Abstract
Purpose of review
Gallstone disease is a major epidemiologic and economic burden worldwide, and the most frequent form is cholesterol gallstone disease.
Recent findings
Major pathogenetic factors for cholesterol gallstones include a genetic background, hepatic hypersecretion of cholesterol, and supersaturated bile which give life to precipitating cholesterol crystals that accumulate and grow in a sluggish gallbladder. Additional factors include mucin and inflammatory changes in the gallbladder, slow intestinal motility, increased intestinal absorption of cholesterol, and altered gut microbiota. Mechanisms of disease are linked with insulin resistance, obesity, the metabolic syndrome, and type 2 diabetes. The role of nuclear receptors, signaling pathways, gut microbiota, and epigenome are being actively investigated.
Summary
Ongoing research on cholesterol gallstone disease is intensively investigating several pathogenic mechanisms, associated metabolic disorders, new therapeutic approaches, and novel strategies for primary prevention, including lifestyles.
Keywords: cholesterol crystallization, gallbladder, mucin gel, supersaturated bile
INTRODUCTION
Gallstones have a prevalence of 10–15% in adults [1] in the United States and Europe. About 75% of adult patients are asymptomatic, but gallstone disease generates major economic and social burdens [1,2] if symptoms or complications occur.
Basic and clinical aspects of gallstone pathogenesis continue to receive attention worldwide [3,4▪,5,6,7▪,8▪]. Housset et al. [9▪] reviewed several functions of the gallbladder in health and disease. The European Society for the Study of the Liver has published exhaustive Clinical Practice Guidelines on prevention, diagnosis, and treatment of gallstones [10▪▪]. A study on 1 064 089 pregnant women [11], associated gallstone disease with adverse maternal and neonatal outcomes including preterm birth, a condition linked with risk of developmental problems [12]. The multivariable logistic regression models within the WHO Multinational mONItoring of trends and determinants in CArdiovascular disease (WHO MONICA) studies in Denmark confirmed a strong association between gallstone disease and insulin resistance, systemic inflammation, and genetic predisposition to obesity or type 2 diabetes [13▪].
Risk factors for gallstone disease include unmodifiable [i.e., aging, female gender, races, and lithogenic (LITH) genes] and modifiable conditions (Table 1). In Western countries, gallstones are comprised mainly of cholesterol in 75–80% of cases, and are often associated with systemic abnormalities [14] (Fig. 1). Primary prevention strategies in the general populations and in study participants at risk are conceivable [21] while studying metabolic pathways [22–26].
Table 1.
Exogenous risk factors associated with any type cholelithiasis
| Metabolic syndrome (Chol) |
| Physical inactivity |
| Insulin resistance |
| Diabetes mellitus |
| Obesity (visceral) |
| Nonalcoholic fatty liver disease |
| Dietary factors (Chol) |
| High carbohydrate intake |
| High calorie intake |
| High glycemic load |
| Low fiber intake |
| High heme iron intake |
| Increased enterohepatic circulation of bilirubin |
| Liver cirrhosis (Chol, Pigm) |
| Ileal resections (Pigm) |
| Crohn’s disease (Chol, Pigm) |
| Medications (Chol) |
| Hormone replacement therapy |
| Octreotide |
| Fibrates |
| Calcineurin inhibitors |
| Defective gallbladder motility |
| See Table 2 |
FIGURE 1.
