Abstract
In humans and mice, susceptibility to infections and autoimmunity increases with age, due to age-associated changes in innate and adaptive immune responses. Aged innate cells also have reduced activity, which leads to decreased naïve T cell and B cell responses. Aging innate cells also contribute to an overall heightened inflammatory environment. Naïve T cell and B cells undergo cell intrinsic age-related changes that lead to reduced effector and memory responses. However, previously established B and T cell memory responses persist with age. One dramatic change is the appearance of a newly recognized population of age-associated B cells (ABC) with a unique CD21−CD23− phenotype. Here we discuss the discovery and origins of the naïve phenotype IgD+ versus activated CD11c+Tbet+ ABC, with a focus on their protective and pathogenic properties. In humans and mice, antigen-experienced CD11c+Tbet+ ABC increase with autoimmunity and also appear in response to bacterial and viral infections. However, our analyses indicate that CD21− CD23− ABC include resting naïve progenitor ABC expressing IgD. Like generation of CD11c+Tbet+ ABC, the naïve ABC response to pathogens depends on TLR stimulation, making this a key feature of ABC activation. We put forward a potential map of the development of distinct subsets from the putative naïve ABC. We suggest defining the signals that can harness the response of naïve ABC may contribute to protection against pathogens in the elderly, while CD11c+Tbet+ ABC may be useful targets for therapeutic strategies to counter autoimmunity.
Keywords: age-associated B cells, B lymphocytes, autoimmunity, memory B cells, aging
I. Immune Responses to Respiratory Infections in the Aged
With age, humans become more susceptible to infections, leading to increased global morbidity and mortality.1,2 Lower respiratory tract infections (LRTI) are the most common cause for hospitalizations in people over the age of 65 and 90% LRTI-related deaths are within the elderly population.1,3 Many viruses and bacteria cause LRTI such as Streptococcus pneumoniae, influenza and more recently SARS-CoV-2.1,3,4 According to the CDC, over half of influenza-related hospitalizations and deaths in the US, are in patients over the age of 65.5 Unfortunately, current vaccination strategies often fail to induce sufficient immunity in aged populations leaving them vulnerable to future outbreaks.6–8 Aged males and females often show distinct susceptibility to infections, tumors and autoimmunity.8 Susceptibility to autoimmune disorders increases more among aged females, while there is a higher incidence of tumors in aged males8. Aged males are more highly susceptible to respiratory infections such as influenza and in the current COVID pandemic.4,8 Understanding the mechanisms involved in responses of the aged immune system should provide insights that can inform development of new strategies to improve vaccine efficacy and therapeutic intervention in the elderly.
Previous studies have established that age-related changes in human and murine immune systems increase susceptibility towards infections.8 Aged innate cells express lower levels of toll-like receptors (TLR) which are one class of pathogen recognition receptors (PRR), and thus, in the aged, infections that stimulate these PRR produce reduced levels of type I interferon and other inflammatory mediators during responses to pathogens and reduce ability to clear the infection.9,10 Aged neutrophils and macrophages exhibit decreased migration and phagocytosis.8,9,11 As animals age, immature monocytes constitutively secrete higher levels of inflammatory cytokines in the absence of infection increasing basal levels of inflammation during aging.8 Macrophages from aged animals express lower MHC-II and costimulatory molecule levels leading to a decreased ability to presentation antigen to T cells.6 Although aged dendritic cells (DC) secrete proinflammatory cytokines, they have defects in migration, phagocytosis, and antigen presentation to T cell during infection.12–14 Therefore, unchecked inflammatory responses by immature monocytes and DC and poor function of neutrophils, macrophages and DC leads to an increase in tissue injury and impaired response of T cells, and hence less effective pathogen clearance.8,9 The increased proinflammatory environment that develops in the absence of infection may also contribute to age-related autoimmune disorders.8
In the adaptive immunity, T and B cells undergo multiple changes with age that play an even greater role in impaired immune responses. The aged thymus produces less naïve CD4 T cells, leading to a decrease in the CD4 TCR repertoire and a progressive reduction of the naïve CD4 T cell compartment (Figure 1).15–18 When stimulated the aged naïve CD4 T cells proliferate less and produce less IL-2 contributing to a decrease in CD4 T cell effector responses, especially reduced formation of T follicular helper cells (TFH).19 One key change is a reduced response of CD4 T cells to IL-6, produced by interacting APC, which acts as a signal 3 for TFH and memory CD4 T cell generation.20,21 The limited TFH generation causes a dramatic decrease in germinal center B cell (GCB) responses, which leads to fewer Ab-secreting cells (AbSC), long-lived PC (LLPC) and memory B cells (Bmem) (Figure 1).6,16,17,20,22–24 Unlike young naïve CD4 T cells, aged naïve CD4 T cells are poor at developing into new T cell memory cells.25 Naïve CD8 T cells have similar defects and their poor response impairs development of cytotoxic effectors that kill infected targets. As with CD4 cells, memory CD8 T cells generated early in life remain more competent.26 Thus, it is response to new or previously unseen pathogens that is mostly hampered by age.
Figure 1: Age-Associated Changes in T cell and B cells.
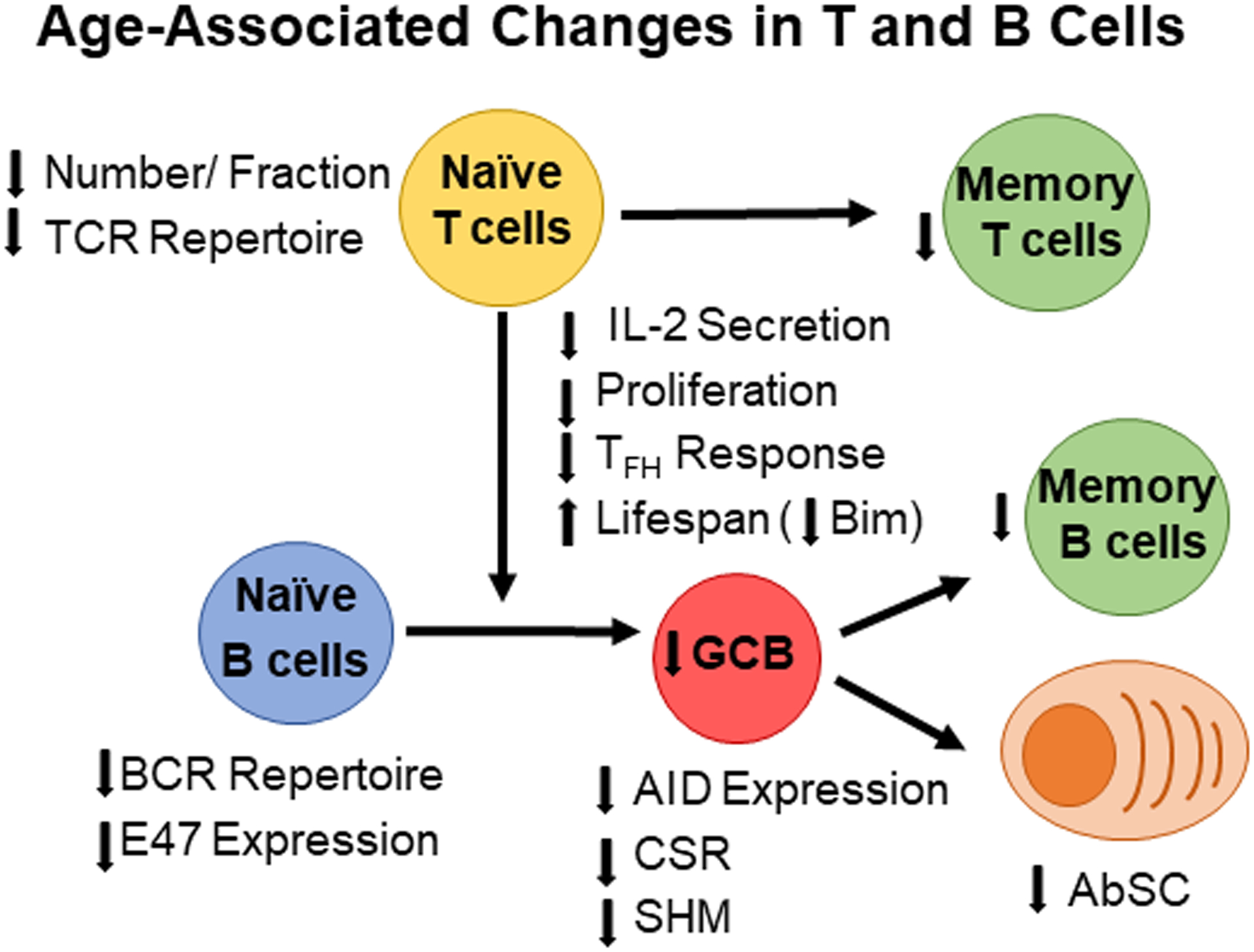
With age, the number of naïve B cells and T cells decreases leading to a smaller TCR and BCR populations and repertoires. Upon pathogen exposure, naïve CD4 T cells secrete less IL-2 leading to lower effector T cell responses including T follicular helper cells (Tfh). Lower Tfh responses decrease GCB responses, which leads to fewer memory B cells and antibody-secreting cells towards newly encountered pathogens. However, memory B cells from previous immune responses accumulate with age leading to a larger memory B cells pool in the aged.
