Abstract
This report documents a rare case of COVID-19-associated constrictive pericarditis (CP) in the setting of a recent COVID-19 infection. A 55-year-old man with a history of hypertension and gout presented with acute hypoxic respiratory failure and was diagnosed with COVID-19 pneumonia with progression to acute respiratory distress syndrome. His hospital course was complicated by a large pericardial effusion; an emergent bedside transthoracic echocardiography was concerning for cardiac tamponade, so pericardiocentesis was performed. A workup with cardiac magnetic resonance imaging showed changes consistent with a diagnosis of CP. Viral and idiopathic aetiologies are the most common cause of CP in the developed world, with COVID-19 now a proposed predisposing viral illness. The virus induces systemic inflammation and pericardial changes that can lead to CP physiology. Imaging modalities including echocardiogram and cardiac magnetic resonance play an integral role in confirming the diagnosis.
Keywords: cardiovascular medicine, pericardial disease, COVID-19, cardiovascular system, infectious diseases
Background
Coronavirus disease 2019 was declared a pandemic in March 2020, and as of January 2021, the WHO has confirmed 88 million reported cases and over 1.9 million deaths globally from the start of the pandemic.1 While pre-existing cardiovascular diseases are linked with higher mortality in those who contract the virus, cardiologists discovered that COVID-19 can lead to the development of new diagnoses in individuals without pre-existing disease. Thus far, a wide range of cardiovascular complications have been documented including myocardial injury, heart failure, arrhythmias, acute pericarditis and cardiac tamponade.2 3 This case represents a rarely documented presentation of constrictive pericard (CP) found in an individual recently infected with COVID-19. This case contributes to the growing evidence and extent of cardiovascular injury precipitated by a known COVID-19 infection.
Case presentation
A 55-year-old man with a previous medical history of hypertension, gout and obesity presented with COVID-19 pneumonia, which progressed to SARS requiring ventilatory support on day 2 of hospitalisation. He was started on broad-spectrum antibiotics, including vancomycin and piperacillin/tazobactam for possible underlying bacterial pneumonia. He also received tocilizumab, ramdesivir and convalescent plasma on days 3, 6 and 8, respectively. He continued to be ventilator dependent and underwent tracheostomy on day 30. He was eventually weaned off ventilatory support on day 44 and decannulated on day 74.
His prolonged hospital course was complicated by acute kidney injury requiring continuous veno-venous hemofiltration, a catheter-associated internal jugular vein clot, a ventilator-associated pneumonia, Enterococcus faecalis bacteremia, Clostridium difficile colitis and multiple decubitus ulcers, including a large stage IV sacral ulcer and sacral osteomyelitis requiring operative debridement and a 6-week course of antibiotics.
On day 128 of admission, he developed significant nausea, vomiting, diarrhoea and was tachycardic with a heart rate around 130 beats/min. He also endorsed abdominal distension and pain but denied chest discomfort, palpitations, dyspnoea or orthopnea.
Investigations
His white cell count was elevated at 14.6×109/L with 81% neutrophils. He had a haemoglobin of 86 g/L (baseline approximately 120) and a thrombocytosis of 527×109/L. A serum chemistry was within normal limits and a lactate was elevated at 3.0 mmol/L. Inflammatory markers were elevated with a C reactive protein of 180 mg/L and erythrocyte sedimentation rate of 100 mm/hour. He had an upper respiratory tract viral panel including SARS-CoV-2 PCR, and a repeat stool Clostridium difficile PCR, all of which came back negative. An ECG showed normal sinus rhythm with low voltage and nonspecific T-wave changes in the inferior leads.
An abdominal CT was done due to concern for ileus in the setting of recent Clostridium difficile colitis. The image did not show obstruction but incidentally revealed a moderate to large pericardial effusion with mass effect on the heart chambers and enhancement of the pericardium concerning for pericarditis with cardiac tamponade physiology (figure 1).
Figure 1.
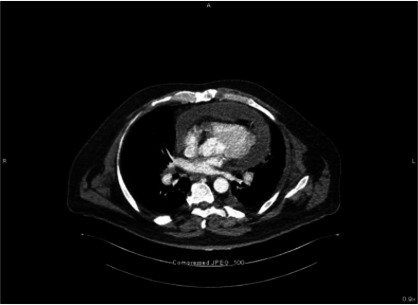
Axial view of CT image demonstrating pericardial effusion.
An emergent bedside transthoracic echocardiography (TTE) showed circumferential pericardial fluid with right ventricular collapse, also concerning for tamponade. He underwent emergent pericardiocentesis with drain placement. A total of 750cc of serosanguineous fluid was removed immediately and around 20cc over the next 24 hours. A repeat TTE showed a trivial effusion with persistent ventricular interdependence. Tissue Doppler studies were not available.