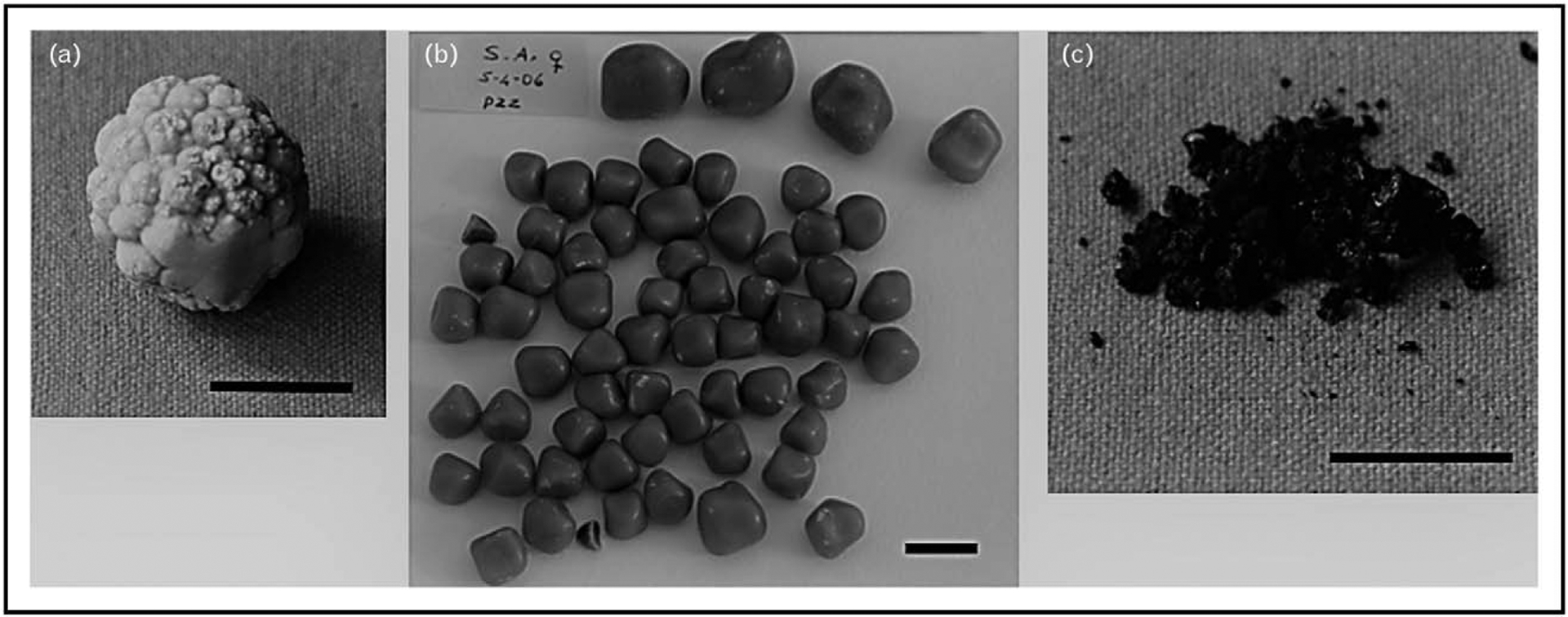
(a) Solitary cholesterol gallstone showing a spheroidal modular surface. (b) Multiple cholesterol gallstones showing a multifaceted surface. Cholesterol content in both cases is more that 75%. (c) Pigment gallstones. Black, soft, friable, and easily pulverized material contains mainly calcium bilirubinate, calcium carbonate, and phosphate. A tiny amount of cholesterol (less than 10% cholesterol) can be found. The black horizontal lines at the bottom are equal to 1 cm.
Five primary defects play a critical role in the pathogenesis of cholesterol gallstones: LITH genes and genetic factors; hepatic hypersecretion of cholesterol, resulting in supersaturated gallbladder bile; rapid phase transitions of cholesterol in bile, with the precipitation of solid cholesterol crystals; impaired gallbladder motility with hypersecretion and accumulation of mucin gel in the gallbladder lumen and immune-mediated gallbladder inflammation; and intestinal factors involving absorption of cholesterol, slow intestinal motility, and altered gut microbiota [7▪] (Fig. 2).
FIGURE 2.