Like naïve T cells, generation of pre and pro-B cells in the bone marrow (BM) decreases with age due to changes in the BM microenvironment.27,28 Although the total number of peripheral B cell remain the consistent, follicular B cells (FOB), which are the B cells that initiate GCB, decrease in the spleen.28 Due to a diminishing immature B cell pool in aged mice, splenic B cells are not replenished with new mature B cells and aged FOB consists of longer-lived, older naïve B cells, which express a reduced antibody repertoire (Figure 1).28–30 Like FOB, marginal zone B cells (MZB), which are usually T-independent, decrease with age due to changes in anatomical structure in the marginal zone.28,31 These structural changes also prevent MZB from interacting with macrophages in the marginal zone decreasing the MZB response to blood-borne pathogens.31
Intrinsic changes in B cells also contribute to decrease in GCB responses, class switch recombination (CSR) and somatic hypermutation (SHM) (Figure 1).28 Studies show that human and murine aged B cells express less E47, a transcription factor that plays a role in Aicda transcription.32,33 Aicda gene encodes for activation-induced cytidine deaminase (AID), an enzyme required for CSR and SHM in B cells.33 Therefore, lower E47 expression in aged B cells leads to lower expression of AID, which decreases isotype-switching and affinity maturation (Figure 1).33 Decrease in CSR and SHM negatively impact production of antigen-specific GCB, antibodies, and memory B cells, which all play critical roles in protection clearing infections and providing long-term immunity to prevent re-infection.16,28 Since naïve T and B cells are most impaired by aging, it is unsurprising that vaccines given to the elderly, including that for influenza, are often ineffective and provide only short-term protection, requiring yearly immunization.6
II. Discovery of Aged Associated B cells (ABC)
Although the numbers of splenic B cells remain roughly constant between youth and old age in mice, the subset composition changes.28 In young humans and mice, naïve splenic B cells are composed mostly of naïve FOB and MZB subpopulations, but these decline with age while isotype-switched IgG+ memory B cells accumulate.8,23,34–36 This shift from naïve to antigen-experienced B cells contributes to a decreasing immunoglobulin repertoire with age, and leads to poor responses to new pathogens.28–30 In 2011, studies by Michael Cancro and colleagues, identified a novel subset of splenic B cells that emerges in unimmunized aged mice.34 Unlike FOB (CD23+) and MZB (CD21+), this new B cell subset lacks CD23 and CD21. They were called “age associated B cells” (ABC) (Figure 2).34,37 Flow cytometry analysis revealed that CD21−CD23− ABC maintain surface IgD expression, but do not express CD86 and MHC II (Table 1).23,28,34,38 We find that CD21−CD23− ABC are a heterogenous population composed IgD+ and IgD− CD21− CD23− ABC.23 In the absence of intentional immunization, the IgD+ B cells have a phenotype consistent with unswitched naïve B cells, while the IgD− B cells have a phenotype of more antigen-experienced B cells.28,36,39 Therefore, we proposed that CD21−CD23− ABC are composed of resting, unswitched naïve B cells (IgD+) and isotype-switched memory B cells (IgD).23 We believe these 2 subsets comprise the resting compartment of ABC (Table 1).
Figure 2: B cell responses shift to PR-dependent mechanisms with age.
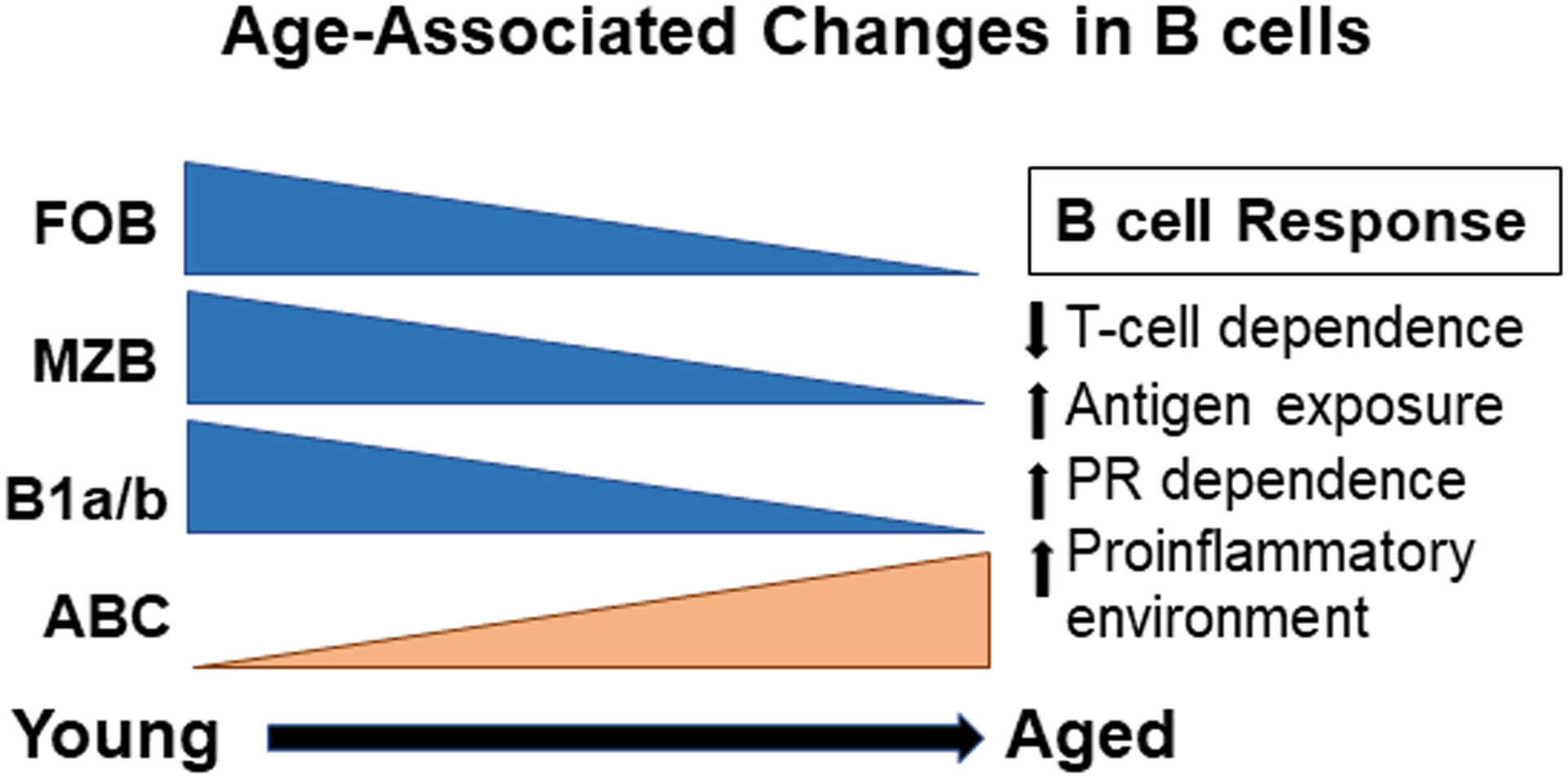
With age, humans and mice experience higher antigen exposure and an increased proinflammatory environment. Furthermore, the number of FOB, MZB and B1b/a cells decrease as ABC accumulate with age. This can be replicated in chronically infected and autoimmune-prone young mice, which accumulate CD11c+Tbet+ ABC. T-cell responses also decrease leading to a higher dependence on PR signals. Unlike, T-cell dependent FOB, ABC can be T-independent, and their activation depends on higher Ag exposure and PR signals. Thus, they can mount a primary response to new pathogens, which may provide protection.
