Abstract
Diastereoselective fluorination of N-Boc (R) and (S)-2,2-dimethyl-4-((arylsulfonyl)methyl)oxazolidines, and a previously unknown diastereoselective epimerization at the fluorine-bearing carbon atom alpha to the sulfone was realized. Diastereoselectivities of both reactions were excellent for benzothiazolyl sulfones, allowing access to two enantiomerically pure diastereomers from one chiral precursor. To demonstrate synthetic utility, the benzothiazolyl sulfones were converted to diastereomerically pure (S,S) and (R,S)-benzyl sulfones via sulfinate salts and to amino acids. To understand the diastereoselectivities, DFT analysis was performed.
Graphical Abstract

Fluoroorganic chemistry has been a focus of intense research in the past decade due to the interesting properties of fluoroorganic compounds, making them desirable candidates as pharmaceuticals, materials, and agrochemicals, to name a few.1 Diastereoselective introduction of fluorine atom into a molecule by asymmetric induction via an existing stereogenic center, either as part of the molecule or by a removable chiral auxiliary, has been extensively studied in electrophilic fluorinations alpha to a carbonyl moiety.2a However, diastereoselective C-F bond formation alpha to a sulfone has received scarce attention.2 We have been involved in the electrophilic fluorination of heteroaryl sulfones,3,4 and this prompted our interest in exploring the effect of a stereogenic center on diastereoselectivity of fluorination alpha to a sulfone moiety. As a chiral entity, we chose the 2,2-dimethyloxazolidine unit, which can be further converted to various derivatives, including unnatural amino acids.5 In this context, several sulfone-derived amino acids have shown biological activity, such as S-aryl cysteine S,S-dioxides that inhibit mammalian kynureninase,6a,b and α-amino β-sulfone hydroxamates that were shown to be potent inhibitors of MMP enzymes.6c,d Asymmetric induction by a 2,2-dimethyloxazolidine unit has been explored in reactions of Garner’s aldehyde,7 but there are limited reports of fluorination alpha to its stereogenic center. Deoxofluorination with DAST alpha to the stereogenic center of a 2,2-dimethyloxazolidine moiety has been reported, but only one diastereoisomer could be synthesized via this route.8 Alternatively, a 1:1 diastereoisomeric mixture of fluorinated product was synthesized by desilylation–fluorination of an allylsilane derivative.9
We initially focused on a 1,3-benzothiazol-2-yl sulfone, N-Boc-protected (R)-4-((benzo[d]thiazol-2-ylsulfonyl)methyl)-2,2-dimethyloxazolidine. Synthesis commenced from either (R)-Garner aldehyde or from d-serine (Scheme 1, also see the Supporting Information (SI)), to give the common intermediate (S)-4-(hydroxymethyl)-2,2-dimethyloxazolidine. A reaction of this intermediate with benzo[d]thiazole-2-thiol under Mitsunobu conditions gave sulfide (R)-1a that was oxidized to sulfone (R)-2a with (NH4)2Mo7O24•4H2O/H2O2. Fluorination under heterogeneous conditions previously reported by us3,4 (LDA, solid NFSI addition, PhMe) resulted in a highly diastereoselective reaction, with a product ratio ≥97:3 (yield of 60%, 65% based upon recovered starting material). To unequivocally determine configuration at the new stereogenic center, 3a was crystallized (EtOH) and analyzed crystallographically. This showed the major diastereoisomer to be (R,R)-3a. Similarly, (S)-2a, synthesized from l-serine, upon fluorination gave (S,S)-3a (X-ray structure shown in Scheme 1), along with a trace of (S,R)-3a. In several repetitions, the isolated yields ranged from 56 to 64%, with some recovered starting. The amounts of the minor diastereomer were trace to 3% and barely detectable by 1H NMR.
Scheme 1. Highly Diastereoselective Fluorination of N-Boc- Protected (R)- and (S)-4-((Benzo[d]thiazol-2-ylsulfonyl)methyl)-2,2-dimethyloxazolidine and X-ray Structures of the Productsa.
aThermal ellipsoid are at the 50% probability level. N: blue; S: yellow; O: red; F: green
Next, the influence of base on the diastereomer ratio in the fluorination of (S)-2a was studied (Table 1).