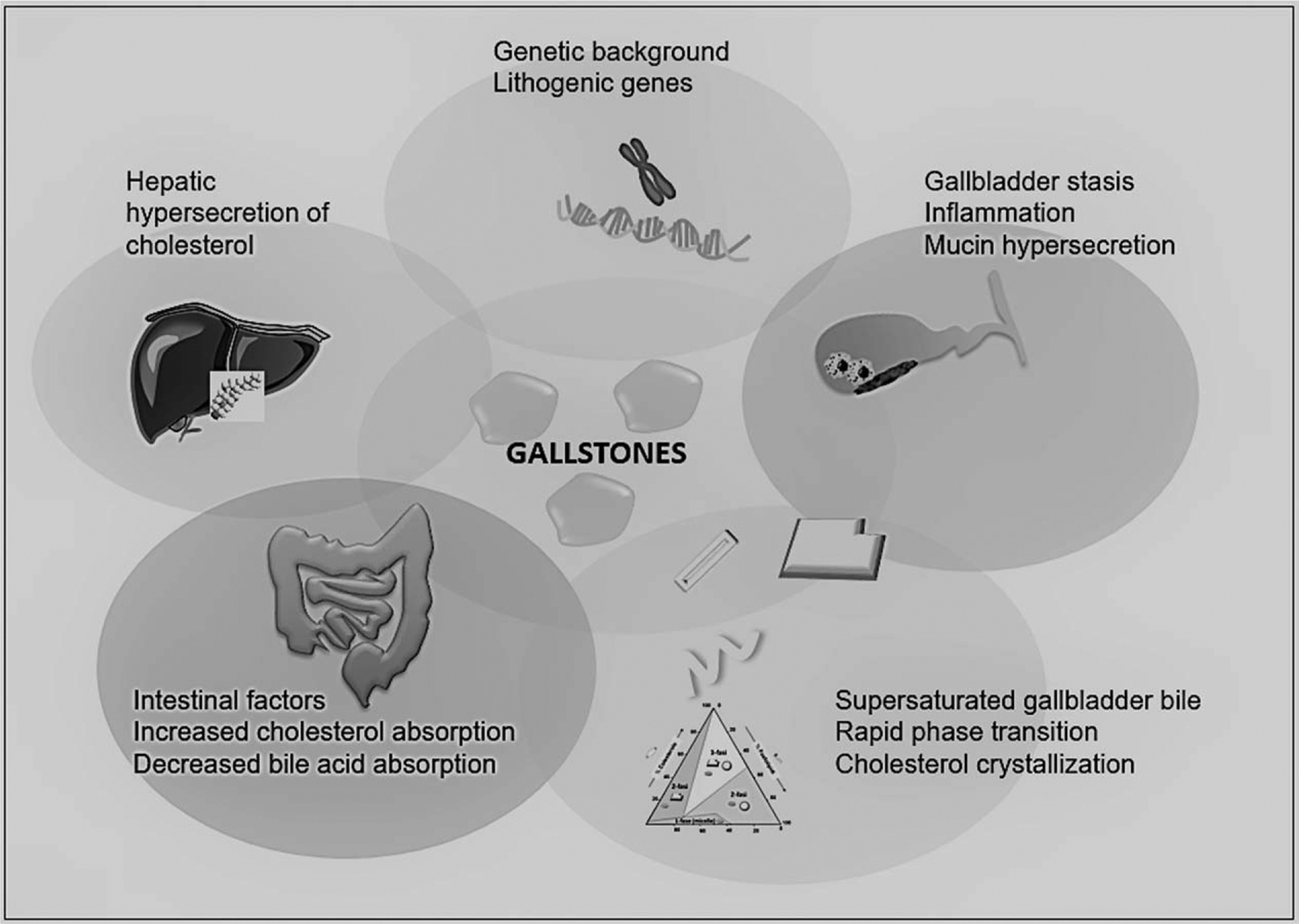
Pathogenetic factors involved in the formation of cholesterol gallstones. Five primary defects are involved. The primary cause of cholesterol gallstone formation originates from increased hepatic hypersecretion of cholesterol. The genetic predisposition is largely involved in this process.