Table 1:
Comparison of resting vs. activated ABC phenotypes
| Markers | Resting ABC | Activated ABC | References |
|---|---|---|---|
| CD21 | − | − | 34,40 |
| CD23 | − | − | 34,40 |
| IgD | + | − | 34,40 |
| (Memory: IgD−) | |||
| T-bet | + | + | 40, 46,48 |
| CD80 | N/A | + | 40,56 |
| CD86 | − | + | 34,40 |
| MHC II | − | + | 34,40 |
| CD11c | + | + | 40,41,46.48 |
| CD11b | N/A | + | 40,41,48 |
The table shows (+)-positive or (−)-negative expression of various markers associated with ABC in resting and activated states, based on the listed references. Resting ABC are composed of IgD+ and IgD− (naïve & memory) subsets that lack CD80 and CD86 expression. Meanwhile, active ABC express these two costimulatory markers show decreased IgD expression suggesting they are an active isotype-switched subset.
Philippa Marrack and her colleagues described another distinct ABC population that lacked CD21 expression but expressed CD11c and CD11b and accumulated in young autoimmune-prone mice.40 Unlike the resting CD21−CD23− ABC, CD11c+CD11b+ ABC express elevated CD86, MHC-II and Fas, suggesting that they are likely activated and/or atypical memory B cells (Table 1).40,41 Further gene array analysis has revealed that this population expresses high levels of T-bet, a transcription factor associated with IgG2a/c+ memory B cells during viral infections.40–45 Later studies revealed that CD21−CD23−ABC also express CD11c and T-bet mRNA suggesting that this population is possibly related to the more activated CD11c+CD11b+ ABC subset.46 However, whether CD11c+CD11b+ ABC arise from CD21−CD23−ABC and if they represent a small or larger fraction of the presumed ABC subset remains unclear (Figure 3).
Figure 3: Proposed relationship between naive and primed ABC.
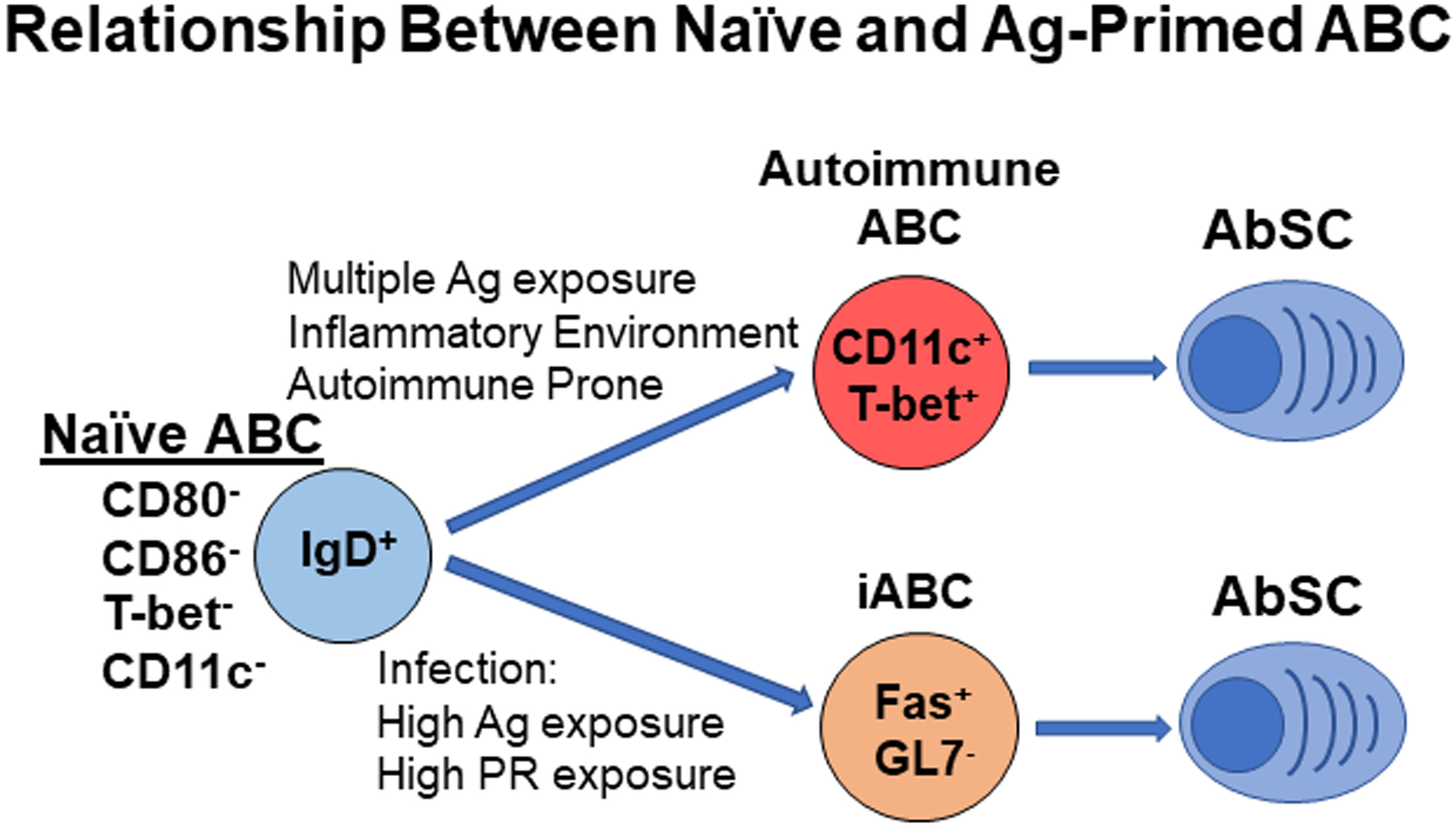
Our studies show that with high Ag and PR exposure, naïve ABC can become active ABC which express Fas and differentiate into Ab-producing cells. We termed this subset as induced age associated B cells (iABC). Although autoimmune ABC can accumulate in young mice, we propose that this primed subset may also arise from naïve ABC with multiple Ag exposures and heightened inflammatory environment. Like iABC effectors, this autoimmune ABC differentiates into Ab-producing cells which produces autoantibodies.
