Abstract
Objective
Patients with short bowel syndrome (SBS) and colon in continuity have better adaptation potential compared with patients with jejunostomy. Adaptation may involve enhanced postprandial secretion of the enteroendocrine hormones glucagon-like peptide (GLP)-1 and GLP-2 which are normally degraded by dipeptidyl peptidase (DPP)-4. Nevertheless, some patients with SBS with colon in continuity suffer from high-volume faecal excretions and have been shown to benefit from treatment with GLP-2. Therefore, we aimed to evaluate efficacy of sitagliptin, a DPP-4 inhibitor, on reducing faecal excretions in this patient group.
Design
In an open-label, case series, proof-of-concept pilot study, 100 mg oral sitagliptin was given two times per day for 8 weeks to patients with SBS with ≥50% colon in continuity with or without the need for parenteral support (PS). To assess intestinal function, metabolic balance studies were done at baseline and following 8 weeks of treatment.
Results
Of the 10 patients planned for enrolment, 8 patients were included; 7 patients completed the study. Although postprandial endogenous GLP-2 concentrations increased by 49 hours×pmol/L (39, 105; p=0.018) (median (min, max)), sitagliptin did not significantly reduce median faecal wet weight (−174 g/day (−1510, 675; p=0.176)) or increase intestinal wet weight absorption. However, heterogeneity in the treatment effect was observed: intestinal wet weight absorption increased in all four patients with intestinal failure. One patient achieved a reduction in PS by 500 mL per administration day.
Conclusion
Following this negative, small pilot study, larger, placebo-controlled, studies are needed to establish the therapeutic potential of DPP-4 inhibition in patients with SBS.
Keywords: intestinal failure, short bowel syndrome, gut hormones, glucagen-like peptides, intestinal absorption
Summary box.
What is already known about this subject?
Compared with patients with jejunostomies, patients with short bowel syndrome (SBS) and colon in continuity have better potential for intestinal adaptation due to enhanced endogenous responses of glucagon-like peptide (GLP)-1, GLP-2, and peptide YY (PYY) which are normally degraded by dipeptidyl peptidase (DPP)-4. In a subgroup of these patients, who suffer from high volume faecal output, treatment with GLP-2 has been shown to improve intestinal absorption.
To identify clinical trials which aimed to investigate the effect of a DPP-4 inhibitor on intestinal absorption in patients with SBS, we searched PubMed and MEDLINE for publications between 1 January 1990 and 30 June 2019 with the search terms ‘short bowel syndrome’, ‘colon in continuity’, ‘dipeptidyl peptidase-4 inhibitor’, ‘intestinal adaptation’, and ‘adults’. The search did not retrieve any clinical trials investigating the impact of a DPP-4 inhibitor on intestinal absorption and adaptation in adult patients with SBS and a colon in continuity. Hence, the current paper represents a first-in-class pilot study in this patient population.
Sitagliptin (Januvia) is a DPP-4 inhibitor that decreases the breakdown of the endogenously secreted intact GLP-1, GLP-2 and PYY. Because of its glucose-regulatory properties, sitagliptin is approved by the authorities in the EU and the USA in the treatment of patients with type 2 diabetes.
What are the new findings?
Results from this study showed that although postprandial endogenous total GLP-2 concentrations increased in patients with SBS and ≥50% of colon in continuity following 8 weeks of treatment with oral intake of 100 mg sitagliptin two times per day, the median faecal wet weight output did not change significantly. No significant changes were seen in total GLP-1, total glucose-dependent insulinotropic peptide and total PYY which was consistent with previous studies.
Summary box.
How might it impact on clinical practice in the foreseeable future?
Our findings from this open-label, case series, proof-of-concept pilot study suggest that treatment with sitagliptin was safe and well- tolerated in patients with SBS and ≥50% of colon in continuity. However, it remains to be established whether treatment with sitagliptin might have a therapeutic potential in this subgroup of patients or other subpopulations of SBS.
Introduction
In patients with short bowel syndrome (SBS) and distal intestinal resection, hormonal replacement therapy with a variety of the so-called ‘pro-adaptive’ or ‘brake’ hormones, normally secreted from the L cells in the distal intestine in response to meals, can ameliorate the pathophysiological consequences of SBS.1–6 In this respect, treatment with glucagon-like peptide (GLP)−2 has received special attention. GLP-2 has been shown to inhibit gastric hypersecretion7 and acellerated gastric emptying,8 increase intestinal mucosal surface area,2 6 9 blood flow,10 and barrier function,11 thereby enhancing nutrient and fluid absorption in both preclinical and clinical SBS settings.2 12 Teduglutide, a GLP-2 analogue has been approved by the authorities in the EU and the USA for the treatment of adult and paediatric patients with SBS.13
The beneficial effects of ‘pro-adaptive’ hormones do not seem to be solely attributed to GLP-2 and its analogues. Overall, complex and yet not fully understood neuroendocrine interactions seem to exist between multiple and simultaneously secreted enteroendocrine hormones. Hormones, such as GLP-1 and peptide YY (PYY), are secreted from the same L-cells as GLP-2, and they are also believed to affect gastrointestinal (GI) secretions and transit time as well as intestinal absorption.14 15 For instance, GLP-1 analogues have been shown to reduce bowel movements and faecal output as well as to increase intestinal wet weight and energy absorption in patients with jejunostomy.3 16
Along with elevated plasma GLP-2, high plasma concentrations of GLP-1 and PYY have been proposed to contribute to the GI brake mechanism in patients with SBS that have colon in continuity by slowing accelerated gastric emptying.15 17 18 Consequently, these patients usually have a better adaptation potential and intestinal function compared with patients with a jejunostomy.18 After small bowel resection, the colon adapts by increasing the absorption of water and electrolytes,19 20 and colonic fermentation of malabsorbed carbohydrates results in increased production of short-chain fatty acids serving as an energy source.21 22 In addition, a colon in continuity is believed to slow the intestinal transit of chyme through a colonic brake mechanism and thereby increase exposure between the chyme and the intestinal mucosa.4
The intact form of GLP-1, GLP-2, and PYY have short half-lives of only a few minutes due to an enzymatic breakdown by dipeptidyl peptidase (DPP)-4. Sitagliptin (Januvia) is a DPP-4 inhibitor that inhibits the breakdown of endogenously secreted intact GLP-1, GLP-2 and PYY by up to 90% 12–24 hours after oral administration.23 Sitagliptin is approved by the US Food and Drug Administration and the European Medicines Agency in the treatment of type 2 diabetes (100 mg tablet per day). Sitagliptin has glucose-regulatory properties through an increased incretin effect: that is, it increases circulating levels of intact endogenous GLP-1 and glucose-dependent insulinotropic peptide (GIP) concentrations and suppresses glucagon secretion.24
This open-label pilot study aimed to evaluate the effect of sitagliptin on faecal wet weight output in patients with SBS who have ≥50% of colon in continuity. By its assumed effects on increasing the plasma concentrations and the half-life of endogenously secreted L cell hormones, our hypothesis was that a cascade of proadaptive effects would result in a decrease in faecal wet weight output, an increase in intestinal absorption of wet weight, energy and electrolytes, as well as an increase in urine production, which could lead to a reduction in the need for parenteral support (PS).