Table 1.
Effect of Base on the Fluorination of (S)-2aa
| entry | base | mono 3a:difluoro product ratiob | (S,S)-3a/(S,R)-3a isomer ratiob |
|---|---|---|---|
| 1 | LDA | no difluoro | ≥97:3c |
| 2 | LHMDS | 74:26 | 88:12d |
| 3 | NaHMDS | 72:28 | 45:55d |
| 4 | MDA | no difluoro | 22:78e |
| 5 | MDA | no difluoro | 16:84f |
| 6 | MDA | no difluoro | 15:85g |
Reactions were performed in PhMe, base (1.2 equiv) was added to (S)-2a at −78 °C, after 12 min solid NFSI was added (1.4 equiv); −78 °C 1.5 h, then rt 1.5 h.
Determined by 19F NMR of the crude reaction mixture.
In several repetitions, the amount of (S,R)-3a ranged from 3% to trace amounts.
Product not isolated.
Isolated yield of 3a was 27% and of (S)-2a was 45%.
To (S)-2a at −78 °C, MDA was added, after 1 h 15 min NFSI was added; −78 °C 1.5 h, then rt 1.5 h. Isolated yield of 3a was 30% and of (S)-2a was 46%.
To (S)-2a at −50 °C, MDA was added, after 1 h 15 min NFSI was added; −50 °C 1.5 h, then rt 1.5 h. Isolated yield of 3a was 27% and of (S)-2a was 42%.
From Table 1, certain observations emerge. Whereas competing difluorination was not observed with LDA and iPr2NMg (MDA), it was seen with LHMDS and NaHMDS. Diastereoselectivity of the fluorination depended on the metal ion, with Li as a counterion favoring (S,S)-3a as the major isomer.
We then assessed whether epimerization at the new stereogenic center could be accomplished under basic conditions. With LDA or n-BuLi, in PhMe, decomposition of 3a occurred. However, when the (R,R)-3a and the (S,S)-3a were independently exposed to NaHMDS in PhMe, a mixture of diastereomers resulted from each; (R,R)-3a/(R,S)-3a = 1:10 and (S,S)-3a/(S,R)-3a = 1:9, respectively. The stereochemistry at the epimerized stereogenic carbon was confirmed by X-ray crystallography (Figure 1, crystals from EtOH).
Figure 1.
Products from the epimerization of (R,R)-3a to (R,S)-3a (top) and of (S,S)-3a to (S,R)-3a (thermal ellipsoids are at the 50% probability level). N: blue; S: yellow; O: red; F: green).
To assess the effect of the aryl moiety on the diastereoselectivity of the fluorination and epimerization, a series of sulfones was synthesized and subjected to fluorination (Scheme 2 and Table 2). Yields of the fluorination step were moderate to excellent.
Scheme 2.
Synthesis and Fluorination of N-Boc Protected (S)-4-((Arylsulfonyl)methyl)-2,2-dimethyloxazolidines
Table 2.
Diastereoselectivitiesa and Yields in the Fluorination and Subsequent Epimerization Reactions
 | |||
|---|---|---|---|
| entry | Het/Ph | fluorination:b F-isomers dr (S,S)-:(S,R)-3 | epimerizationc of (S,S)-3 + (S,R)-3: base, % yield, dr (S,S)-:(S,R)-3 |
| 1 | 2a: BT | ≥97:3 | NaHMDS, 76, 10:90 |
| 2 | 2b: 2-Py | 78:22 | NaHMDS, 87, 6:94 |
| 3 | 2c: 4-Py | 88:12 | NaHMDS, 85, 4:96 |
| 4 | 2d: Ph | 96:4 | NaHMDS, 63, 8:92 |
| 5 | 2e: N-Me-Im | 62:38 | NaHMDS, 83, 17:83 |
| 6 | 2a: BT | ≥97:3 | LHMDS, 89, 2:98 |
| 7 | 2e: N-Me-Im | 62:38 | LHMDS, 84, 10:90 |
| 8 | 2e: N-Me-Im | 17:83d | LHMDS, 81, 4:96 |
| 9 | 2a: BT | 2:98d | NaHMDS, 65, 10:90 |
| 10 | 2e: N-Me-Im | 4:96d | NaHMDS, 76, 15:85 |
Determined by 19F NMR of the crude reaction mixture.