III. Requirements for ABC development and/or differentiation
Several studies from Cancro’s group suggest that CD21−CD23−ABC arise from antigen-experienced FOB that accumulate with age.34,46 When young CD23+-enriched FOB were adoptively transferred into unimmunized WT hosts they divided more compared to FOB transferred into CD40 −/− and MHC-II −/− hosts.34,46 Moreover, the highly divided fraction of donor FOB cells lacked CD21 and CD23 expression, but expressed high levels of T-bet.46 In addition, aged CD154 −/− mice, which lack CD40L on T cells did not form CD21−CD23−ABC. Since CD4 T cell activation requires MHC II-bound peptides on antigen presenting cells, and CD40 binding to CD40L mediates T and B cell interactions, this data suggests that generation of CD21−CD23−Tbet+ ABC is T-cell dependent.37,46 Further studies to evaluate if ABC are antigen-experienced quantified the number of somatic mutations in resting ABC in unimmunized aged mice and compared it to aged FOB, MZB, and GCB. This study revealed that CD21−CD23−ABC contain more somatic mutations in their heavy and light chains compared to aged FOB and MZB, but not as many as GCB, which undergo multiple rounds of affinity maturation.46 This indicates that CD21−CD23−ABC have undergone at least some affinity maturation consistent with the hypothesis they are antigen-experienced memory B cells. However, this ABC subset has a diverse B cell receptor (BCR) repertoire similar to aged FOB and MZB, suggesting that the subset in aged mice, housed in SPF conditions, had undergone minimal clonal selection indicating a more naïve like status.46 Moreover, reports indicate that CD21−CD23−ABC mostly express IgD, and contain a separate population of IgD− B cells and they occur equally in germ-free vs. specific pathogen-free (SPF) aged mice (Kugler-Umana and Swain, unpublished results), implying there may be multiple subsets of ABC.23 We propose that the majority of resting ABC in SPF mice are indeed naïve and that they develop by an intrinsic pathway, independent of foreign and commensal microbe exposure. We suggest that the CD21−CD23−ABC may also contain a fraction of memory B cells that accumulate in T-cell dependent manner. Thus, we propose, in very old mice (over 18 months) resting ABC are dominant naïve B cell population.
While FOB are T-cell dependent, MZB. B1b, and B1a cells are T-cell independent B cell subsets that rapidly become antibody-secreting cells in response to BCR crosslinking and TLR signaling39. However, these subsets decrease in number and activity.8,27,31 Studies shows that CD21−CD23− ABC divide more with in vitro with TLR7 and TLR9 stimulation compared to aged FOB and MZB.34 Our own studies indicate that in aged mice, the resting ABC are the major B cell population that responds to influenza infection independent of T cells (Figure 3). We transferred FACS-sorted resting phenotype ABC into influenza-infected RAG−/− host and studied their differentiation and activity.23 We found infection caused naïve ABC to express Fas and become influenza-specific Ab-secreting cells (AbSC).23 We termed this subset as induced age-associated B cells (iABC). Further studies show that iABC formation from donor ABC depends on TLR7 and/or other endosomal TLR expressed by host cells (Kugler-Umana and Swain, unpublished results). TLR7 is an endosomal sensor capable of detecting ssRNA which we believe is how influenza infection stimulates the ABC response. This suggests that resting ABC can mount an influenza-specific response, without T cells, but dependent on TLR pathways.
Like activation of CD21−CD23−ABC, MZB and B1b subsets, initial CD11c+CD11b+ ABC generation also requires TLR signaling (Figure 3).40 Studies indicate that unimmunized aged TLR7−/− and Myd88−/− aged mice do not accumulate the CD11c+CD11b+ ABC subset compared to aged WT, TLR4−/− and TLR3−/− mice. Moreover, repeated administration of TLR7 agonists increases the number of ABC in young mice compared to repeated administration of TLR3, TLR4 and TLR9 agonists suggesting that chronic TLR7 stimulation is particularly important for CD11c+CD11b+ ABC generation.40 Further in vitro and in vivo studies also shows that, in both cases, TLR7 stimulation synergizes with BCR signaling to generate CD11c+ B cells from young B cells that express T-bet which are presumably the Ag-experienced B cells.42 Additionally, overexpression of T-bet also leads to an increase of CD11c and CD11b expression in B cells.42 Although TLR7 stimulation plays an important role in generating these active ABC, CD11c+CD11b+ ABC also require IL-21.47,48 Adding IL-21 in vitro in addition to TLR7 or 9 stimulation also led to an increase in CD11c+T-bet+ B cells from cultured FOB, compared to TLR7 or 9 stimulation alone.47 Later studies investigated the molecular pathways that contribute to IL-21 dependent CD11c+CD11b+ ABC generation. Their studies revealed that young SWAP-70 and DEF6 double knockout (DKO) mice develop lupus and prematurely accumulate CD11c+CD11b+T-bet+ ABC which lack CD21 and CD23 expression.48 Additionally, they show that this increase in ABC is due to the absence of regulation, by the SWAP-70 and DEF6 proteins, of IL-21 mediated IRF5 accessibility to key targets involved in ABC formation.48 This suggests that accumulation of CD11c+CD11b+T-bet+ ABC also depends on IRF5 signaling.48 Altogether these studies support the idea that the ABC with the activated CD11c+/CD11b+, T-bethi phenotype arise from a combination of Ag or auto-Ag recognition, IL-21 and TLR7 stimulation. Although ABC generation depends on IL-21 in the context of autoimmunity, whether IL-21 dependence holds true in aged mice where T cell help is deficient and ABC activation is T-cell independent, remains unclear.16,23
IV. Role of ABC in autoimmunity (mouse and human)
Multiple studies suggest that both ABC populations play a role autoimmune pathogenesis (Table 2).38,47,49,50 Prior to becoming mature B cells, pro-B cells express a surrogate light chain (SLC), which along with μ heavy chain form a functional pre-BCR, that is required for B cell selection.51,52 Without SLC, the pro-B cell pools shift to SLClow pro-B cells and allows for the introduction of autoreactive B cells into the peripheral B cell pool.51,52 Studies show that CD21−CD23−ABC secrete TNF-α that reduces SLChigh pro-B cells in aged mice, which increases the pool of SLClow pro-B cells and autoantibodies.35,51,52 Therefore, this ABC subset may aid the development of autoreactive B cells and thus contribute to rising autoimmunity with age.
Table 2:
Comparison of ABC-like subsets in autoimmunity and chronic infections
| Condition | Phenotype | Location | Function | References |
|---|---|---|---|---|
| Rheumatoid Arthritis (RA) (human) |
T-bet+CD11c+IgD−IgM−CD21−CD23−CD11c+IgG+ | Blood, synovial joint tissues | Local Inflammation, Autoantibody production | 40,57 |
| Systemic Lupus (SLE) (human, mouse) |
T-bet+CD11c+IgD−IgM−CD21−CD23−CD38−IgG+ | Blood, spleen, kidneys | T cell activation, autoantibody production | 50,54,56 |
| Multiple Sclerosis (MS) (human) |
IgD−IgM−CD21−CD11c+IgG+ | Blood | autoantibody production | 55 |
| HIV (human) |
T-bet+CD85j+CD11c+IgD−IgM−CD21−IgG+ | Blood | Antibody production | 63 |
| HCV (human) |
T-bet+IgD−IgM−CD21−IgG+ | Blood | Antibody production | 64 |
Table shows phenotype, distribution, location and function of ABC-like populations in different autoimmune diseases and chronic infections based on the listed references.
