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N. Engl. J. Med. 2019;380(25):2418–2428. doi: 10.1056/NEJMoa1716734. [DOI] [PubMed] [Google Scholar]
- 4.Kwong R.Y., Ge Y., Steel K. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J. Am. Coll. Cardiol. 2019;74(14):1741–1755. doi: 10.1016/j.jacc.2019.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le D.E., Bin J.P., Coggins M.P. Relation between myocardial oxygen consumption and myocardial blood volume: a study using myocardial contrast echocardiography. J. Am. Soc. Echocardiogr. 2002;15(9):857–863. doi: 10.1067/mje.2002.121275. [DOI] [PubMed] [Google Scholar]
- 6.McCommis K.S., Goldstein T.A., Abendschein D.R. Roles of myocardial blood volume and flow in coronary artery disease: an experimental MRI study at rest and during hyperemia. Eur. Radiol. 2010;20(8):2005–2012. doi: 10.1007/s00330-010-1740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson R.F., Wyche K., Christensen B.V., Zimmer S., Laxson D.D. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82(5):1595–1606. doi: 10.1161/01.cir.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 8.Kramer C.M., Barkhausen J., Bucciarelli-Ducci C. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020;22(1):17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu A., Wijesurendra R.S., Francis J.M. Adenosine stress and rest T1 mapping can differentiate between ischemic, infarcted, remote, and normal myocardium without the need for gadolinium contrast agents. JACC Cardiovasc. Imaging. 2016;9(1):27–36. doi: 10.1016/j.jcmg.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piechnik S.K., Neubauer S., Ferreira V.M. State-of-the-art review: stress T1 mapping—technical considerations, pitfalls and emerging clinical applications. Magn. Reson. Mater. Phys. Biol. Med. 2018;31(1):131–141. doi: 10.1007/s10334-017-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Assen M., van Dijk R., Kuijpers D., Vliegenthart R., Oudkerk M. T1 reactivity as an imaging biomarker in myocardial tissue characterization discriminating normal, ischemic and infarcted myocardium. Int. J. Cardiovasc. Imag. 2019;35(7):1319–1325. doi: 10.1007/s10554-019-01554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah R., Sree Raman K., Walls A. Gadolinium-free cardiovascular magnetic resonance stress T1 mapping in patients with chronic kidney disease. JACC Cardiovasc. Imaging. 2019;12(10):2083–2085. doi: 10.1016/j.jcmg.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Yimcharoen S., Zhang S., Kaolawanich Y., Tanapibunpon P., Krittayaphong R. Clinical assessment of adenosine stress and rest cardiac magnetic resonance T1 mapping for detecting ischemic and infarcted myocardium. Sci. Rep. 2020;10(1):14727. doi: 10.1038/s41598-020-71722-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen K.L., Bandettini W.P., Shanbhag S. Safety and tolerability of regadenoson CMR. Eur. Heart J. Cardiovasc. Imaging. 2014;15(7):753–760. doi: 10.1093/ehjci/jet278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz-Menger J., Bluemke D.A., Bremerich J. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020;22(1):19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piechnik S.K., Ferreira V.M., Dall’Armellina E. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J. Cardiovasc. Magn. Reson. 2010:1269. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira V.M., Piechnik S.K., Dall’Armellina E. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012;14(1):42. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Werys K, Popescu IA, et al. Quality assurance of quantitative cardiac T1-mapping in multicenter clinical trials – a T1 phantom program from the hypertrophic cardiomyopathy registry (HCMR) study. Int. J. Cardiol. 10.1016/j.ijcard.2021.01.026. [DOI] [PMC free article] [PubMed]
- 19.Carapella V., Puchta H., Lukaschuk E. Standardized image post-processing of cardiovascular magnetic resonance T1-mapping reduces variability and improves accuracy and consistency in myocardial tissue characterization. Int. J. Cardiol. 2020:298128–298134. doi: 10.1016/j.ijcard.2019.08.058. [DOI] [PubMed] [Google Scholar]
- 20.Mahmod M., Piechnik S.K., Levelt E. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J. Cardiovasc. Magn. Reson. 2014;16(1):92. doi: 10.1186/s12968-014-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira V.M., Piechnik S.K., Dall’Armellina E. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J. Cardiovasc. Magn. Reson. 2014;16(1):36. doi: 10.1186/1532-429X-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piechnik S.K., Ferreira V.M., Lewandowski A.J. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J. Cardiovasc. Magn. Reson. 2013;15(1):13. doi: 10.1186/1532-429X-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohnen S., Prüßner L., Vettorazzi E. Stress T1-mapping cardiovascular magnetic resonance imaging and inducible myocardial ischemia. Clin. Res. Cardiol. 2019;108(8):909–920. doi: 10.1007/s00392-019-01421-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G., Linke A., Xu X. Comparative profile of vasodilation by CVT-3146, a novel A2A receptor agonist, and adenosine in conscious dogs. J. Pharmacol. Exp. Ther. 2003;307(1):182–189. doi: 10.1124/jpet.103.053306. [DOI] [PubMed] [Google Scholar]
- 25.Vasu S., Bandettini W.P., Hsu L.Y. Regadenoson and adenosine are equivalent vasodilators and are superior than dipyridamole- a study of first pass quantitative perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013;15(1):85. doi: 10.1186/1532-429X-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieu H.D., Shryock J.C., von Mering G.O. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J. Nucl. Cardiol. 2007;14(4):514–520. doi: 10.1016/j.nuclcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Nakamori S., Fahmy A., Jang J. Changes in myocardial native T1 and T2 after exercise stress: a noncontrast CMR pilot study. JACC Cardiovasc. Imaging. 2020;13(3):667–680. doi: 10.1016/j.jcmg.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Levelt E, Piechnik SK, Liu A, et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 19: 10.1186/s12968-017-0397-8 [DOI] [PMC free article] [PubMed]
- 29.Arnold J.R., Karamitsos T.D., Bhamra-Ariza P. Myocardial oxygenation in coronary artery disease. J. Am. Coll. Cardiol. 2012;59(22):1954–1964. doi: 10.1016/j.jacc.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 30.Uren N.G., Camici P.G., Melin J.A. Effect of aging on myocardial perfusion reserve. J. Nucl. Med. 1995;36(11):2032–2036. [PubMed] [Google Scholar]
- 31.Nickander J., Themudo R., Sigfridsson A. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males – an adenosine stress cardiovascular magnetic resonance study. Sci. Rep. 2020;10(1):10380. doi: 10.1038/s41598-020-67196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwood J.P., Motwani M., Maredia N. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the clinical evaluation of magnetic resonance imaging in coronary heart disease (CE-MARC) trial. Circulation. 2014;129(10):1129–1138. doi: 10.1161/CIRCULATIONAHA.112.000071. [DOI] [PubMed] [Google Scholar]