Abstract
This report documents a case of sinus bradycardia in a hospitalised 27-month-old girl with a history of moderate persistent asthma, recent suspected viral respiratory infection and suspicion for multisystem inflammatory syndrome in children (MIS-C). This patient developed profound sinus bradycardia during her hospitalisation despite an overall well clinical appearance and good outcome. Reports of bradycardia related to COVID-19 infection are few but growing in number. In this article, we discuss what has been observed in the literature about bradycardia in relation to COVID-19 and MIS-C. We also propose sinus bradycardia as a potential sign of MIS-C with recent respiratory symptoms, which would warrant close follow-up of such patients.
Keywords: arrhythmias, COVID-19, infections
Background
A Kawasaki-like entity suspected to be related to SARS-CoV-2 infection in children was first observed in the UK in April 2020 and in the USA in April to May of 2020.1 After several cases describing multisystem involvement, hypotension and elevated inflammatory markers in PCR-positive or antibody-positive patients with coronavirus were discovered, a health advisory was issued defining multisystem inflammatory syndrome in children (MIS-C).2 The cardiovascular system is one of the many systems that can be affected in this process, with a clinical spectrum including arrhythmia, myocardial dysfunction and coronary artery aneurysm. Only rare reports of sinus bradycardia in these patients exist. The following case describes a 27 months old initially suspected of having an asthma exacerbation secondary to viral respiratory infection and ultimately found to meet criteria for MIS-C, who developed profound, persistent bradycardia.
Case presentation
A 27-month-old overall healthy girl with a history of moderate persistent asthma presented to her primary care clinic for 2 days of fever and poor oral intake in the setting of 1–2 weeks of intermittent increased work of breathing. By parents’ report, her dyspnoea would improve only transiently with intermittent albuterol use at home. She was directly admitted to the general paediatric ward from her primary care clinic due to concerns for acute asthma exacerbation secondary to viral upper respiratory infection. This was initially managed with albuterol, inhaled corticosteroids and a two-dose course of dexamethasone. On admission, she was febrile and tachycardic but otherwise haemodynamically stable.
On hospital day 3, the patient became notably bradycardic with heart rates of 30–50 beats per minute, both at rest and when awake without any associated perfusion changes. She was otherwise haemodynamically stable. She was transferred to the paediatric intensive care unit for telemetry and notably maintained normal perfusion and overall well appearance despite her bradycardia.
Investigations
Preliminary lab work early in the patient’s hospital course was notable for negative COVID-19 PCR, mild lactate elevation, D-dimer elevation (897 ng/mL; reference = <229), C reactive protein (CRP) elevation (261 mg/L; reference = <5), mild ferritin elevation (178.0 ng/mL; reference = 13–150) and mild transaminitis. Mild acute kidney injury was suspected with initial creatinine of 0.63 mg/dL. B-type Natriuretic Peptide (NT proBNP), troponin, Erythrocyte sedimentation rate (ESR) and procalcitonin were within normal range. An ECG on the day of admission showed normal sinus rhythm with normal intervals.
The patient’s respiratory status improved with the above medications over the course of 24 hours; however, inflammatory markers remained elevated on trended labs drawn on hospital day 1. Concern for MIS-C increased, and while repeat COVID-19 PCR was negative, an SARS-CoV-2 antibody panel was sent and was reactive (index = 152; reference = </=1), suggesting exposure or prior infection. A respiratory viral panel PCR obtained by nasopharyngeal swab was negative. All results considered, this patient did meet criteria for a definition of MIS-C.2 With this diagnosis, initial echocardiography was obtained on hospital day 2 and showed normal cardiac anatomy, function and coronary vasculature.