Fluorinations were performed in PhMe, base (1.2 equiv) was added to (S)-2 at −78 °C, after 12 min solid NFSI was added (1.4 equiv); −78 °C 1.5 h, then rt 1.5 h.
Epimerization of mixtures of 3 (dr shown in column 3): base (1.5 equiv) was added to 3 at −78 °C, −78 °C 6.5 h, −78 °C → −20 °C in 20 min, then quench.
Diastereomer mixture of F-isomers was obtained in epimerization.
Fluorination proceeded diastereoselectively in all cases (Table 2, entries 1–7, vide infra). Diastereoselectivity depended on the aryl moiety: excellent for benzothiazolyl (3a) and for phenyl (3d), good for 2- and 4-pyridyl (3b and 3c), and moderate for N-methyl-2-imidazolyl (3e).
Epimerization of fluorinated substrates 3 was also tested by exposure of diastereomeric mixtures, isolated in the fluorination reaction, to NaHMDS in PhMe (Table 2, entries 1–5). Epimerization with NaHMDS gave (S,R)-3 as the major diastereoisomer in all cases, with high diastereoselectivities for 3a–d (entries 1–4 in Table 2). The lowest distereoselectivity was observed for the epimerization of 3e, with a (S,S)-3e:(S,R)-3e dr = 17:83 (entry 5). Because diastereoselectivity in the initial fluorination reaction depended on the counterion, with the highest diastereoselectivity observed with Li bases, we also tested epimerization reactions of 3a and 3e with LHMDS (Table 2, entries 6–8). Indeed, with LHMDS instead of NaHMDS, a higher diastereoselectivity was observed for the epimerization of the (S,S)-3a + (S,R)-3a mixture (dr = ≥97:3), resulting in a (S,S)-3a + (S,R)-3a mixture with dr = 2:98 (compare entry 6 to entry 1). Isomerization of the (S,S)-3e + (S,R)-3e mixture (dr = 62:38) with LHMDS also resulted in an increased diastereoselectivity, as compared to epimerization with NaHMDS (compare entry 7 to entry 5). Repeated epimerization of the (S,S)-3e + (S,R)-3e mixture (dr = 17:83) with LHMDS gave a (S,S)-3e + (S,R)-3e mixture with dr = 4:96 (entry 8). Finally, exposure of epimerized mixture (S,S)-3a + (S,R)-3a (dr = 2:98) to NaHMDS (entry 9) gave a product mixture with dr = 10:90, comparable to the result in entry 1. Similarly, upon exposure to NaHMDS, the (S,S)-3e + (S,R)-3e mixture (dr = 4:96, obtained by epimerization with LHMDS) reverted to a (S,S)-3e + (S,R)-3e mixture with dr = 15:85, (compare entry 10 to entry 5).
To assess whether any loss of chirality occurred at the initial stereogenic center upon fluorination, the four stereoisomers of 3a were isolated and shown to separate on Chiralpak IC-3 (see the SI). Fluorination of (S)-2a afforded crude mixture of (S,S)-3a/(S,R)-3a that was purified by column chromatography. Both diastereomers were collected together in order to prevent any potential loss of enantiomers via self-disproportionation.10 The HPLC trace did not show the presence of (R,R)-3a. Similarly, (R,R)-3a was subjected to epimerization to (R,S)-3a by NaHMDS, and HPLC analysis of the crude mixture did not show presence of (S,R)-3a.
