Abstract
A novel coronaravirus, identified as SARS-CoV-2, spread throughout the world in 2020. The COVID-19 pandemic has led to many discoveries and clinical manifestations. A young patient is presented with new, self-resolving neutropenia presenting weeks after a prolonged hospital stay for COVID-19 pneumonia. Workup included analysis for underlying infection, nutritional abnormalities, malignancy, medication and toxin exposure, all of which were negative. From 2020 to the present, few reports have described neutropenia associated with a recent COVID-19 infection. In particular, no reports have described a delayed presentation of neutropenia. The authors would like to propose that the significant inflammatory response associated with COVID-19 is likely what led to this patient’s postviral neutropenia. Furthermore, in young healthy patients, bone marrow biopsy may be deferred and a watchful-waiting approach may be taken to assess for neutropenia resolution.
Keywords: COVID-19, haematology (incl blood transfusion), medical management, haematology (drugs and medicines), infectious diseases
Background
Neutropenia has a variety of aetiologies, including medications, malignancy and infection, and its association with SARS-CoV-2 has not been clearly described in recent publications. After an extensive evaluation for other potential causes, we propose a case of self-resolving neutropenia after recovering from COVID-19. We suggest that the significant inflammatory response resulting from the viral infection may have precipitated the bone marrow suppression seen in this patient. A literature review has found that there have been cases of neutropenia seen in acute COVID-19 infection, usually related to immunosuppression, but no clear cases of neutropenia initially presenting several weeks after the resolved infection.1 2
Case presentation
A 22-year-old male US Army soldier of Native American descent presented to the emergency room in Hawaii with worsening shortness of breath for several days approximately 2 weeks after discharge from a hospital in Arizona for a SARS-CoV-2-related pneumonia. He spent 17 days in the hospital, where he received treatment with a 5-day course of intravenous remdesivir (200 mg loading dose, followed by 100 mg every following day) and a 10-day course of 6 mg intravenous dexamethasone per day. During the prior hospitalisation, he had been on several days of bilevel positive airway pressure for acute respiratory distress syndrome (ARDS), was eventually transitioned to high-flow nasal cannula and, before his discharge, was maintaining oxygen saturations of >95% on room air. At the time of his current presentation to the emergency room, he was afebrile, maintaining oxygen saturation on room air and had a normal physical examination, but a routine complete blood count (CBC) found that he was profoundly neutropenic, with an absolute neutrophil count of 450/mL (1500–7500/mL). The patient’s lymphocytes were also noted to be low at 650/mL (100–480/mL). All other cell lines were normal, and he was admitted to the hospital for observation and further evaluation of his neutropenia.
Investigations
While hospitalised, the patient underwent an extensive workup for his new neutropenia. There were no malignancies noted in his family history. He had all routine childhood vaccinations, denied any illicit drug use, and was not on any medications or supplements. Recent travel included his hospitalisation in Arizona, where he had gone elk hunting after discharge. Chart review found that his CBCs had always been normal in the years before his current presentation, and his CBC at the time of discharge from his recent hospitalisation was normal. One week before his current admission, he had presented to the emergency room for shortness of breath with normal laboratory and physical examination findings, including a normal neutrophil count of 2750/mL.
To find any possible pathology for his new neutropenia, a targeted workup included analysis for underlying malignancy, infections, toxins, autoimmune conditions and nutritional deficiencies. On admission, a chest X-ray found persistent peripheral airspace opacities that were improving from his recent COVID-19 infection. Initial labs, including renal function, electrolytes, coagulation profile, procalcitonin and hepatic function, were normal. Inflammatory markers were elevated, including lactate dehydrogenase at 281 U/L (121–247 U/L), C reactive protein (CRP) at 2.14 mg/dL (0–0.5 mg/dL) and erythrocyte sedimentation (ESR) rate at 24 mm/hour (0–10 mm/hour). However, ESR and CRP were both noted to be trending downward from his recent COVID-19 pneumonia-associated admission. Toxin workup included a negative urine drug screen and a normal copper level of 96 μg/dL (63–121 μg/dL). Autoimmune workup included a negative antinuclear antibody. Folate and B12 were normal.
