Abstract
A 32-year-old woman presented with an incidental finding of hypokalaemia on routine bloods at 9 weeks of a second pregnancy, on a background of lifelong salt craving. Her previous pregnancy was uncomplicated. She had no previous significant medical or family history. Venous blood gases showed a hypokalaemic, normochloraemic metabolic alkalosis. Urinary potassium was elevated. Escalating doses of oral supplementation of potassium, magnesium, sodium and potassium-sparing diuretics were required through the course of pregnancy, in response to regular electrolyte monitoring. These were later weaned and completely stopped post partum. Delivery was uneventful with no maternal or neonatal complications. Genetic testing performed post partum showed heterogenous mutation of SCL12A3 gene.
Keywords: fluid electrolyte and acid-base disturbances, pregnancy
Background
Gitelman syndrome is an inherited autosomal recessive salt-wasting renal tubulopathy.1 2 Presentation can be precipitated by stressors such as pregnancy. While maternal and fetal outcomes are largely favourable, regular monitoring and replacement of serum electrolytes is essential. Here, we report a case presentation of Gitelman syndrome diagnosed in the first trimester of the second pregnancy that was managed on an outpatient basis with an uncomplicated delivery and good neonatal outcome.
Case presentation
A 32-year-old fit and healthy gym instructor presented with an incidental finding of serum potassium of 2.1 mmol/L (normal level 3.5–4.5 mmol/L) on routine bloods at 9 weeks pregnancy. She was otherwise asymptomatic although reported lifelong salt craving. Her previous pregnancy abroad was unremarkable, although delivery was complicated by neonatal meconium aspiration requiring neonatal unit admission and ventilation. While booking blood tests were performed in the first pregnancy, electrolytes were not measured.
Venous blood gas showed a pH of 7.507 (normal level 7.350–7.450), potassium of 2.6 mmol/L, with a calcium of 1.13 mmol/L (normal level 1.13–1.32 mmol/L), bicarbonate of 28.8 mmol/L (normal level 22–28 mmol/L) and chloride of 102 mmol/L (normal level 95–108 mmol/L). On examination, her blood pressure was 108/22. ECG showed normal sinus rhythm. General examination was unremarkable. Due to severe hypokalaemia, she was admitted with 40 mmol of potassium replaced intravenously—post-replacement potassium was 3.0 mmol/L. She was subsequently discharged on the same day with oral potassium supplements in the form of Sando-K at a dose of two tablets, three times a day.
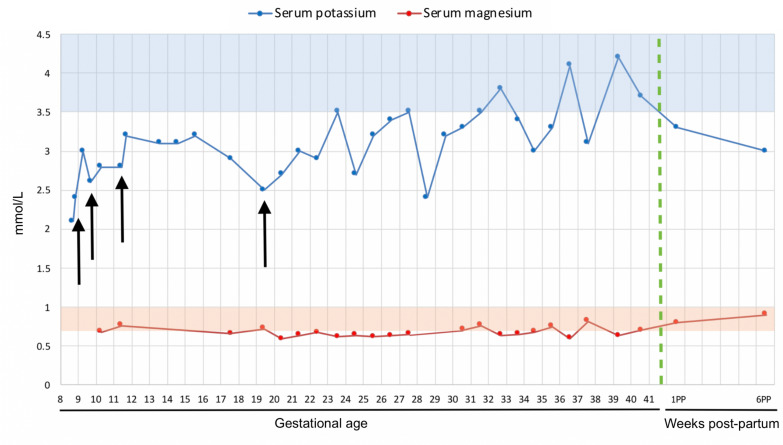
Despite this, repeat blood tests a week later (gestational age 9 weeks, 5 days) showed a potassium of 2.6 mmol/L. Serum magnesium levels were found to be 0.68 mmol/L (normal level 0.7–1.0 mmol/L) and bicarbonate 30 mmol/L (normal level 22–29 mmol/L). Amiloride, a potassium-sparing diuretic, at 2.5 mg one time a day was therefore commenced. Serum electrolytes were monitored every 2 weeks (figure 1).
Figure 1.
Chart showing antepartum and post-partum trend of potassium and magnesium. Regions shaded blue and orange indicate the normal ranges of potassium and magnesium, respectively. Black arrows indicate points where treatment was initiated or changed. The green dotted line indicates date of delivery.