The CD11c+CD11b+T-bet+ ABC accumulate at a faster rate in several young mouse models for SLE such as Mer −/− mice, NZBxWF1, and SWAP-70 −/−DEF6 −/− double knock out mice compared to young wild-type mice40,53. These ABC can make more autoantibodies against chromatin, nuclear ribonuclear proteins (nRNP) and cardiolipin compared to FOB, suggesting they directly contribute to autoimmunity40,48,53. One possibility is that the autoimmune environment may mimic the aging environment leading to the generation of this subset even in the young. Many studies on CD11c+CD11b+T-bet+ ABC function have been done in mouse models of autoimmunity. Several indicate that CD11chi T-bethi ABC secrete IFNγ and can present self-antigen to T cells.40,42,44,50,54 Furthermore, the presence of CD11chi T-bethi ABC correlate with kidney damage and early mortality in SLE-prone mice.50
According to a study by Marrack, healthy human patients across different ages and sexes did not have CD11chiCD21low ABC cells.40 However, this ABC population increased with age in peripheral blood among aged RA (Rheumatoid Arthritis) patients (Table 2).40 In contrast, CD21−CD23− ABC and CD11b+CD11c+T-bet+ ABC develop in healthy WT aged mice and CD21−CD23− ABC develop in germ-free mice (Kugler-Umana and Swain, unpublished results).34,40,41 Human ABC are a CD11chiCD21low subset that express activation markers such as CD80 and CD86, lack IgD and IgM expression, and express IgG.40,55 Given this phenotype and human exposure to multiple infections and non-infectious Ag, it makes sense that the human ABC populations are most likely isotype-switched activated memory B cells.39,40 Like murine CD11b+CD11c+T-bet+ ABC, CD11c+T-bet+ ABC accumulate in the blood of young SLE patients and correlate with SLE symptoms (Table 2).40,50,56 Also like human ABC found in aged RA patients, this autoimmune-associated B cell population lacks surface IgD and IgM expression and a fraction express IgG.40,56 The CD11c+ autoimmune B cells express IL-21 inducible genes suggesting this subset is IL-21 dependent in human SLE as well.48,54 Recent studies have also identified CD11c+T-bet+ ABC in inflamed synovial tissues of aged RA patients and CD11c+ B cells in nephritic kidneys in young SLE patients suggesting that this subset may contribute to local inflammation (Table 2).56,57 Although human ABC lack CD21 expression, they are not equivalent to the murine CD21−CD23− naïve predominant ABC population since the human ABC do not express surface IgD and do express CD80 and CD86.23,34 Unlike human ABC increase in aged RA patients, in MS patients this population decreases with age.40,55 Furthermore, unlike the 2011 RA study, in this 2016 MS study there is a significant increase of CD11chiCD21low ABC in aged healthy patients compared to young patients.40,55 This suggests that human ABC are not associated with all autoimmune conditions and there is a need to collect more data from healthy aged patients. Furthermore, the progenitor of the different human subsets of distinct phenotype is unclear and they may not all be derived from bone fide ABC. Studies in mice show that the activated ABC subset in human autoimmune disease may be a good therapeutic target. B cell specific T-bet deletion in young SLE model mice reduced development of splenic CD11chiT-bethi ABC, and led to decreased spontaneous GCB formation, which reduced autoantibody production and kidney damage. T-bet targeting or the depletion of the activated ABC subset could be a useful therapy for autoimmunity.50
V. Role of ABC in Bacterial and Viral infections (mouse and human)
Several ABC-like populations have been identified in bacterial and viral infections. Initial studies identified a CD11c+IgM+ B cell population in E. muris-infected young mice, which were described as unswitched memory B cells, which express T-bet.58–60 To study their secondary responses without the interference of neutralizing antibodies, this subset was sorted and transferred into E. muris challenged vs. naive hosts.60 With E. muris challenge, this T-bet+ memory B cell subset differentiated into AbSC, suggesting they could provide protection against E. muris reinfection.60 Similar to E. muris infection, CD11c+T-bet+ ABC accumulate in young mice infected with gammaherpesvirus 68 (gHV68), mouse CMV, lymphocyte choriomeningitis virus, and vaccinia.42 To determine if such ABC contributed to anti-ghV68 antibody production and ensure that T-bet deletion is restricted to B cells, they made mixed bone chimeras containing a mixture of bone marrow cells from μMT mice and either T-bet −/− mice or WT mice.42 Results showed that the B cell T-bet−/− chimeras had higher ghV68 viral titers and less ghV68-specific antibodies, suggesting that T-bet+ B cells play a role in viral clearance.42 This data coincides with reports that show that T-bet drives NP-specific IgG2a+ memory B cells and influenza-specific AbSC cells formation.43,47,61 Meanwhile, a recent study indicates that T-bethi influenza-specific memory B cells are restricted to the spleen, blood and bone marrow in humans and mice and that they produce anti-HA stalk antibodies in mice, indicating that they may have potential to contribute to protective responses.62
In humans, T-bet+ B cells accumulate in chronically-infected HIV-positive patients, chronically-infected aged Hepatitis C (HCV) patients and patients immunized with live yellow fever or vaccinia vaccine.63,64 Antiviral treatment of chronic HCV infection reduced the number T-bet+ B cells, suggesting that chronic infection is needed to sustain this subset.64 Like the CD11c+T-bet+ ABC in autoimmune patients and autoimmune-prone mice, this subset lacks CD21, IgD, and IgM expression, but does express IgG, CD86 and CD95 indicating these are isotyped-switched memory B cells.63,64 Furthermore, in HIV-positive patients, a large frequency of HIV-specific B cells expressed T-bet, which correlates with the production of HIV-specific IgG1 antibodies in the serum.63 More recently, studies indicate that rhinovirus infection produces T-bet+ memory B cell in human subjects, which infiltrate nasal tissue and make heterotypic IgG responses.65 This suggests that T-bet+ B cells may play a role in inducing and/or mediating human antigen-specific B cell responses. One major challenge to understanding the role of different subsets, is that T-bet can be expressed by conventional B cells as well as ABC B cells and so great care must to taking in designing experiments that ascribe functions to ABC.
VI. Positive ABC Role in Overcoming Aged Defects and Shared Strategies of T and B Aging
Based on the literature in the field and our data discussed above, we propose that ABC, defined as the lineage not expressing CD21 and CD23, are a lineage of B cells with subsets at different differentiation stages. We find that in unimmunized aged mice, the majority of ABC are resting naive B cells expressing sIgD, and/or IgM. This population increases with age and is more prominent in females (Figure 2).34 In contrast, in autoimmunity and chronic infection, activated memory ABC population(s) develop and accumulate.38 The activated populations are likely diverse and functionally heterogenous, having developed under different conditions. Although many studies have focused on the activated, T-bethi ABC as mediators of autoimmunity, the activated ABC induced by infections may play critical positive roles in protective immunity. At least in aged mice they are the major naïve B cell subset that responds to new pathogens.23 Furthermore, recent studies indicate that a T-bethi B cells may play a role in producing anti-HA stalk antibodies during influenza infection.62 This coincides with reports that shows that broadly-neutralizing antibodies are maintained as we age and accumulate with each subsequent vaccination.66–68 Since ABC accumulate with age, we propose that this age-dependent subset may be an important source of these broadly neutralizing antibodies. This may be particularly important given that aged immune systems do not generate effective Ab responses from naïve FOB cells and naïve CD4 do not develop TFH in aged mice, unless pathogen recognition (PR) signals are high.17,20,21 Therefore, understanding ABC generation and activation may provide a strategy for a universal influenza vaccine in aged populations.
We should also consider that TFH that drive T-cell dependent B cell responses, decrease with age leading to poor primary memory B cell formation and antibody responses towards new pathogens. However, our studies show that ABC can respond in the absence of TFH and CD4 T cells to produce AbSC.20 Unlike FOB, in vitro TLR7 and TLR9 stimulation activates CD21−CD23−ABC and in vivo chronic TLR7 stimulation induces CD11c+Tbet+ABC.34,40 This suggests that primary aged B cells responses become less T-cell dependent and depend more on PR pathways. This may be a useful strategy to circumvent the poor T cell responses in the aged (Figure 4). By defining the factors required for protective ABC responses, we may learn how to harness the positive potential of ABC in anti-pathogen immunity.
Figure 4: Proposed Vaccine Strategies to Enhance Response in the Elderly.
