Abstract
A 62-year-old woman was referred to our department for further investigation of anaemia. Blood test showed macrocytic anaemia. Oesophagogastroduodenoscopy (OGD) revealed proximal-predominant gastric atrophy and flat elevated lesion in the gastric body. Several days after OGD, she complained of gait disturbance and was diagnosed with subacute combined degeneration of the spinal cord. Furthermore, laboratory tests showed positive for both anti-parietal cell and anti-intrinsic factor antibodies, as well as increased serum gastrin level and decreased pepsinogen I level, which confirmed the diagnosis of autoimmune gastritis (AIG). Anaemia and neurological symptoms were improved after vitamin B12 supplementation. Subsequently, the patient underwent gastric endoscopic submucosal dissection; histopathological examination revealed gastric adenoma. AIG can cause gastric neoplasms and vitamin B12 deficiency, with the latter resulting in pernicious anaemia and neurological disorders. These diseases are treatable but potentially life-threatening. This case highlights the importance of early diagnosis of AIG and proper management of its comorbidities.
Keywords: stomach and duodenum, endoscopy, spinal cord, vitamins and supplements
Background
Autoimmune gastritis (AIG) is a non-self-limiting chronic inflammatory disorder characterised by the destruction of gastric parietal cells, affecting the oxyntic mucosa and resulting in mucosal atrophy. The inflammation is restricted to the corpus and fundus glands of the stomach, sparing the antrum, which distinguishes AIG from other conditions leading to atrophic gastritis, such as Helicobacter pylori (HP) infection.1 AIG can cause gastric neoplasms and vitamin B12 deficiency, and is associated with other autoimmune diseases, such as thyroiditis and type 1 diabetes mellitus.2
AIG prevalence has been estimated to be ~0.5%–4.5% globally, varying widely owing to different diagnostic criteria, ethnical and demographical setting.1 In Japan, the prevalence of AIG was assumed to be low. However, reports of AIG are increasing as HP prevalence has decreased due to the wide adoption of eradication therapy.3–5
Vitamin B12 is an essential cofactor related to DNA and cell metabolism, and vitamin B12 deficiency is a relatively common but serious condition.6 The mechanism of vitamin B12 deficiency caused by AIG is malabsorption due to decreased production of intrinsic factor from gastric parietal cells because of chronic immune-mediated inflammation. Vitamin B12 deficiency manifests as megaloblastic anaemia, commonly termed pernicious anaemia, and neurological disorders, such as subacute combined degeneration (SCD) of the spinal cord.7 These diseases are treatable, but delayed treatment may lead to irreversible neurological impairment.8
Case presentation
A 62-year-old woman was referred to our department for further investigation of anaemia. She had history of hypothyroidism but taking no medication on her initial visit. The patient’s family history was unremarkable. Her diet was balanced, and she consumed no alcohol. This was the first time she was pointed out anaemia by family physician. On physical examination, she was afebrile with a regular heart rate of 72 beats/min and blood pressure of 110/56 mm Hg. Conjunctival pallor was absent. No remarkable findings were observed in the craniocervical region, chest, abdomen or limbs. Also, neurological findings were unremarkable.
Investigations
Laboratory data showed that the red cell count was mildly low at 2.9×1012/L (normal range 3.8–5.0×1012/L), haemoglobin level was within the normal range at 125 g/L (115–16 g/L), and mean corpuscular volume was high at 124.5 fL (85–102 fL). Serum iron level was within the normal range, but vitamin B12 level was low at <30 pg/mL (249–938 pg/mL). The folic acid level was high at 18.7 ng/mL (3.6–12.9 ng/mL). Oesophagogastroduodenoscopy (OGD) showed proximal-predominant gastric atrophy (figure 1A, B). Magnifying endoscopy with narrow-band imaging (NBI) showed almost normal surface structure in the antrum (figure 1C). In the gastric body, round pits indicative of normal gastric fundic glands were not observed, suggesting severe atrophy of the gastric mucosa (figure 1D). Moreover, we found a flat elevated lesion at the lesser curvature of the gastric body (figure 2A, B). The lesion was 12 mm in size and presented slightly irregular surface and vessel patterns with a demarcation line on NBI with magnifying endoscopy (figure 2C, D). We performed endoscopic biopsies on this lesion and the background mucosa of the antrum and gastric body. The rapid urease test was negative.