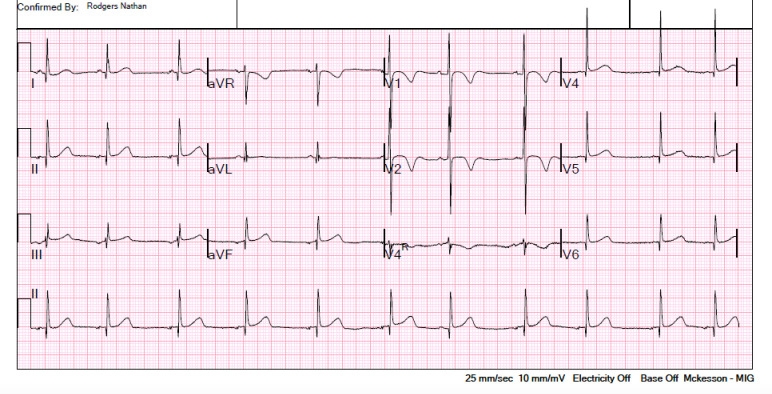
When the patient developed bradycardia on hospital day 3, initial ECG showed sinus bradycardia with a rate of 63 beats per minute (figures 1 and 2). Serial ECGs on subsequent hospital days revealed ongoing sinus bradycardia with respiratory variation. Labs were again trended and revealed further D-dimer elevation (2868 ng/mL) and new NT proBNP elevation (840 pg/mL; reference= 0–450). Serial lab draws thereafter demonstrated subsequent improvement in CRP, NT proBNP, D-dimer, ferritin and transaminases. Troponin, fibrinogen and procalcitonin remained within normal range with serial checks.
Figure 1.
Initial ECG tracing on hospital day 3, when the patient first developed bradycardia.
Figure 2.
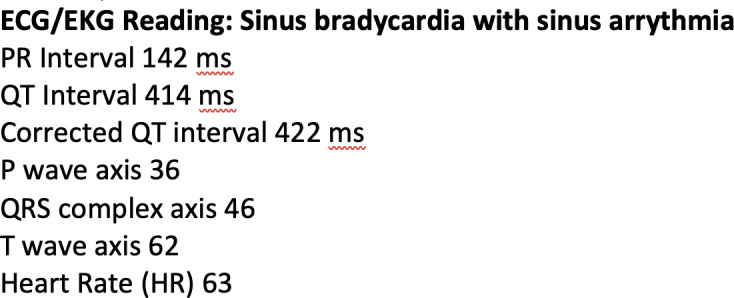
ECG reading from hospital day 3.
Treatments
The primary team consulted our MIS-C collaborative group, which included infectious disease, haematology and cardiology consultants on hospital day 1 given the patient’s antibody-positive status and elevated inflammatory markers. Intravenous immunoglobulin (IVIG) therapy, prednisolone therapy, enoxaparin therapy and aspirin therapy were recommended. The patient tolerated her IVIG infusion that evening without complications. Two additional doses of IVIG were given on hospital days 3 and 4, respectively.
Outcome and follow-up
A follow-up echocardiogram 1 week from initial imaging again revealed normal cardiac anatomy, function and vasculature; however, it demonstrated new small pericardial effusion. During her cumulative 7 days of cardiac and laboratory monitoring, she had no other clinical or haemodynamic instability aside from her persistent bradycardia. She did however have mild hypertension throughout with systolic blood pressures in the 110–120 range, thought to be related to systemic steroid therapy and activity with blood pressure checks. She had no further fevers after the day of admission. She was ultimately discharged on a 5-day prednisolone taper as well as high-dose aspirin (40 mg/kg/day) with plans for close follow-up in infectious disease, cardiology and haematology, as well as with her primary pulmonologist. On follow-up visit with cardiology 4 days after her discharge, she was feeling clinically well overall. Her bradycardia had resolved with a documented heart rate of 130 during that visit and an age-appropriate blood pressure.
Discussion
There is limited description of sinus bradycardia in the setting of acute COVID-19 infection or MIS-C in the literature, although reports are growing in number. Reported cases of cardiac complications related to COVID-19 primarily involve the adult population. One case series involving adults documented bradycardia in four adult patients ages 55–78 during hospitalisations for PCR-positive COVID-19 infection.3 Others have written of bradycardia in adults with or without atrioventricular heart block as one of multiple cardiac complications in the setting of severe respiratory illness attributed to COVID-19.4–10 Two papers speak specifically to associations between COVID-19 and relative bradycardia in adults, seen in states of high metabolic demand such as increased body temperature during which tachycardia would be expected.11–13 One study found that one-third of adult patients with severe COVID-19 infection but overall stable vital signs developed sinus bradycardia at some point during the course of their illness.4
Additionally, there are reports of adult patients infected with COVID-19 who develop bradycardia related to medications such as lopinavir–ritonavir,14 hydroxychloroquine and other QT-interval-prolonging medications15 and of course sedating medications.16 Still, another case described bradycardia found with renal failure and electrolyte disturbances in the setting of BRASH syndrome in a COVID-19-positive adult patient.17
With regard to the paediatric population, a single paper mentions sinus bradycardia in one of seventeen patients diagnosed with MIS-C, the outcome for whom is not detailed.18 Atrio-ventricular block has also been observed in children with multisystem inflammatory syndrome.19
Proposed explanations for the acute cardiac manifestations of COVID-19 include hypoxic injury, ischaemic injury, acute viral myocarditis, right heart strain, takotsubo cardiomyopathy and abnormal electrolyte regulation, none of which were seen in our patient.20 21 MIS-C is considered a second phase of disease involving hyperinflammatory state and postviral immune response, the exact mechanism of which is ill-defined.
