Abstract
Introduction
Prospective studies investigating flavonoid intake and dementia risk are scarce. The aims of this study were to examine associations between flavonoid intake and the risk of incident dementia and to investigate whether this association differs in the presence of lifestyle risk factors for dementia.
Methods
We examined associations in 55,985 participants of the Danish Diet, Cancer, and Health Study followed for 23 years. The Phenol‐Explorer database was used to estimate flavonoid intakes. Information on incident dementia and dementia subtypes was obtained using Danish patient and prescription registries. Hazard ratios (HRs) were calculated using restricted cubic splines in multivariable‐adjusted Cox proportional hazards models.
Results
For incident dementia, moderate compared to low intakes of flavonols (HR: 0.90 [0.82, 0.99]), flavanol oligo+polymers (HR: 0.87 [0.79, 0.96]), anthocyanins (HR: 0.84 [0.76, 0.93]), flavanones (HR: 0.89 [0.80, 0.99]), and flavones (HR: 0.85 [0.77, 0.95]) were associated with a lower risk. For vascular dementia, moderate intakes of flavonols (HR: 0.69 [0.53, 0.89]) and flavanol oligo + polymers (HR: 0.65 [0.51, 0.83]) were associated with lower risk. Flavonoid intakes were not significantly associated with Alzheimer's disease or unspecified dementia. The inverse association between total flavonoid intake and incident dementia was stronger in “ever” smokers than in “never” smokers and in those without hypercholesterolemia versus those with hypercholesteremia. Furthermore, the inverse association of vascular dementia with a moderate total flavonoid intake was stronger in “ever” smokers and those who were “normal” to “overweight” versus “never” smokers or those who were “obese,” respectively.
Conclusion
A moderate intake of flavonoid‐rich foods may help to reduce dementia risk.
Keywords: Alzheimer's disease, dementia, flavonoid subclasses, flavonoids, prospective study, vascular dementia
1. INTRODUCTION
The number of dementia cases (>50 million worldwide) is predicted to reach 152 million by 2050. 1 With coordinated action to address known risk factors, the 2020 Lancet Commission on Dementia estimated that up to 40% of these cases could be prevented. 2 Prevention is now recognized as an important strategy particularly given the limited efficacy of pharmacological treatment approaches. The first line of defense proposed against the development and progression of dementia is lifestyle‐related factors. Dietary components, particularly those that influence vascular risk factors, could be important. 3
Cardiovascular risk factors such as smoking, diabetes, and hypertension are strongly associated with a higher risk of cognitive impairment and dementia. 4 Guidelines recommend targeting midlife cardiovascular risk factors for prevention of dementia. 5 , 6 The significantly lower risk of cardiovascular disease (CVD) observed with higher dietary flavonoid intake 7 , 8 could therefore be important in dementia prevention. Flavonoids, a class of polyphenolic compounds found in abundance in plant‐derived foods and beverages such as fruits, vegetables, dark chocolate, tea, and red wine, have consistently been associated with cardiovascular health. 9 There is also a growing body of evidence for cognitive benefits. 10 Previous studies have observed that total flavonoid intake is associated with lower cognitive decline 11 , 12 and improved executive function in adults with mild to moderate Alzheimer's disease (AD) 13 (the most common form of dementia). A protective effect of high flavonoid intake on CVD risk, its associated risk factors, and cognitive function provides a strong rationale to investigate the relationship between flavonoid intake and incident dementia. No firm conclusions can be drawn from the few prospective studies to date that have investigated flavonoid intake and dementia. 14 , 15 , 16 , 17
Flavonoids are categorized into six main subclasses based on their chemical structure: flavonols, flavanols, flavanones, flavones, anthocyanins, and isoflavones. 9 As structural variations between the flavonoid subclasses and respective major compounds influence bioavailability and biological activity it is informative to investigate the relationships between particular flavonoid subclasses and dementia, 18 particularly for those flavonoid subclasses including flavanols, anthocyanins, and flavanones, for which there is evidence in the literature for a neuro‐protective effect. 10 Furthermore, there is evidence that the association between flavonoid intake and CVD may be modified by risk factors for CVD including smoking and alcohol intake; 7 , 19 whether the same holds true for flavonoids and dementia warrants further investigation.
Thus, the primary aims of this study were to investigate associations between incident dementia and intakes of (1) total flavonoids, (2) flavonoid subclasses, and (3) flavonoid compounds (where significant associations were observed for a particular subclass), in the Danish Diet, Cancer, and Health cohort. Secondary aims were to investigate whether these associations differed according to the presence of known risk factors for both CVD and dementia, that is, diabetes, hypertension, obesity, high cholesterol, alcohol, and smoking.
2. METHODS
2.1. Study population
From December 1993 through May 1997, 160,725 men and women from the greater areas of Copenhagen and Aarhus were invited to participate in the Danish Diet, Cancer, and Health Study. The study recruited 57,053 participants between the ages of 50 and 65 years who had no previous cancer diagnosis. Of these, 56,468 completed a food frequency questionnaire (FFQ). All Danish residents living in Denmark since 1968 are assigned a civil registry number, a unique and permanent 10‐digit number allowing cross‐linking of participants to nationwide registers such as the Civil Registration System, the Integrated Database for Labour Market Research, the Danish National Prescription Registry, and the Danish National Patient Register (DNPR). The DNPR holds information on all patients discharged from non‐psychiatric hospitals since 1977 as well as all patient contact with psychiatric inpatient, emergency department, and outpatient specialty clinics since 1995. 20 This information includes one primary diagnosis and one or more secondary diagnoses defined by the International Classification of Diseases (ICD): the 8th revision (ICD‐8) until 1993 and the 10th revision (ICD‐10) from 1994. 21 The Danish prescription registry holds information on all filled prescriptions from Danish pharmacies since 1994. Each drug is classified by the anatomical therapeutic chemical (ATC) code. 22 In the present study, participants were excluded if they had implausible energy intakes (<2092 kJ/d [<500 kcal/d] and >20,920 kJ/d [>5000 kcal/d]; n = 205); if they had missing covariates or were extreme outliers (n = 214); or if they at the time of recruitment had a diagnosis of dementia (n = 64). A dementia diagnosis consisted of a prior diagnosis of AD (ICD‐8: 29009 and 29010, ICD‐10: F00 and G30), vascular dementia (ICD‐8: 29309 and 29319, ICD‐10: F01), or unspecified dementia (ICD‐8: 29011, 29018, and 29019, ICD‐10: F02 and F03.9); see Figure S1 in supporting information.
This study was approved by the Danish Data Protection Agency (Ref no 2012‐58‐0004 I‐Suite nr: 6357, VD‐2018‐117).
2.2. Exposures
For this study the exposures of interest were intakes of total flavonoids; flavonoid subclasses; and, if relevant, the individual flavonoid compounds within the respective subclass, only where mean intakes of the subclasses were >5 mg/d. Calculations of flavonoid intake in this cohort have been described previously. 7 Briefly, estimates of the flavonoid content of all foods and beverages in the FFQ were obtained from the Phenol‐Explorer database. 23 All 219 flavonoid compounds were grouped into 10 subclasses based on their chemical structure (flavonols, flavanol monomers, flavanol oligo + polymers, flavanones, flavones, anthocyanins, isoflavones, dihydrochalcones, dihydroflavonols, and chalcones). Data used here are those derived from analyses by normal phase chromatography. 24 Total flavonoid intake was calculated by summing each of the 219 flavonoid compounds. The content of flavonoids was expressed as aglycone equivalents in mg/100 g food fresh weight. 24
RESEARCH IN CONTEXT
Systematic review: The beneficial effect of a higher habitual flavonoid intake on cardiovascular disease risk, its risk factors, and cognitive function provides a strong rationale to investigate the relationship between the range of different flavonoids present in the habitual diet and dementia. Few prospective studies have investigated flavonoid intake and dementia, with conflicting results reported.
Interpretation: Our findings suggest that a higher habitual intake of specific flavonoid subclasses, particularly the flavonols and flavanol oligo + polymers, was associated with a lower risk of incident, particularly vascular, dementia. The associations were non‐linear; moderate flavonoid intakes, within readily achievable daily dietary intakes, were associated with a lower risk of dementia.
Future directions: The current findings require replication in other populations to confirm the association between intakes of different flavonoids and dementia. Clinical trials and mechanistic studies to investigate the role of specific flavonoid subclasses on long‐term cognitive health are also warranted.