Figure 1.
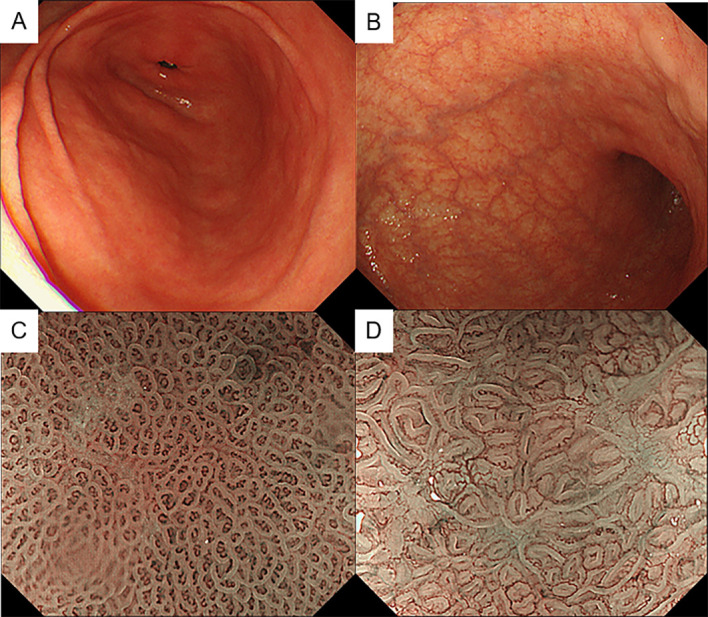
(A, B) Endoscopic images of the antrum (A) and gastric body (B) observed by white-light imaging. (C, D) Magnifying endoscopic images of the antrum (C) and gastric body (D) observed by narrow-band imaging.
Figure 2.
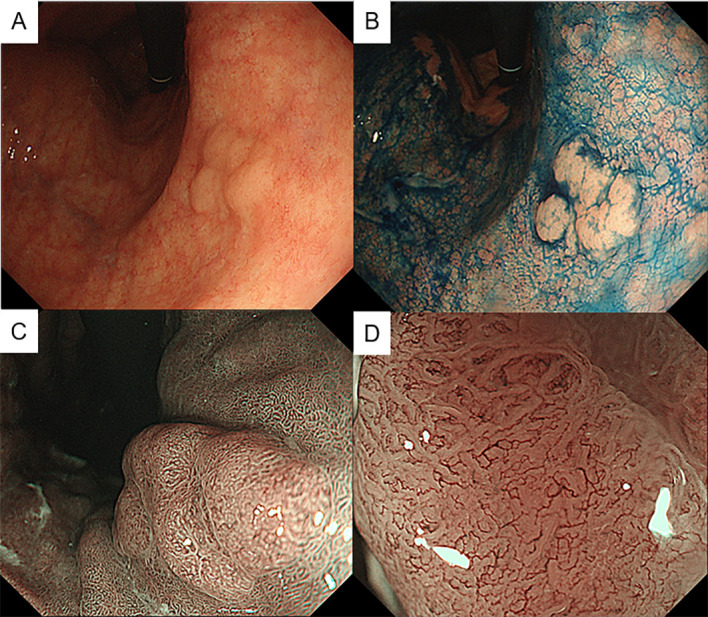
Endoscopic images of the flat elevated lesion at the lower gastric body observed by white-light imaging (A), chromoendoscopy with indigo carmine (B) and magnifying endoscopy with narrow-band imaging (C, D).