Methods
Study protocol
This was a single-centre, open-label, case series, proof-of-concept pilot study. All procedures followed the ethical standards of the Helsinki Declaration and the International Council for Harmonization Good Clinical Practice (ICH-GCP). Prior to enrolment, all patients had given written consent to participate in the study. The study was performed at the Department of Medical Gastroenterology and Hepatology, Rigshospitalet, Denmark.
Adult patients (≥18 years and ≤90 years) with SBS and either intestinal insufficiency (II) or intestinal failure (IF)25 were recruited. Patients were required to have ≥50% of colon in continuity. Other key inclusion criteria included: faecal wet weight output of ≥1.0 kg/day as reported by the patient following previous home measurements; small intestinal length <200 cm (as ascertained from operation notes and the Copenhagen IF database); stable body weight (<5% change within the last 3 months prior to inclusion) and stable PS in patients with SBS-IF.
Procedures
Administration of sitagliptin
The patients received oral sitagliptin 100 mg tablets twice a day for a total of 8 weeks. The total daily dose was twice the dose used in the treatment of patients with type 2 diabetes26 and was used in this study to compensate for the large malabsorption experienced by most patients with SBS. Patients were asked to return all used and unused medicine at the last metabolic balance study for evaluation of compliance.
Study design
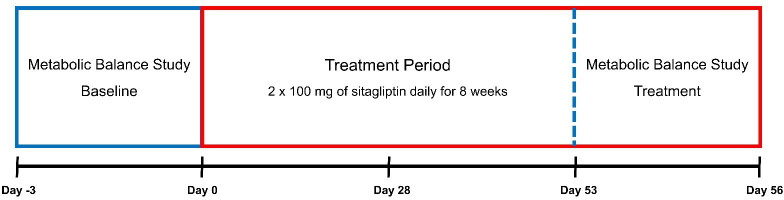
The patients served as their own controls and were hospitalised Monday through Friday for a 72-hour metabolic balance study prior to and on the eighth week of treatment (figure 1). Every fortnight during the treatment period, the patients adhered to a fixed drinking menu and measured 48-hour urine volumes in order to assess the hydration status and determine whether a reduction in PS volume was possible. A reduction in PS volume was done if the 48-hour urine volume was 10% larger than the baseline urine production, and/or if patients had developed symptoms of fluid retention. Patients, in whom PS reduction occurred during the treatment period, were required to return to their baseline PS programme at the end of the seventh treatment week (starting on day 53) to have comparable metabolic balance studies. For safety and tolerability purposes, an outpatient visit was planned after 4 weeks of treatment (on day 28) to assess physical examination, vital signs and ECG, as well as safety blood samples, including hemoglobin A1c (HbA1c), and assessment of adverse events (AEs). AEs were assessed on an ongoing basis either as reported by the patient or when asked for by the investigator during the study.
Figure 1.
Study design. Day −3, admission for baseline metabolic balance study; Day 0, first dosing; Day 28, safety outpatient visit; Day 53, admission for treatment metabolic balance study; Day 56, end of study.
Metabolic balance studies
Each patient underwent two 72-hour metabolic balance studies. Patients were required to urinate and defecate before the start of each metabolic balance study. Dietary intake of solids was ad libitum, while an individual drinking menu (specifying type and amount of fluids) was established for each patient. All dietary solids and fluids were prepared in duplicate portions; one for oral intake and one for laboratory analyses as described elsewhere.27 Urine and faecal output as well as exact replicas of the dietary intake were collected in respective buckets. The collected samples were weighed and analysed for energy by bomb calorimetry (Oxycon Pro; Jaeger, Hoechberg, the Netherlands), carbohydrate by Englyst’s method,28 nitrogen by Kjeldahl’s method,29 and fat by a modified Van de Kamer titration technique.30 The content of sodium and potassium was analysed using flame photometry, while calcium and magnesium concentrations were determined by atomic absorption spectrophotometry analysis.31
Wet weight was defined as the combined amount of fluid, macronutrients, micronutrients, and electrolytes, while absolute intestinal absorption was defined as the difference between dietary intake and faecal excretion. Relative absorption was calculated as (absolute absorption / dietary intake) × 100. Concomitant medication and PS were kept constant during both metabolic balance studies (table 1).