2.3. Study outcomes
Our primary outcome, incident dementia, was defined as a dementia‐related health‐care visit or a prescription for an anti‐dementia drug. 25 About two thirds of all dementia cases are diagnosed in the secondary care setting and are recorded in the DNPR. 26 Dementia health‐care visits were defined as a hospitalization or an outpatient visit with a primary or secondary diagnosis code for AD (ICD‐10: F00, G30), vascular dementia (ICD‐10: F01), or unspecified dementia (ICD‐10: F02 and F03.9) recorded in the DNPR. These diagnoses, for total dementia, have a positive predictive value (PPV) of 85.8% in the DNPR. 27 To capture those treated in the primary sector, we included information on claimed prescriptions for anti‐dementia drugs (ATC code: N06D). Secondary outcomes were hospitalization or outpatient visit for each dementia subtype discretely.
2.4. Covariates
At study enrolment, data on age, sex, duration of education (≤7 years, 8–10 years, ≥11 years), smoking habits, alcohol intake, daily activity, and diet were collected by self‐administered questionnaires. Anthropometry, including height and weight, and blood pressure were measured and a blood sample taken for determination of serum cholesterol levels. Average annual income (defined as household income after taxation and interest, for the value of the Danish currency in 2015) over 5 years was used as a representation of socioeconomic status. ICD‐8 and ICD‐10 codes were used to determine prevalent chronic kidney disease, ischemic heart disease, ischemic stroke, peripheral artery disease, heart failure, atrial fibrillation, and cancers. For treatment of diabetes mellitus, both self‐reported data and data on filled prescriptions for insulin and non‐insulin medication were used. Presence of hypertension was defined as at least one of (1) systolic blood pressure ≥140 mmHg, (2) self‐reported hypertension, (3) self‐reported use of blood pressure‐lowering medication. Hypercholesterolemia was defined as at least one of (1) non‐fasting serum cholesterol ≥6.2 mmol/L, (2) self‐reported hypercholesterolemia, (3) self‐reported statin use.
2.5. Statistical analysis
Participants’ follow‐up time was based on the date of study enrollment to, whichever came first, prescription of dementia medication, the date of dementia health‐care visit, death, emigration, or end of follow‐up (August 2017).
Cox proportional hazards models with restricted cubic splines were used to investigate relationships between flavonoid intake and the primary and secondary outcomes. Using restricted cubic splines, we allowed the association to be non‐linear. All hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained from the model with the exposure fitted as a continuous variable; HR estimates were reported for the median intake in each quintile with the first quintile median as the reference point, representing the lowest flavonoid intake, and were graphed over a fine grid of x values. For visual simplicity, the graphs of HRs derived from the fitting of cubic splines had x‐axis values restricted to intakes within three standard deviations of the mean for each exposure. Throughout, cause‐specific hazards models were used censoring individuals at death, rather than treating death as a competing risk. 28 Cox proportional hazards assumptions were tested using log‐log plots of the survival function versus time and assessed for parallel appearance. Potential confounders of flavonoid intake and dementia were chosen a priori as covariates. Three models of adjustment were used: (1a) minimally adjusted: age and sex; (1b) multivariable‐adjusted: age, sex, body mass index [BMI], smoking status (current/former/never), physical activity (total daily metabolic equivalent), pure alcohol intake (g/d), duration of education, and socioeconomic status (income); (2) multivariable‐adjusted: energy and potential dietary confounders; covariates in model 1b plus energy, intakes (g/d) of fish, red meat, processed meat, polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids.
To examine possible effect modification, additional analyses were stratified by known dementia risk factors, namely: diabetes (present/absent), hypertension (present/absent), BMI (above/below 30 kg/m2), hypercholesterolemia (present/absent), alcohol (above/below 20 g/d), and smoking (ever/never). When stratifying by alcohol intake we excluded all participants with an alcohol intake of zero (n = 1293), due to potential underlying pathologies or habits such as medication use in which alcohol intake is contraindicated (for example anxiolytics, antidepressants, and hypnotics). As there is the potential for residual confounding, when stratifying by smoking status, alcohol intake, and BMI, the corresponding continuous variables were included in the model where appropriate (smoking pack‐years, alcohol intake, and BMI, respectively). To examine the robustness of the associations, we conducted a sensitivity analysis using only dementia‐related health‐care visits as the outcome. All analyses were undertaken using STATA/IC 14.2 (StataCorp LLC) and R statistics (R Core Team, 2019). 29 Statistical significance was set at P ≤ 0.05 (two‐tailed) for all tests.
3. RESULTS
This population of 55,985 Danish citizens, with a median (interquartile range [IQR]) age of 56 (52–60) years at study entry, had a median (IQR) follow‐up of 21 (20–22) years. During a maximum of 23 years of follow‐up (1,073,280 person‐years), 2955 individuals received a diagnosis of dementia, 1348 participants had a health‐care visit for AD, 329 for vascular dementia, and 1338 for unspecified dementia, with some participants receiving a diagnosis of two or more types of dementia.
3.1. Baseline characteristics
The median (IQR) total flavonoid intake was 494 (286–804) mg/d. The median (IQR) intakes for the flavonoid subclasses are presented in Table 1. The baseline characteristics of the study population overall and by quintiles of total flavonoid intake are shown in Table 2. Compared to participants in the lowest quintile of total flavonoid intake, those in higher quintiles were more likely to be female, have a lower BMI, be more physically active, have never smoked, have a higher degree of education, and have a higher income. Those in the higher quintiles were also less likely to be hypertensive or hypercholesteremic, and tended to have a lower prevalence of diabetes and CVD, which in themselves are risk factors for dementia. Furthermore, participants in the higher quintiles consumed more fish; ate less red and processed meat; and, as expected, ate more fruits and vegetables.
TABLE 1.
Flavonoid subclasses, natural sources, and intake per day
| Flavonoid subclass | Natural sources 23 | Intake (mg/d) a |
|---|---|---|
| Flavonols | Tea (black), fruit (berries and pome), cocoa, and onions | 38 [23–73] |
| Flavanol monomers | Tea (black and green), wine, cocoa, chocolate, fruit (pome, drupe, tropical, and berries), nuts, and beans | 66 [25–268] |
| Flavanol oligo+polymers | Tea (black and green), wine, cocoa, fruit (pome, drupe, tropical, and berries), and beans | 255 [157–395] |
| Flavones | Olive oil, olives, herbs, and whole grain wheat | 5 [3–8] |
| Flavanones | Citrus fruit and herbs | 17 [8–38] |
| Anthocyanins | Pomegranate, berries, and red wine | 20 [11–50] |
| Isoflavones | Soybeans, soy foods, and beans | <1 |
Median [interquartile range].
TABLE 2.