This complex scenario puts the studies on cholesterol gallstone disease at the frontline of ongoing research involving treatments and prevention strategies [4▪].
LITH GENE, GENE–ENVIRONMENT INTERACTIONS, AND EPIGENETIC FACTORS
The prevalence of gallstones is high in the case of family history [27,28] and in specific ethnic groups [15,29]. Predisposing genetic factors (Lith genes) identified in mouse models [30,31] are involved in the synthesis, transport, and metabolism of cholesterol and bile acids [32,33]. In humans, the genetic susceptibility to gallstones has been explored by genome-wide association study (GWAS) [34,35,36▪]. The ATP-binding cassette transporters G5 and G8 (ABCG5/G8) are responsible for hepatic cholesterol secretion. Two major variants (ABCG5-R50C and ABCG8-D19H) are associated with gallstones in German, Chilean, Chinese, and Indian populations [37–43]. Carriers of CG genotype of ABCG8 rs11887534 are also at higher risk of gallstone disease, gallbladder and bile duct cancer, compared to carriers of the GG genotype [44]. The increased susceptibility to gallstone disease is also linked with three variants of the Farnesoid X receptor (FXR) gene (rs35724, rs11110385, rs11110386 [45]), as well as polymorphisms of apolipoprotein E4 allele [46], mucin genes [47] and fibroblast grow factor receptor 4 (FGFR4) [48]. The polymorphism rs3758650 (mucin-like protocadherin gene) predicts the development of symptomatic gallstones [49].
A recent large-scale GWAS (8720 cases, 55 152 controls, European ancestry) searching for single-nucleotide polymorphisms associated with gallstone disease [36▪] identified four distinct loci (SULT2A1, TM4SF4, GCKR, and CYP7A1) encoding enzymes involved in cholesterol metabolism/transport, and in sulfonylation of bile acids or hydroxysteroids. The previously detected locus ABCG8 [34], involved in cholesterol efflux [50], was also confirmed. Another contributing gene is the ABCB4 in patients [51] and in mice [52] with gallstones because the ATP-binding cassette transporter B4 (ABCB4) is responsible for hepatic phospholipid secretion and its mutations or knockout lead to a lack of phospholipids in bile.
Another large study from Rodriguez et al. [53▪] on 15 241 women of European ancestry identified two new loci associated with gallbladder disease (GCKR rs1260326:T>C and TTC39B rs686030:C>A), and detected four independent single-nucleotide polymorphisms effects in ABCG8 rs4953023:G>A, ABCG8 rs4299376:G(>)T, ABCG5 rs6544718:T>C, and ABCG5 rs6720173:G>C in conditional analyses taking genotypes of rs4953023:G>A as a covariate.
However, studies on twin pairs show that genetic factors are responsible for no more than 25–30% of symptomatic gallstones [54,55]. Environmental factors and gene–environment interactions can affect gene expression through epigenetic mechanisms [16], which also involve fat storage and insulin resistance [56]. These factors primarily include microRNAs (a large class of tiny, noncoding RNAs) [57]: 114 miRNA target genes are identified and regulate gallstone-related pathways [58]. An inverse correlation has been shown between expression levels of miR-210 and its potential target gene, ATP11A, in human gallstones. The interaction involves the regulation of the ATP-binding cassette ABC transporters pathway of cholesterol [58]. At a cellular level, the miRNA miR-122 regulates cholesterol homeostasis [57]. High circulating levels of miR-122 (3.07-fold higher than in controls) were also detected in obese patients, where risk factors for cholesterol gallstones include insulin resistance [59]. Singh et al. [60▪] reported the epigenetic roles of mammary serine protease inhibitor and thrombospondin 11-methylated genes in gallbladder cancer, but not in gallstone disease, in Indian population.