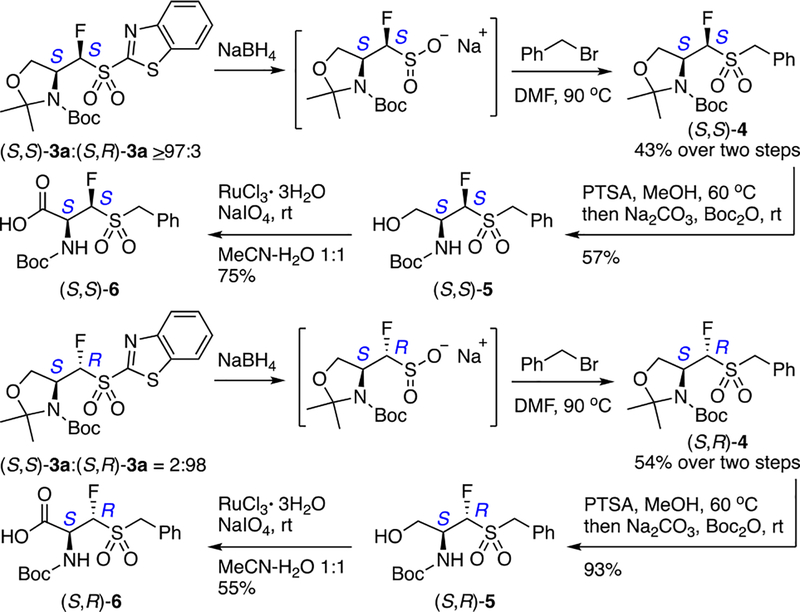
As the highest diastereoselectivity was achieved with benzothiazole-derived sulfones 3a, we wanted to gain some preliminary insight into potential further transformations. Benzothiazole sulfones have been originally described as protected sulfinate salts11 and have gained renewed attention recently.12,13 Therefore, we subjected (S,S)- and (S,R)-3a to reaction with NaBH4, and the crude sulfinate salts were converted to diastereomerically pure sulfones (S,S)-4 and (S,R)-4 (Scheme 3). No chirality erosion at the fluorine-bearing stereogenic center was observed by 19F NMR, indicating the potential use of the isomers of 3a as versatile, chirally-defined, synthetic building blocks. As a proof of principle, sulfones (S,S)- and (S,R)-4 were converted to N-Boc amino acids 6 (see the SI for details).
Scheme 3.
Conversion of 3a to Benzyl Sulfones (S,S)-4 and (S,R)-4 via Sulfinate Salts and to Amino Acids
To gain some insight into the highly diastereoselective fluorination and epimerization reactions, we performed preliminary DFT computations on the Li carbanions derived from (R)-2a, (R,R)-3a and its epimer (R,S)-3a at the B3LYP/6–311++g(2d,2p) level (Figure 2).
Figure 2.
Li carbanions: generated from (R)-2a, upper figures; generated from (R,R)-3a and its epimer (R,S)-3a, lower figures. Li: purple; N: blue; S: yellow; O: red; F: green.
The difference in stability of the two Li carbanions, I and II, generated by proton abstraction from (R)-2a is 19.3 kcal/mol, whereas that for carbanion III generated by proton abstraction from (R,R)-3a and IV generated from (R,S)-3a is 19.1 kcal/mol. Models of more stable carbanions I and III show close proximity of Li to the benzothiazole nitrogen atom, the carbanion, and the carbonyl oxygen atom of the Boc group (Figure 2). Intramolecular chelation of Li in carbanions alpha to a sulfone functionality, containing a preexisting stereogenic center, has previously been shown to result in diastereoselective alkylations by favoring one mode of attack via a less-hindered approach of the electrophile.14–17 In the present case, it is plausible that electrophile approach to the more stable carbanion I, with lithium chelated by the benzothiazole nitrogen atom and the Boc oxygen atom, is not favored from the sterically congested, concave side, and attack occurs preferentially from a less-hindered side, anti to large benzothiazole moiety and syn to sulfone oxygen atoms.18 This would explain the highly diastereoselective fluorination. Isomerization of the newly formed fluorinated stereogenic center in (R,R)-3a could plausibly be rationalized in a similar manner. Capture of a proton by carbanion III generated from (R,R)-3a (showing Li chelation to the benzothiazole nitrogen and Boc oxygen atoms, Figure 2), from a less-hindered side, would result in inversion at the fluorine-bearing stereogenic center, to give (R,S)-3a.19
In summary, metalation–electrophilic fluorination alpha to a sulfone moiety in chiral N-Boc protected (R)- and (S)-2,2-dimethyl-4-((arylsulfonyl)methyl)oxazolidines proceeded with good to excellent diastereoselectivity. Diastereoselectivities depend on the base used and on the aryl/heteroaryl moiety and were best with LDA and benzothiazolyl-derived sulfones. Previously unknown base-induced epimerization at the fluorine-bearing stereogenic center alpha to a sulfone proceeded with good to excellent diastereoselectivity and was the highest for benzothiazolyl sulfones. This chemistry allows for stereodiversity in that from a single chiral precursor either of the two fluorinated, enantiomerically pure diastereomers, can be synthesized. No chirality scrambling occurs at the original stereocenter in the fluorination or epimerization steps. Utility of these chiral, benzothiazole sulfone building blocks was shown by their conversion to benzyl sulfones, via intermediate sulfinate salts, without erosion of chirality at the fluorine-bearing carbon atom. Diastereomeric benzyl sulfones were further converted to N-Boc protected amino acids. On the basis of computational models of the carbanions derived from benzothiazolyl sulfones, chelation of Li by benzothiazole N and the Boc O atoms could plausibly account for the diastereoselectivities observed in metalation–fluorination and in the base-induced epimerization, by approach of the electrophile from a less-hindered side.