Baseline characteristics of study population
| Total population n = 55,985 | Total flavonoid intake quintiles | |||||
|---|---|---|---|---|---|---|
| Q1 n = 11,197 | Q2 n = 11,197 | Q3 n = 11,197 | Q4 n = 11,197 | Q5 n = 11,197 | ||
| Total flavonoid intake (mg/d) | 494 [286–804] | 173 [127–212] | 320 [286–356] | 494 [441–548] | 726 [659–804] | 1201 [1024–1435] |
| Sex n (% male) | 26,626 (47.6) | 6469 (57.8) | 5733 (51.2) | 5329 (47.6) | 4988 (44.5) | 4107 (36.7) |
| Age (y) | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 56 [52–60] | 55 [52–59] |
| BMI (kg/m2) | 25.5 [23.3–28.2] | 26.1 [23.8–28.9] | 25.9 [23.6–28.5] | 25.6 [23.3–28.3] | 25.3 [23.2–27.9] | 24.9 [22.7–27.4] |
| MET score | 56.5 [37.0–84.8] | 51.0 [32.0–78.0] | 55.5 [36.3–84.0] | 57.5 [38.3–85.0] | 58.3 [38.5–87.0] | 60.0 [39.5–88.5] |
| Smoking status, n (%) | ||||||
| Never | 19,648 (35.1) | 2716 (24.3) | 3743 (33.4) | 3991 (35.6) | 4458 (39.8) | 4740 (42.3) |
| Former | 16,134 (28.8) | 2692 (24.0) | 3026 (27.0) | 3264 (29.2) | 3594 (32.1) | 3558 (31.8) |
| Current | 20,203 (36.1) | 5789 (51.7) | 4428 (39.5) | 3942 (35.2) | 3145 (28.1) | 2899 (25.9) |
| Education, n (%) | ||||||
| ≤7 y | 18,438 (32.9) | 5120 (45.7) | 4263 (38.1) | 3603 (32.2) | 3024 (27.0) | 2428 (21.7) |
| 8–10 y | 25,789 (46.1) | 4887 (43.6) | 5257 (47.0) | 5335 (47.6) | 5315 (47.5) | 4995 (44.6) |
| ≥11 y | 11,730 (21.0) | 1184 (10.6) | 1673 (14.9) | 2255 (20.1) | 2851 (25.5) | 3767 (33.6) |
| Mean household income, n (%) | ||||||
| ≤3,94,700 DKK/y | 13,886 (24.8) | 3342 (29.8) | 2744 (24.5) | 2709 (24.2) | 2574 (23.0) | 2517 (22.5) |
| 3,94,701–5,70,930 DKK/y | 14,000 (25.0) | 3268 (29.2) | 2998 (26.8) | 2714 (24.2) | 2601 (23.2) | 2419 (21.6) |
| 5,70,931–7,58,297 DKK/y | 14,049 (25.1) | 2915 (26.0) | 3032 (27.1) | 2900 (25.9) | 2620 (23.4) | 2582 (23.1) |
| >7,58,297 DKK/y | 14,050 (25.1) | 1672 (14.9) | 2423 (21.6) | 2874 (25.7) | 3402 (30.4) | 3679 (32.9) |
| Hypertensive | 24,913 (44.5) | 5355 (47.8) | 5098 (45.5) | 5047 (45.1) | 4838 (43.2) | 4575 (40.9) |
| Hypercholesterolemic | 28,590 (51.1) | 6052 (54.1) | 5784 (51.7) | 5830 (52.1) | 5617 (50.2) | 5307 (47.4) |
| Comorbidities, n (%) | ||||||
| Diabetes | 1177 (2.1) | 279 (2.5) | 219 (2.0) | 253 (2.3) | 215 (1.9) | 211 (1.9) |
| Heart failure | 220 (0.4) | 56 (0.5) | 54 (0.5) | 39 (0.3) | 40 (0.4) | 31 (0.3) |
| Ischemic stroke | 778 (1.4) | 218 (1.9) | 151 (1.3) | 145 (1.3) | 130 (1.2) | 134 (1.2) |
| Ischemic heart disease | 2192 (3.9) | 586 (5.2) | 422 (3.8) | 435 (3.9) | 396 (3.5) | 353 (3.2) |
| Peripheral artery disease | 498 (0.9) | 171 (1.5) | 114 (1.0) | 84 (0.8) | 61 (0.5) | 68 (0.6) |
| Atrial fibrillation | 277 (0.5) | 57 (0.5) | 55 (0.5) | 59 (0.5) | 49 (0.4) | 57 (0.5) |
| CKD | 202 (0.4) | 43 (0.4) | 33 (0.3) | 44 (0.4) | 41 (0.4) | 41 (0.4) |
| Cancer | 246 (0.4) | 55 (0.5) | 42 (0.4) | 60 (0.5) | 33 (0.3) | 56 (0.5) |
| Medication use, n (%) | ||||||
| Insulin treated | 380 (0.7) | 78 (0.7) | 65 (0.6) | 86 (0.8) | 80 (0.7) | 71 (0.6) |
| Antihypertensive | 6893 (12.3) | 1360 (12.1) | 1421 (12.7) | 1405 (12.5) | 1360 (12.1) | 1347 (12.0) |
| Statin | 1073 (1.9) | 262 (2.3) | 210 (1.9) | 221 (2.0) | 212 (1.9) | 168 (1.5) |
| HRT | ||||||
| Never | 15,965 (28.5) | 2605 (23.3) | 3047 (27.2) | 3268 (29.2) | 3268 (29.2) | 3777 (33.7) |
| Current | 8816 (15.7) | 1293 (11.5) | 1569 (14.0) | 1698 (15.2) | 2001 (17.9) | 2255 (20.1) |
| Former | 4547 (8.1) | 821 (7.3) | 844 (7.5) | 895 (8.0) | 934 (8.3) | 1053 (9.4) |
| NSAID | 18,138 (32.6) | 3538 (31.8) | 3528 (31.7) | 3636 (32.7) | 3636 (32.6) | 3800 (34.2) |
| Aspirin | 7086 (12.7) | 1391 (12.4) | 1366 (12.2) | 1434 (12.8) | 1392 (12.4) | 1503 (13.4) |
| Dietary characteristics | ||||||
| Energy (kj/d) | 9500 [7857–11,369] | 8609 [7027– 10,387] | 9262 [7718–11,000] | 9752 [8140–11,587] | 9936 [8320–11,830] | 9929 [8258–11,891] |
| Total fish intake (g/d) | 38 [25–55] | 33 [22–49] | 38 [25–54] | 34 [27–57] | 41 [28–59] | 40 [27–57] |
| Red meat intake (g/d) | 78 [57–107] | 80 [58–108] | 81 [59–110] | 80 [58–110] | 78 [57–107] | 72 [52–99] |
| Processed meat intake (g/d) | 25 [14–40] | 28 [17–45] | 26 [16–42] | 25 [14–40] | 23 [14–38] | 20 [11–34] |
| Dietary fiber intake (g/d) | 20 [16–25] | 16 [13–20] | 19 [16–23] | 21 [17–25] | 22 [18–27] | 23 [19–29] |
| Saturated FA (g/d) | 31 [24–39] | 29 [23–37] | 31 [24–39] | 32 [24–40] | 32 [25–41] | 32 [24–41] |
| Polyunsaturated FA (g/d) | 13 [10–17] | 12 [9–16] | 13 [10–17] | 14 [10–18] | 14 [11–18] | 14 [10–18] |
| Monounsaturated FA (g/d) | 27 [21–35] | 26 [20–34] | 27 [21–35] | 28 [22–35] | 28 [22–35] | 27 [21–34] |
| Fruit intake (g/d) | 171 [95–281] | 87 [44–140] | 162 [97–237] | 193 [114–300] | 224 [139–360] | 240 [141–390] |
| Vegetable intake (g/d) | 161 [105–231] | 114 [71–170] | 150 [99–211] | 168 [114–235] | 184 [127–253] | 196 [135–272] |
| Alcohol intake (g/d) | 13 [6–31] | 11 [3–24] | 13 [6–25] | 15 [6–34] | 14 [7–32] | 13 [6–32] |
Note: Data expressed as median [IQR] unless otherwise stated.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DKK, Danish Krone; FA, fatty acids; HRT, hormone replacement therapy; IQR, interquartile range; MET, metabolic equivalent; NSAID, nonsteroidal anti‐inflammatory drug.
3.2. Associations between total flavonoid intake and incident dementia and dementia subtypes
The non‐linear inverse association between total flavonoid intake and incident dementia (Figure 1) did not reach statistical significance at any level of flavonoid intake (Table 3). However, after adjusting for demographic and lifestyle factors (Model 1b), participants in quintiles (Q) 2 and 3 had a 24% (HR [95% CI]: 0.76 [0.62, 0.93]) and 30% (0.70 [0.54, 0.91]) lower risk of vascular dementia, respectively, compared to participants in Q1 (Table 4). Although total flavonoid intake appeared to be non‐linearly inversely associated with AD, this did not reach statistical significance at any level of flavonoid intake (Figure 1). Total flavonoid intake did not appear to be associated with unspecified dementia (Figure 1).
FIGURE 1.
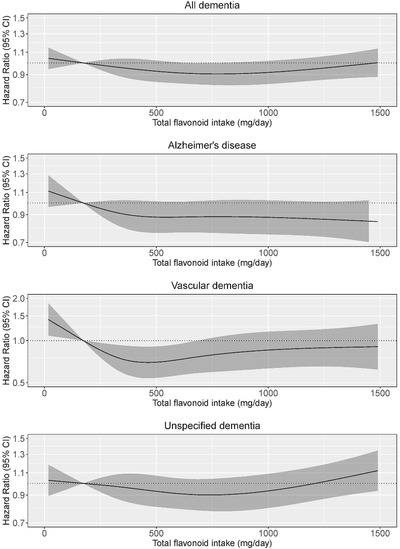
Cubic spline curves depicting the association between total flavonoid intake and both total dementia incidence (n = 2955) and the incidence of dementia subtypes; Alzheimer's disease (n = 1348), vascular dementia (n = 329), and unspecified dementia (n = 1338). Hazard ratios are based on Cox proportional hazards models adjusted for age, sex, body mass index, smoking status, physical activity, alcohol intake, education, and socioeconomic status (income), and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile (173 mg/d).
TABLE 3.
