Abstract
The COVID-19 pandemic has led to a rise in cases of Guillain-Barré syndrome (GBS). This autoimmune sequela is a manifestation of the neurotropism potential of the virus. At present, knowledge regarding the pathophysiology, clinical features, management and outcomes of the condition is still evolving. This paper presents the case of a 22-year-old pregnant patient who came in with a history of upper respiratory tract symptoms followed by acroparaesthesia and progressive ascending weakness. She was confirmed to have COVID-19 and GBS and was subsequently managed with intravenous immunoglobulin (IVIg) followed by supportive therapy. To the authors’ knowledge and based on their literature search, this is the first reported case of GBS in a COVID-19 confirmed pregnant patient who received IVIg.
Keywords: neurology, peripheral nerve disease, pregnancy
Background
Since the first reported case of COVID-19, new knowledge has continuously surfaced about the novel coronavirus. Although its manifestations are mostly respiratory in nature, reports have confirmed the agent’s propensity for neurotropism. This report offers novel insights regarding the management, challenges and outcomes of COVID-19 associated Guillain-Barré syndrome (GBS) in a pregnant patient.
Case presentation
We present the case of a 22-year-old gravida 2 para 0 (0-0-1-0) at 20 weeks age of gestation (AOG), who presented with colds and non-productive cough without associated fever, dyspnoea, abdominal pain, vomiting or diarrhoea. A week after the onset of these symptoms, she developed acroparaesthesias followed by bilateral lower extremity weakness, which eventually progressed to bilateral upper extremity weakness, dysphonia and dysphagia. Her medical, family and personal-social history were all unremarkable.
She was initially admitted at a local hospital and was managed as a case of hypokalaemic period paralysis after noting that her serum potassium was 2.18 mEq/L. However, her weakness persisted despite electrolyte correction. GBS was then suspected, but her nasopharyngeal swab came out positive for SARS-CoV-2 RNA warranting her to be transferred to a COVID-19 referral centre where neurological examination revealed facial diplegia, poor gutturals, hypotonic areflexic quadriparesis and decreased sensation over the distal arms and legs. Proximal muscle strength was Medical Research Council (MRC) 3/5, while distal muscle strength was MRC 1/5.
Nerve conduction studies were deferred initially as there was no dedicated machine at the COVID-19 wards. Lumbar tap revealed no albuminocytological dissociation—white cell count: 0 and total protein 12 mg/dL (normal value: 12–60 mg/dL). Given that her clinical profile was highly suggestive of GBS, she was started on a 5-day course of intravenous immunoglobulin (IVIg) at 0.4 g/kg/day. There were no adverse reactions observed during the therapy, and she subsequently reported less dyspnoea and plateauing of weakness. During her hospitalisation, her blood pressure was noted to spike to 140–150/90–100 mm Hg. Dysautonomia from GBS was deemed unlikely as she did not present with other haemodynamic fluctuations. She did not complain of headache or visual disturbances or present with signs of pulmonary oedema. Liver enzymes revealed to be thrice elevated (Aspartate Aminotransferase (AST): 117 IU/L, Alanine Transaminase (ALT): 115 IU/L). For these, she was diagnosed with pre-eclampsia without severe features and was started on methyldopa 250 mg/tablet, one tablet twice a day and aspirin 100 mg/tablet, one tablet once a day. On testing negative for SARS-CoV-2, electrodiagnostic examination was done and showed absent sural, median, ulnar and radial sensory potentials. All compound muscle action potential (CMAP) amplitudes were significantly reduced, and CMAP latencies and conduction velocities were prolonged. F waves were absent, and temporal dispersions were observed supporting a predominantly demyelinating pattern (table 1). Additionally, denervation changes were noted during electromyography, which suggested secondary axonal loss.
Table 1.
Result of nerve conduction studies
| Motor nerve conduction studies | ||||
| Nerve | Latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | F latency (ms) |
| Left median | ||||
| Wrist | NR | NR | – | NR |
| Elbow | NR | NR | ||
| Left ulnar | ||||
| Wrist | 3.10 (<3) | 0.77 (>6) | 48.9 (>50) | NR |
| Elbow | 8.00 | 0.56 | ||
| Left peroneal | ||||
| Ankle | 5.10 (<5) | 0.07 (>2) | 34.4 (>40) | |
| Below Knee | 14.1 | 0.06 | ||
| Left tibial | ||||
| Ankle | 5.60 (<6) | 0.47 (>6) | 37.1 (>40) | NR |
| Below Knee | 15.4 | 0.17 | ||
| Right peroneal | ||||
| Ankle | NR | NR | – | |
| Below Knee | NR | NR | ||
| Right tibial | ||||
| Ankle | 5.30 (<6) | 0.25 (>6) | 42.8 (>40) | NR |
| Below Knee | 14.4 | 0.11 | ||
| Sensory nerve conduction studies | ||||
| Nerve | Latency (ms) | Amplitude (µV) | Conduction velocity (m/s) | |
| Left median | NR | NR | – | |
| Left ulnar | NR | NR | – | |
| Left radial | NR | NR | – | |
| Left sural | NR | NR | – | |
| Right sural | NR | NR | – | |
Normal values in parentheses.