ALTERED BILE LIPID COMPOSITION
Cholesterol gallstones originate from the precipitation of solid cholesterol crystals mainly from multilamellar vesicles in a concentrated bile supersaturated with cholesterol, in which cholesterol cannot be solubilized by micelles and vesicles [8▪].
Insulin resistance promotes the formation of cholesterol gallstone by stimulating activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase [61] (the rate-limiting step in cholesterol synthesis) and activating the genes involved in cholesterol secretion: ABCG5 and ABCG8 (in concert with dys-regulation of the liver transcription factor forkhead box protein O1) [4▪]. These molecular pathways [25], together with a condition of gallbladder stasis and autonomic neuropathy [62], can account for the high gallstone prevalence in diabetic patients. Gallstone prevalence is markedly higher in women than in men and estrogen enhances cholesterol synthesis (while decreasing bile acid synthesis) by upregulation of estrogen receptor α and the G protein-coupled receptor 30 [63,64].
The nuclear receptors FXR and liver X receptor (LXR) act as bile acid sensors and govern important pathways of cholesterol and bile acid metabolism. FXR knockout mice fed a lithogenic diet show high susceptibility to cholesterol gallstones in parallel with decreased expression of the hepatocyte bile acid transporter Abcb11 and phospholipid transporter Abcb4 [65]. Hepatic insulin resistance influences expression of FXR [25,66,67], and activation of LXR promotes biliary cholesterol secretion because hepatic ABCG5 and ABCG8 are upregulated [68]. The liver-specific disruption of the insulin receptor in LIRKO mice model increases susceptibility to cholesterol gallstones, with mechanisms involving disinhibition of the forkhead box protein O1 signaling cascade, increased expression of the cholesterol transporters Abcg5/g8, and a consequent increase of biliary cholesterol secretion. Changes of Abcg8 expression are also found in humans: the risk of gallstone disease is increased in twins with a heterozygous or homozygous ABCG8 D19H genotype [69]. Furthermore, the characteristics of the insulin resistance syndrome in men were linked with the Q604E polymorphism of the ABCG5 gene [70].
Recent studies have discussed factors modulating bile composition and cholesterol solubilization [4▪,71▪]. The multiligand class B scavenger receptor CD36 promotes cellular free fatty acid uptake and modulates both hepatic and intestinal cholesterol metabolism. Xie et al. [72▪] found that germline Cd36 knockout mice are protected against diet-induced gallstones compared with wild-type mice. Also, Cd36 knockout mice crossed into the susceptible phenotype of congenic gallstone-susceptible liver fatty acid binding protein knockout mice are protected against lithogenic diet-induced gallstones. CD36-modified gallstone susceptibility through a reduction in biliary cholesterol secretion and changes of the bile acid pool by shifting to more hydrophilic species. Notably, gallbladder contractility is also improved as tensiometric changes of gallbladder smooth muscle strips in response to methacholine and potassium chloride.
Biliary aquaporins (AQPs) also play a role in bile concentrating function [73]. Asai et al. [74▪] show that hepatic levels of the transcription factor hypoxia-inducible factor 1α subunit (HIF1A) promote cholesterol gallstone formation in the animal model. Suppression of hepatic AQP8 with decreased water secretion from hepatocytes are involved. In the same study, the activation of HIF1A in human gallstone patients with and nonalcoholic liver steatosis was also shown.