The pericardial fluid analysis was significant for a white blood cell count of 0.988× 109/L (neutrophils 75%, lymphocytes 7%, eosinophils 1%), a red blood cell count of 121×109/L, pH 7.20, a lactate dehydrogenase level of 1223 IU/L, glucose 118 mg/dL, amylase <30 U/L, and total protein 6.1 g/dL. The fluid culture, acid-fast bacilli stain and culture came back negative. Fluid cytology showed heavy infiltrate of polymorphonuclear leukocytes entrapped in fibrin, some lymphocytes and blood consistent with an acute inflammatory process. No malignant cells were seen. Fluid COVID-PCR is not available at our centre.
Cardiac magnetic resonance (CMR) was performed to evaluate for anatomic and physiologic signs of pericardial disease. It revealed a small pericardial effusion, with pericardial circumferential thickening (measuring 4 mm in diameter) as well as late gadolinium enhancement of the pericardium. There was no late gadolinium enhancement of the myocardium. There was paradoxical septal motion at rest and with deep inspiration as well as right to left septal displacement with deep inspiration consistent with ventricular interdependence. However, his left ventricle size and function were normal with an ejection fraction of 57% (figure 2).
Figure 2.
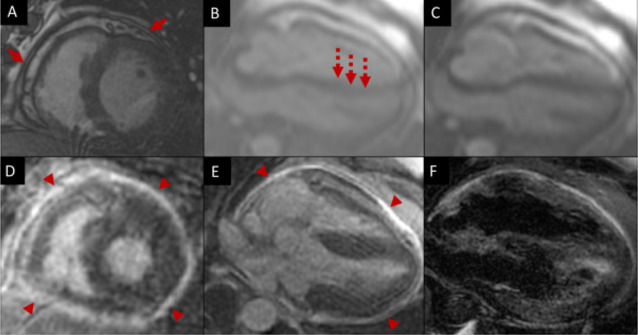
Cardiac magnetic resonance of a 55-year-old man with constrictive pericarditis after COVID-19 illness. (A) MR-echo sequence of left ventricle in short axis with mild left ventricular hypertrophy, small pericardial effusion and pericardial thickening (red arrows). (B and C) MR-echo sequence of the four-chamber with end-diastolic septal flattening (B) with inspiration consistent with ventricular interdependence (dashed arrow). The septum normalises in (C). (D and E) Late gadolinium enhancement imaging of the left ventricular short axis (D) and four-chamber.(E) with marked circumferential pericardial enhancement (arrow heads) consistent with pericarditis. (F) Double inversion recovery with fat saturation showing small pericardial fluid.
Treatment
After therapeutic pericardiocentesis, the patient was started on medical therapy for CP. The treatment recommendation was for high-dose Ibuprofen and Colchicine for 2–3 months before repeating an echocardiogram to reassess for effusion and cardiac function as an outpatient.
Outcome and follow-up
After treatment, the patient remains stable on medical therapy. He continues to be asymptomatic, and his physical examination is largely unremarkable. He completed his stay at an acute rehabilitation unit before returning home.