Differential diagnosis
We considered megaloblastic anaemia due to vitamin B12 deficiency as a differential diagnosis of macrocytic anaemia. Because there was no history of unbalanced diet or anorexia, the suspected aetiology of vitamin B12 deficiency was impaired absorption. OGD showed mucosal atrophy predominantly in the gastric body, and the rapid urease test was negative. Based on these findings, we suspected AIG with macrocytic anaemia due to vitamin B12 deficiency. Based on the endoscopic findings, the flat elevated lesion in the gastric body was suspected to be early gastric cancer.
Treatment
We planned endoscopic submucosal dissection (ESD) for the gastric lesion. Moreover, after additional laboratory tests such as autoantibodies against intrinsic factor and parietal cells were completed, vitamin B12 supplementation was also planned.
Outcome and follow-up
Several days after OGD, the patient complained of gait disturbance. Neurological examinations revealed sensory disturbance and loss of deep tendon reflexes in bilateral lower limbs, positive Romberg sign and ataxic gait. T2-weighted MRI showed high-intensity areas in the posterior column of the thoracic spinal cord (figure 3). On the basis of these findings, neurologist diagnosed her with SCD of the spinal cord. Additional laboratory tests showed positive for anti-parietal cell and anti-intrinsic factor antibodies, increased serum gastrin level and decreased pepsinogen I level and I/II ratio. Serum anti-HP antibody was negative at <3.0 U/mL (latex agglutination turbidimetric immunoassay, normal range <10 U/mL). Anaemia and neurological symptoms improved after vitamin B12 supplementation. Subsequently, she underwent gastric ESD for gastric tumour, and histopathological examination revealed gastric adenoma with negative margins (figure 4A). Gastric mucosa surrounding the tumour presented severe atrophy with intestinal metaplasia (figure 4B). Histopathological findings of the biopsy sample taken from the antrum revealed almost normal pyloric mucosa (figure 4C). Based on laboratory tests and endoscopic and histopathological findings, we diagnosed her with AIG. The patient has undergone maintenance therapy with vitamin B12 supplementation and regular endoscopic surveillance. During a follow-up period of 3 years, neither haematological nor neurological relapse has been observed. OGD showed no emergence of new gastric lesions.
Figure 3.
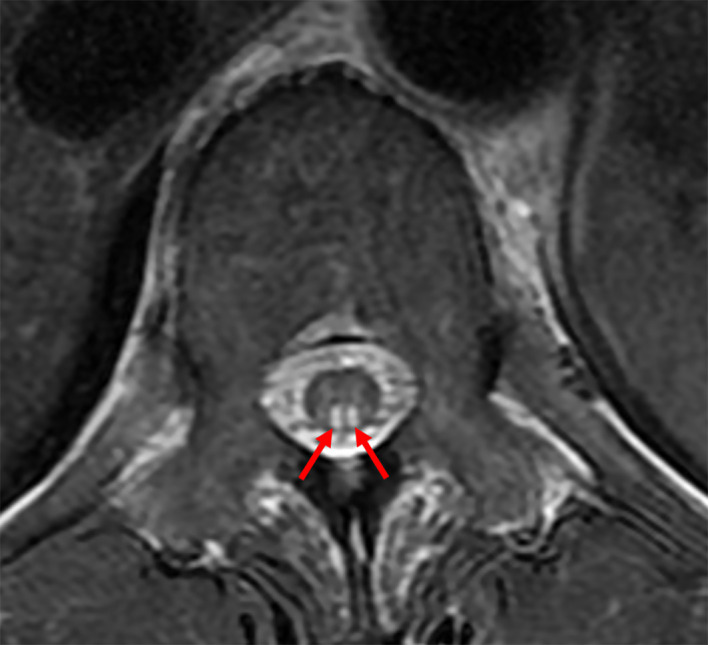
MRI showing a high-intensity area in the posterior column of the thoracic spinal cord on T2-weighted imaging.
Figure 4.