NR, no response.
Outcome and follow-up
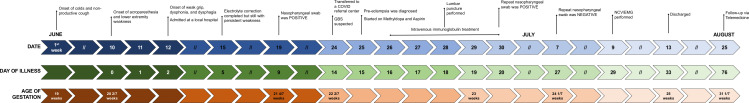
She was discharged on day 33 of illness, day 13 post-IVIG and at 25 weeks AOG with improved proximal muscle strength of 4/5 MRC and distal muscle strength of 2/5 MRC. Follow-up 1 month postdischarge revealed resolution of bulbar symptoms and gradual increase in her ability to perform her activities of daily living with minimal assistance. She then continued her follow-up with adult neurology, rehabilitation medicine and obstetrics-gynaecology. At 37 weeks AOG, her pregnancy was successfully delivered via assisted-vaginal delivery. Figure 1 shows the timeline of pertinent events in the patient’s illness.
Figure 1.
Timeline of pertinent events in the course of the patient’s illness and hospitalisation. Top row: specific date of events. Middle row: day of illness from onset of Guillain-Barr symptoms. Bottom row: age of gestation.
Discussion
GBS is the most common cause of acute to subacute flaccid paralysis worldwide. Its pathophysiology centres on molecular mimicry wherein the antibodies against an invading pathogen attach to epitopes on neuronal cell membranes leading to a cascade of complement activation and destruction.1 2 Although not commonly implicated, human coronaviruses have been shown to display this autoimmune sequela.
SARS-CoV-2 mainly infects the respiratory system by using the spike glycoprotein to bind to the ACE-2 receptor in the respiratory epithelium. Its neurotropic and neuroinvasive potential also comes from the same spike protein as this binds to the ACE-2 receptor and the sialic acid component of cell membrane glycoproteins and gangliosides.3 4 The virus also indirectly produces antibodies such as GD1b, GQ1b and GT1b, which act against neuronal surface glycoproteins or gangliosides.3 4 Peripheral demyelination is then worsened by the dysregulated cytokine and chemokine formation.3
The systematic review done by Uncini and colleagues5 showed a total of 42 published cases of COVID-19 associated GBS. Males comprised the majority. The youngest patient was a 21-year-old man, while the oldest was a 77-year-old woman. The most common neurological feature was hyporeflexia followed only by sensory disturbances and tetraparesis. Classically, GBS follows a postinfectious pattern with a preceding non-specific infection 1–4 weeks prior to the onset of neurological symptoms. Most of the reported cases followed this pattern as the interval from COVID-19 infection to the onset of GBS ranged from 3 to 28 days.5 The lumbar tap done for the reported cases also predominantly showed albuminocytological dissociation and undetectable SARS-CoV-2 viral RNA among the cerebrospinal fluid (CSF) samples which were tested. Acute inflammatory demyelinating polyradiculoneuropathy was the most common electrodiagnostic feature documented, and intravenous immunoglobulin was given in majority of the cases. They, however, displayed mixed outcomes.5
In this paper, the patient also displayed a postinfectious pattern as her neurological symptoms occurred 7 days after the onset of her viral symptoms. However, albuminocytological dissociation was absent. The said phenomenon is classically associated with GBS beginning 10–14 days from the illness onset. Its peak is then observed at 4–6 weeks. CSF total protein is usually not elevated during the first few days of the illness; however, as much as 10% of cases display normal values throughout the course of the disease. Review of available literature has not revealed potential mechanisms or differentials for this phenomenon.6 7
At that time, her CSF was also not examined for the presence of SARS-CoV-2 viral RNA as there were no diagnostic centres able to perform the said test. The patient is also the youngest among the reported female cases and to the authors’ knowledge, the only COVID-positive pregnant patient with GBS who received IVIG.
GBS in pregnancy is rare with cases occurring mostly during the second and third trimester or the first month postpartum because of the predominance of Th1 cells that produce proinflammatory cytotoxic cytokines during these periods.2 8 Prior to the advent of immunotherapies, obstetric patients experienced significant morbidity and mortality with 20% becoming disabled, 35% requiring ventilatory support and 13% succumbing to complications. Premature deliveries also increased in severe cases.9 10 The rapidity of disease onset, severity of paralysis and duration of peak paralysis portended poor prognosis.11
Management of GBS in pregnancy is mainly supportive but IVIg and total plasma exchange, considered equally effective, may be given to alter the natural course of the disease.12 13 Therapy offers maximal benefit if started within the first 2 weeks from symptom onset, although it may be given as late as 4 weeks.2 14 The standard IVIg regimen of 0.4 g/kg/day for 5 days is usually preferred because of better availability and simplicity. However, the randomised trials that evaluated their efficacy excluded obstetric patients; hence, outcome data for this population were mainly derived from case reports.2 12
COVID-19 is known for its thrombotic complications because of the resulting dysregulated immune response.15 As such, treatment modalities to address this have been looked into as a potential treatment. Current studies for the use of IVIg in the treatment of COVID-19 showed that its administration offered reduced mortality rates especially if given early.16 17 Sakoulas and colleagues15 even mentioned that IVIg can diminish the coagulopathy brought about by COVID-19 in contrast to the initial concern that the treatment will increase thrombotic events in these patients.