Hazard ratios of incident dementia by quintiles of flavonoid intake
| Flavonoid intake quintiles | |||||
|---|---|---|---|---|---|
| Q1 (n = 11,197) | Q2 (n = 11,197) | Q3 (n = 11,197) | Q4 (n = 11,197) | Q5 (n = 11,197) | |
| Total flavonoids | |||||
| No. events | 602 | 595 | 604 | 577 | 577 |
| Intake (mg/d)a | 173 (6–251) | 320 (251–394) | 495 (394–601) | 726 (601–908) | 1201 (908–3552) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.94 (0.87, 1.01) | 0.88 (0.8, 0.97) | 0.85 (0.77, 0.93) | 0.87 (0.79, 0.96) |
| Model 1b | ref. | 0.96 (0.89, 1.04) | 0.93 (0.84, 1.02) | 0.91 (0.82, 1.00) | 0.95 (0.85, 1.05) |
| Model 2 | ref. | 0.95 (0.88, 1.02) | 0.91 (0.82, 1.01) | 0.89 (0.80, 0.98) | 0.93 (0.83, 1.04) |
| Flavonols | |||||
| No. events | 639 | 597 | 559 | 606 | 554 |
| Intake (mg/d) a | 15 (0–20) | 26 (20–32) | 38 (32–50) | 66 (50–82) | 116 (83–251) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.91 (0.85, 0.97) | 0.86 (0.79, 0.94) | 0.85 (0.78, 0.94) | 0.86 (0.78, 0.95) |
| Model 1b | ref. | 0.93 (0.88, 1.00) | 0.90 (0.82, 0.99) | 0.92 (0.83, 1.01) | 0.94 (0.85, 1.04) |
| Model 2 | ref. | 0.91 (0.85, 0.98) | 0.87 (0.79, 0.96) | 0.89 (0.80, 0.98) | 0.92 (0.82, 1.02) |
| Flavanol monomers | |||||
| No. events | 613 | 572 | 594 | 623 | 553 |
| Intake (mg/d) a | 14 (0–21) | 30 (21–45) | 66 (45–115) | 260 (115–281) | 473 (281–916) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.97 (0.93, 1.01) | 0.92 (0.83, 1.02) | 0.89 (0.81, 0.98) | 0.89 (0.81, 0.98) |
| Model 1b | ref. | 0.98 (0.94, 1.03) | 0.96 (0.87, 1.06) | 0.96 (0.87, 1.06) | 0.97 (0.88, 1.07) |
| Model 2 | ref. | 0.98 (0.94, 1.02) | 0.95 (0.85, 1.05) | 0.96 (0.87, 1.06) | 0.97 (0.88, 1.08) |
| Flavanol oligo + polymers | |||||
| No. events | 595 | 644 | 556 | 594 | 566 |
| Intake (mg/d) a | 91 (0–136) | 178 (136–217) | 255 (217–302) | 359 (302–434) | 536 (434–2254) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.92 (0.85, 1.00) | 0.86 (0.79, 0.94) | 0.81 (0.74, 0.89) | 0.83 (0.75, 0.91) |
| Model 1b | ref. | 0.95 (0.88, 1.03) | 0.91 (0.83, 1.00) | 0.87 (0.79, 0.96) | 0.89 (0.80, 0.99) |
| Model 2 | ref. | 0.94 (0.87, 1.02) | 0.89 (0.81, 0.98) | 0.85 (0.76, 0.94) | 0.87 (0.77, 0.97) |
| Anthocyanins | |||||
| No. events | 640 | 589 | 504 | 577 | 645 |
| Intake (mg/d) a | 5 (0–10) | 13 (10–17) | 20 (17–24) | 36 (24–53) | 71 (53–397) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.83 (0.77, 0.90) | 0.79 (0.72, 0.88) | 0.86 (0.78, 0.95) | 0.99 (0.9, 1.10) |
| Model 1b | ref. | 0.87 (0.80, 0.94) | 0.84 (0.76, 0.93) | 0.91 (0.82, 1.00) | 1.03 (0.92, 1.15) |
| Model 2 | ref. | 0.86 (0.79, 0.93) | 0.83 (0.75, 0.92) | 0.90 (0.81, 0.99) | 1.01 (0.90, 1.13) |
| Flavanones | |||||
| No. events | 658 | 541 | 551 | 573 | 632 |
| Intake (mg/d) a | 3 (0–6) | 9 (6–13) | 17 (13–26) | 32 (26–49) | 70 (49–564) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.91 (0.85, 0.97) | 0.86 (0.77, 0.95) | 0.89 (0.81, 0.98) | 0.98 (0.89, 1.08) |
| Model 1b | ref. | 0.93 (0.87, 0.99) | 0.89 (0.80, 0.99) | 0.93 (0.84, 1.02) | 1.01 (0.92, 1.12) |
| Model 2 | ref. | 0.92 (0.86, 0.98) | 0.87 (0.78, 0.98) | 0.91 (0.83, 1.01) | 0.99 (0.89, 1.10) |
| Flavones | |||||
| No. events | 619 | 552 | 559 | 587 | 638 |
| Intake (mg/d) a | 2 (0–3) | 4 (3–4) | 5 (4–6) | 7 (6–9) | 11 (9–51) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.84 (0.77, 0.92) | 0.82 (0.74, 0.90) | 0.84 (0.76, 0.92) | 0.91 (0.82, 1.00) |
| Model 1b | ref. | 0.87 (0.79, 0.95) | 0.85 (0.77, 0.95) | 0.88 (0.80, 0.97) | 0.95 (0.86, 1.05) |
| Model 2 | ref. | 0.86 (0.78, 0.94) | 0.84 (0.75, 0.93) | 0.86 (0.78, 0.95) | 0.92 (0.82, 1.03) |
Notes: Hazard ratios (95% CI) for incident dementia during 23 years of follow‐up, obtained from restricted cubic splines based on Cox proportional hazards models. Model 1a adjusted for age and sex; Model 1b adjusted for age, sex, BMI, smoking status, physical activity, alcohol intake, education, and socioeconomic status (income); Model 2 adjusted for all covariates in model 1b plus energy, intakes of fish, red meat, processed food, polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids.
Median; range in parentheses (all such values).
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
TABLE 4.