Discussion
Studies demonstrate widespread immune system activation and cytokine storm with elevated inflammatory biomarkers in individuals with severe COVID-19 infections.2 The virus enters cells by hijacking and tightly binding to the human angiotensin-converting enzyme 2 receptor, a membrane protein expressed in tissues of the lung, heart, kidney and intestine. This mechanism, in addition to the inflammatory response, links the virus to known downstream cardiovascular complications.4 Whether pericarditis in patients infected with COVID-19 results from widespread immune system activation, or is the result of direct viral injury leading to local inflammation, is uncertain. One theory is that persistent pericardial inflammation results from the cytotoxic effect of T and B cell proliferation when the virus’s genetic material embeds within the cardiac tissues—a process called molecular mimicry.5 García-Cruz et al demonstrated viral particles within the pericardial fluids using electronic microscope.6 While there is no validated SARS-CoV2 PCR test for pericardial fluid, there is documentation of pericardial fluid testing positive for SARS-COV2 PCR.7–12
CP can result from any inflammatory process, often in the setting of pericardial injury.5 In developed countries, the most common aetiologies are idiopathic or viral (42%–49%), postsurgical (11%–37%), postradiation therapy (9%–31%), connective tissue and postinfectious (3%–7%), while malignancy, trauma, drugs, asbestosis, sarcoid and uremia account for <10%. In developing countries, tuberculosis remains the primary cause of CP.13 Risk of progression to more severe disease is lowest in viral or idiopathic pericarditis, compared with the other causes.5
This case report is a very rare documentation of CP resulting from a COVID-19 infection. Previously published case reports included post-COVID pericarditis and tamponade, which can present similarly, but are distinguished by their physiology. Most cases present acutely at the time of diagnosis and up to 2 weeks after the first positive COVID-19 test.3 7–10 14–19 There are no clear common risk factors for those who developed symptomatic acute pericarditis. Some had cardiovascular disease including recent myocardial infarctions, but many were healthy or had controlled chronic diseases.6–8 11
While there are only a handful of case reports describing COVID-19-associated pericarditis or pericardial effusion, there are likely more subclinical or missed cases. In some of the early studies from China, pericardial effusion was found in 4.8%–6% of patients admitted for COVID-19 and up to 16% among those with severe disease.20 21 In a study of 26 patients who underwent CMR imaging for cardiac complaints, 7 patients were found to have pericardial effusions.22 In another prospective observational cohort study, Puntmann et al studied 100 unselected patients with no specific indication for CMR at least 2 weeks after initial diagnosis with severe symptomatic COVID-19 infection. Median time between positive COVID-19 testing and CMR was 71 days. Twenty-two of these patients had late gadolinium enhancement of the pericardium, with 20 patients having more than 10 mm pericardial effusion. A healthy control of 50 patients had no pericardial enhancement or effusion.23
In our case, the history of COVID-19 respiratory disease, persistently elevated inflammatory biomarkers, imaging findings of ventricular interdependence and pericardial thickening, make COVID-19-induced pericardial disease our primary differential diagnosis. A SARS-COV2 PCR analysis of the pericardial fluid would help confirm the diagnosis, however, this test was not readily available. Coinfection with other common viruses can lead to a similar presentation, but a respiratory viral panel resulted negative for the most common viruses at the time of his admission. Furthermore, the patient has no known history of tuberculosis exposure or prior cardiac surgery, malignancy or radiation. He had no other findings suggestive of autoimmune disease. In addition, he lacks associated signs and symptoms characteristic of alternative diagnoses. However, in the months leading to this diagnosis, he suffered from other causes of inflammation including: colitis, Klebsiella osteomyelitis and Enterococcus faecalis bacteremia in addition to COVID-19 pneumonia. The time course from onset of pericardial inflammation to constrictive disease is variable, and the 4-month interval between his COVID-19 infection and progression to pericardial disease falls within a reasonable timeframe.
Imaging findings on echo, CT and CMR help distinguish CP from other pericardial disease pathologies including acute pericarditis and restrictive disease. Particularly, a thickened pericardium >3 mm and ventricular interdependence point towards CP, whereas a pericardium of <3 mm in thickness and myocardial involvement is more suggestive of a restrictive disease pathology.5 Both have elevated filling pressures with normal systolic function. However, in CP, right ventricular end diastolic pressure increases at the expense of left ventricular pressures during inspiration. This is not typically seen in restrictive disease.13
Medical therapy remains the mainstay of treatment for mildly symptomatic CP. Treatment usually includes colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs) including ibuprofen.5 Most cases progress to a chronic disease requiring surgical intervention with pericardiectomy—the surgical removal of both the parietal and epicardial layers of the pericardium—as the only definitive treatment.3 One study found that 17% of CP cases resolved without surgery after about 8 weeks of medical therapy alone. However, most cases progressed to a chronic disease requiring definitive treatment with surgical intervention. Although the safety of NSAIDs has been questioned in the literature, there is no conclusive evidence of harm.24 25 Many of the reported cases of acute pericarditis in the setting of COVID-19 infection used colchicine with and without NSAIDs with overall good outcomes, except for one case reporting worsening pericardial effusion and clinical status requiring surgical pericardiectomy.7 9 10 15 16 18 19 26 Prior to definitive therapy with surgical intervention, diuretic medications can offer symptom relief.13
Learning points.
The COVID-19 virus can induce systemic inflammation that compromises the cardiovascular system and leads to constrictive pericardial disease.
The constrictive pericard (CP) diagnosis is based upon distinct physiology that is similar to other pericardial disease processes. Imaging is essential (primarily echocardiogram and cardiac magnetic resonance) to confirm the diagnosis.
Viral and idiopathic aetiologies are the most common cause of CP in the developed world, with COVID-19 now a possible predisposing condition.
Acknowledgments
The authors acknowledge the dedication of all providers who cared for the patient described in the report and aided in his recovery.