Treatment was subsequently escalated 2 weeks later (gestational age 11 weeks, 5 days) to 5 mg of amiloride once daily. Slow sodium tablets were initiated at two tablets, three times a day. Repeat serum potassium was 3.2 mmol/L. Urinary potassium was 145 mmol/day (normal level 25–125 mmol/day).
Eight weeks later (gestational age 19 weeks, 5 days), serum potassium was found to be 2.5 mmol/L and serum magnesium 0.72 mmol/L. Doses of amiloride, Sando-K and slow sodium tablets were therefore increased to 10 mg one time a day; two tablets four times a day; and two tablets four times a day, respectively. Eplerenone was also commenced at 25 mg one time a day.
Weekly blood tests for electrolytes were subsequently performed until delivery at a gestational age of 41 weeks and 4 days. No further changes to medications were made.
Serial growth scans at 12, 24, 28, 32 and 36 weeks showed a normal sized baby around the 50th centile, with normal liquor volume, although a breech position was noted on scans at 24, 28 and 36 weeks. Labour was induced via a membrane sweep performed at a gestational age of 41 weeks and 2 days, with an uncomplicated normal cephalic vaginal delivery 2 days later.
Genetic testing performed post partum via next generation sequencing demonstrated heterozygosity for a missense mutation c.3089A>G, p.Gln1030Arg.
Differential diagnosis
Hypokalaemic, normochloraemic metabolic alkalosis have multiple differentials, such as vomiting, diarrhoea, use of laxatives or diuretics or primary hyperaldosteronism—several of these were eliminated through the history, clinical observations and electrolyte panel.
The two main differentials to consider were therefore Bartter and Gitelman syndrome. However, Bartter syndrome often presents at an earlier age with more severe symptoms such as growth retardation, polyuria and polydipsia—akin to the effects of loop diuretics. By contrast, Gitelman syndrome is often asymptomatic and usually diagnosed incidentally.3
In this case, diagnosis was determined through genetic testing which showed heterozygosity for SCL12A3 gene mutation.
Outcome and follow-up
She was gradually weaned off her medications post partum, and was medication-free 5 weeks after. Serum potassium levels 6 weeks post partum were 3.0 mmol/L, and magnesium 0.9 mmol/L. Further blood tests 12 weeks post partum showed complete normalisation of serum electrolytes, with a potassium of 4.2 mmol/L and magnesium of 0.87 mmol/L.
Discussion
Gitelman syndrome is an autosomal recessive salt-wasting renal tubulopathy that is characterised by hypokalaemic metabolic alkalosis with hypomagnesaemia, with a prevalence of 1:40 000.1 2 This is due to mutations in the SCL12A3 gene that encode the thiazide-sensitive sodium chloride co-transporter found in the distal convoluted tubule.4 Over 350 mutations have been discovered so far, of which missense mutations made up 59%. Analysis of 448 cases by Vargas-Poussou et al showed that 70% had two affected alleles, of which 25% were homozygous and 74.9% compound heterozygous. However, 18% of cases only had one detectable mutant allele—47% of these were found to have large rearrangements on screening by MPLA or QMPSF.5 Notably, the method of next generation sequencing used in this case is known to not detect large deletions and duplication variants—therefore it is possible that genetic variants may be present, although not profiled.
Alternatively, clinically manifest Gitelman syndrome in a heterozygote could be explained by epigenetic modifications, silent polymorphisms6 7 or the effects of proteins such as lysine deficient protein kinase 1 and 4 affecting the expression of the thiazide-sensitive sodium chloride co-transporter in the distal nephron.8 Interestingly, a study conducted by Xia et al found that individuals with a single heterozygous mutation of SCL12A3 demonstrated a milder degree of hypokalaemia compared with homozygotes or individuals with complex heterozygous mutations,9 and may therefore require a stressor such as pregnancy to provoke clinically evident tubulopathy.
For instance, physiological changes during pregnancy result in elevation of renin and aldosterone levels—despite this, excessive urinary potassium loss does not occur due to tubular adaptations for potassium reabsorption,10 11 which may be in part caused by anti-mineralocorticoid effects of progesterone.12 However, it has been postulated that these compensatory mechanisms are insufficient in Gitelman syndrome.13 Pregnancy therefore can act as a stressor precipitating a new presentation of tubulopathy.