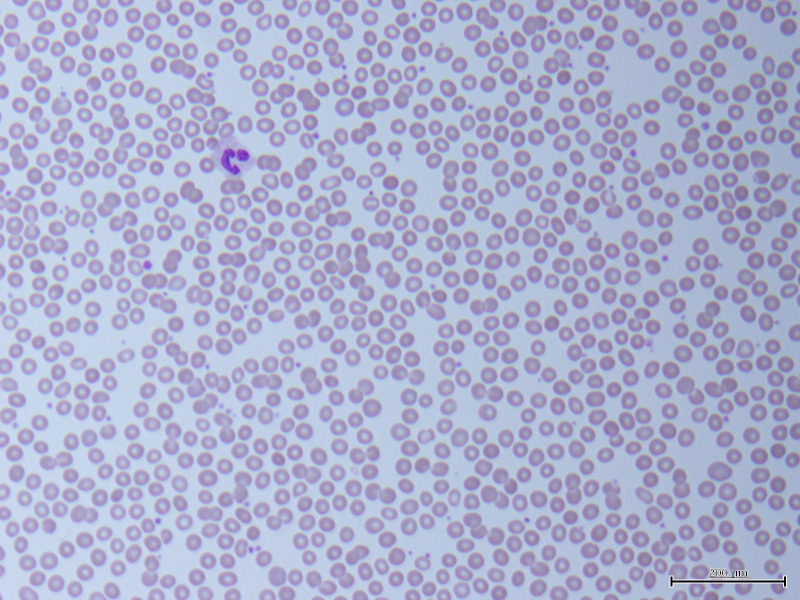
A peripheral blood smear was remarkable for severe neutropenia (figure 1). No obvious abnormal cells or any evidence of solid or haematolymphoid neoplasia was seen. A reticulocyte panel found that he had a mildly elevated bone marrow response with a reticulocyte percentage of 2.54% (0.5%–2.3%). Flow cytometry to rule out acute leukaemias, lymphoma, lymphoproliferative disorders and plasma cell dyscrasias demonstrated no specific immunophenotypical abnormalities and no evidence of B-cell or T-cell lymphoproliferative disorders. Further malignancy workup included a CT of the chest without contrast, which found evidence of mosaic attenuation in bilateral lungs consistent with resolving COVID-19 pneumonia but no suspicious nodules or masses.
Figure 1.
Peripheral blood smear (×400) demonstrating rare neutrophils, suggestive of severe neutropenia. Red blood cells appeared normochromatic, normocytic and without anisopoikilocytosis, variant morphologies or changes to central pallor. Platelets presented in normal numbers with rare giant forms.
The infectious workup included SARS-CoV-2 PCR obtained from the nasopharynx, which continued to be positive, while other respiratory viruses including influenza A, influenza B, respiratory syncytial virus PCR and infectious mononucleosis (heterophile antibody) were negative. Blood and urine cultures were negative. Epstein-Barr virus (EBV), cytomegalovirus (CMV) DNA, HIV 1/2 antibodies, HIV p24 antigen and QuantiFERON tuberculosis screening were all negative. Fungal analysis, including Aspergillus galactomannan antigen, Brucella IgG, and Coccidioides IgG and IgM were tested due to geographical exposure and elk hunting in his recent history, all of which were negative.
Differential diagnosis
When evaluating new neutropenia, potential aetiologies include infection, medication or toxin exposure, autoimmune or inflammatory disease destructive processes, nutritional deficiencies, congenital predisposition or underlying malignancy. In our young patient, many of these aetiologies were eliminated, with laboratory and radiology imaging analysis being relatively unremarkable. Infectious agents were assessed with blood and urine cultures, as well as serum markers for possible bacterial, viral and fungal culprits for his neutropenia. Malignancy was ruled out by careful physical examination, collection of history, review of bodily systems and CT chest imaging. Medication review eliminated any obvious causes for his neutropenia due to the time course of his presentation. The dexamethasone he received for his recent COVID-19 infection, more than 2 weeks before his current hospital admission, would have caused a demargination of his neutrophils, leading to an increased neutrophil count rather than neutropenia.3 Remdesivir has been studied in SARS-CoV-2-infected patients, with no adverse effects of neutropenia directly correlating to the drug being described in the literature.4 5 After an extensive workup, his very recent COVID-19 pneumonia was determined to have been the most likely the culprit for his neutropenia.
