Abstract
Background and aim:
Several gallstone patients complain of dyspeptic symptoms, irrespective of the presence of typical colicky pain. Symptoms often persist after a cholecystectomy. Systematic studies on dyspepsia and dynamic gastrointestinal motor function are missing in gallstone patients with preserved gallbladder or after a cholecystectomy.
Materials and methods:
Forty-six gallstone patients (age 55 ± 2 years; 15M, 31F) and 24 cholecystectomized patients (age 57 ± 2 years; 6M, 18F) (no difference in type and volume of gallstones between the two groups) were compared against a group of 65 healthy controls (age 51 ± 2 years; 30M, 35F). Dyspepsia occurring in the prior months was assessed by a questionnaire, gastric and gallbladder emptying by functional ultrasonography and orocecal transit time by a hydrogen breath test using a lactulose-enriched standard liquid meal.
Results:
Gallstone patients had significantly greater dyspepsia, fasting and residual gallbladder volumes, and slower gallbladder emptying, gastric emptying and small intestinal transit time than controls. In cholecystectomized patients, gastric emptying further delayed, compared to gallstone patients and controls.
Conclusion:
Gallstone patients with the gallbladder “in situ” or after a cholecystectomy display dyspeptic symptoms. Symptoms are associated with multiple gastrointestinal motility defects involving the gallbladder, stomach and small intestine. After cholecystectomy, gastric emptying worsens.
Keywords: breath test, cholelithiasis, dyspepsia, motility, orocecal transit time, small intestine, ultrasonography
1 |. INTRODUCTION
Gallstone patients are amongst the most admitted patients to European hospitals,1 and the socioeconomic costs of symptomatic uncomplicated or complicated gallstone disease are always on the rise. In developed countries, about 20% of adults have gallstones,2 and the incidence rate increases by 0.60%–1.39% per year.3 Gallstone prevalence increases along with aging and is higher in women than in men.4 Cholesterol gallstones are mainly made of cholesterol monohydrate crystals, and in the Western society, they account for approximately 75%–80% of the gallstones.4–6 In the remaining 20%–25%, gallstones are a black pigment formed by polymerized calcium bilirubinate or brown pigment stones growing in the gallbladder or in the infected extrahepatic or intrahepatic bile ducts, respectively.7
Cholesterol gallstones frequently include several metabolic abnormalities, ranging from insulin resistance, visceral adiposity expansion, overweight, obesity, type 2 diabetes, or metabolic syndrome,8,9 coronary heart disease,10 risks for cardiovascular disease and cancer. Other abnormalities are independent of the aforementioned risk factors.10–12
Appropriate therapy, either medical or mainly surgical (cholecystectomy), is necessary in about 20% of those who suffer from gallstones, because of colicky pain or complications (such as acute cholecystitis, gallbladder empyema and acute pancreatitis)13,14 developing during a lifetime. Gallstone patients may also complain of vague or specific dyspeptic symptoms, likely unrelated to the physical presence of gallstones.4 Instead, dyspeptic symptoms might originate from a different mechanism including motility defects (ie intestine, stomach and oesophagus), food intolerance, changes of gut microbiota, self-perception, etc.
Nonspecific gastrointestinal symptoms can also appear in about 10% of patients after cholecystectomy, but the origin of these disturbances is still unclear.15 The aim of this study was therefore to evaluate the coexistence of motor disorders of the gastrointestinal tract along with dyspeptic symptoms, in patients with a gallbladder “in situ” and in a group of matched patients after a cholecystectomy.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
The study comprised 46 gallstone patients with the gallbladder “in situ” and 24 cholecystectomized patients operated for symptomatic gallstone disease. A group of 65 matched healthy subjects served as the control group. The three subgroups were homogeneous for age and body mass index (Table 1). Epidemiological and clinical features and ultrasonographic appearance of gallstones stones were highly suggestive of cholesterol cholelithiasis.
TABLE 1.
Clinical characteristics of enrolled subjects
| Control subjects | Gallstone patients | Cholecystectomized patients | |
|---|---|---|---|
| Number | 65 | 46 | 24 |
| Age (y) | 51 ± 2 | 55 ± 2 | 57 ± 2 |
| Males:Females | 30:35 | 15:31 | 6:18 |
| BMI (kg/m2) | 25.0 ± 0.3 | 26.4 ± 0.6 | 25.4 ± 0.8 |
BMI, body mass index.
Data expressed as mean ± standard error.
Gallstones in the group with the gallbladder “in situ” were small and occupied invariably less than 30% of the fasting gallbladder volume, a safe condition that prevents major mechanical obstructions during postprandial gallbladder motility studies.16–19 Invariably, fasting gallbladder volume has to be greater than 12 mL, a safe cut-off ruling out hypoplasic, contracted, severely or chronically inflamed gallbladders.20 In addition, the gallbladder wall thickness had to be less than 3 mm to rule out ongoing inflammatory changes, and the gallbladder shape had to be regularly pear-shaped, to simplify volume measurements.
All gallstone patients (either with gallbladder “in situ” or cholecystectomized) had been symptomatic, that is, describing one or more episodes of typical colicky pain in the last 18–24 months.13,21 None of the gallstone patients had developed pain in the last eight weeks (a condition potentially able to interfere with motility studies, due to a persisting inflamed gallbladder wall). Cholecystectomized patients had undergone a laparoscopic cholecystectomy within 18–24 months before recruitment. The macroscopic analysis of stones, as well as the microscopic analysis of bile, confirmed that stones were mainly made of cholesterol.22
None of enrolled patients had diabetes or any other clinical or pharmacological condition potentially able to influence the indices of gallbladder, gastric and intestinal motility examined in the study.
Healthy subjects had a negative record for major surgeries, for drug intake in the last week capable of modifying the gallbladder and gastrointestinal motor function, and pregnancy was absent in all women.