The mainstay of management comprises of potassium and magnesium replacement. In instances of severe persistent hypokalaemia despite oral supplementation, potassium-sparing diuretics,14–17 renin-angiotensin system antagonists18 and non-steroidal anti-inflammatory medications,19 20 can be used in combination;21 the first is the only viable option during pregnancy. Additionally, due to ongoing metabolic alkalosis, chloride should concurrently be supplemented with potassium. Hypomagnesaemia should also be corrected as it can aggravate and cause refractory hypokalaemia.22 Sodium replacement is also crucial to reverse secondary hyperaldosteronism that occurs in Gitelman syndrome1—a compensatory mechanism due to the inactivation of thiazide-sensitive sodium chloride co-transporters. This effect is compounded by raised aldosterone levels in pregnancy. Salt supplementation therefore is essential to dampen the effects of hyperaldosteronism and reduce potassium wasting.
Gitelman syndrome in pregnancy is associated with largely favourable maternal outcomes, although with wide variability in the extent of electrolyte disturbance and subsequent management.23–31 For instance, a dramatic rise in electrolyte requirements in Gitelman syndrome during pregnancy requiring multiple admissions for intravenous replacement have been described in three case reports. Of these, two were diagnosed with Gitelman syndrome prior to pregnancy, one of which was on oral potassium and magnesium supplements, while the other only required oral potassium supplements; while pregnant the former required 39 admissions with escalating frequency to biweekly for intravenous supplementation of magnesium and potassium,27 while the latter had three admissions for intravenous potassium and magnesium replacement.30 Interestingly, in the case described by McCarthy et al, electrolyte supplementation requirement returned to prepregnancy levels following delivery.27 The third case was diagnosed following a second pregnancy with persistent hypokalaemia and hypomagnesaemia requiring regular intravenous potassium during the last 4 months of pregnancy and in the postnatal period, although there was also a history of hypokalaemia requiring oral supplementation and intravenous replacement during labour during the first pregnancy as well.13 While the patient in this case report did not require frequent admission for intravenous electrolyte replacement, these three cases demonstrate the extreme of management required during the intrapartum phase.
By contrast, there are some instances of Gitelman syndrome in pregnancy requiring higher doses of oral supplementation without the need for repeated admission for intravenous replacement. Mathen et al and Raffi et al both describe cases of established Gitelman syndrome on regular potassium, magnesium or potassium sparing diuretic prior to conception requiring escalating doses of their regular medications.
Neither patient required admission during prior to delivery for intravenous electrolyte replacement. Interestingly, following delivery, oral supplementation requirement returned to baseline for both patients.31 32
Similar to the patient described in this case report, Daskalakis et al describe a case of Gitelman syndrome that was diagnosed in the intrapartum phase, requiring a single admission for intravenous potassium and magnesium at 10 weeks gestation, with oral potassium and magnesium supplementation commenced for the duration of pregnancy.23 This suggests that pregnancy can act as a stressor to aggravate pre-existing disease or precipitate the presentation of quiescent disease, with increasing electrolyte requirements during the intrapartum period and a return to preconception levels following delivery. Of note, sodium supplementation was not described in any of the cases—it would be interesting to observe the impact of sodium replacement on the severity of electrolyte requirements in future cases.
With regards to fetal outcomes, uneventful neonatal postpartum outcomes were described for several of the abovementioned.23 27 30–32 However, oligohydramnios has been reported in six cases23–25 27 29 along with one case of fetal demise26—whether this was dependent on maternal or fetal factors is indeterminate. Neonatal presentation has been reported in a pair of prematurely born twins noted to have a tendency towards hypokalaemia from the third week of life; both pairs of twins were placed on oral potassium supplements, with one set of twins also treated with indomethacin.33 There is a further case of Gitelman diagnosed at 5 months due to failure to thrive and severe dehydration managed with oral potassium and magnesium supplementation.34 However, Gitelman is usually diagnosed in early adulthood or later childhood. Despite this, it should be noted that it is not a benign disease and can present with tetanic episodes or short stature at school age.35 However, there is yet to be any cases diagnosed in the prenatal stage, in part due to a lack of clear indicators for prenatal screening and fetal surveillance for Gitelman syndrome, although this may well change with growing awareness of the potential consequences of paediatric disease.
Patient’s perspective.