Table 1.
Individual baseline characteristics
| Patient ID |
Sex/age (years) | Diagnosis | Anatomy | Time since last intestinal resection (years) | SBS classification | Concomitant medication | Parenteral Support | Body weight | BMI | Dietary intake | Faecal output | Wet weight absorption | Urine production | ||||||
| SB length (cm) | Colon | IF/II | Proton pump inhibitor | Codeine | Loperamide | Fluid (ml/day) | (kg) | (kg/m2) | Wet weight (g/day) | Energy (MJ/day) | Wet weight (g/day) | Energy (MJ/day) | Absolute (ml/day) |
Relative (%/day) |
(ml/day) | ||||
| 1 | M/68 | Ileus | 50 | Anastomosis with transverse colon | 12 | IF | Yes | Yes | Yes | 1000 | 53.8 | 19.5 | 1737 | 6.4 | 383 | 3.1 | 1353 | 78 | 1520 |
| 2 | M/40 | CD | 65 | Jejuno-ascendo-anastomosis | 15 | IF | No | No | No | 2667 | 74.3 | 24.0 | 3513 | 7.9 | 1990 | 4.6 | 1523 | 43 | 3273 |
| 3 | F/56 | MVD | 85 | Anastomosis with transverse colon | 2.3 | IF | No | No | No | 1833 | 57.8 | 19.5 | 3553 | 13.0 | 3480 | 8.6 | 73 | 2 | 1023 |
| 4 | M/69 | CD | 100 | Anastomosis with transverse colon | 1.5 | IF | Yes | No | No | 3000 | 75.5 | 25.5 | 2997 | 10.9 | 3357 | 5.9 | −360 | −12 | 1692 |
| 5 | M/35 | CD | UK | Ileo-ascendo-anastomosis | 4.6 | II | No | Yes | No | 0 | 73.3 | 19.9 | 4827 | 18.5 | 983 | 6.0 | 3843 | 80 | 2607 |
| 6 | F/20 | MVD | 70 | Anastomosis with transverse colon | 11 | II | No | No | No | 0 | 61.9 | 22.5 | 4353 | 11.2 | 1087 | 4.9 | 3267 | 75 | 2290 |
| 7 | F/43 | CD | 200 | Anastomosis with transverse colon | 5 | II | Yes | Yes | No | 0 | 92.1 | 30.8 | 2973 | 14.4 | 483 | 2.6 | 2490 | 84 | 1177 |
| 8 | M/50 | RC | 190 | Anastomosis with transverse colon | 6 | II | Yes | Yes | No | 0 | 74.5 | 23.0 | 3022 | 10.8 | 460 | 1.7 | 2562 | 85 | 1007 |
BMI, body mass index; CD, Crohn’s Disease; F, female; IF, intestinal failure; II, Intestinal insufficiency; M, male; MVD, mesenteric vascular disease; RC, rectal cancer; SB, small bowel; SBS, short bowel syndrome; UK, unknown.
Body weight and body composition
Body weight was measured each morning before breakfast and after urination and defecation and was calculated based on a 3-day average. Lean body mass, fat mass and bone mineral density (BMD) were measured once during each of the metabolic balance studies by dual-energy X-ray absorptiometry (Norland XR-36 Dual energy X-ray Absorptiometry (DXA) densitometer, Norland Corp, Fort Atkinson, Wisconsin, USA).
Hormone analyses
Plasma concentrations of GLP-1, GLP-2, PYY, GIP and glucagon were measured in fasting state and at fixed time points after ingestion of a standardised breakfast (0, 2, 5, 10, 15, 20, 30, 45, 60, 120, and 180 min) using radioimmunoassays (see supplemental material and figures 1–6 for details on hormone analyses). The standardised breakfast contained 773 kcal (3250 kJ) and had an energy ratio of 11% protein (22 g), 46% carbohydrate (87 g), and 43% fat (38 g) as described previously. The patients had 15 min to consume the breakfast.3
bmjgast-2021-000604supp001.pdf (592.9KB, pdf)
Outcomes
The primary end point of the study was the absolute change from baseline in faecal wet weight output following treatment with sitagliptin. Key secondary endpoints included changes from baseline in: (1) Intestinal absorption of wet weight, energy and macronutrients, (2) Urine volume, (3) The need for PS volume, (4) Oral wet weight intake, (5) Body weight, (6) Body composition (fat mass, fat-free mass and BMD (spine)), and (7) Plasma concentrations of endogenous GLP-1, GLP-2, PYY, GIP and glucagon after a standardised meal stimulation.
Statistical analysis
A power calculation could not be performed for this study as no previous studies examining sitagliptin on patients with SBS exist. The sample size was chosen based on the magnitude of effect of GLP-2 treatment on reductions of faecal output as observed in previous studies.2 9 Adequate power was expected regarding the primary end point by having the patients serve as their own control.
IBM SPSS Statistics V.25 was used for the statistical analyses. A value of p<0.05 was considered statistically significant. A non-parametrical test, the Wilcoxon signed-rank test was used to assess the effect of sitagliptin on change from baseline. Unless specified otherwise, data are presented as median (min, max).
Area under the curve (AUC) was used when analysing the postprandial hormone profiles. Spearman’s correlation coefficient was used to estimate correlations.
Results
Baseline demographics are presented in table 1.