All participants signed a written informed consent before entry. The studies had approval of the Human Subjects Committees at the Bari University Medical School (Bari, Italy), as per the ethical guidelines of the 1975 Declaration of Helsinki.
2.2 |. Assessment of dyspepsia
Dyspepsia was quantified by a specific questionnaire according to Buckley et al23 with a score describing severity, frequency and duration of four different symptoms: pain, epigastric burning, belching and a sense of postprandial fullness (maximum score = 48). The score referred to the worst perception of symptoms over the past 12 months in control, gallstone patients and cholecystectomized patients. In gallstone patients, the description of the symptom score was independent on episodes of colicky pain (if any). The value of 8 represented a normal upper limit.24
2.3 |. Test meal
The standard test meal (Nutridrink®; Nutricia, Milano, Italy) consisted of 200 mL liquid suspension containing 12 g (20%) protein, 11.6 g (19%) fat and 36.8 g (61%) carbohydrates for a total of 300 kcal, 1260 kJ, 455 mOsm/L, energy density 1.5 Kcal/mL, pH 6.5.
2.4 |. Ultrasonographic studies of gallbladder and gastric emptying
The ultrasound equipment consisted of a 3.5 MHz convex probe (AU 450, Ansaldo-Hitachi, Genoa and Noblus Hitachi, Japan). The study included the simultaneous examination of gallbladder and gastric emptying.18,25–29 Subjects had to fast for at least 12 hours, and measurements were taken in a seated position for 120 minutes. Immediately before and during the examination, subjects could not smoke and drink coffee or soft drinks to avoid interference with the gallbladder and gastric kinetics.
The gallbladder volume was measured according to the ellipsoid formula30,31 as fasting gallbladder volume, postprandial volumes taken every 10–15 minutes and minimum residual volume during 120 minutes. Gallbladder half-emptying time was the time when the gallbladder reached 50% emptying.
For the stomach, the fasting antral area was measured (mean of two measurements at times −5 and 0 minutes before the start of the meal). Postprandial antral areas were calculated immediately after ingestion of the test meal and then at regular time intervals (5–15 minutes) for 120 minutes. Gastric half-emptying time was the time when the stomach reached 50% emptying and calculated from the emptying curve (area/time).26–29
2.5 |. Orocecal transit time
Subjects followed a diet restricted in fermentable foods and were in “wash-out” for at least 10 days from drugs or manoeuvres (ie enemas) that could interfere with the intestinal microbiota or intestinal kinetics. A portable detector (EC60 Gastrolyzer®, Bedfont, UK) was used to measure the values of hydrogen (H2) in the fasted breath and at 10 minutes intervals up to 240 minutes after ingestion of 10 g of crystalline lactulose contained in 15 mL Duphalac® (Mylan, Milano, Italy), dissolved in the standard liquid meal. Orocecal transit time (OCTT) was the time required to observe an increase in H2 in the expired air by at least 10 parts per million (ppm) on the minimum H2 fasting level, and for at least three consecutive time points.27,32–34
2.6 |. Statistical analysis
The results are mean ± standard errors (SEM). The one-way ANOVA followed by the Fisher’s LSD multiple comparison test was used to assess the differences between control, gallstone patients and cholecystectomized patients. Student’s t test compared gallbladder motility between control and gallstone patients. Significant differences were considered with a probability (P) of less than 0.05.35 Calculations were performed with the NCSS 10 Statistical Software (2015) (NCSS, LLC. Kaysville, UT, USA, ncss.-com/software/ncss).
3 |. RESULTS
3.1 |. Dyspeptic symptoms
The score of dyspepsia in gallstone patients and cholecystectomized patients was almost threefold increased and significantly (P < 0.0001) greater than the score of the control group, but comparable between the two groups of patients (Table 2).
TABLE 2.
Results from the questionnaire for dyspepsia and gastrointestinal motility
| Control subjects | Gallstone patients | Cholecystectomized patients | |
|---|---|---|---|
| Number | 65 | 46 | 24 |
| Dyspepsia score | 5.6 ± 0.3 | 14.9 ± 1.3* | 15.2 ± 2.0* |
| Gallbladder motility | |||
| Fasting volume (mL) | 23.6 ± 0.9 | 31.6 ± 2.2* | — |
| Residual volume (mL) | 6.4 ± 0.4 | 10.4 ± 0.9* | — |
| Residual volume (%) | 27.1 ± 1.3 | 34.7 ± 2.7* | — |
| Half‐emptying time (T50, min) | 22.7 ± 0.9 | 44.3 ± 3.1* | — |
| Stomach (antrum) motility | |||
| Fasting area (cm2) | 3.6 ± 0.1 | 3.4 ± 0.2 | 3.4 ± 0.1 |
| Max. postprandial area (cm2) | 12.2 ± 0.3 | 12.1 ± 0.3 | 11.7 ± 0.4 |
| Residual postprandial area (%) | 4.8 ± 1.2 | 12.8 ± 2.0* | 24.2 ± 4.3*,# |
| Half‐emptying time (T50, min) | 28.5 ± 1.3 | 44.3 ± 3.1* | 69.2 ± 14.8*# |
| Small intestine motility | |||
| OCTT (min) | 111.4 ± 5.5 | 166.1 ± 9.2* | 176.8 ± 11.1* |
OCTT, orocecal transit time.
Data expressed as mean ± standard error. Significantly different from control:
P < 0.05;
significantly different from gallstone P < 0.05.
The ingestion of the test meal was uneventful, and during the motility studies, the compliance was excellent in all subjects
3.2 |. Gallbladder motility
The functional ultrasonographic study of time-dependent changes of gallbladder volume in the groups of gallstone patients and control showed that both fasting and postprandial volumes increased continuously by about 33% in gallstone patients throughout the emptying curve. Consequently, the residual (minimal) volume increased by 66% (absolute value) and 29% (normalized value) and half-emptying time almost doubled, as compared to the controls (Table 2, Figure 1A–C).