Hazard ratios of vascular dementia by quintiles of flavonoid intake
| Flavonoid intake quintiles | |||||
|---|---|---|---|---|---|
| Q1 (n = 11,197) | Q2 (n = 11,197) | Q3 (n = 11,197) | Q4 (n = 11,197) | Q5 (n = 11,197) | |
| Total flavonoids | |||||
| No. events | 85 | 63 | 61 | 57 | 63 |
| Intake (mg/d) a | 173 (6–251) | 320 (251–394) | 495 (394–601) | 726 (601–908) | 1201 (908–3552) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.70 (0.57, 0.86) | 0.61 (0.47, 0.78) | 0.66 (0.51, 0.86) | 0.74 (0.55, 0.98) |
| Model 1b | ref. | 0.76 (0.62, 0.93) | 0.70 (0.54, 0.91) | 0.78 (0.59, 1.03) | 0.88 (0.65, 1.19) |
| Model 2 | ref. | 0.75 (0.61, 0.92) | 0.68 (0.52, 0.89) | 0.76 (0.56, 1.01) | 0.85 (0.62, 1.18) |
| Flavonols | |||||
| No. events | 88 | 72 | 49 | 54 | 66 |
| Intake (mg/d) a | 15 (0–20) | 26 (20–32) | 38 (32–50) | 66 (50–82) | 116 (83–251) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.71 (0.59, 0.85) | 0.60 (0.47, 0.78) | 0.70 (0.54, 0.91) | 0.75 (0.56, 1.00) |
| Model 1b | ref. | 0.76 (0.64, 0.92) | 0.69 (0.53, 0.89) | 0.83 (0.63, 1.10) | 0.91 (0.67, 1.23) |
| Model 2 | ref. | 0.73 (0.60, 0.88) | 0.64 (0.48, 0.84) | 0.79 (0.59, 1.05) | 0.86 (0.63, 1.19) |
| Flavanol monomers | |||||
| No. events | 81 | 67 | 64 | 56 | 61 |
| Intake (mg/d) a | 14 (0–21) | 30 (21–45) | 66 (45–115) | 260 (115–281) | 473 (281–916) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.84 (0.74, 0.96) | 0.67 (0.50, 0.90) | 0.75 (0.57, 0.99) | 0.78 (0.58, 1.03) |
| Model 1b | ref. | 0.88 (0.77, 1.00) | 0.75 (0.55, 1.01) | 0.89 (0.67, 1.20) | 0.93 (0.69, 1.26) |
| Model 2 | ref. | 0.87 (0.77, 1.00) | 0.73 (0.54, 1.00) | 0.90 (0.66, 1.21) | 0.94 (0.69, 1.27) |
| Flavanol oligo+polymers | |||||
| No. events | 90 | 64 | 53 | 62 | 60 |
| Intake (mg/d) a | 91 (0–136) | 178 (136–217) | 255 (217–302) | 359 (302–434) | 536 (434–2254) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.63 (0.51, 0.78) | 0.57 (0.45, 0.72) | 0.63 (0.49, 0.82) | 0.69 (0.52, 0.91) |
| Model 1b | ref. | 0.70 (0.57, 0.86) | 0.65 (0.51, 0.83) | 0.74 (0.56, 0.97) | 0.81 (0.60, 1.09) |
| Model 2 | ref. | 0.68 (0.55, 0.85) | 0.63 (0.49, 0.82) | 0.71 (0.53, 0.94) | 0.77 (0.56, 1.06) |
| Anthocyanins | |||||
| No. events | 71 | 69 | 49 | 62 | 78 |
| Intake (mg/d) a | 5 (0–10) | 13 (10–17) | 20 (17–24) | 36 (24–53) | 71 (53–397) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.80 (0.63, 1.02) | 0.74 (0.55, 1.01) | 0.79 (0.60, 1.04) | 0.98 (0.72, 1.32) |
| Model 1b | ref. | 0.90 (0.71, 1.15) | 0.88 (0.65, 1.19) | 0.91 (0.68, 1.22) | 1.08 (0.79, 1.50) |
| Model 2 | ref. | 0.90 (0.71, 1.15) | 0.88 (0.64, 1.19) | 0.92 (0.68, 1.23) | 1.08 (0.78, 1.50) |
| Flavanones | |||||
| No. events | 85 | 51 | 67 | 62 | 64 |
| Intake (mg/d) a | 3 (0–6) | 9 (6–13) | 17 (13–26) | 32 (26–49) | 70 (49–564) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.86 (0.70, 1.05) | 0.78 (0.57, 1.06) | 0.81 (0.61, 1.07) | 0.88 (0.66, 1.18) |
| Model 1b | ref. | 0.91 (0.75, 1.11) | 0.87 (0.63, 1.19) | 0.91 (0.69, 1.21) | 0.97 (0.73, 1.31) |
| Model 2 | ref. | 0.91 (0.74, 1.11) | 0.86 (0.62, 1.18) | 0.90 (0.68, 1.20) | 0.96 (0.71, 1.29) |
| Flavones | |||||
| No. events | 68 | 61 | 65 | 61 | 74 |
| Intake (mg/d) a | 2 (0–3) | 4 (3–4) | 5 (4–6) | 7 (6–9) | 11 (9–51) |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.87 (0.66, 1.14) | 0.84 (0.62, 1.14) | 0.84 (0.63, 1.12) | 0.92 (0.68, 1.25) |
| Model 1b | ref. | 0.97 (0.73, 1.27) | 0.96 (0.71, 1.30) | 0.98 (0.73, 1.30) | 1.05 (0.78, 1.44) |
| Model 2 | ref. | 0.98 (0.74, 1.29) | 0.98 (0.74, 1.29) | 1.00 (0.74, 1.36) | 1.09 (0.77, 1.52) |
Notes: Hazard ratios (95% CI) for vascular dementia during 23 years of follow‐up, obtained from restricted cubic splines based on Cox proportional hazards models. Model 1a adjusted for age and sex; Model 1b adjusted for age, sex, BMI, smoking status, physical activity, alcohol intake, education, and socioeconomic status (income); Model 2 adjusted for all covariates in model 1b plus energy, intakes of fish, red meat, processed food, polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids.
Median; range in parentheses (all such values).
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
3.3. Associations between flavonoid subclass intakes and incident dementia
Associations between intakes of flavonols, flavanol oligo + polymers, anthocyanins, flavanones, and flavones and incident dementia were non‐linear (Table 3 and Figure 2). For the aforementioned subclasses, the lowest risks seen for moderate intakes (Q3 and Q4). No evidence of an association between the flavanol monomers and incident dementia was observed. Similar associations were also observed after further adjustments for potential dietary confounders (Model 2).
FIGURE 2.
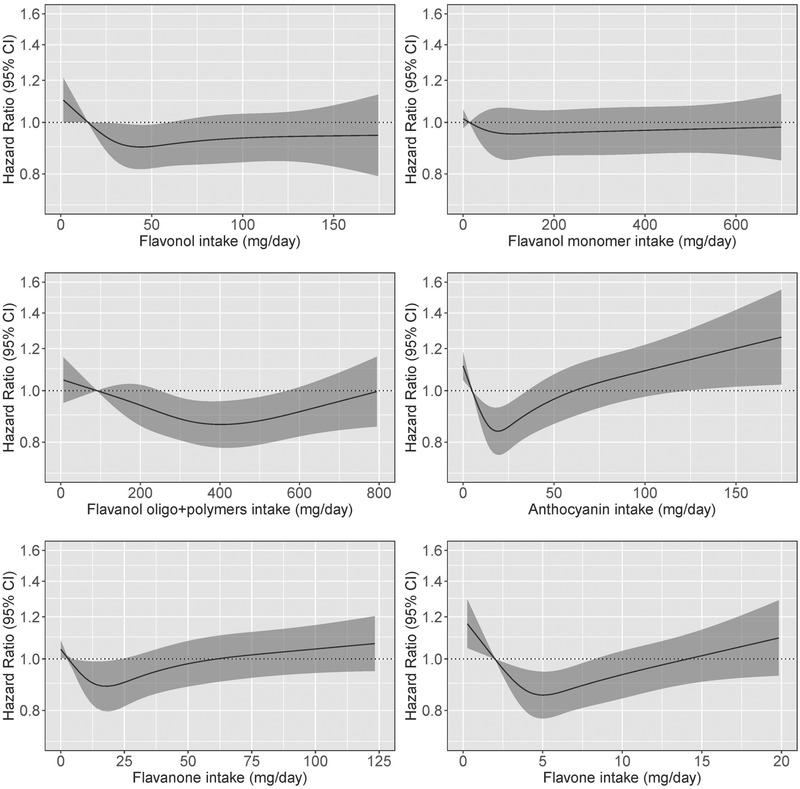
Hazard ratios based on cubic spline curves to depict the association between flavonoid subclass intakes (mg/d) and all cases of incident dementia among participants of the Danish Diet, Cancer, and Health cohort. Hazard ratios are based on Cox proportional hazards models adjusted for age, sex, body mass index, smoking status, physical activity, alcohol intake, education, and socioeconomic status (income), and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile
3.4. Associations between major flavonoid compound intakes and incident dementia
Associations between intakes of flavonoid compounds and incident dementia were assessed only for the subclasses significantly associated with a lower risk of dementia in at least one intake quintile (flavonols, flavanol oligo + polymers, anthocyanins, flavanones, and flavones). Intakes of quercetin, procyanidin trimers, hesperidin, apigenin, delphinidin, and malvidin were associated with incident dementia (Figure S1). For all aforementioned compounds, the lowest risks were seen for moderate intakes (participants in Q3 and Q4; Table S1 in supporting information).
3.5. Associations between total flavonoid intakes and incident dementia stratified by risk factors for dementia
The association between total flavonoid intake and incident dementia, stratified by risk factors for dementia, is presented in Figure 3. The association appeared to differ by smoking and hypercholesterolemia status in that there was a statistically significant lower risk of incident dementia at a moderate total flavonoid intake for “ever” smokers (Q3 vs. Q1 HR [95% CI]: 0.89 [0.80, 0.99]) and those without hypercholesterolemia (Q4 vs. Q1 HR [95% CI]: 0.84 [0.72, 0.96]), but not for “never” smokers (Q3 vs. Q1: 1.07 [0.87, 1.31], or those with hypercholesterolemia (Q4 vs. Q1 HR [95% CI]: 1.00 [0.87, 1.14]), after multivariable adjustments (Model 1b).
FIGURE 3.