Of the 10 patients planned to be included in the study, 8 patients with SBS and ≥50% of colon in continuity were included in the study. All were of Caucasian descent; four with SBS-II and four with SBS-IF. One patient (ID No. 5, table 1) discontinued due to AEs (hot flushes and flare-up of a depression). Patient recruitment took place between November 2014 and October 2016 and further enrolment of patients was discontinued due to lack of patients to fit the inclusion criteria and study design within the planned recruitment period. The study laboratory analyses were completed in March 2019. A total of seven patients completed the study and were included in the statistical analyses of the results.
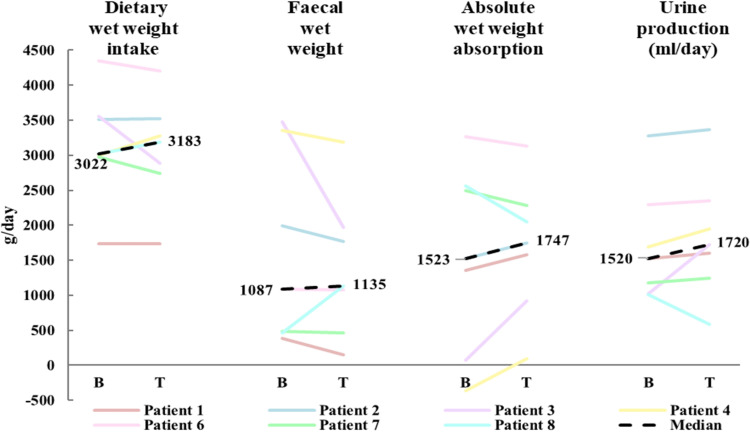
Following 8 weeks of treatment with sitagliptin, the median reduction from baseline in absolute wet weight output of −174 g/day (−1510, 675) did not reach statistical significance (p=0.176). Although individual data suggested that six out of seven patients had a reduction in absolute faecal wet weight output ranging from −1510 g/day to −10 g/day (figure 2), a clinically relevant reduction in faecal wet weight output (approx. 500 g/day6 9 32) was only achieved in one patient (ID No. 3).
Figure 2.
Median (n=7) changes from baseline (B) to treatment (T) in dietary wet weight intake, faecal wet weight output, absolute wet weight absorption, and urine production.
Treatment with sitagliptin did not significantly affect median values of total dietary intake (−7 g/day (−663, 276); p=0.672), wet weight absorption (223 g/day (−514, 847); p=0.398) (online supplemental figure 7) or urine production (80 mL/day (−427, 697); p=0.176). The reduction in faecal wet weight output was largest in patients with SBS-IF (n=4, range −1510 g/day to –174 g/day) compared with patients with SBS-II (n=3, range −23, 675 g/day). A trend towards positive correlation (r=0.72, p=0.055) was observed between baseline faecal wet weight output and change in intestinal absorption (online supplemental figure 8).
A reduction in PS volume of 500 mL per administration day was only achieved in one out of four patients with SBS-IF (ID No. 3) based on an increase in urine production of 697 mL/day. For the same patient, faecal wet weight output decreased by 1510 g/day, total dietary intake decreased by 663 g/day and wet weight absorption increased by 847 g/day. Moreover, body weight increased by 1.7 kg, lean body mass by 200 g and fat mass by 1000 g.
For the entire group of patients, changes observed regarding median absorption of electrolytes, macronutrients and energy were minor and did not reach statistical significance (tables 2 and 3).
Table 2.
Wet weight and electrolytes
| Baseline | Treatment | Effect | P value | ||
| Wet weight (g/day) | Dietary intake | 3022 (1737, 4353) | 3183 (1730, 4208) | −7 (−663, 276) | 0.672 |
| Faeces | 1087 (383, 3480) | 1135 (153, 3183) | −174 (−1510, 675) | 0.176 | |
| Absolute absorption | 1523 (−360, 3267) | 1747 (90, 3132) | 223 (−514, 847) | 0.398 | |
| Urine (mL/day) | 1520 (1007, 3273) | 1720 (580, 3367) | 80 (−427, 697) | 0.176 | |
| Sodium (mmol/day) | Dietary intake | 133 (53, 194) | 130 (46, 216) | −11 (−64, 87) | 0.237 |
| Faeces | 41 (16, 296) | 92 (6, 272) | −5 (−55, 90) | 0.866 | |
| Absolute absorption | 37 (−163, 170) | 51 (−147, 132) | −3 (−85, 41) | 0.553 | |
| Urine | 156 (34, 217) | 132 (46, 178) | 2 (−39, 12) | 1 | |
| Potassium (mmol/day) | Dietary intake | 70 (39, 112) | 69 (37, 124) | −2 (−27, 27) | 0.933 |
| Faeces | 49 (14, 96) | 30 (9, 86) | −15 (−46, 12) | 0.063 | |
| Absolute absorption | 10 (−15, 80) | 39 (−10, 80) | 18 (−25, 73) | 0.31 | |
| Urine | 51 (25, 79) | 41 (27, 75) | 1 (−24, 17) | 0.672 | |
| Calcium (mmol/day) | Dietary intake | 29 (11, 70) | 23 (10, 62) | −3 (−19, 4) | 0.091 |
| Faeces | 28 (15, 60) | 31 (5, 47) | −7 (−21, 12) | 0.31 | |
| Absolute absorption | −2 (−20, 22) | −1 (−19, 21) | −1 (−9, 10) | 0.499 | |
| Urine | 4 (0, 7) | 3 (1, 9) | 0 (−3, 2) | 0.866 | |
| Magnesium (mmol/day) | Dietary intake | 15 (10, 27) | 17 (9, 22) | −1 (−5, 4) | 0.31 |
| Faeces | 17 (10, 21) | 17 (3, 24) | −3 (−6, 7) | 0.31 | |
| Absolute absorption | 0 (−3, 7) | 0 (−7, 6) | −1 (−3, 5) | 0.866 | |
| Urine | 3 (1, 8) | 3 (0, 9) | 0 (0, 1) | 0.916 | |
Data are presented as median (min, max).