FIGURE 1.
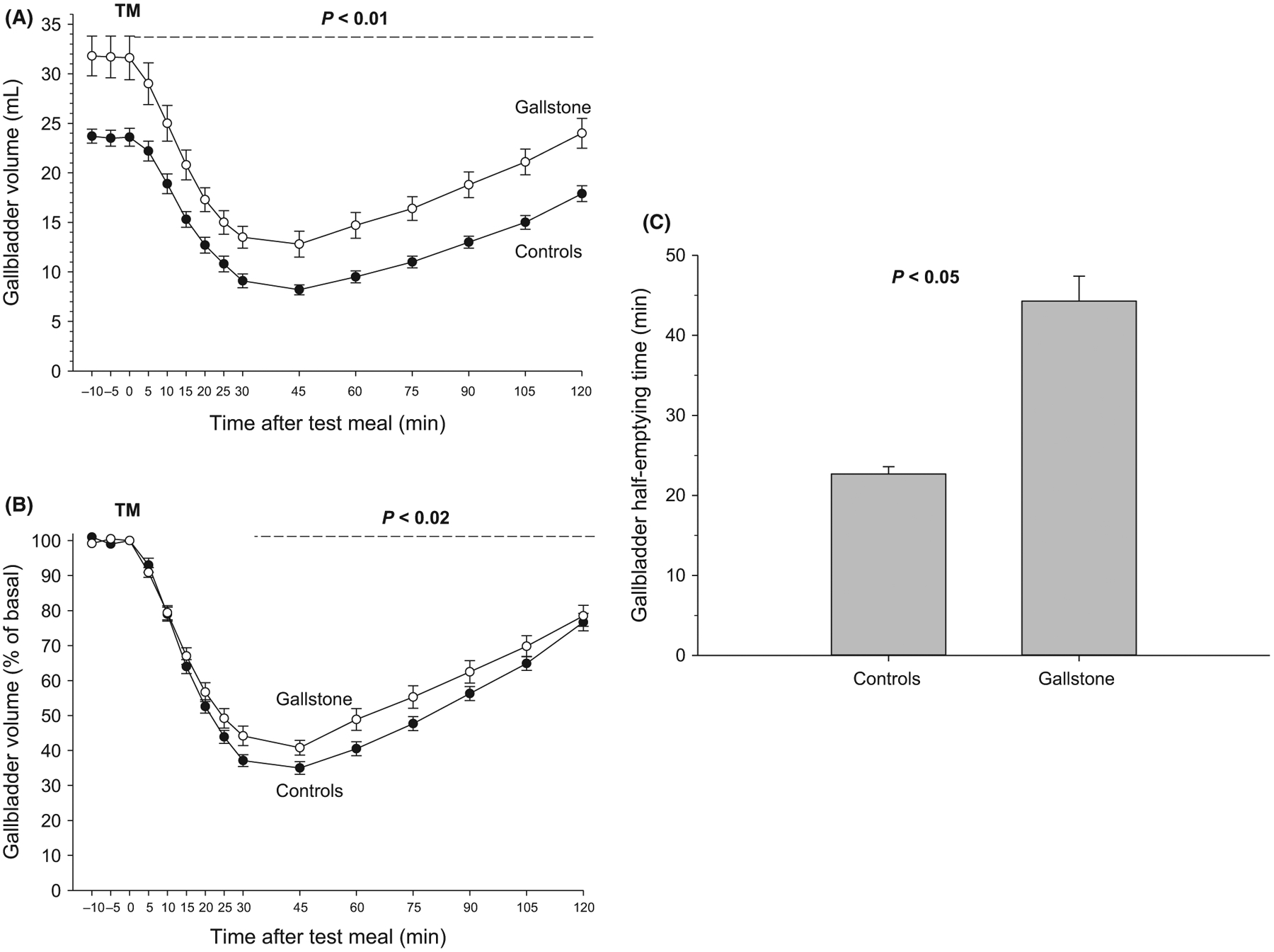
Gallbladder motility studies. Time-dependent changes of fasting and postprandial gallbladder volumes and half-emptying time in 46 gallstone patients and 65 control healthy subjects. Symbols indicate means, and vertical lines are standard errors. TM = test meal administered. A, Absolute values in mL with significant difference (P < 0.01) between the two curves between 0 and 120 min; B, Percentage of fasting gallbladder volume with significant difference (P < 0.02) between 30 and 75 min; C, Half-emptying time in min. Significant difference (P < 0.05) between groups
3.3 |. Gastric motility
The functional ultrasonographic study of time-dependent changes of antral areas appears in Table 2, Figure 2A–C. Fasting antral areas were comparable across the three groups (mean range 3.4–3.6 cm2). Following the ingestion of the 200 mL liquid test meal, antral dilation invariably occurred within 5 minutes and was comparable across groups (mean range 11.7–12.2 cm2). Gastric emptying was incomplete, delayed throughout the 120 minutes in both gallstone and cholecystectomized patients, as compared to the control group. However, the gastric emptying defects were more evident in cholecystectomized patients. Overall, in patients the score of dyspeptic symptoms was unrelated to the timing of antral emptying (half-emptying time, data not shown).
FIGURE 2.