INTESTINAL ABSORPTION OF CHOLESTEROL
Gallstone patients display an imbalance between absorption and synthesis of cholesterol: increased biliary cholesterol secretion from high dietary cholesterol and decreased bile acid synthesis and pool, all driving bile supersaturation [75]. The small intestine absorbs dietary cholesterol and reabsorbs the secreted biliary cholesterol [76], with variable absorption efficiency [77,78]. Intestinal factors depend on expression of sterol transport proteins and on dietary cholesterol [76,79]), which are regulated by multiple genes [76] and determined by the balance between influx and efflux of intraluminal cholesterol molecules crossing the brush border membrane of the enterocyte [76]. In mice, high-cholesterol diets and high intestinal cholesterol absorption efficiency are two independent risk factors for cholesterol gallstone formation [80]. Dysfunctional cholesterol transporters expressed on the enterocyte brush border membrane can account for such defects. In animal models, cholesterol uptake is altered with variants of the Niemann-Pick type C1-like protein (NPC1L1) transporter [81]. Ezetimibe, the potent NPC1L1 selective inhibitor, reduces the amount of cholesterol reaching the liver through the enterolymphatic circulation of cholesterol by inhibiting intestinal cholesterol absorption and the biliary cholesterol saturation. In this scenario, cholesterol gallstone formation is prevented in ezetimibe-treated gallstone-susceptible mice, even fed a lithogenic diet [82–84]. Furthermore, gallstone patients show significantly lower cholesterol absorption [85,86], and higher or unchanged [86] de novo synthesis of cholesterol [85]. This metabolic trait could precede gallstone formation in risk groups [85]. Insulin resistance also affects cholesterol homeostasis by reducing intestinal cholesterol absorption while increasing cholesterol synthesis, and the effect is independent on obesity [87,88].
Osteopontin (OPN) is a soluble cytokine and a matrix-associated protein expressed in several tissues and body fluids and is involved in cholesterol homeostasis [89]. Lin et al. [90▪] reported that OPN knockout mice are protected against lithogenic diet-induced gallstone formation because of reduced expression of intestinal NPC1L1 and intestinal cholesterol absorption.
GUT MICROBIOTA
Intestinal dysbiosis occurs in cholesterol gallstone patients and might play an important role in the pathogenesis of gallstone disease. Wu et al. [91] studied the composition of bacterial communities of gut, bile, and gallstones from 29 cholesterol gallstone patients and the gut of 38 healthy study participants, analyzing 299 217 bacterial 16S ribosomal RNA gene sequences. They found significant increment of intestinal bacterial phylum Proteobacteria and decrement of Faecalibacterium spp., Lachnospira spp., and Roseburia spp. Others reported increased amount of Gram-positive anaerobic bacteria with elevated 7α-dehydroxylation activity in the cecum of gallstone patients, a finding linked with increased concentrations of the hydrophobic and lithogenic secondary bile acid deoxycholate [92].
Gallstone patients undergoing cholecystectomy show microbial diversity of gut microbiota and the genus Roseburia spp. is reduced compared with that in controls. Moreover, the microbiota from gallstone patients is enriched with uncultivated genus Oscillospira spp. This last genus is positively correlated with the concentration of the secondary bile acids and negatively correlated with the primary bile acids, whereas the phylum Bacteroidetes shows an opposite trend [93].
Wang et al. [94▪] recently found that mice fed a lithogenic diet and forming gallstones display reduced microbiota richness and α diversity with lower levels of Firmicutes and decreased ratio of Firmicutes to Bacteroidetes.
The microbiota is also affected by environmental toxics introduced with food. This step might influence pathogenetic factors of gallstones. Liu et al. [95▪▪] found abnormal gut microbiota (as abundance and composition) after 8-week exposure to organochlorine pesticides such as dichlorodiphenyldichloroethylene (P,p’-DDE) and β-hexachlorocyclohexane. These changes include bile acid composition, enhanced hydrophobicity, decreased expression of genes regulating bile acid reabsorption in the terminal ileum, and a compensatory increase in expression of genes involved in the synthesis of hepatic bile acids.
GALLBLADDER MOTILITY
Several clinical conditions are associated with defective gallbladder motility (Table 2), which is another risk factor for cholesterol gallstones [15,96,97]. About one-third of cholesterol gallstone patients display enlarged fasting and postprandial residual gallbladder volumes with delayed emptying [98–100]. This defect antedates gallstone formation and is not affected by the presence of gallstones [98,101–103], unless chronic gallbladder inflammation and/or mechanical obstruction exist [98]. Sustained supersaturation of cholesterol in bile enhances the absorption of cholesterol into gallbladder muscularis propria, reduces back diffusion of cholesterol into bile, and inhibits action potentials and Ca2+currents [104]. In animals on lithogenic diet, Tharp et al. [105] demonstrated that curbing the accumulation of triacylglycerol in the gallbladder wall increases its contractile strength and prevents gallstone formation.