Table 3.
Energy and macronutrients
| Baseline | Treatment | Effect | P value | ||
| Energy (MJ/day) | Dietary intake | 11 (6.4, 14.4) | 11.3 (5.9, 12.5) | −0.5 (−3.1, 1.6) | 0.735 |
| Faeces | 4.6 (1.7, 8.6) | 4.1 (0.9, 7.3) | −0.4 (−2.2, 1.1) | 0.237 | |
| Absolute absorption | 5.1 (3.3, 11.8) | 6.0 (3.2, 9.5) | 0.4 (−2.6, 1.7) | 0.612 | |
| Carbohydrate (MJ/day) | Dietary intake | 4.2 (2.4, 6.1) | 4.3 (1.6, 5.1) | −0.4 (−1.2, 0.9) | 0.176 |
| Faeces | 0.5 (0.2, 1.2) | 0.6 (0.2, 0.9) | −0.1 (−0.3, 0.1) | 0.446 | |
| Absolute absorption | 3.6 (2.1, 5.7) | 3.7 (1.4, 4.8) | −0.3 (−0.9, 0.7) | 0.176 | |
| Protein (MJ/day) | Dietary intake | 2.2 (1.0, 2.7) | 2.1 (0.9, 2.4) | −0.1 (−0.6, 0.5) | 0.249 |
| Faeces | 1.1 (0.4, 2.3) | 1.1 (0.2, 1.7) | −0.07 (−0.6, 0.1) | 0.398 | |
| Absolute absorption | 0.6 (0.4, 1.7) | 0.8 (0.03, 1.8) | −0.1 (−0.4, 0.4) | 0.612 | |
| Fat (MJ/day) |
Dietary intake | 3.4 (2.7, 5.5) | 4.0 (2.4, 5.4) | −0.2 (−0.6, 0.7) | 0.735 |
| Faeces | 2.2 (0.8, 4.2) | 2.1 (0.5, 4.0) | −0.2 (−1.7, 0.4) | 0.237 | |
| Absolute absorption | 1.1 (0.5, 4.2) | 0.8 (0.5, 4.1) | 0.01 (−0.3, 0.8) | 0.866 | |
Data are presented as median (min, max).
No significant changes were observed for median values of body weight, lean body mass, fat mass, or BMD (online supplemental table 1). Moreover, no significant changes were observed in median urine production, creatinine clearance, plasma creatinine, plasma urea, or plasma aldosterone. Four out of seven patients experienced an increase in creatinine clearance ranging from 7 mL/min to 21 mL/min after treatment. However, the median change of 7 mL/min (−9, 21) did not reach statistical significance (p=0.271).
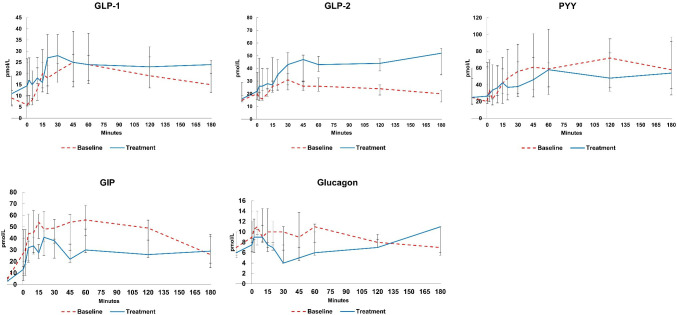
The median AUC0-3h (Area under the curve from 0 to 3 hours) for intact GLP-2 increased significantly by 49 (39, 105) h×pmol/L (p=0.018) after treatment with sitagliptin corresponding to a relative increase of 67%. Although a trend for an increase in AUC0-3h for total GLP-1 (p=0.063) and a decrease for AUC0-3h for total GIP (p=0.091), were observed, the changes did not reach statistical significance. The fasting values of total GLP-1, intact GLP-2 and total GIP did not change following treatment with sitagliptin. No changes were observed in the median fasting value or AUC0-3h for total PYY or glucagon (figure 3 and table 4).
Figure 3.
Postprandial hormone profiles at baseline and after treatment. GIP, glucose-dependent insulinotropic peptide; GLP, glucagon like peptide; PYY, peptide YY.
Table 4.
Fasting and postprandial hormone profiles
| Hormone | Baseline | Treatment | Effect | P value | |
| Total GLP-1 | Fasting value (pmol/L) |
9 (4,16) | 11 (5, 34) | 0 (−5, 30) | 0.345 |
| AUC (h×pmol/L) |
67 (28, 90) | 71 (46, 117) | 20 (−20, 31) | 0.063 | |
| Intact GLP-2 | Fasting value (pmol/L) |
15 (9, 22) | 16 (10, 53) | 1 (−2, 32) | 0.201 |
| AUC (h×pmol/L) |
75 (47, 103) | 125 (89, 184) | 49 (39, 105) | 0.018 | |
| Total PYY | Fasting value (pmol/L) |
25 (6, 83) | 25 (6, 85) | 0 (−56, 19) | 0.686 |
| AUC (h×pmol/L) |
212 (30, 432) | 146 (70, 293) | 25 (−227, 79) | 0.612 | |
| Total GIP | Fasting value (pmol/L) |
5 (1, 8) | 3 (1, 6) | −2 (−4, 4) | 0.288 |
| AUC (h×pmol/L) |
158 (17, 204) | 99 (28, 190) | −44 (61, 37) | 0.091 | |
| Glucagon | Fasting value (pmol/L) |
7 (3, 12) | 6 (2, 15) | −1 (−7, 7) | 0.389 |
| AUC (h×pmol/L) |
29 (16, 46) | 25 (18, 33) | −1 (−17, 4) | 0.398 |
Data are presented as median (min, max).