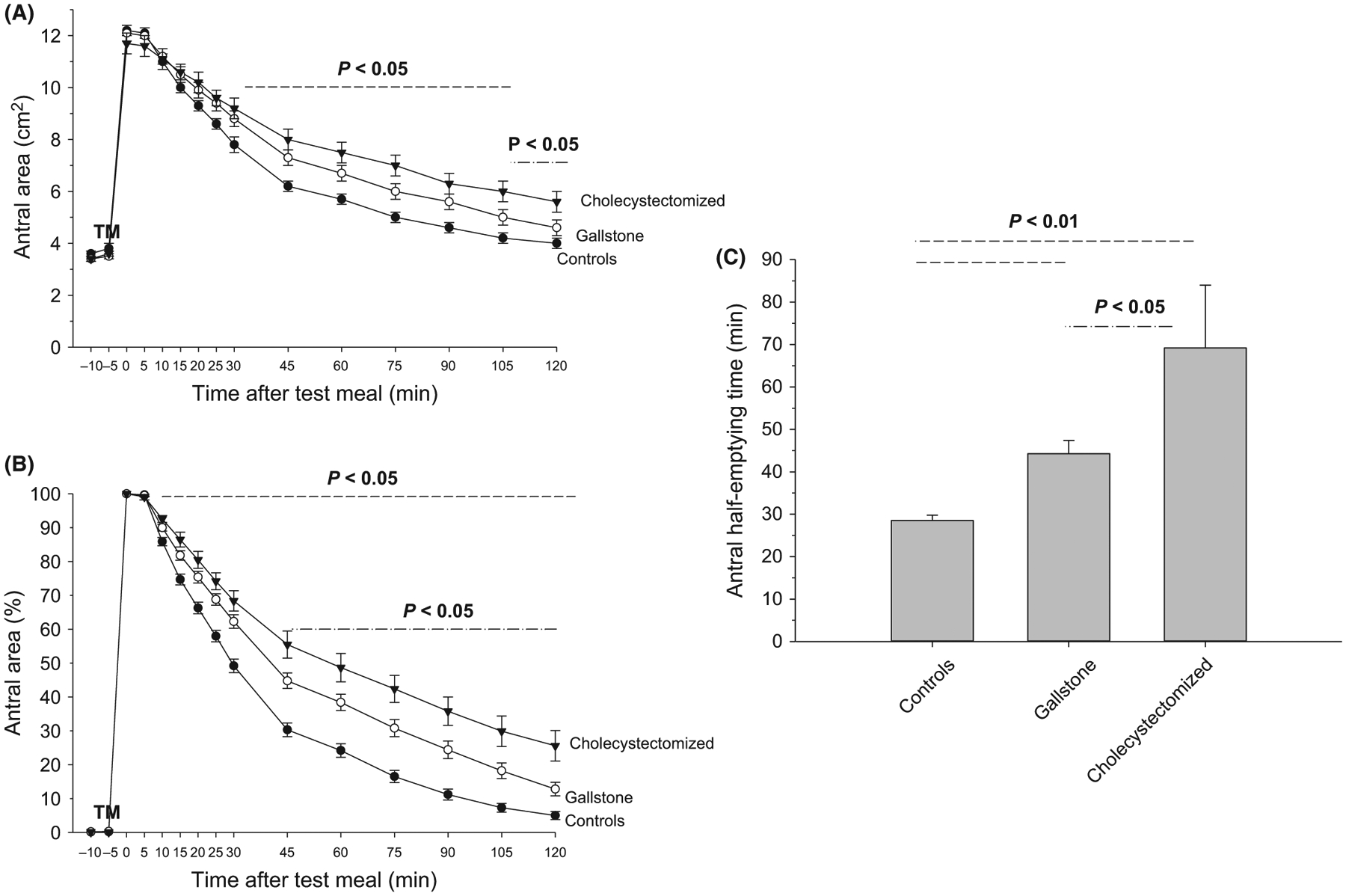
Gastric emptying studies. Time-dependent changes of fasting and postprandial antral areas and half-emptying time in 46 gallstone patients, 24 cholecystectomized patients and 65 control healthy subjects. Symbols indicate means, and vertical lines are standard errors. TM = test meal administered. A, Absolute values in cm2 with significant difference (P < 0.05) between gallstone patients, cholecystectomized patients and controls (----) from 25 to 105 min, and between gallstone patients and cholecystectomized patients (-.-.-) between 105 and 120 min; B, Percentage of maximal antral area with significant difference (P < 0.05) between gallstone patients, cholecystectomized patients and controls (----) between 10 and 120 min, and between gallstone patients and cholecystectomized patients (-.-.-) between 45 and 120 min; C, Half-emptying time in min. Significant differences P < 0.01 vs controls (---) and P < 0.05 vs gallstone patients (-.-.-)
3.4 |. Orocecal transit time
For the small intestine, concentrations of H2 in expired air showed a progressive time-dependent increment in the three groups. This finding is a reliable marker of bacterial fermentation of lactose in the distal gut. However, the extent of H2 production by gut microbiota was greater in the control group rather than gallstone and cholecystectomized patients (Figure 3). OCTT was 50%–59% delayed in gallstone and cholecystectomized patients, as compared to healthy controls (Table 2, Figure 3A, B). A trend towards a progressively reduced area under curve (AUC) of H2 levels in exhaled air was recorded between controls, gallstone and cholecystectomized patients, although the difference between the three subgroups was not significant (Figure 3C).
FIGURE 3.

Intestinal transit. A, Time-dependent curves of H2 levels (ppm) in exhaled air in 46 gallstone patients, 24 cholecystectomized patients and 65 control healthy subjects. Symbols indicate means, and vertical lines are standard errors. The horizontal dotted line indicates the cut-off level to document a significant increase in H2 production (10 ppm); B, Orocecal transit time (OCTT). Significant differences P < 0.05 vs controls (---); C, Area under curve (AUC) of H2 levels in exhaled air (ppm × 240 min)
4 |. DISCUSSION
The present study shows that gallstone patients often complain of dyspeptic symptoms, while exhibiting diffuse motility defects, as compared to healthy subjects. The dysmotility profile involves the gallbladder, the stomach and the small intestine, and deteriorates in cholecystectomized patients, even years after surgery.