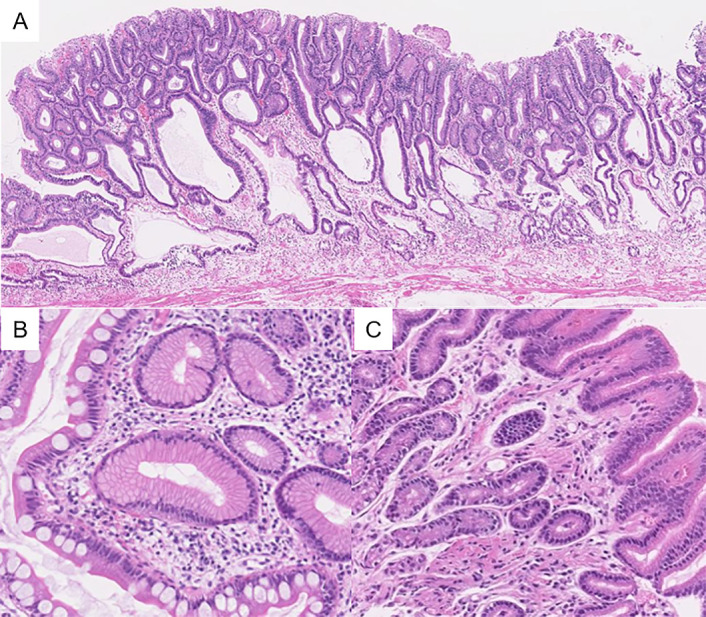
Histopathological images of the gastric adenoma (A) and background mucosa ((B) gastric body, (C) antrum)).
Discussion
Herein, we report a case of AIG that caused gastric adenoma and SCD of the spinal cord. Simultaneous occurrence of these two comorbidities is rare, and both diseases were successfully treated without adverse events or neurological sequelae. This case highlights the importance of early diagnosis of AIG and proper management of its comorbidities.
Miceli et al reported clinical clusters of AIG, and the most relevant factors leading to a diagnosis of AIG were haematological findings related to vitamin B12 deficiency (37.4%), followed by incidental histological evidence in gastric biopsy samples (34.3%), other autoimmune disease (9.1%) and neurological symptoms (6.1%).9 However, the diagnosis of AIG is often challenging due to its wide clinical spectrum ranging from asymptomatic condition to irreversible disease course. Recently, Lenti et al reported that female sex, history of infertility/miscarriage, high education level of the patients and previous misdiagnosis, especially functional dyspepsia, are the risk factors of diagnostic delay in AIG.10 Therefore, it is important to pay more attention to these factors to prevent diagnostic delay.
AIG is diagnosed by laboratory tests, endoscopy and histopathological evaluation.1 Since the discovery of autoantibodies against intrinsic factor and parietal cells in 1960s, these autoantibodies have been used for the diagnosis of AIG. Other laboratory tests, such as serum gastrin and pepsinogen, are helpful in the diagnosis. In AIG, the loss of parietal cells causes achlorhydria and increased secretion of gastrin from G cells.11 In contrast with gastrin, pepsinogen, a peptide secreted by chief cells, decreases as a result of the loss of oxyntic mucosa.12 A previous study reported that patients with AIG had high gastrin and low pepsinogen I levels.13 Recent expert review recommends measurement of antibody for parietal cells, serum gastrin and pepsinogen for first-line serological screening of AIG.1 The characteristic endoscopic findings of AIG are atrophy of the gastric body and fundus with a minimal disease of the antral mucosa.14 Magnifying endoscopy may identify these findings more clearly.15 16 Endoscopic biopsy protocol suggested by the updated Sydney system recommends taking two samples from the antrum, one sample from the incisura angularis and two samples from the corpus, in order to compare histological findings across compartments.17 Histopathological examination assesses the presence, degree and aetiology of inflammation. Because AIG is often associated with enterochromaffin-like cell hyperplasia, immunohistochemical staining for chromogranin or synaptophysin is recommended. In our case, a history of hypothyroidism and macrocytic anaemia with vitamin B12 deficiency were first signs indicative of AIG. Laboratory tests, endoscopic findings and histopathological evaluations confirmed the diagnosis.