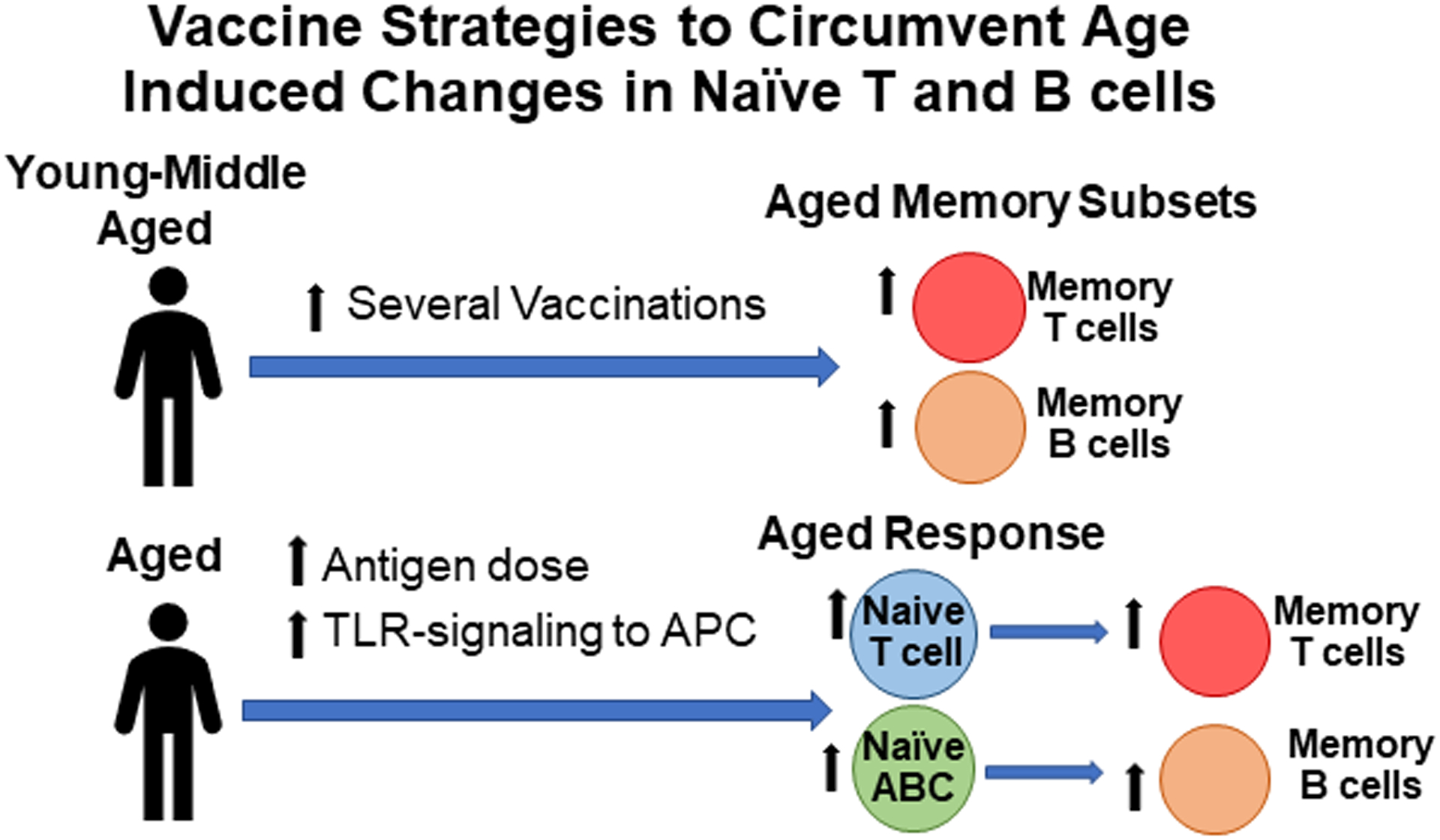
Studies show that memory T and B cell response increase with age and may provide protection against reoccurring infection. One way of enhancing the aged immune system is by increasing exposure to Ag by vaccination in young or middle-aged populations in order to increase the size of the memory repertoire later. Another way of enhancing aged immune responses is by providing higher doses of Ag and TLR-signaling to antigen-presenting cells (APC). These should lead to optimal naïve aged T and B cells responses and help establish new memory T and B cells responses to new pathogens.
Like aged B cells, aged T cells also become more dependent on PR pathways.20,21 Our early studies showed that addition of IL-2 or addition of pro-inflammatory cytokines [IL-1, IL-6, TNF] increased aged CD4 T cell responses.19,69 Indeed we found that APC stimulated with TLR agonists make IL-6 and interact with the aged naïve CD4 T cells, restoring much of the aged naïve CD4 T cells response.17,20,21 We suggest this heightened dependence on PR signaling limits the naïve T cell response to circumstances where a pathogen presents a compelling danger, most likely to prevent un-necessary inflammation and limit development of autoimmunity. This change is thus analogous to the change in the B cells with aging, where the responsive naïve ABC have a strict dependence on TLR7/9, indicating they are restricted by the need for PR signaling. We suggest the increasing dependence on PR pathways is a shared beneficial strategy, for the animal and a key feature of T and B cell aging.
Unsurprisingly most aging studies imply that all aging is harmful and leads to harmful loss of immune functions. However, we argue that loss of certain aspects in adaptive immunity in aging may be part of a beneficial strategy.70,71 The loss of naïve T and B cells with age is largely caused by homeostatic shifts. This is particularly clear for naïve T cells because the thymus undergoes gradual involution and makes less T cells. We propose that this may be an evolutionary strategy to make room for memory T and B cells, which accumulate with exposure to pathogens throughout young life.6 Unlike naïve T cells, memory CD4 cells generated in young mice retain their function with age.72 Our research shows that development of naïve CD4 defects is also a programmed process driven by the increased chronological age of the naïve CD4 population that becomes longer-lived.73,74 Additional studies, where CD4 T cells are depleted from aged mice, indicated that newly generated CD4 T cells do not express key age-related defects and function like young cells.22,75 Furthermore, without a thymus and heterogenous expression of the pro-apoptotic protein BIM, the naïve CD4 T cell population develop aging defects more quickly and become older.73,74 We recently found that loss of naïve CD4 T cells and functional reduction in IL-6 response, occur in germ-free mice just as in conventional mice even though they mice lack exposure to pathogens and to commensal microbes (Swain et al. unpublished). These results suggest that the changes in naive T cells are part of a developmental program and that they have helpful effects.
We suggest that identifying the changes in the aged immune system and strategies to overcome those changes, may provide alternative vaccination strategies in the elderly (Figure 4). Since T and B cell memory cells accumulate and remain responsive with age, one strategy would be to immunize young and middle-aged populations with key vaccines to increase the T and B cell memory pool.6,66,72 Based on our studies of aged naïve T cells, another strategy would be to develop vaccines that provide higher levels of antigen and TLR-signaling and thus drive more optimal CD4 T cells responses including new memory responses to emerging or altered pathogens.17,20,21 Not only should increases in antigen dose and TLR signaling improve naïve CD4 T cell responses, but it may also enhance the naïve ABC responses. Targeting both aged naïve T cells and ABC may lead to establishing new memory cells to emerging or altered pathogens in aged populations.
VII. Conclusions
We conclude that the process of aging leads to major changes in innate and adaptive, T and B cell immunity. We focus on changes in naive CD4 and naïve B cells, that make it difficult to readily vaccinate the elderly to new pathogens and propose different vaccine strategies for aged populations (Figure 4). We discuss that for B cells, there is a shift of naïve B cells from conventional FOB responses which become limited, to a recently described ABC subset (age-associated B cells) and point out the naïve ABC can respond only if sufficient pathogen recognition is present. We draw parallel to the cell intrinsic changes in naïve CD4 T cells, which also become dependent on PR activation of APC. We suggest this may reveal a basic strategy to avoid un-necessary responses with age which can be pathogenic and possibly to avoid dangerous autoimmunity, but to retain sufficient potential to respond to pathogen infection. These new hypotheses need further evaluation but provide a novel framework to think about some of the aging changes that most impact immunity.
ACKOWLEDGEMENTS
This work was supported by funding from grants R21AI128606, R21AG058758, P01AG021600-06 (Haynes) and R01AI118820 to S.L.S and T32 AI007349, R25 GM113686 and T32 AI132152 to O.K.U.