Treatment
While hospitalised, he was monitored with daily CBCs as his neutrophils continued to downtrend with the nadir being 190/mcL. Haematology and oncology assessed the patient and chose a watchful-waiting approach before performing a bone marrow biopsy due to his asymptomatic and haemodynamically stable state. A careful discussion regarding antibiotic and antifungal prophylaxis took place with the assistance of infectious disease, and it was decided not to discharge the patient with prophylaxis to avoid diagnostic confliction if he were to present with fever or infectious symptoms in the days after discharge. The patient’s own choice to avoid antibiotics and antifungals was given strong consideration in this decision. He did not receive any medications while hospitalised. He remained on neutropenic precautions and was discharged with appropriate follow-up, along with strict return precautions for any low-grade fevers, rash or infectious symptoms.
Outcome and follow-up
One week later, the patient returned to the clinic for follow-up, continuing to remain asymptomatic and afebrile. He had remained in his home isolated from other individuals and following strict neutropenic precautions provided to him from the hospital. His CBC in the clinic demonstrated normal neutrophils at 2810/mcL, but borderline residual lymphocytopenia at 930/mcL. He did not receive granulocyte colony-stimulating factor (G-CSF) to produce the neutropenic response. He did not require any antibacterial or antifungal agents as prophylaxis or treatment during this period of time. No bone marrow biopsy was collected, and to date, his CBCs remain normal, and his post-COVID-19 symptoms have continued to improve.
Discussion
The COVID-19 pandemic is a relatively new disease process with remarkable findings occurring daily. To date, we have been able to find rare case reports and no case series describing residual and self-resolving neutropenia related to the virus. Literature review on recent COVID-19 infections describes other cell lines decreasing, including platelets, lymphocytes and general leucopenia.4 A study of 1099 patients in China with SARS-CoV-2 infection found that almost 82.1% had lymphopenia; 36.2% had thrombocytopenia; and 33.7% had leucopenia.6 7 Other studies found that neutropenia can also be found in the initial infection, but no further discussion or case reports describing new neutropenia weeks after resolution of the viral infection.8 One case report that demonstrated neutropenia after COVID-19 infection approached the evaluation of their patient using a method similar to ours, with the same conclusion of the viral infection being the ultimate cause. Unlike our patient, they chose to give their patient G-CSF to improve the neutrophil count.9 We chose to wait to administer G-CSF to see if the patient would produce the appropriate neutrophil response without intervention, which he successfully did. This was done due to the high risk of potentially causing severe complications, to include potential cytokine storm and death.1 Review of current articles at the time of admission included the analysis of cases that had used G-CSF for neutropenia recovery from COVID-10 infection, with fatal complications.10 This was also taken into account when making the decision to hold off on an immediate treatment possibility for his neutropenia.
The profound inflammatory response caused by COVID-19 is the most classic association of the infection and causes significant immune damage to the lungs and other organs, such as the bone marrow.6 11 A potential cause for bone marrow suppression includes the ability of coronaviruses to infect bone marrow cells, resulting in abnormal haematopoiesis and laboratory abnormalities.6 12 Careful monitoring for infectious symptoms rather than reactive treatment may be appropriate in certain situations, such as the young, healthy patient presented in this case.