The extent of dyspeptic symptoms occurring in the previous year was similar in gallstone patients with or without “in situ” gallbladder. Moreover, we confirm that the mean score of dyspepsia is extremely low in control subjects, as shown in previous studies.24,36
The ingestion of the liquid test meal was not associated with dyspeptic symptoms (especially fullness) throughout the observation time. Likely, the moderate volume (215 mL) and the isosmotic/fat composition of the test meal (11.6 g = 19%) fat, 300 kcal was not sufficient to trigger upper gastrointestinal symptoms in these groups of subjects. Larger volumes, higher caloric content, fat content or meal type (ie semisolid vs liquid), might explain the symptomatic response of a test meal.27 This was the case in chronic alcoholic patients,36 scleroderma patients and dyspeptic patients without dyspepsia.24
The delayed gastric emptying can explain only in part these findings. In fact, the extent of dyspepsia (as measured by a score) and the timing of postprandial gastric emptying (ie, half-emptying time) were apparently unrelated in the present group of patients. Additionally, cholecystectomized patients showed the worst indices of gastric emptying, as compared with gallstone patients, although the score of dyspepsia was similar in the two subgroups. In line with these findings, and in accordance with international guidelines, this study confirms that dyspepsia is not specifically related to the presence of gallstones.7,13,37,38 Furthermore, studies conducted on the general population found no greater prevalence of dyspepsia in gallstone patients.4,39 Dyspeptic symptoms might rather originate from multiple coexisting motility defects in gallstone patients, likely persisting after a cholecystectomy. This is the case in this study and in another study, which examined gallstone patients before and after a cholecystectomy.40 Indeed, dyspepsia persisted in a consistent number of patients. Indirectly, we confirm that the colicky pain is a specific symptom clinically linked to the presence of uncomplicated gallstones, and it should be considered in a group of patients who are scheduled for a cholecystectomy.
On the other hand, a study found that cholecystectomy is associated with a series of nonspecific gastrointestinal symptoms, especially in the first three years after surgery. Findings include a wide panel of gastric disorders such as peptic ulcer, hiatus hernia and gastro-oesophageal reflux. Misdiagnosis of some pre-existing diseases, however, is possible.15
The presence of an altered postprandial gastric emptying occurs either with or without “in situ” gallbladder, suggesting that this defect is independent of the physical presence of gallstones, and possibly linked with mechanisms, which also influence gastrointestinal motility at different levels of the gastrointestinal tract.41 The extent and timing of gastric emptying worsen after gallbladder removal, pointing to the presence of altered pathways involving bile flow and/or signalling molecules. At variance with the present study, an early report found improved gastric emptying following cholecystectomy.40 Differences in selection and number of patients, characteristics of study design (ie two separate groups in this study vs pre-postcholecystectomy in the study by Ibrarullah40) and duration of follow-up might account for such differences.
The possibility exists, however, that some significant findings found in the present series of cholecystectomized patients could be partly due to cholecystectomy itself involving neuro-hormonal and anatomic changes. Post-operative ileus, for example, is a predictable, self-terminating event, occurring after most general anaesthetic procedures.42 However, cholecystectomy might induce effects in the medium- and long-term period but studies are somewhat heterogeneous. Symptoms of postcholecystectomy syndrome are likely not related to increased duodenogastric reflux after surgery.43 Cholecystectomy, however, may cause an exaggerated meal-stimulated cholecystokinin (CCK) response, a factor involved in relaxation of the lower oesophageal sphincter and likely increased incidence of gastro-oesophageal reflux.44 In the dog, fed-state motility (but not fasted gastrointestinal motility) was the only parameter improving after laparoscopic cholecystectomy, compared to open cholecystectomy.45 In a small, short-term study performed in five women at baseline, and six months after surgery, laparoscopic cholecystectomy suppressed the normal inhibitory effect of pharmacological doses of CCK on the Oddi sphincter. Sphincter denervation might explain such findings.46 From the above-mentioned results, it is clear that further prospective studies are necessary to enrol a larger number of gallstone patients and examined at different time points before and after gallbladder removal.
All gallstone patients had a small gallstone burden (<30% of fasting volume), a condition unable to affect the gallbladder kinetics directly by obstructing the cystic duct and impeding gallbladder emptying.16,17 Major acute and chronic inflammatory changes of the gallbladder wall were absent, as confirmed by a thin gallbladder wall. All gallstone and cholecystectomized patients had suffered from typical colicky pain without known complications of gallbladder disease (ie acute cholecystitis, empyema, biliary pancreatitis, etc).37,38 However, we found that gallstone patients displayed increased fasting, postprandial volumes and delayed gallbladder emptying, as compared with controls, suggesting the presence of sluggish gallbladder function and ongoing gallbladder stasis.16 Growth of cholesterol gallstones depends on at least five primary pathogenic defects and involves LITH genes and genetic factors, hepatic hypersecretion of cholesterol accumulating in a supersaturated gallbladder bile, rapid phase precipitation of solid cholesterol crystals in bile and gallbladder stasis. This type of visceral stasis is a clear predisposing factor towards accumulation of concentrated supersaturated bile and hypersecretion of mucin gel from the gallbladder epithelium during the immune-mediated gallbladder inflammation. Additional factors involve intestinal cholesterol absorption, defective intestinal motility and changes of the gut microbiota.47,48 The presence of a hypomotile gallbladder, both in the fasting state49,50 and in response to neuro-hormonal postprandial stimuli,16,51,52 plays an important role. Gallbladder hypomotility, in fact, is one of the major pathogenic factors contributing to the accumulation of excess biliary cholesterol in the gallbladder lumen,47,52 as well as precipitation of solid cholesterol crystals, a key step in the further growth of gallstones.48 This study assessed gallbladder motility in patients with cholesterol gallstones, but a type of intermediate motility defect also exists in patients with pigment stones.53,54
Gallbladder kinetics depends on about 60 to 80% of its reduction on vagal cholecystokinin-mediated mechanisms, which lead to expulsion and increased intraluminal concentrations of bile acids (BA).55 BA recirculation increases following cholecystectomy, and this mechanism may contribute to the increased risk for metabolic syndrome observed after gallbladder removal.55 The missing gallbladder function may also explain why several components of metabolic syndrome, such as glucose or lipids homeostasis, are present after a cholecystectomy, mainly due to abnormal metabolic signalling on gene expression, BA/farnesoid X receptor (FXR) and BA/G-coupled bile acid receptor-1 (GPBAR-1) and fibroblast growth factor 19 (FGF19) axis.56
Our results confirm that both gallstone and cholecystectomized patients have a more general impairment of gastrointestinal motility, which also involves the small intestine. Here, we find that OCTT is delayed in response to the test meal containing lactulose, a substrate which becomes fermentable at the level of the cecum, where gut microbiota displays the highest density.57
A previous observation reported a significantly delayed OCTT in gallstone patients, an increased prevalence of small intestinal bacterial overgrowth (SIBO), high levels of serum BA and a positive correlation between OCTT and serum BA levels in patients with both gallstones and SIBO.58 Remarkably, another study found prolonged large intestinal transit and increased levels of biliary deoxycholate in a subgroup of gallstone patients.59
This study also shows a trend towards a progressive decrease of fermentation of lactulose, from controls to gallstone and cholecystectomized patients. Although the difference among average AUC values of H2 in expired air did not reach the statistical significance, this trend suggests a reduced overall fermentation capacity of gut microbiota in patients with gallstone disease (with or without “in situ” gallbladder), as compared to healthy subjects. This hypothesis could be confirmed, at least in part, by previous observations describing relevant qualitative differences in gut microbiota between healthy subjects, gallstone patients and cholecystectomized subjects.60–62 Further studies are certainly required in order to better explore this possibility.