Abbreviations:
- ABC
age-associated B cells
- AbSC
antibody-secreting cells
- AID
activation-induced cytidine deaminase
- APC
Antigen-presenting cell
- BCR
B cell receptor
- BM
bone marrow
- CSR
Class switch recombination
- DC
dendritic cells
- FOB
follicular B cells
- GCB
germinal center B cells
- iABC
induced age-associated B cells
- MZB
marginal-zone b cells
- LLPC
long-lived plasma cell
- LRTI
lower respiratory infection
- nRNP
nuclear ribonuclear proteins
- PR
pathogen-recognition pathway
- PRR
pathogen recognition receptor
- RA
Rheumatoid Arthritis
- SHM
somatic hypermutation
- SLE
systemic lupus
- SPF
Specific pathogen-free
- SLC
surrogate light chain
- TCR
T cell receptor
- Tfh
T follicular cell
- TLR
toll-like receptor
Footnotes
DECLARATION OF ORIGINALITY
All text, figures and tables in the manuscript are the authors’ original work. This manuscript has not been submitted or published previously.
REFERENCES
- 1.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Vol. 377, The Lancet. Lancet Publishing Group; 2011. p. 1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, Adetifa IMO, Adhikari TB, Akibu M, Al Lami FH, Al-Eyadhy A, Alvis-Guzman N, Amare AT, Amoako YA, Antonio CAT, Aremu O, Asfaw ET, Asgedom SW, Atey TM, Attia EF, Avokpaho EFGA, Ayele HT, Ayuk TB, Balakrishnan K, Barac A, Bassat Q, Behzadifar M, Behzadifar M, Bhaumik S, Bhutta ZA, Bijani A, Brauer M, Brown A, Camargos PAM, Castañeda-Orjuela CA, Colombara D, Conti S, Dadi AF, Dandona L, Dandona R, Do HP, Dubljanin E, Edessa D, Elkout H, Endries AY, Fijabi DO, Foreman KJ, Forouzanfar MH, Fullman N, Garcia-Basteiro AL, Gessner BD, Gething PW, Gupta R, Gupta T, Hailu GB, Hassen HY, Hedayati MT, Heidari M, Hibstu DT, Horita N, Ilesanmi OS, Jakovljevic MB, Jamal AA, Kahsay A, Kasaeian A, Kassa DH, Khader YS, Khan EA, Khan MN, Khang YH, Kim YJ, Kissoon N, Knibbs LD, Kochhar S, Koul PA, Kumar GA, Lodha R, Magdy Abd El Razek H, Malta DC, Mathew JL, Mengistu DT, Mezgebe HB, Mohammad KA, Mohammed MA, Momeniha F, Murthy S, Nguyen CT, Nielsen KR, Ningrum DNA, Nirayo YL, Oren E, Ortiz JR, PA M, Postma MJ, Qorbani M, Quansah R, Rai RK, Rana SM, Ranabhat CL, Ray SE, Rezai MS, Ruhago GM, Safiri S, Salomon JA, Sartorius B, Savic M, Sawhney M, She J, Sheikh A, Shiferaw MS, Shigematsu M, Singh JA, Somayaji R, Stanaway JD, Sufiyan MB, Taffere GR, Temsah MH, Thompson MJ, Tobe-Gai R, Topor-Madry R, Tran BX, Tran TT, Tuem KB, Ukwaja KN, Vollset SE, Walson JL, Weldegebreal F, Werdecker A, West TE, Yonemoto N, Zaki MES, Zhou L, Zodpey S, Vos T, Naghavi M, Lim SS, Mokdad AH, Murray CJL, Hay SI, Reiner RC. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018. November 1;18(11):1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline KA, Bowdish DM. Infection in an aging population. Curr Opin Microbiol. 2016;29:63–7. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. February 15;395(10223):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the US. Influenza (Flu). 2020. [Google Scholar]
- 6.McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-cell immunity to influenza in older adults: A pathophysiological framework for development of more effective vaccines. Front Immunol. 2016;7(FEB):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElhaney JE. Influenza vaccine responses in older adults. Vol. 10, Ageing Research Reviews. NIH Public Access; 2011. p. 379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bupp MRG, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Vol. 9, Frontiers in Immunology. Frontiers Media S.A.; 2018. p. 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Vol. 13, Nature Reviews Immunology. Nature Publishing Group; 2013. p. 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai PS, Molony RD, Martinod K, Dong H, Pang IK, Tal MC, Solis AG, Bielecki P, Mohanty S, Trentalange M, Homer RJ, Flavell RA, Wagner DD, Montgomery RR, Shaw AC, Staeheli P, Iwasaki A. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science (80- ). 2016. April 22;352(6284):463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging Impairs Alveolar Macrophage Phagocytosis and Increases Influenza-Induced Mortality in Mice. J Immunol. 2017. August 1;199(3):1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD 2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011. December 1;121(12):4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su K, Osann S, Gupta A, Agrawal S, Agrawal J-N, Cao H. Phosphoinositide 3-Kinase-Signaling Pathway Dendritic Cells in Elderly Humans: A Role of Altered Innate Immune Functioning of. J Immunol Ref. 2007;178:6912–22. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal A, Agrawal S, Cao J-N, Su H, Osann K, Gupta S. Altered Innate Immune Functioning of Dendritic Cells in Elderly Humans: A Role of Phosphoinositide 3-Kinase-Signaling Pathway. J Immunol. 2007. June 1;178(11):6912–22. [DOI] [PubMed] [Google Scholar]
- 15.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012. October;24(5):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016. July 25;6(1):25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: Causes, consequences and strategies to circumvent. Vol. 54, Experimental Gerontology. NIH Public Access; 2014. p. 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shifrut E, Baruch K, Gal H, Ndifon W, Deczkowska A, Schwartz M, Friedman N. CD4+ T Cell-Receptor Repertoire Diversity is Compromised in the Spleen but Not in the Bone Marrow of Aged Mice Due to Private and Sporadic Clonal Expansions. Front Immunol. 2013. November 19;4(NOV):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004. May 1;172(9):5194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmakshatriya V, Kuang Y, Devarajan P, Xia J, Zhang W, Vong AM, Swain SL. IL-6 Production by TLR-Activated APC Broadly Enhances Aged Cognate CD4 Helper and B Cell Antibody Responses In Vivo. J Immunol. 2017;198(7):2819–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-Activated Dendritic Cells Enhance the Response of Aged Naive CD4 T Cells via an IL-6-Dependent Mechanism. J Immunol. 2010;185(11):6783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J Immunol. 2008;181(7):4825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swain SL, Kugler-Umana O, Kuang Y, Zhang W. The properties of the unique age-associated B cell subset reveal a shift in strategy of immune response with age. Cellular Immunology. 2017. July 11; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005. January;114(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003. December 9;100(25):15053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011. August;23(4):537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: Composition and function. Vol. 11, Biogerontology. Springer Netherlands; 2010. p. 125–37. [DOI] [PubMed] [Google Scholar]
- 28.Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Vol. 25, Current Opinion in Immunology. NIH Public Access; 2013. p. 504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci U S A. 2008. August 19;105(33):11898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson KL, Wu Y-C, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson B-O, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009. February 1;8(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186(6):3441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180(8). [DOI] [PubMed] [Google Scholar]
- 33.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for Decreased Function of B Cells in Aged Mice and Humans. J Immunol. 2008. March 1;180(5):2741–6. [DOI] [PubMed] [Google Scholar]
- 34.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell. 2013. April;12(2):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colonna-Romano G, Bulati M, Aquino A, Pellicanò M, Vitello S, Lio D, Candore G, Caruso C. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. 2009. October 1;130(10):681–90. [DOI] [PubMed] [Google Scholar]
- 37.Cancro MP. Age-Associated B Cells. Annu Rev Immunol. 2020. April 26;38(1). [DOI] [PubMed] [Google Scholar]
- 38.Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: Key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016. January;269(1):118–29. [DOI] [PubMed] [Google Scholar]