AUC, area under the curve; GIP, glucose-dependent insulinotropic peptide; GLP, glucagon like peptide; PYY, peptide YY.
The median compliance of sitagliptin was 99% (96, 100). Three patients had a 100% compliance. A total of seven out of eight patients experienced at least one AE; most were mild to moderate in severity. No serious AEs were associated with sitagliptin treatment. One patient experienced increased symptoms of depression and decided to discontinue the treatment. Another patient experienced abdominal pain that ceased after the treatment dose was reduced to one tablet per day (100 mg) for 13 days. Subsequent increase to 200 mg/day for the remaining study period was well tolerated.
Four patients (three with SBS-IF and one with SBS-II) experienced oedema during the treatment period. This was objectivised as increase in body weight in two patients (ID No. 3: 1.7 kg and ID No. 7: 2.0 kg). Other side effects included abdominal pain (n=1), upper respiratory tract infection (n=1), headache (n=2), restless legs (n=2), increased bleeding from anal fissure (n=1), dry mouth (n=1), influenza-like symptoms (n=1), diarrhoea (n=1), and fever lasting 1 day (n=1). No safety concerns arose regarding laboratory values, vital signs, or ECGs. No patients were diabetic at baseline. The median HbA1c at baseline was 31 (26, 35) mmol/mol. HbA1c was unchanged following treatment with sitagliptin (−1 (−2, 0) mmol/mol; p=0.059).
Discussion
This is the first single-centre, open-label, case series, proof-of-concept pilot study to assess the therapeutic potential of sitagliptin, a DPP-4 inhibitor, in patients with SBS and ≥50% of colon in continuity. Eight weeks of treatment with oral intake of 100 mg sitagliptin two times per day did not significantly reduce the median faecal wet weight output. No significant changes were observed regarding median intestinal absorption of wet weight, electrolytes and macronutrients, urine production, renal function, body weight or body composition.
Individual data suggested a reduction in faecal wet weight output in six out of seven patients, and an increase in intestinal absorption of wet weight in all four patients with SBS-IF. The incoherence between the number of patients, who experienced a reduction in faecal wet weight output, and the number of patients with an increase in intestinal wet weight absorption might be explained by the decrease in total dietary intake observed in five patients. It is possible that the decrease in total dietary intake occurred due to an increase in the concentration of intact GLP-1,33 which, however, was not measured in the present study. Despite the reduction in total dietary intake, this was not reported as an AE in terms of lack of appetite.
Treatment with sitagliptin was associated with significantly increased postprandial circulating concentration of intact GLP-2. No significant changes in total GLP-1, total GIP and total PYY were observed, which is consistent with findings in a previous study in patients with type 2 diabetes.34 Contrary to our expectation, circulating concentration of glucagon did not decrease as suggested by previous studies35–37 probably due to the small number of patients included in the present study. In murine intestine, real-time PCR analyses have previously shown the highest DPP-4 mRNA expression in the jejunum and ileum, whereas expression has been reported to be low in the proximal and distal colon.38 However, to our knowledge, the exact site of sitagliptin absorption is not known. The comparison of intestinal hormones in the present study with previous findings in patients with type 2 diabetes, does not add to the existing data on site of sitagliptin absorption. Nonetheless, SBS models may be used to address this issue taking into account that the absorption of sitagliptin and the associated intestinal hormone AUCs in patients with SBS are not only affected by a reduced surface area, but also by upper GI hypersecretion, increased GI emptying, first pass metabolism in a probably compromised liver, and finally by the heterogeneity in this patient group. Moreover, postsurgical adaptation-related increase in gut hormones with preserved ileum and/or proximal colon, is a confounder to be considered when comparing intestinal hormone AUCs with other patient groups.
The current perception regarding the enteroendocrine hormone profiles in patients with ileum resections and a colon in continuity is based on findings from studies by Jeppesen et al18 and Nightingale et al.17 In these studies elevated fasting plasma concentrations of GLP-1, GLP-218 and PYY,17 as well as significantly higher meal-stimulated responses, were observed when compared with healthy controls. Our findings in the present study were not consistent with this. Although, in the present study, both the fasting concentration and the meal-stimulated response of GLP-1 were higher compared with healthy controls from the Jeppesen study, they did not reach the high responses as reported in the patients with SBS with colon in continuity.18 Regarding GLP-2, the patients in our study had lower fasting concentration and meal-stimulated response compared with both the healthy controls and the patients with colon in continuity. Although a head-to-head comparison was not performed, both fasting concentration and meal-stimulated response of PYY in our study seem to be slightly higher than the controls, but lower than reported in the patients with colon in continuity by Nightingale et al.17 The preservation of segments of terminal ileum, the ileocecal valve and ascending colon could possibly explain these discrepancies. Six out of seven patients in the Jeppesen et al18 study had preserved ascending colon and four patients had a preserved ileocecal valve. In the Nightingale et al study17 all six patients had preserved ascending colon and two out of six patients had preserved ileocecal valve. On the contrary, no patients in the present study had preserved ileum and ileocecal valve and only two out of seven patients had parts of the ascending colon preserved.