Delay of intestinal transit, associated with gallbladder hypomotility, should be seen in the context of a wide “intestinal disease”,63 since a greater proportion of hepatic bile leads to dihydroxylation, partly mediated by the gut microbiota with a consequent increase of the deoxycholate, strongly hydrophobic and cytotoxic BA. The reuptake in the enterohepatic circle of deoxycholate is able to influence the phenomena of crystallization and nucleation of biliary cholesterol with greater lithogenic effects.64,65
Overall, the results of the present study suggest that patients developing cholesterol cholelithiasis suffer from a type of “pan-enteric” motility defect.63 Abnormalities may involve the oesophagus,66 the stomach,66 the small intestine and the colon as well.67–70 The link between cholesterol cholelithiasis and obesity28,29 and/or diabetes,71 as components of the metabolic syndrome, might further deteriorate the overall profile of gastrointestinal motility.52 Apparently, the motility defect persists (or worsens as in the case of gastric emptying) after a cholecystectomy.
Further studies should involve a larger number of subjects with gallstones of any type and size, in order to avoid possible biases. In this context, studies should focus on existing links between multiple gastrointestinal motility defects, pathogenic pathways of gallstones, and persisting (or worsening) of symptoms and defects after gallbladder removal.
5 |. CONCLUSION
This study shows that gallstone patients with the gallbladder “in situ” or after cholecystectomy display symptoms of dyspepsia. Symptoms are associated with multiple gastrointestinal motility defects involving increased fasting and residual gallbladder volume, along with delayed gastric and small intestine transit. After a cholecystectomy, gastric emptying worsens.
ACKNOWLEDGEMENTS
The present paper is written in the context of the FOIE GRAS project, which received funding from the European Union’s Horizon 2020 Research and Innovation programme under the Marie Skłodowska-Curie Grant Agreement No.722619. The authors thank Rosa De Venuto and Paola De Benedictis for their excellent technical assistance. There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Farthing M, Roberts SE, Samuel DG, et al. Survey of digestive health across Europe: final report. Part 1: the burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United European Gastroenterol J. 2014;2:539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. [DOI] [PubMed] [Google Scholar]
- 3.Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol. 2018;34:81–89. [DOI] [PubMed] [Google Scholar]
- 4.Attili AF, Carulli N, Roda E, et al. Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.). Am J Epidemiol. 1995;141:158–165. [DOI] [PubMed] [Google Scholar]
- 5.Attili AF, Capocaccia R, Carulli N, et al. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology. 1997;26:809–818. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20:1–19. [PubMed] [Google Scholar]
- 7.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers. 2016;2:16024. 10.1038/nrdp.2016.24 [DOI] [PubMed] [Google Scholar]
- 8.Di Ciaula A, Garruti G, Wang DQ-H, Portincasa P. Role of insulin resistance in the formation of cholesterol gallstones. In: Wang DQ-H, Portincasa P, eds. Gallstones – Recent Advances in Epidemiology, Pathogenesis, Diagnosis and Management. New York, NY: Nova Science Publishers; 2017:357–372. [Google Scholar]
- 9.Shabanzadeh DM, Skaaby T, Sorensen LT, Eugen-Olsen J, Jorgensen T. Metabolic biomarkers and gallstone disease - a population-based study. Scand J Gastroenterol. 2017;52:1270–1277. [DOI] [PubMed] [Google Scholar]
- 10.Lv J, Qi L, Yu C, et al. Gallstone disease and the risk of ischemic heart disease. Arterioscler Thromb Vasc Biol. 2015;35:2232–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Xu M, Heianza Y, et al. Gallstone disease and increased risk of mortality: two large prospective studies in US men and women. J Gastroenterol Hepatol. 2018;33:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shabanzadeh DM, Sorensen LT, Jorgensen T. Gallstone disease and mortality: a cohort study. Int J Public Health. 2017;62:353–360. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. Electronic address eee. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146–181. [DOI] [PubMed] [Google Scholar]
- 14.Portincasa P, Di Ciaula A, de Bari O, Garruti G, Palmieri VO, Wang DQ. Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol. 2016;10:93–112. [DOI] [PubMed] [Google Scholar]
- 15.Isherwood J, Oakland K, Khanna A. A systematic review of the aetiology and management of post cholecystectomy syndrome. Surgeon. 2018, in press, 10.1016/j.surge.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 16.Portincasa P, Di Ciaula A, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430–440. [DOI] [PubMed] [Google Scholar]
- 17.Portincasa P, Di Ciaula A, Palmieri V, Vendemiale G, Altomare E, Palasciano G. Sonographic evaluation of gallstone burden in humans. Ital J Gastroenterol. 1994;26:141–144. [PubMed] [Google Scholar]