Viral aetiologies are a relatively common cause of neutropenia, likely related to bone marrow suppression or peripheral destruction. The most common viruses associated with neutropenia tend to be EBV, CMV, viral hepatitis, influenza viruses, measles and parvovirus species.13–15 It has been postulated that in certain viral-associated bone marrow suppression, the host’s immune response may play a larger role in the cell lines suppressed than the actual viral cytotoxicity.15 In the patient presented, he had a significant inflammatory response, manifesting itself as ARDS, along with his persistently elevated inflammatory markers that were trending downward when he presented weeks after his prolonged COVID-19 hospitalisation. This inflammatory response may have been responsible for the delayed, yet transient, neutropenia.
We propose that like other viral infections, neutropenia can be a complication of COVID-19 infection. A watchful-waiting approach for resolution may be appropriate in certain situations, especially in young, asymptomatic patients, before performing a bone marrow biopsy.
Learning points.
Neutropenia is a complication in many viral illnesses.
COVID-19 may be associated with transient and self-resolving neutropenia, including delayed presentations of neutropenia following resolving infection.
Evaluation approaches for neutropenia should include careful assessment for nutritional deficiencies, autoimmune disorders, inflammatory diseases, infections, malignancy, medication and toxin exposure.
Neutropenia following COVID-19 infection may be due to the significant inflammatory response caused by the viral infection causing bone marrow suppression.
A watchful-waiting approach for resolution of neutropenia may be appropriate with young, healthy patients after careful evaluation for any underlying causes before performing a bone marrow biopsy.
Acknowledgments
The authors would like to thank Dr. Salvatore Mignano in the Tripler Army Medical Center Pathology Department for his assistance in obtaining and interpreting peripheral smear imaging.
Footnotes
Contributors: All authors participated in the writing of this manuscript. VMFM wrote the majority of the manuscript, edited, performed literature review and was active in caring for the patient. JO edited the manuscript, assisted with background research and cared for the patient. VM and JO obtained both verbal and virtual written consent from the patient. JM assisted with background research, writing the manuscript and editing. JR assisted with editing the final manuscript and background research.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hernandez JM, Quarles R, Lakshmi S, et al. Pancytopenia and profound neutropenia as a sequela of severe SARS-CoV-2 infection (COVID-19) with concern for bone marrow involvement. Open Forum Infect Dis 2021;8:ofab017. 10.1093/ofid/ofab017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Issa N, Lacassin F, Camou F. First case of persistent pancytopenia associated with SARS-CoV-2 bone marrow infiltration in an immunocompromised patient. Ann Oncol 2020;31:1418–9. 10.1016/j.annonc.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa M, Terashima T, D'yachkova Y, et al. Glucocorticoid-Induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation 1998;98:2307–13. 10.1161/01.cir.98.21.2307 [DOI] [PubMed] [Google Scholar]
- 4.Bishara D, Kalafatis C, Taylor D. Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents. Ther Adv Psychopharmacol 2020;10:204512532093530. 10.1177/2045125320935306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy 2020;40:416–37. 10.1002/phar.2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol 2020;99:1205–8. 10.1007/s00277-020-04019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galbraith MD, Kinning KT, Sullivan KD, et al. Seroconversion stages COVID19 into distinct pathophysiological states. medRxiv 2020. 10.1101/2020.12.05.20244442. [Epub ahead of print: 07 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Pereira P, Iturrate I, de La Cámara R, et al. Can COVID-19 cause severe neutropenia? Clin Case Rep 2020;8:3349–51. 10.1002/ccr3.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taha M, Sharma A, Soubani A. Clinical deterioration during neutropenia recovery after G-CSF therapy in patient with COVID-19. Respir Med Case Rep 2020;31:101231. 10.1016/j.rmcr.2020.101231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 2020;9:221–36. 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med 2000;172:248–52. 10.1136/ewjm.172.4.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baranski B, Young N. Hematologic consequences of viral infections. Hematol Oncol Clin North Am 1987;1:167–83. 10.1016/S0889-8588(18)30671-3 [DOI] [PubMed] [Google Scholar]
- 15.Kurtzman G, Young N. Viruses and bone marrow failure. Baillieres Clin Haematol 1989;2:51–67. 10.1016/S0950-3536(89)80007-1 [DOI] [PubMed] [Google Scholar]