Footnotes
Contributors: JKB co-authored the manuscript after caring for the patient during the COVID-19 pandemic. She wrote the summary, background, case, investigations and discussion sections. She saw the manuscript through to publication. She is guarantor. MA also co-authored the manuscript. He contributed to the case presentation, investigation, and discussion sections. He was essential to the acquisition, analysis and interpretation of the images. CMT contributed to the revision and intellectual content. She supported the conception and design of the paper. AF made the initial diagnosis while caring for the patient. He contributed first hand data via chart records, physical exam and imaging studies. He also interpreted the imaging studies. ADC also provided the authoring team with images and reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO . COVID-19 Weekly epidemiological update, 2021. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-12-january-2021 [Accessed 19 Jan 2021].
- 2.Lazaridis C, Vlachogiannis NI, Bakogiannis C, et al. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hellenic J Cardiol 2020;61:381–95. 10.1016/j.hjc.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asif T, Kassab K, Iskander F, et al. Acute pericarditis and cardiac tamponade in a patient with COVID-19: a therapeutic challenge. Eur J Case Rep Intern Med 2020;7:001701. 10.12890/2020_001701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020;12:372. 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler Y, Charron P, Imazio M. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Cruz E, Manzur-Sandoval D, Lazcano-Díaz EA, et al. Cardiac tamponade in a patient with myocardial infarction and COVID-19: electron microscopy. Case Reports 2020;2:2021–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer F, Dagrenat C, Couppie P, et al. Pericardial effusion in patients with COVID-19: case series. Eur Heart J Case Rep 2020;4:1-7. 10.1093/ehjcr/ytaa287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farina A, Uccello G, Spreafico M, et al. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur J Intern Med 2020;76:100–1. 10.1016/j.ejim.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond TT, Das A, Manzuri S, et al. Pediatric COVID-19 and pericarditis presenting with acute pericardial tamponade. World J Pediatr Congenit Heart Surg 2020;11:802–4. 10.1177/2150135120949455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox K, Prokup JA, Butson K, et al. Acute effusive pericarditis: a late complication of COVID-19. Cureus 2020;12:e9074. 10.7759/cureus.9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purohit R, Kanwal A, Pandit A, et al. Acute myopericarditis with pericardial effusion and cardiac tamponade in a patient with COVID-19. Am J Case Rep 2020;21:e925554. 10.12659/AJCR.925554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua A, O'Gallagher K, Sado D, et al. Life-Threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J 2020;41:2130. 10.1093/eurheartj/ehaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch TD. Constrictive pericarditis: diagnosis, management and clinical outcomes. Heart 2018;104:725–31. 10.1136/heartjnl-2017-311683 [DOI] [PubMed] [Google Scholar]
- 14.Karadeniz H, Yamak BA, Özger HS, et al. Anakinra for the treatment of COVID-19-Associated pericarditis: a case report. Cardiovasc Drugs Ther 2020;34:883–5. 10.1007/s10557-020-07044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Kumar J, Daly C, et al. Acute pericarditis as a primary presentation of COVID-19. BMJ Case Rep 2020;13:e237617. 10.1136/bcr-2020-237617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tung-Chen Y. Acute pericarditis due to COVID-19 infection: an underdiagnosed disease? Medicina Clinica (English Ed.). 2020;155:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blagojevic NR, Bosnjakovic D, Vukomanovic V, et al. Acute pericarditis and severe acute respiratory syndrome coronavirus 2: case report. Int J Infect Dis 2020;101:180–2. 10.1016/j.ijid.2020.09.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naqvi SGZ, Naseeb U, Fatima K, et al. Acute pericarditis and pericardial effusion in a hypertensive COVID-19 patient. Cureus 2020;9. 10.7759/cureus.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz-Martínez Y, Cabeza-Ruiz LD, Vásquez-Lozano SH, et al. Pericarditis in a young internal medicine resident with COVID-19 in Colombia. Travel Med Infect Dis 2020;37:101863. 10.1016/j.tmaid.2020.101863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020;295:210–7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;55:327–31. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13:2330–9. 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19).. JAMA Cardiology 2020;5:1265–73. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell B, Moss C, Rigg A, et al. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience 2020;14. 10.3332/ecancer.2020.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imazio M, Brucato A, Lazaros G, et al. Anti-Inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med 2020;21:625–9. 10.2459/JCM.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 26.Amoozgar B, Kaushal V, Mubashar U, et al. Symptomatic pericardial effusion in the setting of asymptomatic COVID-19 infection: a case report. Medicine 2020;99:e22093. 10.1097/MD.0000000000022093 [DOI] [PMC free article] [PubMed] [Google Scholar]