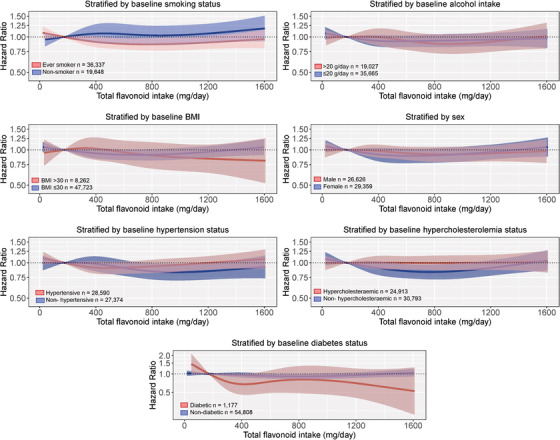
Multivariable‐adjusted association between total flavonoid intake and incident dementia stratified by baseline smoking status, alcohol intake, body mass index (BMI), sex, diabetes status, cholesterol levels, and hypertension status. Hazard ratios are based on Cox proportional hazards models and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile (173 mg/d). All analyses were standardized for age, sex, BMI, smoking, physical activity, alcohol intake, education, and socioeconomic status (income)
3.6. Associations between flavonoid subclass intakes and vascular dementia
Associations between flavonoid subclasses and vascular dementia appeared to be non‐linear (Table 4 and Figure 4). Intakes of the flavonol and flavanol oligo + polymer subclasses were associated with incident vascular dementia, with the lowest risks observed for participants in Q3. No evidence of an association between the anthocyanin, flavanol monomer, flavanone, or flavone subclasses and incident dementia was observed.
FIGURE 4.
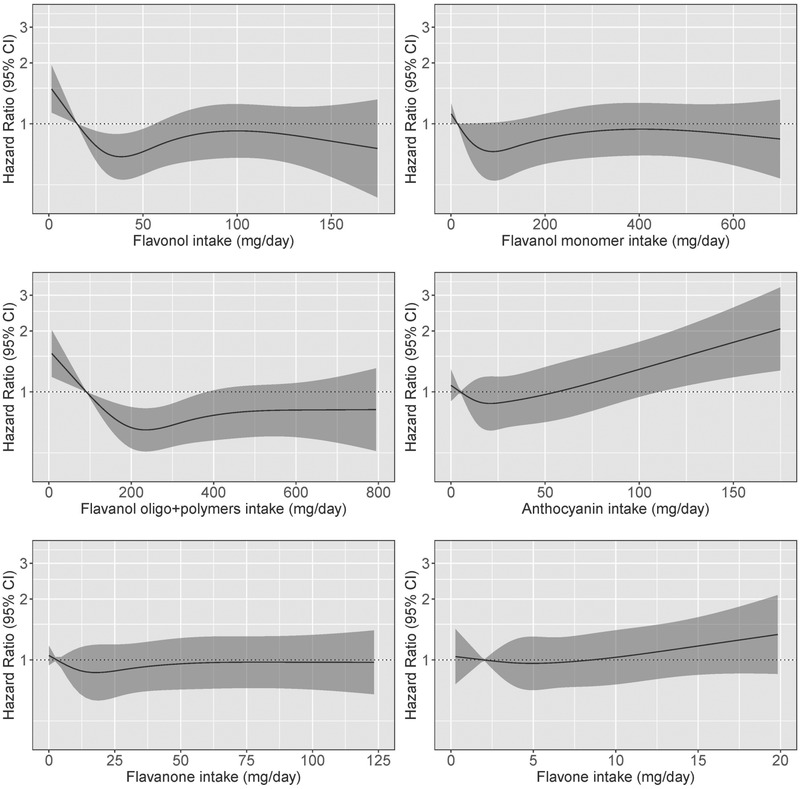
Hazard ratios based on cubic spline curves to depict the association between flavonoid subclass intakes (mg/d) and all cases of vascular dementia among participants of the Danish Diet, Cancer, and Health cohort. Hazard ratios are based on Cox proportional hazards models adjusted for age, sex, body mass index, smoking status, physical activity, alcohol intake, education, and socioeconomic status (income), and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile
3.7. Associations between total flavonoid intakes and vascular dementia stratified by risk factors for dementia
The association between total flavonoid intake and vascular dementia, stratified by risk factors for dementia, is presented in Figure 5. The association appeared to differ by smoking status, alcohol intake, BMI, and hypocholesteremia; there was a statistically significant lower risk of vascular dementia with a moderate total flavonoid intake for “ever” smokers (Q3 vs. Q1 HR [95% CI]: 0.70 [0.53, 0.91]) and those who were “normal” to “overweight” (Q3 vs. Q1: 0.64 [0.48, 0.85], but not for “never” smokers [Q3 vs. Q1: 0.95 [0.48, 1.89]) or those who were “obese” (Q3 vs. Q1: 1.14 [0.60, 2.18]), after multivariable adjustments (Model 1b). Furthermore, the association appeared to be “U‐shaped” in high alcohol consumers and participants who were not hypercholesteremic. We did not stratify by baseline diabetes status as there were very few cases of vascular dementia in this subgroup (n = 18).
FIGURE 5.
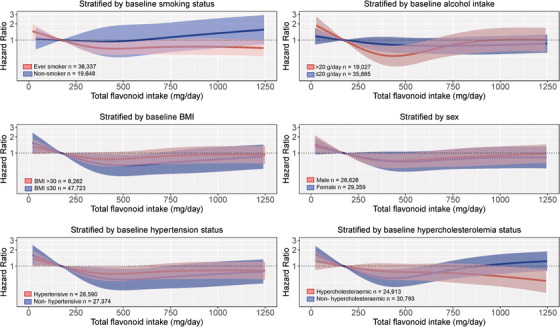
Multivariable‐adjusted association between total flavonoid intake and vascular dementia stratified by baseline smoking status, alcohol intake, body mass index (BMI), sex, diabetes status, hypertension status, and cholesterol levels. Hazard ratios are based on Cox proportional hazards models and are comparing the specific level of flavonoid intake (horizontal axis) to the median intake for participants in the lowest intake quintile (173 mg/d). All analyses were standardized for age, sex, BMI, smoking, physical activity, alcohol intake, education, and socioeconomic status (income)
3.8. Sensitivity analysis
Defining incident dementia using only dementia‐related health‐care visits (n = 2413) the observed association with total flavonoid intakes was stronger (Figure S3 in supporting information); compared to those in Q1, the risk of incident dementia was statistically significantly lower for participants in Q2–4 (HR [95% CIs] for Q2: 0.91 [0.84, 0.99]; Q3: 0.86 [0.77, 0.96]; Q4: 0.86 [0.77, 0.96]; Q5: 0.89 [0.79, 1.01])
4. DISCUSSION
Primary prevention of dementia through modification of lifestyle‐related factors, such as diet, is now recognized to be of importance. 3 There is the potential to lower the risk of developing dementia with evidence‐based dietary guidelines focusing on specific dietary components, especially in high‐risk populations. In this prospective cohort study of 55,985 Danish men and women, moderate intakes of flavonols, flavanol oligo + polymers, anthocyanins, flavanones, and flavones were associated with a lower risk of incident dementia. Additionally, we observed that moderate intakes of flavonols and flavanol oligo + polymers were significantly associated with a 24% to 30% lower risk of vascular dementia. The association between total flavonoid intake and incident dementia appeared to differ by smoking and hypercholesterolemia status while the association between total flavonoid intake and vascular dementia appeared to differ by smoking status, alcohol intake, BMI, and hypocholesteremia.