Supplementary Material
ACKNOWLEDGMENT
Support of this work by NSF Grant No. CHE-1565754 and PSC CUNY awards to B.Z. is gratefully acknowledged. Infrastructural support at CCNY was provided by NIH Grant No. G12MD007603 from the NIMHD. We thank Dr. Andrew Poss (Honeywell) for a sample of NFSI and Dr. Michelle Neary (The CUNY X-ray Facility, Hunter College) for X-ray analysis of (S,S)-3a. We are grateful to the CUNY HPCC at the CSI funded by NSF Grant Nos. CNS-0958379, CNS-0855217, and ACI 1126113.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b01358.
Experimental details and compound data, and 1H and 13C spectra (PDF). CCDC: 1586437 (R,R-3a), 1586438 (R,S-3a), 1586439 (S,R-3a), 1586440 (S,S-3a).
REFERENCES
- (1).(a) Kirsch P Modern Fluoroorganic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2004. [Google Scholar]; (b) Laali KK, Ed. Modern Organofluorine Chemistry–Synthetic Aspects; Bentham Science Publishers: San Francisco, 2006; Vol. 2. [Google Scholar]; (c) Bégué J-P; Bonnet-Delpon D Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons, Inc.: Hoboken, NJ, 2008. [Google Scholar]; (d) O’Hagan D Chem. Soc. Rev 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]; (e) Liang T; Neumann CN; Ritter T Angew. Chem., Int. Ed 2013, 52, 8214–8264. [DOI] [PubMed] [Google Scholar]; (f) Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA J. Med. Chem 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]; (g) Thematic issue on organofluorine chemistry: Chem. Rev 2015, 115, 563–1306.25627818 [Google Scholar]
- (2).(a) Ma J-A; Cahard D Chem. Rev 2008, 108, PR1–PR43. [DOI] [PubMed] [Google Scholar]; (b) Iwasaki Y; Shimizu M; Hirosawa T; Yamada S Tetrahedron Lett 1996, 37, 6753–6754. [Google Scholar]
- (3).Zajc B; Kumar R Synthesis 2010, 1822–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Recent examples:; (a) Kumar R; Pradhan P; Zajc B Chem. Commun 2011, 47, 3891–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mandal SK; Ghosh AK; Kumar R; Zajc B Org. Biomol. Chem 2012, 10, 3164–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kumar R; Zajc BJ Org. Chem 2012, 77, 8417–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kumar R; Singh G; Todaro LJ; Yang L; Zajc B Org. Biomol. Chem 2015, 13, 1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Banerjee S; Sinha S; Pradhan P; Caruso A; Liebowitz D; Parrish D; Rossi M; Zajc BJ Org. Chem 2016, 81, 3983–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Qiu X-L; Meng W-D; Qing F-L Tetrahedron 2004, 60, 6711–6745. [Google Scholar]; (b) Qiu X-L; Qing F-L Eur. J. Org. Chem 2011, 2011, 3261–3278. [Google Scholar]