I didn’t have any symptoms as such, when my potassium was 2.1 mmol/L. I was still regularly teaching around 10 or more fitness classes a week (including a spin and weight class). I ran the London Marathon too in 4 hours with still no symptoms. Since giving birth, although my levels seem to be levelling out—I don’t feel any different. I also don’t believe I experienced it in my first pregnancy—I have always enjoyed salt and additional salt on already salty food. I lived abroad for my first pregnancy.
Learning points.
Physiological stressors such as pregnancy can unmask inherited tubulopathies such as Gitelman syndrome.
Sodium supplementation, alongside potassium and magnesium if required, is crucial to the management of Gitelman syndrome.
Doses of electrolyte supplementation may need to be escalated in pregnancy with Gitelman syndrome.
Regular monitoring of electrolytes to titrate replacement must be performed.
Footnotes
Contributors: ML performed collection of clinical data and wrote up the manuscript after a review of the relevant literature. DG is the senior author who reviewed and supervised the writing of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Knoers NVAM, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis 2008;3:22. 10.1186/1750-1172-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 1966;79:221–35. [PubMed] [Google Scholar]
- 3.Al Shibli A, Narchi H, Bartter NH. Bartter and Gitelman syndromes: spectrum of clinical manifestations caused by different mutations. World J Methodol 2015;5:55–61. 10.5662/wjm.v5.i2.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 1996;12:24–30. 10.1038/ng0196-24 [DOI] [PubMed] [Google Scholar]
- 5.Vargas-Poussou R, Dahan K, Kahila D, et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 2011;22:693–703. 10.1681/ASN.2010090907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reissinger A, Ludwig M, Utsch B, et al. Novel NCCT gene mutations as a cause of Gitelman's syndrome and a systematic review of mutant and polymorphic NCCT alleles. Kidney Blood Press Res 2002;25:354–62. 10.1159/000068695 [DOI] [PubMed] [Google Scholar]
- 7.Riveira-Munoz E, Chang Q, Godefroid N, et al. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 2007;18:1271–83. 10.1681/ASN.2006101095 [DOI] [PubMed] [Google Scholar]
- 8.Yang C-L, Angell J, Mitchell R, et al. Wnk kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 2003;111:1039–45. 10.1172/JCI17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia M-F, Bian H, Liu H, et al. Hypokalemia, hypomagnesemia, hypocalciuria, and recurrent tetany: Gitelman syndrome in a Chinese pedigree and literature review. Clin Case Rep 2017;5:578–86. 10.1002/ccr3.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sealey JE, Itskovitz-Eldor J, Rubattu S, et al. Estradiol- and progesterone-related increases in the renin-aldosterone system: studies during ovarian stimulation and early pregnancy. J Clin Endocrinol Metab 1994;79:258–64. 10.1210/jcem.79.1.8027239 [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Sinosich MJ, Saunders DM, et al. Potassium regulation and progesterone-aldosterone interrelationships in human pregnancy: a prospective study. Am J Obstet Gynecol 1986;155:349–53. 10.1016/0002-9378(86)90824-0 [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich EN, Lindheimer MD. Effect of administered mineralocorticoids or ACTH in pregnant women. attenuation of kaliuretic influence of mineralocorticoids during pregnancy. J Clin Invest 1972;51:1301–9. 10.1172/JCI106926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koudsi L, Nikolova S, Mishra V. Management of a severe case of Gitelman syndrome with poor response to standard treatment. BMJ Case Rep 2016;2016. 10.1136/bcr-2015-212375. [Epub ahead of print: 17 Feb 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colussi G, Rombolà G, De Ferrari ME, et al. Correction of hypokalemia with antialdosterone therapy in Gitelman's syndrome. Am J Nephrol 1994;14:127–35. 10.1159/000168701 [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Yoshida M, Nakayama M, et al. Eplerenone improved hypokalemia in a patient with Gitelman's syndrome. Intern Med 2012;51:83–6. 10.2169/internalmedicine.51.5723 [DOI] [PubMed] [Google Scholar]