While there is a growing body of evidence that particular dietary patterns, such as the Mediterranean diet, are associated with a lower risk of dementia 30 and reduced dementia‐related neuropathology, 31 to our knowledge few prospective studies to date have investigated flavonoid intake and dementia. In the Paquid study, flavonoid intake (mean, 14.4 mg/d) was associated with a 51% (relative risk: 0.49, 95% CI: 0.26, 0.92) lower risk of dementia (66 incident cases) in 1367 subjects over the age of 65 and followed up for 5 years. 14 Lefèvre‐Arbogast et al. identified a pattern of polyphenol intake (mean, 1071 mg/d) associated with a 50% (HR: 0.50, 95% CI: 0.32, 0.80) lower risk of dementia (256 incident cases) in 1329 older adults followed for 12 years in the Three‐City Study. 17 Conversely, flavonoid intake at baseline (mean, 28.5 mg/d) was not associated with dementia (465 incident cases) in 5395 participants of the Rotterdam Study after a mean of 9.6 years follow‐up. 15 Flavonoid intake (estimated using a mean intake of tea as a proxy, 2 to 6 mg/d), was not associated with dementia (235 incident cases) after 30 years of follow‐up in 2459 men and women enrolled in the Honolulu‐Asia Aging Study. 16 More recently, participants of the Framingham Heart Study offspring cohort (2801 followed for 19.7 years, 193 incident cases of dementia), individuals with the highest flavonol, anthocyanins, and flavonoid polymers intake (based on a cumulative average across five exams) had a 46%, 76%, and 42% lower risk of dementia, respectively, compared to participants with the lowest intakes. 32 Evidence from observational studies investigating flavonoid‐rich foods and risk of dementia indicate potential protective effects for vegetables, particularly green leafy vegetables; fruit, particularly berries; and coffee and tea. 33 The present study is the largest study to date investigating flavonoid intake and risk of dementia (2955 incident cases diagnosed during follow‐up). It is, however, difficult to compare results between our study and previous studies due to inconsistencies in the number of different flavonoid subclasses considered and methods of calculation. For reference, median and mean flavonoid intakes in the present study were 494 and 596 mg/d, respectively, calculated using estimates of 219 compounds. Our results are consistent with recent flavonoid intake calculations using the United States Department of Agriculture and Phenol‐Explorer databases. 24 , 34 , 35
Of dementia subtypes, a statistically significant association was only observed between total flavonoid intake and vascular dementia. Compared to those with low flavonoid intakes, a 24% to 30% lower risk of vascular dementia was observed for participants with moderate flavonoid intakes. Vascular dementia can be a consequence of stroke and microvascular disease, and is estimated to account for 15% to 20% of all dementia cases. 36 It is important to note, however, that AD (the most common form of dementia) and vascular dementia are not distinct, and there is an overlap in underlying pathology; cerebrovascular dysfunction is prominent in AD 37 and pure vascular dementia (dementia caused solely by vascular pathology) is relatively rare. 38 There is a growing body of evidence supporting the beneficial effects of flavonoids on cardiovascular health and by extension, cerebrovascular health. 9 , 39 While a wide range of potential biological mechanisms have been reported for flavonoids and flavonoid‐rich foods, 40 benefits of specific flavonoid subclasses on vascular health could be mediated by enhanced nitric oxide bioavailability, 9 increased expression of heme‐oxygenase‐1, 41 inhibition of angiotensin‐converting enzyme activity, 42 effects on cholesterol, 43 and improved inflammatory status, 44 or a complex milieu of all these beneficial effects.
Interestingly, while no association was observed between total flavonoid intake and incident dementia, a non‐linear inverse association was observed for specific flavonoid subclasses and some of their individual flavonoid compounds. This is not surprising given the structural variations between the flavonoid subclasses, and their respective major compounds, which influences bioavailability and biological activity. 18 Evidence for a threshold after which higher flavonoid intakes afford no added benefit, is not new and has been seen for other outcomes in the same cohort. 7 , 19 Furthermore, our study suggests a U‐shaped association between intakes of several flavonoid subclasses and incident dementia. We hypothesize that this is driven by the co‐occurrence of other, potentially detrimental, dietary factors (e.g., added sugar in anthocyanin‐rich fruit juice/squash) in flavonoid‐rich foods. A lower risk of dementia was observed at moderate intakes of the flavonols, flavanol oligo + polymers, anthocyanins, flavanones. and flavones subclasses. These findings support a growing body of evidence in the literature that flavonols, flavanols, anthocyanins, and flavanones are the most neuro‐protective of all the flavonoids subclasses, improving measures of cognitive function and limiting cognitive decline. 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 A moderate intake of these flavonoid subclasses is well within achievable daily dietary limits: one cup of tea, one apple, one orange, 50 g of blueberries, and 50 g of dark chocolate would provide these flavonoid subclasses and more than 500 mg of total flavonoids.
The association between total flavonoid intake and both incident and vascular dementia was only present in current and former smokers; there was no association in participants who had never smoked. Smoking is strongly associated with a higher risk of dementia. 54 A recent meta‐analysis of 37 studies observed a 30% higher risk of all‐cause dementia (relative risk: 1.30, 95% CI: 0.18, 0.45) for participants who currently smoke compared to those who have never smoked. 54 It is proposed that smoking increases the risk of vascular dementia via detrimental effects on cerebrovascular health. 55 In the current study, the inverse association of total flavonoid intake with incident dementia observed in participants who smoked or had ever smoked is likely related to the protective effect of flavonoids on vascular health. Of note, a randomized controlled trial conducted in healthy individuals who smoked observed that flavonoid‐rich grape juice intake restored vascular function. 56 That the association between total flavonoid intake and vascular dementia was only present in participants who were “normal” to “overweight” at baseline could be related to differences in gut microbiome composition, 57 which plays a crucial role in flavonoid metabolism. 58 However, as the number of participants who were “obese” was low and associated confidence intervals are wide, this requires further investigation. Furthermore, the presence of an association between total flavonoid intake and vascular dementia, but not incident dementia, in participants who were hypercholesteremic at baseline requires further investigation taking into consideration treatment or changes in hypercholesteremia status during follow‐up. Given the low number of vascular dementia cases in this cohort, and that associations were stratified over several effect modifiers thereby increasing the likelihood of spurious associations, these stratified analyses should be interpreted with caution and viewed as exploratory.
Our cohort study has a number of strengths. The 23‐year duration of follow‐up with very limited loss to follow‐up allowed for the accumulation of a large number of incident cases despite the protracted nature of the disease. The length of follow‐up and age of enrolment (∼50 years) also allowed for the examination of flavonoid intake in association with mid‐life risk factors. This is important as substantial evidence indicates that mid‐life risk factors (hypertension, obesity, cholesterol, smoking, and physical activity) are important for predicting dementia risk in later life. 4
As this is an observational study a number of limitations apply. We are unable to infer causality or rule out residual or unmeasured confounding factors. Flavonoids could also be a surrogate marker of a healthier diet and lifestyle. However, the association between a higher flavonoid intake and a lower risk of vascular dementia was still present after adjustment for lifestyle factors and other indicators of a healthy diet. While the 23‐year duration of follow‐up allowed for the long preclinical phase of dementia to be considered, dietary intake data was only captured at baseline and dietary habits may have changed over the 23 years of follow‐up. Any such changes, however, would likely have attenuated the observed association. Due to the number of flavonoid subclasses and their individual flavonoid compounds making up the subclasses, a number of statistical tests were performed. However, these tests were hypothesis driven given the current knowledge on the bioavailability, bioefficacy, and mechanistic understanding of the different subclasses. Additionally, other than a dementia diagnosis (study exclusion criteria), there was no information on cognitive impairment at baseline, nor detailed information at follow‐up (beyond a diagnosis of dementia in the DNPR or prescription for an anti‐dementia drug in the DNPR). Additionally, dementia incidence may have been underestimated as participants who did not take medication for the treatment of dementia and who did not have a health‐care visit with a dementia diagnosis code would have been missed. Further, no studies are available providing PPVs for dementia diagnoses defined as either a dementia‐related health‐care visit or a prescription for an anti‐dementia drug. However, the association between total flavonoid intake and incident dementia was stronger in a sensitivity analysis using only dementia‐related health‐care visits as the outcome, which has a PPV of 85.8% in the DNPR. 27 This may be because the inclusion of a redeemed prescription for an anti‐dementia drug in the outcome may decrease specificity, particularly if such drugs are used off‐label in Denmark. Dementia can only be definitively diagnosed post mortem, although brain imaging increases diagnostic accuracy significantly. This raises the issue of individuals being incorrectly classified (particularly with respect to dementia subtypes). The ICD‐10 registration of subtypes of dementia is underreported in the DNPR and thus subtype analysis should be interpreted with caution. 26 Finally, the participants in this study were most likely White, meaning that caution should be taken when extrapolating these findings to other ethnicities. Further studies in other populations are required to confirm our findings.
In conclusion, in this large prospective cohort study of Danish men and women we observed that a moderate and achievable dietary intake of total flavonoids was associated with significantly lower risk of vascular dementia. Specific subclasses, flavanol oligo + polymers and flavonols, were associated with a lower risk of both incident and vascular dementia. Our results suggest that small changes to the habitual diet, encouraging consumption of flavonoid‐rich foods among low‐flavonoid consumers may help to lower the risk of dementia.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The Danish Diet, Cancer, and Health Study was funded by the Danish Cancer Society, Denmark. FD is funded by The Danish Heart Foundation (Grant number 17‐R115‐A7443‐22062) and Gangstedfonden (Grant number A35136), Denmark. NPB is funded by a National Health and Medical Research Council Early Career Fellowship (Grant number APP1159914), Australia. The salary of JRL is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID: 102817). The salary of JMH is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship, Australia (Grant number APP1116937). SRRS is supported by a BrightFocus Foundation Fellowship, USA.