The persistently gastric inflammation contributes to the development of gastric neoplasms including neuroendocrine tumours (NETs), gastric adenomas and carcinomas. It is well known that HP eradication therapy improves gastritis and leads to reduction of the incidence of gastric cancer and its related mortality.18 On the other hand, there has been no established treatment for AIG. In a previous large cohort study in Sweden, AIG was associated with a 3-fold increased risk of gastric carcinoma and a 13-fold increased risk of NET.19 A long-term single centre prospective study including a total of 282 patients in Italy reported that 12 patients (4.3%) had gastric neoplasms at the time of first OGD.20 Among the 270 patients, 17 newly gastric neoplasms (6.3%) were diagnosed in a 19-year follow-up period, of which 10 patients developed neuroendocrine or glandular dysplasia and 7 patients developed NET. No patient developed invasive adenocarcinoma. Additionally, the risk of developing gastric neoplasms was greater in patients with more severe atrophy. A recent multicentre study of AIG in Japan reported that the prevalence of gastric adenoma, adenocarcinoma and NET was 0.8%, 9.8% and 11.4%, respectively.5 This was a retrospective study, therefore, the results need to be deliberately interpreted because there was a selection bias, and HP infection was diagnosed only by the positive serum antibody against HP, with 7.8% of the total enrolled patients. Nevertheless, based on these prevalence data, careful endoscopic observation and adequate surveillance for gastric neoplasms will be required for patients with AIG.
The clinical goal of managing AIG is proper administration of vitamin B12 to prevent haematological and neurological comorbidities. A previous study reported that neuropsychiatric disorders caused by vitamin B12 deficiency can be present even in the absence of anaemia or macrocytosis,21 suggesting that neurological symptoms are not always correlated with the degree of anaemia. Similarly, the patient in this case developed SCD of the spinal cord despite having only mild anaemia. Other studies have reported that SCD of the spinal cord can be improved by the treatment of vitamin B12 therapy, but some neurological impairments may persist.22 23 Moreover, these studies also reported that the neurological outcome was associated with the duration of illness. Thus, early diagnosis and intervention of vitamin B12 deficiency is essential to prevent irreversible neurological impairment. In our case, initial treatment by vitamin B12 led to complete resolution of the patient’s neurological symptoms, and maintenance treatment has been effective for the prevention of the relapse.
Although little is known about pathogenesis of AIG, recent proteomics study have demonstrated a different proteomics signature of AIG from HP-associated gastritis, which includes the decreased abundance of proteins involved in the tricarboxylic acid cycle and the increased abundance of those involved in cytoskeleton and cadherin binding.24 In addition, 90% of the decreased proteins and 50% of the increased proteins were also found in gastric cancer, suggesting that these proteomics alternations may be part of the progression to gastric carcinogenesis. Thus, recent advances in understanding of molecular mechanism of AIG will contribute to resolve the clinical issues, such as diagnosis in early stage and development of the treatment.
In conclusion, we presented a case of AIG concomitant with gastric adenoma and SCD of the spinal cord. To improve the patient’s clinical outcome, physicians should remember the diagnostic approaches of AIG and proper management of its comorbidities.
Learning points.
Macrocytic anaemia and history of thyroiditis are diagnostic clues for autoimmune gastritis (AIG).
Proximal-predominant gastric atrophy is a diagnostic indicator for AIG.
Identifying AIG-related gastric neoplasms at an early stage would allow for curative resection by minimally invasive treatment.
Neurological symptoms caused by vitamin B12 deficiency are not always correlated with the severity of associated anaemia.