- (6).(a) Dua RK; Taylor EW; Phillips RS J. Am. Chem. Soc 1993, 115, 1264–1270. [Google Scholar]; (b) Drysdale MJ; Reinhard JF Bioorg. Med. Chem. Lett 1998, 8, 133–138. [DOI] [PubMed] [Google Scholar]; (c) Becker DP; DeCrescenzo G; Freskos J; Getman DP; Hockerman SL; Li M; Mehta P; Munie GE; Swearingen C Bioorg. Med. Chem. Lett 2001, 11, 2723–2725. [DOI] [PubMed] [Google Scholar]; (d) Becker DP; Barta TE; Bedell L; DeCrescenzo G; Freskos J; Getman DP; Hockerman SL; Li M; Mehta P; Mischke B; Munie GE; Swearingen C; Villamil CI Bioorg. Med. Chem. Lett 2001, 11, 2719–2722. [DOI] [PubMed] [Google Scholar]
- (7).(a) Liang X; Andersch J; Bols MJ Chem. Soc., Perkin Trans. I 2001, 2136–2157. [Google Scholar]; (b) Passiniemi M; Koskinen AM P. Beilstein J. Org. Chem. 2013, 9, 2641–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).De Jonghe S; Van Overmeire I; Van Calenbergh S; Hendrix C; Busson R; De Keukeleire D; Herdewijn P Eur. J. Org. Chem 2000, 2000, 3177–3183. [Google Scholar]
- (9).Teare H; Huguet F; Tredwell M; Thibaudeau S; Luthra S; Gouverneur V ARKIVOC, 2007, (x), 232–244. [Google Scholar]
- (10).Soloshonok VA Angew. Chem. Int. Ed 2006, 45, 766–769. [DOI] [PubMed] [Google Scholar]
- (11).Ueno Y; Kojima A; Okawara M Chem. Lett 1984, 2125–2128. [Google Scholar]
- (12).Aziz J; Messaoudi S; Alami M; Hamze A Org. Biomol. Chem 2014, 12, 9743–9759. [DOI] [PubMed] [Google Scholar]
- (13).(a) Prakash GKS; Ni C; Wang F; Hu J; Olah GA Angew. Chem. Int. Ed 2011, 50, 2559–2563. [DOI] [PubMed] [Google Scholar]; (b) Zhou Q; Ruffoni A; Gianatassio R; Fujiwara Y; Sella E; Shabat D; Baran PS Angew. Chem. Int. Ed 2013, 52, 3949–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He Z; Tan P; Ni C; Hu J Org. Lett 2015, 17, 1838–1841. [DOI] [PubMed] [Google Scholar]; (d) Day JJ; Neill DL; Xu S; Xian M Org. Lett 2017, 19, 3819–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dehmlow EV; Pieper S; Neumann B; Stammler H-G Liebigs Ann./Recueil 1997, 1997, 1013–1018. [Google Scholar]
- (15).Enders D; Müller SF; Raabe G; Runsink J Eur. J. Org. Chem 2000, 2000, 879–892. [Google Scholar]
- (16).Gais H-J In Organosulfur Chemistry in Asymmetric Synthesis; Toru T; Bolm C, Eds.; Wiley: Weinheim, 2008, pp. 375–398. [Google Scholar]
- (17).Hellmann G; Hack A; Thiemermann E; Luche O; Raabe G; Gais H-J Chem. Eur. J 2013, 19, 3869–3897. [DOI] [PubMed] [Google Scholar]
- (18).Approach of electrophile anti to a bulky substituent and syn to sulfone oxygens in carbanions alpha to sulfone has been reported, see reference 16 and 17.
- (19).Substitution reactions of organolithiums with electrophiles were reported to proceed more commonly with retention of configuration; however, inversion has been observed as well.; (a) Clayden J Stereoselective and Stereospecific Substitution Reactions of Organolithiums. In Organolithiums: Selectivity for Synthesis; Baldwin JE; Williams RM, Eds.; Tetrahedron Organic Chemistry Series Vol 23; Elsevier Science Ltd: Oxford, UK, 2002; pp. 241–271. [Google Scholar]; (b) Gawley RE Tetrahedron Lett 1999, 40, 4297–4300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.