Bondonno CP, Bondonno NP, Dalgaard F, et al. Flavonoid intake and incident dementia in the Danish Diet, Cancer, and Health cohort. Alzheimer's Dement. 2021;7:e12175. 10.1002/trc2.12175
Catherine P. Bondonno and Nicola P. Bondonno contributed equally to this study.
REFERENCES
- 1. Patterson C. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. London, UK: Alzheimer's Disease International (ADI); 2018. [Google Scholar]
- 2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 4. Bendlin B, Carlsson C, Gleason C, et al. Midlife predictors of Alzheimer's disease. Maturitas. 2010;65:131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369:2275‐2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455‐532. [DOI] [PubMed] [Google Scholar]
- 7. Bondonno NP, Dalgaard F, Kyrø C, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hertog MG, Kromhout D, Aravanis C, et al. Flavonoid intake and long‐term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381‐386. [PubMed] [Google Scholar]
- 9. Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev. 2015;73:216‐235. [DOI] [PubMed] [Google Scholar]
- 10. Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104:S40‐S7. [DOI] [PubMed] [Google Scholar]
- 11. Caldwell K, Roodenrys S, Charlton K, Richards R, Morgan O. Dietary flavonoid intake and cognitive performance in older adults with Alzheimer's type dementia. J Aging Res Clin Pract. 2016;5:93‐97. [Google Scholar]
- 12. Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Letenneur L, Proust‐Lima C, Le Gouge A, Dartigues J‐F, Barberger‐Gateau P. Flavonoid intake and cognitive decline over a 10‐year period. Am J Epidemiol. 2007;165:1364‐1371. [DOI] [PubMed] [Google Scholar]
- 14. Commenges D, Scotet V, Renaud S, Jacqmin‐Gadda H, Barberger‐Gateau P, Dartigues J‐F. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357‐363. [DOI] [PubMed] [Google Scholar]
- 15. Devore EE, Grodstein F, van Rooij FJ, et al. Dietary antioxidants and long‐term risk of dementia. Arch Neurol. 2010;67:819‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late‐life incident dementia: the Honolulu‐Asia Aging Study. Am J Epidemiol. 2004;159:959‐967. [DOI] [PubMed] [Google Scholar]
- 17. Lefèvre‐Arbogast S, Gaudout D, Bensalem J, et al. Pattern of polyphenol intake and the long‐term risk of dementia in older persons. Neurology. 2018;90:e1979‐e88. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez‐Mateos A, Vauzour D, Krueger CG, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014;88:1803‐1853. [DOI] [PubMed] [Google Scholar]
- 19. Dalgaard F, Bondonno NP, Murray K, et al. Associations between habitual flavonoid intake and hospital admissions for atherosclerotic cardiovascular disease: a prospective cohort study. Lancet Planet Health. 2019;3:e450‐e9. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin epidemiol. 2015;7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30‐33. [DOI] [PubMed] [Google Scholar]
- 22. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38‐41. [DOI] [PubMed] [Google Scholar]
- 23. Neveu V, Perez‐Jiménez J, Vos F, et al. Phenol‐Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez‐Jimenez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220‐1228. [DOI] [PubMed] [Google Scholar]
- 25. Fann JR, Ribe AR, Pedersen HS, et al. Long‐term risk of dementia among people with traumatic brain injury in Denmark: a population‐based observational cohort study. Lancet Psychiatry. 2018;5:424‐431. [DOI] [PubMed] [Google Scholar]
- 26. Phung TKT, Waltoft BL, Kessing LV, Mortensen PB, Waldemar G. Time trend in diagnosing dementia in secondary care. Dement Geriatr Cogn Disord. 2010;29:146‐153. [DOI] [PubMed] [Google Scholar]
- 27. Phung TKT, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220‐228. [DOI] [PubMed] [Google Scholar]
- 28. Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670‐2677. [DOI] [PubMed] [Google Scholar]
- 29. R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3‐900051‐07‐0. URL: http://www.R‐project.org/; 2019. [Google Scholar]
- 30. van de Rest O, Berendsen AA, Haveman‐Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6:154‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rainey‐Smith SR, Gu Y, Gardener SL, et al. Mediterranean diet adherence and rate of cerebral Aβ‐amyloid accumulation: data from the Australian imaging, biomarkers and lifestyle study of ageing. Transl Psychiatry. 2018;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shishtar E, Rogers GT, Blumberg JB, Au R, Jacques PF. Long‐term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am J Clin Nutr. 2020;112:343‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006‐1015. [DOI] [PubMed] [Google Scholar]
- 34. Cassidy A, Rimm EB, O'Reilly ÉJ, et al. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43:946‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ivey KL, Hodgson JM, Croft KD, Lewis JR, Prince RL. Flavonoid intake and all‐cause mortality. Am J Clin Nutr. 2015;101:1012‐1020. [DOI] [PubMed] [Google Scholar]
- 36. Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arter Thromb Vasc Biol. 2019;39:1542‐1549. [DOI] [PubMed] [Google Scholar]
- 37. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction‐the disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15:158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. [DOI] [PubMed] [Google Scholar]
- 39. Hooper L, Kroon PA, Rimm EB, Cohn JS, et al. Flavonoids, flavonoid‐rich foods, and cardiovascular risk: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38‐50. [DOI] [PubMed] [Google Scholar]
- 40. Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr Rev Food Sci Food Saf. 2018;17:1054‐1112. [DOI] [PubMed] [Google Scholar]
- 41. Croft KD, Zhang D, Jiang R, et al. Structural requirements of flavonoids to induce heme oxygenase‐1 expression. Free Rad Biol Med. 2017;113:165‐175. [DOI] [PubMed] [Google Scholar]
- 42. Guerrero L, Castillo J, Quiñones M, et al. Inhibition of angiotensin‐converting enzyme activity by flavonoids: structure‐activity relationship studies. PloS one. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raman G, Avendano EE, Chen S, et al. Dietary intakes of flavan‐3‐ols and cardiometabolic health: systematic review and meta‐analysis of randomized trials and prospective cohort studies. Am J Clin Nutr. 2019;110:1067‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. García‐Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti‐inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537‐552. [DOI] [PubMed] [Google Scholar]
- 45. Bergland AK, Soennesyn H, Dalen I, et al. Effects of anthocyanin supplementation on serum lipids, glucose, markers of inflammation and cognition in adults with increased risk of dementia–a pilot study. Front Genet. 2019;10:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brickman AM, Khan UA, Provenzano FA, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dodd GF, Williams CM, Butler LT, Spencer JP. Acute effects of flavonoid‐rich blueberry on cognitive and vascular function in healthy older adults. Nutr Health Aging. 2019:1‐14. [Google Scholar]
- 48. Kent K, Charlton K, Roodenrys S, et al. Consumption of anthocyanin‐rich cherry juice for 12 weeks improves memory and cognition in older adults with mild‐to‐moderate dementia. Eur J Nutr. 2017;56:333‐341. [DOI] [PubMed] [Google Scholar]
- 49. Mastroiacovo D, Kwik‐Uribe C, Grassi D, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study—a randomized controlled trial. Am J Clin Nutr. 2014;101:538‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neshatdoust S, Saunders C, Castle SM, et al. High‐flavonoid intake induces cognitive improvements linked to changes in serum brain‐derived neurotrophic factor: two randomised, controlled trials. Nutr Healthy Aging. 2016;4:81‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watson AW, Haskell‐Ramsay CF, Kennedy DO, Cooney JM, Trower T, Scheepens A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase‐B in healthy young adults. J Funct Foods. 2015;17:524‐539. [Google Scholar]
- 52. Willis LM, Shukitt‐Hale B, Joseph JA. Recent advances in berry supplementation and age‐related cognitive decline. Curr Opin Clin Nutr Metab Care. 2009;12:91‐94. [DOI] [PubMed] [Google Scholar]
- 53. Holland TM, Agarwal P, Wang Y, et al. Dietary flavonols and risk of Alzheimer dementia. Neurology. 2020;94:e1749‐e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta‐analysis of prospective cohort studies with investigation of potential effect modifiers. PLOS ONE. 2015;10:e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peters R. Blood pressure, smoking and alcohol use, association with vascular dementia. Exp Gerontol. 2012;47:865‐872. [DOI] [PubMed] [Google Scholar]
- 56. Siasos G, Tousoulis D, Kokkou E, et al. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am J Hypertens. 2013;27:38‐45. [DOI] [PubMed] [Google Scholar]
- 57. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cassidy A, Minihane A‐M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105:10‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