Early diagnosis of AIG may help to prevent irreversible neurological impairment due to subacute combined degeneration of the spinal cord.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Contributors: MT, GS and YS conceived the study. MT and GS acquired clinical data. MT, GS, YS and HN interpreted clinical data. MT, GS and HN wrote the manuscript. HN performed critical revision of the article for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lenti MV, Rugge M, Lahner E, et al. Autoimmune gastritis. Nat Rev Dis Primers 2020;6:56. 10.1038/s41572-020-0187-8 [DOI] [PubMed] [Google Scholar]
- 2.Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol 2013;10:529–41. 10.1038/nrgastro.2013.101 [DOI] [PubMed] [Google Scholar]
- 3.Furuta T, Baba S, Yamade M, et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. Aliment Pharmacol Ther 2018;48:370–7. 10.1111/apt.14849 [DOI] [PubMed] [Google Scholar]
- 4.Notsu T, Adachi K, Mishiro T, et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern Med 2019;58:1817–23. 10.2169/internalmedicine.2292-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terao S, Suzuki S, Yaita H, et al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig Endosc 2020;32:364–72. 10.1111/den.13500 [DOI] [PubMed] [Google Scholar]
- 6.Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ 2014;349:g5226. 10.1136/bmj.g5226 [DOI] [PubMed] [Google Scholar]
- 7.Stabler SP. Clinical practice. vitamin B12 deficiency. N Engl J Med 2013;368:149–60. 10.1056/NEJMcp1113996 [DOI] [PubMed] [Google Scholar]
- 8.Ota K, Yamaguchi R, Tsukahara A, et al. Subacute combined degeneration of the spinal cord caused by autoimmune gastritis. Intern Med 2020;59:2113–6. 10.2169/internalmedicine.4684-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miceli E, Lenti MV, Padula D, et al. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol 2012;10:812–4. 10.1016/j.cgh.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 10.Lenti MV, Miceli E, Cococcia S, et al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment Pharmacol Ther 2019;50:167–75. 10.1111/apt.15317 [DOI] [PubMed] [Google Scholar]
- 11.Strickland RG, Bhathal PS, Korman MG, et al. Serum gastrin and the antral mucosa in atrophic gastritis. Br Med J 1971;4:451–3. 10.1136/bmj.4.5785.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso N, Granada ML, Soldevila B, et al. Serum autoimmune gastritis markers, pepsinogen I and parietal cell antibodies, in patients with type 1 diabetes mellitus: a 5-year prospective study. J Endocrinol Invest 2011;34:340–4. 10.1007/BF03347456 [DOI] [PubMed] [Google Scholar]
- 13.Rembiasz K, Konturek PC, Karcz D, et al. Biomarkers in various types of atrophic gastritis and their diagnostic usefulness. Dig Dis Sci 2005;50:474–82. 10.1007/s10620-005-2461-8 [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Lam-Himlin D, Vemulapalli R. Review of autoimmune metaplastic atrophic gastritis. Gastrointest Endosc 2013;77:284–92. 10.1016/j.gie.2012.09.033 [DOI] [PubMed] [Google Scholar]
- 15.Yagi K, Aruga Y, Nakamura A, et al. Regular arrangement of collecting venules (Rac): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophago-gastric adenocarcinoma. J Gastroenterol 2005;40:443–52. 10.1007/s00535-005-1605-0 [DOI] [PubMed] [Google Scholar]
- 16.Yagi K, Nakamura A, Sekine A, et al. Features of the atrophic corpus mucosa in three cases of autoimmune gastritis revealed by magnifying endoscopy. Case Rep Med 2012;2012:1–4. 10.1155/2012/368160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. 10.1097/00000478-199610000-00001 [DOI] [PubMed] [Google Scholar]
- 18.Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113–21. 10.1136/gutjnl-2020-320839 [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993;71:745–50. [DOI] [PubMed] [Google Scholar]
- 20.Miceli E, Vanoli A, Lenti MV, et al. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther 2019;50:1172–80. 10.1111/apt.15540 [DOI] [PubMed] [Google Scholar]
- 21.Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988;318:1720–8. 10.1056/NEJM198806303182604 [DOI] [PubMed] [Google Scholar]
- 22.Shevell MI, Rosenblatt DS. The neurology of cobalamin. Can J Neurol Sci 1992;19:472–86. 10.1017/S0317167100041676 [DOI] [PubMed] [Google Scholar]
- 23.Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med 2006;21:1063–8. 10.1111/j.1525-1497.2006.00525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Repetto O, De Re V, Giuffrida P, et al. Proteomics signature of autoimmune atrophic gastritis: towards a link with gastric cancer. Gastric Cancer 2021. 10.1007/s10120-020-01148-3. [Epub ahead of print: 23 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]