The safety profile of sitagliptin in our study was comparable to what has already been shown in treatment of patients with type 2 diabetes and does not seem to add to existing safety data on sitagliptin.39 However, the most common AE was oedema, which has not been reported as a known AE of sitagliptin. This is probably indicative of the positive physiological effects of sitagliptin on fluid and sodium absorption indirectly achieved by the increased circulating concentrations of the pro-absorptive hormones, intact GLP-1 and GLP-2.2 6 9 32 Other AEs such as respiratory tract infection, fever, restless legs, diarrhoea, anal fissure flare associated with defecation, and dryness of the mouth were reported in relation to sitagliptin treatment. Further studies are needed to assess a direct causality between sitagliptin and these AEs since an increase in the absorption of the concomitant medications leading to these AEs could not be excluded.
This study is limited by the number of patients included. Pilot studies often use a sample size of 12 patients to gain an estimate of precision.40 However, the smaller sample size in the present study is much due to sparsity of patients willing to participate leading to an early termination of the study.
Recruitment of patients with ≥50% of colon in continuity is challenging in our department, since these patients constitute less than 5% of the whole chronic IF population thanks to better potential for spontaneous adaptation.17 18 Therefore, therapeutical approaches should aim to accelerate the functional adaptation in the more acute phase after surgical intestinal resection in this patient population. Another limitation of this study was that a faecal output of ≥1.0 kg/day was only observed in four out of the seven patients included in the analysis, which may impact the results presented in this paper. The protocol did not allow exclusion of patients with lower faecal wet weight output after inclusion, and since this study suggests a possible therapeutic effect in patients with high-volume faecal wet weight output, future studies should include a screening prior to inclusion to make sure this inclusion criterion is met. Finally, this study used a higher-dose treatment than recommended for patients with type 2 diabetes to counteract malabsorption. Future studies using oral medications should include therapeutic drug monitoring and/or pharmacokinetic-pharmacodynamic data to illustrate or justify the need for this dosing regimen.
In conclusion, treatment with 100 mg of sitagliptin two times per day was safe and well tolerated in this open-label, case series, proof-of-concept pilot study. Eight weeks of treatment with sitagliptin did not significantly reduce median faecal wet weight output in patients with SBS and ≥50% of colon in continuity. Furthermore, numerical changes observed regarding median intestinal absorption of wet weight, electrolytes and macronutrients, urine production, renal function, body weight or body composition did not reach statistical significance. Treatment with sitagliptin was associated with significant increase in postprandial concentrations of intact GLP-2, numerical increase in total GLP-1 and numerical decrease in total GIP, but not glucagon, PYY, or HbA1c. Although our individual data suggested a possible effect in patients who suffer from high-volume faecal wet weight output, it remains to be established whether treatment with sitagliptin might have a therapeutic potential in this subgroup of patients with SBS and ≥50% of colon in continuity.
Future studies should conduct feasibility work before the inception of the trial. A larger sample size and more robust study design, preferably a randomised clinical study with a cross-over study design and a placebo arm, should focus on the effect of sitagliptin in patients with SBS with minimal intestinal absorptive capacity. Treatment with sitagliptin as monotherapy as well as additive therapy to other antisecretory and antimotility agents would be of interest. Major topics of interest would include assessing key elements in the pathophysiology of SBS in terms of GI secretions and transit time as well as the intestinotrophic potential of sitagliptin. Value would also be derived from biological efficacy work to assess the effect of DPP-4 inhibitors on intestinal hormones (including GLP-2) in a larger group of patients with a short bowel, or a case series of patients deriving longer-term clinical benefit. Both patients with colon in continuity and/or terminal ileum with a preserved L cell capacity would be of interest.
Acknowledgments
The authors thank Jette Christiansen, Birgitte Schou and Dorte Christensen from the Department of Medical Gastroenterology and Hepatology, Rigshospitalet, for their technical assistance.
Footnotes
Contributors: RMN, MKH and PBJ contributed equally to the conception and design of the research; LMT and HJ contributed to the acquisition and analysis of the data; CBC, JJH and BH contributed to the analysis and interpretation of the data. RMN drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and have read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JJH has received speaker honoraria from MSD. CBC, JJH and BH are supported by grants from the Novo Nordisk Foundation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Any shared data sets containing individual participant data will be in a de-identified or anonymous format. The results of the trial will be made available on one or more public portals according to standard disclosure requirements for clinical trials.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study protocol was approved by the Danish Medicines Agency and the Regional Committee on Health Research Ethics (Project-ID: H-22014066) and registered at clinicaltrialsregister.eu (EudraCT: 2014-001941-25).