- 18.Portincasa P, Colecchia A, Di Ciaula A, et al. Standards for diagnosis of gastrointestinal motility disorders. Section: ultrasonography. A position statement from the Gruppo Italiano di Studio Motilita Apparato Digerente. Dig Liver Dis. 2000;32:160–172. [DOI] [PubMed] [Google Scholar]
- 19.Portincasa P, Moschetta A, Colecchia A, Festi D, Palasciano G. Measurement of gallbladder motor function by ultrasonography: towards for standardization. Dig Liver Dis (già Ital J Gastroenterol Hepatol). 2003;35(Suppl. 3):S56–S61. [DOI] [PubMed] [Google Scholar]
- 20.Fiorucci S, Bosso R, Morelli A. Erythromycin stimulates gallbladder emptying and motilin release by atropine-sensitive pathways. Dig Dis Sci. 1992;37:1678–1684. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfield L, Carulli N, Dowling R, Sama C, Wolpers C. Asymptomatic gallstones: definition and treatment. Gastroenterol Int. 1989;2:25–29. [Google Scholar]
- 22.Portincasa P, van Erpecum KJ, Jansen A, Renooij W, Gadellaa M, vanBerge-Henegouwen GP Behavior of various cholesterol crystals in bile from patients with gallstones. Hepatology. 1996;23:738–748. [DOI] [PubMed] [Google Scholar]
- 23.Buckley MJ, Scanlon C, McGurgan P, O’Morain CA. A validated dyspepsia symptom score. Ital J Gastroenterol Hepatol. 1997;29:495–500. [PubMed] [Google Scholar]
- 24.Di Ciaula A, Covelli M, Berardino M, et al. Gastrointestinal symptoms and motility disorders in patients with systemic scleroderma. BMC Gastroenterol. 2008;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolk MF, van Erpecum KJ, van Berge Henegouwen GP, Kesselring OF, Hopman WP. Gallbladder volume and contraction measured by sum-of-cylinders method compared with ellipsoid and area-length methods. Acta Radiol. 1990;31:591–596. [PubMed] [Google Scholar]
- 26.Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labo G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89:752–759. [DOI] [PubMed] [Google Scholar]
- 27.Diella G, Di Ciaula A, Lorusso MP, et al. Distinct effects of two almond cultivars on agreeability and gastrointestinal motility in healthy subjects: more than mere nutraceuticals. J Gastrointestin Liver Dis. 2018;27:31–39. [DOI] [PubMed] [Google Scholar]
- 28.Di Ciaula A, Wang DQ, Portincasa P. Gallbladder and gastric motility in obese newborns, pre-adolescents and adults. J Gastroenterol Hepatol. 2012;27:1298–1305. [DOI] [PubMed] [Google Scholar]
- 29.Portincasa P, Altomare DF, Moschetta A, et al. The effect of acute oral erythromycin on gallbladder motility and on upper gastrointestinal symptoms in gastrectomized patients with and without gallstones: a randomized, placebo-controlled ultrasonographic study. Am J Gastroenterol. 2000;95:3444–3451. [DOI] [PubMed] [Google Scholar]
- 30.Everson GT, Braverman DZ, Johnson ML, Kern F Jr. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology. 1980;79:40–46. [PubMed] [Google Scholar]
- 31.Dodds WJ, Groh WJ, Darweesh RM, Lawson TL, Kishk SM, Kern MK. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol. 1985;145:1009–1011. [DOI] [PubMed] [Google Scholar]
- 32.Bond JH Jr, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 33.La Brooy SJ, Male PJ, Beavis AK, Misiewicz JJ. Assessment of the reproducibility of the lactulose H2 breath test as a measure of mouth to caecum transit time. Gut. 1983;24:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonfrate L, Krawczyk M, Lembo A, Grattagliano I, Lammert F, Portincasa P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur J Gastroenterol Hepatol. 2015;27:785–796. [DOI] [PubMed] [Google Scholar]
- 35.Hintze J NCSS 10 Statistical Software. Number Cruncher Statistical System (NCSS), LLC. Kaysville, Utah, USA, ncss.com/software/ncss, 2015. [Google Scholar]
- 36.Di Ciaula A, Grattagliano I, Portincasa P. Chronic alcoholics retain dyspeptic symptoms, pan-enteric dysmotility, and autonomic neuropathy before and after abstinence. J Dig Dis.2016;17:735–746. [DOI] [PubMed] [Google Scholar]
- 37.Portincasa P, Wang D. Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, eds. Yamada’s Textbook of Gastroenterology. Hoboken, NJ: Wiley-Blackwell; 2015:1808–1834. [Google Scholar]
- 38.Portincasa P, Wang D. Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, eds. Yamada’s Atlas of Gastroenterology. Hoboken, NJ: Wiley-Blackwell; 2016:335–353. [Google Scholar]
- 39.Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology. 1995;21:655–660. [DOI] [PubMed] [Google Scholar]
- 40.Ibrarullah M, Mittal BR, Aagrawal DK, Das BK, Kaushik SP. Gastric emptying in patients with gallstone disease with or without dyspepsia: effect of cholecystectomy. ANZ J Surg. 1994;64:247–250. [DOI] [PubMed] [Google Scholar]
- 41.Di Ciaula A, Portincasa P. Recent advances in understanding and managing cholesterol gallstones. F1000Res. 2018;7:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowling TE. Does disorder of gastrointestinal motility affect food intake in the post-surgical patient? Proceedings of the Nutrition Society. 2007;53:151–157. [DOI] [PubMed] [Google Scholar]
- 43.Manifold DK, Anggiansah A, Owen WJ. Effect of cholecystectomy on gastroesophageal and duodenogastric reflux. Am J Gastroenterol. 2000;95:2746–2750. [DOI] [PubMed] [Google Scholar]