- 39.Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Vol. 177, Cell. Cell Press; 2019. p. 524–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011. August 4;118(5):1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du SW, Arkatkar T, Al Qureshah F, Jacobs HM, Thouvenel CD, Chiang K, Largent AD, Li Q-Z, Hou B, Rawlings DJ, Jackson SW. Functional Characterization of CD11c + Age-Associated B Cells as Memory B Cells. J Immunol. 2019. December 1;203(11):2817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013. August 20;110(34):E3216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang NS, McHeyzer-Williams LJ, Okitsu SL, Burris TP, Reiner SL, McHeyzer-Williams MG. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORα. Nat Immunol. 2012. June 6;13(6):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015. July 1;195(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, Gunn B, Chen D, Paley MA, Alter G, Reiner SL, Lauer GM, Teijaro JR, Wherry EJ. Cutting Edge: B Cell-Intrinsic T-bet Expression Is Required To Control Chronic Viral Infection. J Immunol. 2016;197(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ. Age-Associated B Cells Express a Diverse Repertoire of V H and Vκ Genes with Somatic Hypermutation. J Immunol. 2017;198(5):1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, Cancro MP. Cutting Edge: IL-4, IL-21, and IFN- Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol. 2016;197(4):1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manni M, Gupta S, Ricker E, Chinenov Y, Park SH, Shi M, Pannellini T, Jessberger R, Ivashkiv LB, Pernis AB. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol. 2018. April 26;19(4):407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubtsova K, Rubtsov AV., Cancro MP, Marrack P. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J Immunol. 2015;195(5):1933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017. April 3;127(4):1392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratliff M, Alter S, McAvoy K, Frasca D, Wright JA, Zinkel SS, Khan WN, Blomberg BB, Riley RL. In aged mice, low surrogate light chain promotes pro-B-cell apoptotic resistance, compromises the PreBCR checkpoint, and favors generation of autoreactive, phosphorylcholine-specific B cells. Aging Cell. 2015. June 1;14(3):382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley RL, Khomtchouk K, Blomberg BB. Age-associated B cells (ABC) inhibit B lymphopoiesis and alter antibody repertoires in old age. Cell Immunol. 2017. November 1;321:61–7. [DOI] [PubMed] [Google Scholar]
- 53.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol Res. 2013. March;55(1–3):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Zhang H, Liu S, Xia F, Kang Z, Zhang Y, Liu Y, Xiao H, Chen L, Huang C, Shen N, Xu H, Li F. Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus. Proc Natl Acad Sci U S A. 2019. September 10;116(37):18550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, Hupperts R, Somers V. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol. 2016;197(12):4576–83. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, Liu H, Manna Z, Goldbach-Mansky R, Hasni S, Siegel R, Sanjuan M, Streicher K, Cancro MP, Kolbeck R, Ettinger R. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c hi T-bet + B cells in SLE. Nat Commun. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Goodman SM, Tabechian D, Hughes LB, Salomon-Escoto K, Watts GFM, Jonsson AH, Rangel-Moreno J, Meednu N, Rozo C, Apruzzese W, Eisenhaure TM, Lieb DJ, Boyle DL, Mandelin AM, Albrecht J, Bridges SL, Buckley CD, Buckner JH, Dolan J, Guthridge JM, Gutierrez-Arcelus M, Ivashkiv LB, James EA, James JA, Keegan J, Lee YC, McGeachy MJ, McNamara MA, Mears JR, Mizoguchi F, Nguyen JP, Noma A, Orange DE, Rohani-Pichavant M, Ritchlin C, Robinson WH, Seshadri A, Sutherby D, Seifert J, Turner JD, Utz PJ, Boyce BF, DiCarlo E, Gravallese EM, Gregersen PK, Moreland L, Firestein GS, Hacohen N, Nusbaum C, Lederer JA, Perlman H, Pitzalis C, Filer A, Holers VM, Bykerk VP, Donlin LT, Anolik JH, Brenner MB, Raychaudhuri S. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yates JL, Racine R, McBride KM, Winslow GM. T Cell–Dependent IgM Memory B Cells Generated during Bacterial Infection Are Required for IgG Responses to Antigen Challenge. J Immunol. 2013;191(3):1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Racine R, Chatterjee M, Winslow GM. CD11c Expression Identifies a Population of Extrafollicular Antigen-Specific Splenic Plasmablasts Responsible for CD4 T-Independent Antibody Responses during Intracellular Bacterial Infection. J Immunol. 2008. July 15;181(2):1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenderes KJ, Levack RC, Papillion AM, Cabrera-Martinez B, Dishaw LM, Winslow GM. T-Bet + IgM Memory Cells Generate Multi-lineage Effector B Cells. Cell Rep. 2018. July 24;24(4):824–837.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone SL, Peel JN, Scharer CD, Risley CA, Chisolm DA, Schultz MD, Yu B, Ballesteros-Tato A, Wojciechowski W, Mousseau B, Misra RS, Hanidu A, Jiang H, Qi Z, Boss JM, Randall TD, Brodeur SR, Goldrath AW, Weinmann AS, Rosenberg AF, Lund FE. T-bet Transcription Factor Promotes Antibody-Secreting Cell Differentiation by Limiting the Inflammatory Effects of IFN-γ on B Cells. Immunity. 2019. May 21;50(5):1172–1187.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, Kostiv M, Rosenfeld AM, Meng W, Christensen SR, Hensley SE, Yewdell J, Canaday DH, Zhu J, McDermott AB, Dori Y, Itkin M, Wherry EJ, Pardi N, Weissman D, Naji A, Prak ETL, Betts MR, Cancro MP. The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity. 2020;52:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, Robb ML, Ostrowski MA, Deeks SG, Slifka MK, Tomaras GD, Moir S, Moody MA, Betts MR. T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI insight. 2017;2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang L-Y, Li Y, Kaplan DE. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat. 2017. May 1;24(5):389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eccles JD, Turner RB, Kirk NA, Muehling LM, Borish L, Steinke JW, Payne SC, Wright PW, Thacker D, Lahtinen SJ, Lehtinen MJ, Heymann PW, Woodfolk JA. T-bet+ Memory B Cells Link to Local Cross-Reactive IgG upon Human Rhinovirus Infection. Cell Rep. 2020. January 14;30(2):351–366.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devarajan P, Swain SL. Original Antigenic Sin: Friend or Foe in Developing a Broadly Cross-Reactive Vaccine to Influenza? Vol. 25, Cell Host and Microbe. Cell Press; 2019. p. 354–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henry C, Zheng NY, Huang M, Cabanov A, Rojas KT, Kaur K, Andrews SF, Palm AKE, Chen YQ, Li Y, Hoskova K, Utset HA, Vieira MC, Wrammert J, Ahmed R, Holden-Wiltse J, Topham DJ, Treanor JJ, Ertl HC, Schmader KE, Cobey S, Krammer F, Hensley SE, Greenberg H, He XS, Wilson PC. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe. 2019. March 13;25(3):357–366.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Paparoditis P, Horton AP, Frühwirth A, McDaniel JR, Jung J, Boutz DR, Hussein DA, Tanno Y, Pappas L, Ippolito GC, Corti D, Lanzavecchia A, Georgiou G. Persistent Antibody Clonotypes Dominate the Serum Response to Influenza over Multiple Years and Repeated Vaccinations. Cell Host Microbe. 2019. March 13;25(3):367–376.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999. October 4;190(7):1013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Vol. 24, Seminars in Immunology. NIH Public Access; 2012. p. 350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haynes L, Swain SL. Why Aging T Cells Fail: Implications for Vaccination. Vol. 24, Immunity. Cell Press; 2006. p. 663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003. December 9;100(25):15053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009. October 27;106(43):18333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010. October 15;185(8):4535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005. March 21;201(6):845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