Overall, this case report has shown that IVIg is a reasonable treatment option for pregnant patients with GBS even if they are COVID-19 positive.
Learning points.
In most cases, the history and physical examination alone play significant roles for us to arrive at the correct impression for patients presenting with progressive weakness.
To our knowledge, our case is the first documented COVID-19 associated Guillain-Barré syndrome (GBS) in a pregnant woman who was treated with intravenous immunoglobulin (IVIg).
The management of COVID-19 associated GBS in pregnancy is mainly supportive with immunotherapies such as IVIg only administered to halt the course of the disease.
Although the theoretical risk of a superimposed prothrombotic state secondary to COVID-19 and IVIg has been raised, studies have showed that the treatment modality is well tolerated.
Further studies are still needed to better understand the features, management and outcomes of COVID-19 associated GBS especially in the obstetric population.
Footnotes
Twitter: @WilsonTuralde
Contributors: JJG: conceptualisation, literature review, manuscript writing, manuscript editing. CWT and VMA: conceptualisation, manuscript review and editing. MAB: performance and analysis of electrodiagnostic test, manuscript review and editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Donofrio PD. Guillain-Barré syndrome. Continuum 2017;23:1295–309. 10.1212/CON.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 2.Pacheco LD, Saad AF, Hankins GDV, et al. Guillain-Barré syndrome in pregnancy. Obstet Gynecol 2016;128:1105–10. 10.1097/AOG.0000000000001716 [DOI] [PubMed] [Google Scholar]
- 3.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 2020;142:14–22. 10.1111/ane.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol neuroinflammation 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uncini A, Vallat J-M, Jacobs BC. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry 2020:1–6 http://www.ncbi.nlm.nih.gov/pubmed/32855289 [DOI] [PubMed] [Google Scholar]
- 6.Gunatilake SSC, Gamlath R, Wimalaratna H. An unusual case of recurrent Guillain-Barré syndrome with normal cerebrospinal fluid protein levels: a case report. BMC Neurol [Internet] 2016;16:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma M, Kes P, Bašić-Kes V, et al. Guillain-Barré syndrome in a patient suffering acute myocardial infarction. Acta Clin Croat 2002;41:255–7. [Google Scholar]
- 8.Meenakshi-Sundaram S, Swaminathan K, Karthik SN, et al. Relapsing Guillain-Barre syndrome in pregnancy and postpartum. Ann Indian Acad Neurol 2014;17:352–4. 10.4103/0972-2327.138527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada H, Noro N, Kato EH, et al. Massive intravenous immunoglobulin treatment in pregnancy complicated by Guillain-Barré syndrome. Eur J Obstet Gynecol Reprod Biol 2001;97:101–4. 10.1016/S0301-2115(00)00481-4 [DOI] [PubMed] [Google Scholar]
- 10.Sharma SR, Sharma N, Masaraf H, et al. Guillain-Barré syndrome complicating pregnancy and correlation with maternal and fetal outcome in North eastern India: a retrospective study. Ann Indian Acad Neurol 2015;18:215–8. 10.4103/0972-2327.150608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hukuimwe M, Matsa TT, Gidiri MF. Guillain-Barré syndrome in pregnancy: a case report. Women’s Heal 2017;13:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuero MR, Varelas PN. Neurologic complications in pregnancy. Crit Care Clin 2016;32:43–59. 10.1016/j.ccc.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Gold R, Stangel M, Dalakas MC. Drug Insight: the use of intravenous immunoglobulin in neurology--therapeutic considerations and practical issues. Nat Clin Pract Neurol 2007;3:36–44. 10.1038/ncpneuro0376 [DOI] [PubMed] [Google Scholar]
- 14.Elovaara I, Hietaharju A. Can we face the challenge of expanding use of intravenous immunoglobulin in neurology? Acta Neurol Scand 2010;122:309–15. 10.1111/j.1600-0404.2009.01317.x [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor 2020;2:e0280. 10.1097/CCE.0000000000000280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herth FJF, Sakoulas G, Haddad F. Use of intravenous immunoglobulin (Prevagen or Octagam) for the treatment of COVID-19: retrospective case series. Respiration 2021;99:1145–53. 10.1159/000511376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao W, Liu X, Hong K. High-Dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol 2021;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]