- 44.McDonnell CO, Bailey I, Stumpf T, Walsh TN, Johnson CD. The effect of cholecystectomy on plasma cholecystokinin. Am J Gastroenterol. 2002;97:2189–2192. [DOI] [PubMed] [Google Scholar]
- 45.Hotokezaka M, Combs MJ, Mentis EP, Schirmer BD. Recovery of fasted and fed gastrointestinal motility after open versus laparoscopic cholecystectomy in dogs. Ann Surg. 1996;223:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luman W, Williams AJ, Pryde A, et al. Influence of cholecystectomy on sphincter of Oddi motility. Gut. 1997;41:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Neuschwander-Tetri BA, Portincasa P. The Biliary System, 2nd ed., Vol. 8. San Rafael, CA: Morgan & Claypool Life Sciences; 2017:i–178. [Google Scholar]
- 48.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolk MF, van Erpecum KJ, Smout AJ, et al. Motor cycles with phase III in antrum are associated with high motilin levels and prolonged gallbladder emptying. Am J Physiol. 1993;264:G596–600. [DOI] [PubMed] [Google Scholar]
- 50.Stolk M, van Erpecum KJ, Samson M, et al. Interdigestive gallbladder emptying, antroduodenal motility and motilin release in cholesterol gallstone patients. In: Stolk M, ed. Pathogenesis of Cholesterol Gallstones. Utrecht: Thesis Universiteit Utrecht; 1993:65–78. [Google Scholar]
- 51.Portincasa P, Di Ciaula A, Palmieri VO, Baldassarre G, van Berge-Henegouwen GP, Palasciano G. Enhancement of gallbladder (GB) emptying in gallstone patients after oral cholestyramine. Neth J Med. 1994;45:A36. [PubMed] [Google Scholar]
- 52.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–2126. [DOI] [PubMed] [Google Scholar]
- 53.Portincasa P, Di Ciaula A, Vendemiale G, et al. Gallbladder motility and cholesterol crystallization in bile from patients with pigment and cholesterol gallstones. Eur J Clin Invest. 2000;30:317–324. [DOI] [PubMed] [Google Scholar]
- 54.Portincasa P, Moschetta A, Berardino M, et al. Impaired gallbladder motility and delayed orocecal transit contribute to pigment gallstone and biliary sludge formation in beta-thalassemia major adults. World J Gastroenterol. 2004;10:2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Ciaula A, Garruti G, Wang DQ, Portincasa P. Cholecystectomy and risk of metabolic syndrome. Eur J Intern Med. 2018;53:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garruti G, Wang DQ, Di Ciaula A, Portincasa P. Cholecystectomy: a way forward and back to metabolic syndrome? Lab Invest. 2018;98:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29 (Suppl 1):1–49. [DOI] [PubMed] [Google Scholar]
- 58.Kaur J, Rana SV, Gupta R, Gupta V, Sharma SK, Dhawan DK. Prolonged orocecal transit time enhances serum bile acids through bacterial overgrowth, contributing factor to gallstone disease. J Clin Gastroenterol. 2014;48:365–369. [DOI] [PubMed] [Google Scholar]
- 59.Shoda J, He BF, Tanaka N, et al. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995;21:1291–1302. [PubMed] [Google Scholar]
- 60.Wu T, Zhang Z, Liu B, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genom. 2013;14:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keren N, Konikoff FM, Paitan Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7:874–880. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Wang J, Li J, et al. Cholecystectomy damages aging-associated intestinal microbiota construction. Front Microbiol. 2018;9:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Erpecum KJ, Van Berge-Henegouwen GP. Gallstones: an intestinal disease? Gut. 1999;44:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Erpecum KJ, Portincasa P, Gadellaa M, van deHeijning B, Renooij W, vanBerge-Henegouwen GP Effects of bile salt hydrophobicity on nucleation behaviour of cholesterol crystals in model bile. Eur J Clin Invest. 1996;26:602–608. [DOI] [PubMed] [Google Scholar]
- 65.Di Ciaula A, Garruti G, Lunardi Baccetto R, et al. Bile acid physiology. Ann Hepatol. 2017;16:s4–s14. [DOI] [PubMed] [Google Scholar]
- 66.Portincasa P, Di Ciaula A, Palmieri VO, Velardi A, vanBerge-Henegouwen GP, Palasciano G. Impaired gallbladder and gastric motility and pathological gastroesophageal reflux in gallstone patients. Eur J Clin Invest. 1997;8:653–661. [DOI] [PubMed] [Google Scholar]
- 67.Dowling RH, Veysey MJ, Pereira SP, et al. Role of intestinal transit in the pathogenesis of gallbladder stones. Can J Gastroenterol. 1997;11:57–64. [DOI] [PubMed] [Google Scholar]
- 68.Veysey MJ, Thomas LA, Mallet AI, et al. Prolonged large bowel transit increases serum deoxycholic acid: a risk factor for octreotide induced gallstones. Gut. 1999;44:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Berge-Henegouwen GP, Portincasa P, van Erpecum KJ. Effect of lactulose and fiber-rich diets on bile in relation to gallstone disease: an update. Scand J Gastroenterol Suppl. 1997;222:68–71. [DOI] [PubMed] [Google Scholar]
- 70.Thomas LA, Veysey MJ, Bathgate T, et al. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806–815. [DOI] [PubMed] [Google Scholar]
- 71.Palasciano G, Portincasa P, Belfiore A, et al. Gallbladder volume and emptying in diabetics: the role of neuropathy and obesity. J Intern Med. 1992;231:123–127. [DOI] [PubMed] [Google Scholar]