- 16.Morton A. Eplerenone in the treatment of Gitelman's syndrome. Intern Med J 2008;38:377. 10.1111/j.1445-5994.2008.01664.x [DOI] [PubMed] [Google Scholar]
- 17.Morton A, Panitz B, Bush A. Eplerenone for Gitelman syndrome in pregnancy. Nephrology 2011;16:349. 10.1111/j.1440-1797.2010.01396.x [DOI] [PubMed] [Google Scholar]
- 18.Brambilla G, Perotti M, Perra S, et al. It is never too late for a genetic disease: a case of a 79-year-old man with persistent hypokalemia. J Nephrol 2013;26:594–8. 10.5301/jn.5000256 [DOI] [PubMed] [Google Scholar]
- 19.Liaw LC, Banerjee K, Coulthard MG. Dose related growth response to indometacin in Gitelman syndrome. Arch Dis Child 1999;81:508–10. 10.1136/adc.81.6.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkins N, Wallis M, McGillivray B, et al. A severe phenotype of Gitelman syndrome with increased prostaglandin excretion and favorable response to indomethacin. Clin Kidney J 2014;7:306–10. 10.1093/ckj/sfu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard A, Vargas-Poussou R, Vallet M, et al. Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome. J Am Soc Nephrol 2015;26:468–75. 10.1681/ASN.2014030293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed F, Mohammed A. Magnesium: the forgotten Electrolyte-A review on hypomagnesemia. Med Sci 2019;7. 10.3390/medsci7040056. [Epub ahead of print: 04 Apr 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daskalakis G, Marinopoulos S, Mousiolis A, et al. Gitelman syndrome-associated severe hypokalemia and hypomagnesemia: case report and review of the literature. J Matern Fetal Neonatal Med 2010;23:1301–4. 10.3109/14767051003678010 [DOI] [PubMed] [Google Scholar]
- 24.de Arriba G, Sánchez-Heras M, Basterrechea MA. Gitelman syndrome during pregnancy: a therapeutic challenge. Arch Gynecol Obstet 2009;280:807–9. 10.1007/s00404-009-0994-3 [DOI] [PubMed] [Google Scholar]
- 25.de Haan J, Geers T, Berghout A. Gitelman syndrome in pregnancy. Int J Gynaecol Obstet 2008;103:69–71. 10.1016/j.ijgo.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Lakhi N, Jones J, Govind A. Fetal demise despite normalisation of serum potassium in Gitelman syndrome. Case report and literature review. Aust N Z J Obstet Gynaecol 2010;50:301–2. 10.1111/j.1479-828X.2010.01156.x [DOI] [PubMed] [Google Scholar]
- 27.McCarthy FP, Magee CN, Plant WD, et al. Gitelman's syndrome in pregnancy: case report and review of the literature. Nephrol Dial Transplant 2010;25:1338–40. 10.1093/ndt/gfp688 [DOI] [PubMed] [Google Scholar]
- 28.Kwan TK, Falk MC. Second pregnancy outcome in a patient with Gitelman syndrome without the use of parenteral electrolyte supplementation. Aust N Z J Obstet Gynaecol 2011;51:94–5. 10.1111/j.1479-828X.2010.01248.x [DOI] [PubMed] [Google Scholar]
- 29.Jones JM, Dorrell S. Outcome of two pregnancies in a patient with Gitelman's syndrome-a case report. J Matern Fetal Investig 1998;8:147–8. [PubMed] [Google Scholar]
- 30.Talaulikar GS, Falk MC. Outcome of pregnancy in a patient with Gitelman syndrome: a case report. Nephron Physiol 2005;101:p35–8. 10.1159/000086418 [DOI] [PubMed] [Google Scholar]
- 31.Raffi F, Fairlie FM, Madhuvrata P, et al. Pregnancy with Gitelman's syndrome. Obstet Med 2011;4:39–41. 10.1258/om.2010.100046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathen S, Venning M, Gillham J. Outpatient management of Gitelman's syndrome in pregnancy. BMJ Case Rep 2013;2013. 10.1136/bcr-2012-007927. [Epub ahead of print: 25 Jan 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tammaro F, Bettinelli A, Cattarelli D, et al. Early appearance of hypokalemia in Gitelman syndrome. Pediatr Nephrol 2010;25:2179–82. 10.1007/s00467-010-1575-1 [DOI] [PubMed] [Google Scholar]
- 34.Nandi M, Pandey G, Sarkar S. Gitelman syndrome in an infant. Indian J Nephrol 2015;25:316. 10.4103/0971-4065.156904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettinelli A, Bianchetti MG, Girardin E, et al. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr 1992;120:38–43. 10.1016/S0022-3476(05)80594-3 [DOI] [PubMed] [Google Scholar]