From a clinical standpoint, our paediatric patient appeared well throughout her hospital course in spite of her aberrant inflammatory markers and bradycardia. Telemetry showed no heart block nor additional types of arrhythmia, and serial echocardiograms demonstrated normal systolic function. Moreover, clinical and laboratory evaluatory evaluation suggested adequate perfusion.
As to the aetiology of our patient’s bradycardia, multiple explanations including medication side effects, organic medical conditions and inherited dysrhythmia were considered. Slowed heart rate has been documented as an adverse reaction during IVIG infusion.22 In our patient, we thought an IVIG-induced bradycardia less likely given (1) the bradycardia did not manifest in conjunction with an infusion, but rather 24–36 hours after IVIG infusion and (2) the bradycardia was persistent for multiple days on telemetry, irrespective of whether IVIG was being given.
Of the other medications given throughout her course, bradycardia has been observed as a side effect of dexamethasone and other steroid medications. This has been documented in oncological patients for whom steroids are used for nausea and vomiting, in patients with severe Kawasaki disease treated in part with prednisolone and in other populations.23–25 Proposed mechanisms for steroid-induced dysrhythmia are several. With their intrinsic mineralocorticoid activity causing retention of sodium, hypertension can ensue, which may prompt baroreceptor-mediated reflex bradycardia. This may have been a contributor for our patient whose blood pressures were elevated from an age-appropriate baseline throughout her course, although notably she was still on prednisolone therapy during her follow-up office visit at which point her bradycardia and hypertension had both resolved. Another proposed mechanism involves a steroid-induced decrease in the sensitivity of myocardial alpha-adrenergic and beta-adrenergic receptors, dampening a heart rate response.26 Despite the possible cardiovascular effects of steroid therapy, the benefits of continuing prednisolone on discharge in this paediatric patient with inflammatory disease were thought to outweigh the risks, especially given her demonstrated normal cardiac output and reassuring heart rate variability with activity.
Our patient did not require any haemodynamic support nor sedating or anxiolytic medications throughout her course, these representing other common classes of medication, which can induce bradycardia. We did consider other organic causes of bradycardia and tested preliminarily for hypothyroidism; thyroid stimulating hormone (TSH) was found to be within normal limits (1.13 mlU/L; reference range = 0.27–4.20). There was no known family history of arrhythmia noted and no indication of heart block or long QT syndrome on 12-lead ECG or telemetry. Other causes of sinus bradycardia, such as hypothermia, sinus pause, sinoatrial exit block or premature atrial contractions with compensatory pauses, were not evident.
Learning points.
Sinus bradycardia may be a sign of multisystem inflammatory syndrome in children in otherwise well-appearing children with recent history of respiratory symptoms and fever.
Even if PCR for COVID-19 is negative, antibody testing and trending of inflammatory markers should be considered in these patients.
If there is evidence of exposure or recent infection on antibody testing, cardiology referral and follow-up may be indicated.
Footnotes
Twitter: @abjorklund20
Contributors: TCH took care of the patient, wrote up the case report, contributed to the literature review and critically edited and approved the final manuscript. Along with TMS, TCH is responsible for overall content as guarantor. ARB took care of the patient, contributed to the literature review and helped write and critically edited and approved the final manuscript. TMS took care of the patient, contributed to the literature review and critically edited and approved the final manuscript. NR took care of the patient and critically edited and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . CDCHAN-00432 [Internet]. (US): CDC Health Alert Network, 2020. Available: https://emergency.cdc.gov/han/2020/han00432.asp [Accessed Oct 2020].