References
- 1.Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 1999;45:559–63. 10.1136/gut.45.4.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 2001;120:806–15. 10.1053/gast.2001.22555 [DOI] [PubMed] [Google Scholar]
- 3.Hvistendahl M, Brandt CF, Tribler S, et al. Effect of liraglutide treatment on jejunostomy output in patients with short bowel syndrome: an open-label pilot study. JPEN J Parenter Enteral Nutr 2018;42:112–21. 10.1177/0148607116672265 [DOI] [PubMed] [Google Scholar]
- 4.Nightingale JM, Kamm MA, van der Sijp JR, et al. Disturbed gastric emptying in the short bowel syndrome. Evidence for a 'colonic brake'. Gut 1993;34:1171–6. 10.1136/gut.34.9.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeppesen PB, Gabe SM, Seidner DL, et al. Factors associated with response to Teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology 2018;154:874–85. 10.1053/j.gastro.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 6.Naimi RM, Hvistendahl M, Enevoldsen LH, et al. Glepaglutide, a novel long-acting glucagon-like peptide-2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol 2019;4:354–63. 10.1016/S2468-1253(19)30077-9 [DOI] [PubMed] [Google Scholar]
- 7.Wøjdemann M, Wettergren A, Hartmann B, et al. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab 1999;84:2513–7. 10.1210/jcem.84.7.5840 [DOI] [PubMed] [Google Scholar]
- 8.Wøjdemann M, Wettergren A, Hartmann B, et al. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol 1998;33:828–32. 10.1080/00365529850171486 [DOI] [PubMed] [Google Scholar]
- 9.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005;54:1224–31. 10.1136/gut.2004.061440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremholm L, Hornum M, Andersen UB, et al. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 2011;168:32–8. 10.1016/j.regpep.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brubaker PL, Izzo A, Hill M, et al. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol Endocrinol Metab 1997;272:E1050–8. 10.1152/ajpendo.1997.272.6.E1050 [DOI] [PubMed] [Google Scholar]
- 13.SPC R. Revestive: summary of product characteristics, 2016. [Google Scholar]
- 14.Savage AP, Adrian TE, Carolan G, et al. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987;28:166–70. 10.1136/gut.28.2.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marathe CS, Rayner CK, Jones KL, et al. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res 2011;2011:1–10. 10.1155/2011/279530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel D, Basseri B, Low K, et al. Efficacy of the glucagon-like peptide-1 agonist exenatide in the treatment of short bowel syndrome. Neurogastroenterol Motil 2011;23:739–e328. 10.1111/j.1365-2982.2011.01723.x [DOI] [PubMed] [Google Scholar]
- 17.Nightingale JM, Kamm MA, van der Sijp JR, et al. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the 'colonic brake' to gastric emptying. Gut 1996;39:267–72. 10.1136/gut.39.2.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeppesen PB, Hartmann B, Thulesen J, et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 2000;47:370–6. 10.1136/gut.47.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladefoged K, Olgaard K. Sodium homeostasis after small-bowel resection. Scand J Gastroenterol 1985;20:361–9. 10.3109/00365528509091665 [DOI] [PubMed] [Google Scholar]
- 20.Hylander E, Ladefoged K, Jarnum S. Calcium absorption after intestinal resection. The importance of a preserved colon. Scand J Gastroenterol 1990;25:705–10. 10.3109/00365529008997596 [DOI] [PubMed] [Google Scholar]
- 21.Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet 1994;343:373–6. 10.1016/S0140-6736(94)91220-3 [DOI] [PubMed] [Google Scholar]
- 22.Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 1998;43:478–83. 10.1136/gut.43.4.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinclair EM, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology 2005;20:357–65. 10.1152/physiol.00030.2005 [DOI] [PubMed] [Google Scholar]
- 24.Kim JH. Effects of sitagliptin on insulin and glucagon levels in type 2 diabetes mellitus. Diabetes Metab J 2015;39:304–6. 10.4093/dmj.2015.39.4.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015;34:171–80. 10.1016/j.clnu.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 26.Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther 2006;28:55–72. 10.1016/j.clinthera.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 27.Jeppesen PB, Mortensen PB. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut 2000;46:701–6. 10.1136/gut.46.5.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englyst HN, Cummings JH. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984;109:937–42. 10.1039/an9840900937 [DOI] [PubMed] [Google Scholar]
- 29.Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern" (New method for the determination of nitrogen in organic substances). Zeitschrift für analytische Chemie 1883;22:366–83. [Google Scholar]
- 30.Berstad A, Erchinger F, Hjartholm A-S. Fecal fat determination with a modified titration method. Scand J Gastroenterol 2010;45:603–7. 10.3109/00365521003611299 [DOI] [PubMed] [Google Scholar]
- 31.Jeppesen PB, Staun M, Tjellesen L, et al. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut 1998;43:763–9. 10.1136/gut.43.6.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt CF, Hvistendahl M, Naimi RM, et al. Home parenteral nutrition in adult patients with chronic intestinal failure: the evolution over 4 decades in a tertiary referral center. JPEN J Parenter Enteral Nutr 2017;41:1178–87. 10.1177/0148607116655449 [DOI] [PubMed] [Google Scholar]
- 33.Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 2001;86:4382–9. 10.1210/jcem.86.9.7877 [DOI] [PubMed] [Google Scholar]
- 34.Aaboe K, Knop FK, Vilsbøll T, et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2010;12:323–33. 10.1111/j.1463-1326.2009.01167.x [DOI] [PubMed] [Google Scholar]
- 35.Murai K, Katsuno T, Miyagawa J-ichiro, et al. Very short-term effects of the dipeptidyl peptidase-4 inhibitor sitagliptin on the secretion of insulin, glucagon, and incretin hormones in Japanese patients with type 2 diabetes mellitus: analysis of meal tolerance test data. Drugs R D 2014;14:301–8. 10.1007/s40268-014-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otsuka Y, Yamaguchi S, Furukawa A, et al. Addition of sitagliptin or metformin to insulin monotherapy improves blood glucose control via different effects on insulin and glucagon secretion in hyperglycemic Japanese patients with type 2 diabetes. Endocr J 2015;62:133–43. 10.1507/endocrj.EJ14-0148 [DOI] [PubMed] [Google Scholar]
- 37.Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006;91:4612–9. 10.1210/jc.2006-1009 [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara K, Inoue T, Yorifuji N, et al. Combined treatment with dipeptidyl peptidase 4 (DPP4) inhibitor sitagliptin and elemental diets reduced indomethacin-induced intestinal injury in rats via the increase of mucosal glucagon-like peptide-2 concentration. J Clin Biochem Nutr 2015;56:155–62. 10.3164/jcbn.14-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MSD . Januvia-Summary of product characteristics, 2012. [Google Scholar]
- 40.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287–91. 10.1002/pst.185 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000604supp001.pdf (592.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Any shared data sets containing individual participant data will be in a de-identified or anonymous format. The results of the trial will be made available on one or more public portals according to standard disclosure requirements for clinical trials.