- 3.Amaratunga EA, Corwin DS, Moran L, et al. Bradycardia in patients with COVID-19: a calm before the storm? Cureus 2020;12:e8599. 10.7759/cureus.8599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu L, Gong L, Jiang Z, et al. Clinical analysis of sinus bradycardia in patients with severe COVID-19 pneumonia. Crit Care 2020;24:257. 10.1186/s13054-020-02933-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Xu L, Zhu W, et al. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin Cardiol 2020;43:1054. 10.1002/clc.23422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman JP, Abrams MP, Kushnir A, et al. Cardiac electrophysiology consultative experience at the epicenter of the COVID-19 pandemic in the United States. Indian Pacing Electrophysiol J 2020;20:250–6. 10.1016/j.ipej.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol 2020;59:329–36. 10.1007/s10840-020-00789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kir D, Mohan C, Sancassani R. Heart brake: an unusual cardiac manifestation of COVID-19. JACC Case Rep 2020;2:1252–5. 10.1016/j.jaccas.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino G, Pascariello G, Bernardi N, et al. Sinus node dysfunction in a young patient with COVID-19. JACC Case Rep 2020;2:1240–4. 10.1016/j.jaccas.2020.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraiwa H, Goto Y, Nakamura G, et al. Relative bradycardia as a clinical feature in patients with coronavirus disease 2019 (COVID-19): a report of two cases. J Cardiol Cases 2020;22:260–4. 10.1016/j.jccase.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capoferri G, Osthoff M, Egli A, et al. Relative bradycardia in patients with COVID-19. Clin Microbiol Infect 2021;27:295–6. 10.1016/j.cmi.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeuchi K, Saito M, Yamamoto S, et al. Relative bradycardia in patients with mild-to-moderate coronavirus disease, Japan. Emerg Infect Dis 2020;26:2504–6. 10.3201/eid2610.202648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyls C, Martin N, Hermida A, et al. Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit: risk of bradycardia. Circ Arrhythm Electrophysiol 2020;13:e008798. 10.1161/CIRCEP.120.008798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel J, Patel R, Rodriguez L-M, et al. Cardiovascular considerations of experimental hydroxychloroquine therapy on patients diagnosed with COVID-19: a case series review. Cureus 2020;12:e9151. 10.7759/cureus.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Zhong Z, Li G, et al. [A comparative study on clinical effects of dexmedetomidine and midazolam on patients with severe coronavirus disease 2019 on non-invasive ventilation]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:677–80. 10.3760/cma.j.cn121430-20200305-00187 [DOI] [PubMed] [Google Scholar]
- 17.Prabhu V, Hsu E, Lestin S, et al. Bradycardia, renal failure, atrioventricular nodal blockade, shock, and hyperkalemia (BRASH) syndrome as a presentation of coronavirus disease 2019. Cureus 2020;12:e7816. 10.7759/cureus.7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020;324:294–6. 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionne A, Mah DY, Son MBF, et al. Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics 2020;146:e2020009704. 10.1542/peds.2020-009704 [DOI] [PubMed] [Google Scholar]
- 20.Babapoor-Farrokhran S, Rasekhi RT, Gill D. Arrhythmia in COVID-19. SN Compr Clin Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021;180:307–22. 10.1007/s00431-020-03766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raheja H, Kumar V, Hollander G, et al. Intravenous immunoglobulin-induced profound bradycardia in a patient with idiopathic thrombocytopenic purpura. Am J Ther 2018;25:e572–4. 10.1097/MJT.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 23.Paw Cho Sing E, Schechter T, Ali M, et al. Safety of dexamethasone for nausea and vomiting prophylaxis in children receiving hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2018;40:e278–82. 10.1097/MPH.0000000000001186 [DOI] [PubMed] [Google Scholar]
- 24.Üsküdar Cansu D, Bodakçi E, Korkmaz C. Dose-Dependent bradycardia as a rare side effect of corticosteroids: a case report and review of the literature. Rheumatol Int 2018;38:2337–43. 10.1007/s00296-018-4167-1 [DOI] [PubMed] [Google Scholar]
- 25.Nagakura A, Morikawa Y, Sakakibara H, et al. Bradycardia associated with prednisolone in children with severe Kawasaki disease. J Pediatr 2017;185:106–11. 10.1016/j.jpeds.2017.02.074 [DOI] [PubMed] [Google Scholar]
- 26.Hall ED, Plaster M, Braughler JM. Acute cardiovascular response to a single large intravenous dose of methylprednisolone and its effects on the responses to norepinephrine and isoproterenol. Proc Soc Exp Biol Med 1983;173:338–43. 10.3181/00379727-173-41653 [DOI] [PubMed] [Google Scholar]