Table 2.
Conditions associated with defective gallbladder motility
| Physiological, dietary, and metabolic factors |
| Pregnancy |
| Obesity |
| Insulin resistance, diabetes mellitus |
| Rapid body weight loss (bariatric surgery for morbid obesity, and very low calorie diet) |
| Increased biliary cholesterol secretion |
| Physical inactivity (men>women) |
| Westernized diet: high calorie, low fiber, high-refined carbohydrate, and high lipids |
| Total parenteral nutrition |
| Gastrointestinal diseases |
| Irritable bowel syndrome |
| Primary sclerosing cholangitis |
| Acute hepatitis A |
| Chronic pancreatitis |
| Liver cirrhosis |
| Neural factors |
| Neural damage after total gastrectomy, and spinal cord injury |
| Drugs and hormones |
| Inhibition of CCK release by somatostatin, somatostatinoma, therapy with somatostatin analogues (octreotide), celiac disease |
| Estrogens and oral contraceptives |
| Oral bile acid therapy |
| Use of 5-hydroxytryptamine inhibitors |
The lipid-induced gallbladder lipotoxicity [106–108] is revealed to be associated with defective smooth muscle contractility and relaxation [109,110], whereas excessive cholesterol absorption may lead to cell proliferation and inflammation in the gallbladder mucosa and lamina propria [97,111]. Dysfunctional gallbladder motility provides sufficient time for cholesterol nucleation and gallstone growth [102,112] and predisposes to gallstone recurrence after successful extracorporeal shock-wave lithotripsy and/or oral bile acid dissolution therapy [113,114].
Endogenous CCK regulates postprandial gallbladder emptying [115–117] by activating CCK-1 receptors (CCK-1R) that are located on gallbladder myocytes [102,118]. Wang et al. [119▪] confirmed that CCK knockout mice fed a lithogenic diet have defective postprandial gallbladder emptying and show rapid cholesterol crystallization and gallstone formation. Mice also had enlarged fasting gallbladder volume, sluggish intestinal transit time, and increased intestinal cholesterol absorption with supersaturated bile. Devazepide, a CCK-1R antagonist produces similar outcomes [120▪].
Under lithogenic conditions, the signaling transduction decoupling of the CCK-1R deteriorates [112,121–123] as CCK binding to CCK-1R is not followed by G protein activation [121,124–126]. Indeed, tensiometric studies on isolated gallbladder smooth muscle strips show more severe dysfunction in patients with cholesterol stones than those with pigment stones [98]. Also, polymorphisms in the CCK1-R gene [127] and decreased density of CCK-1R [128] may be associated with cholesterol cholelithiasis in humans. Defective gallbladder motility is observed in lean, nondiabetic, gallstone-free study participants with insulin resistance [129], whereas changes in CCK-1R density is evident in patients with gallstone and type 2 diabetes [130]. Impaired gallbladder motility is also found in women with polycystic ovary syndrome, a condition where insulin resistance often exists [131]. In these cases, gallbladder dysmotility is ameliorated by metformin treatment [132].
Villanacci et al. [133▪] recently explored by immunohistochemistry the main cell components of gallbladder intrinsic innervation in patients with cholesterol stones and in acalculous gallbladders. Neurons, enteric glial cells, mast cells, and interstitial cells of Cajal (ICC), were markedly decreased in gallstone patients. This study integrates the findings of Tan et al. [134] relating decreased stem cell factor/ckit signaling pathway with depletion of ICC and defective gallbladder motility in gallstone patients.
Impaired gallbladder motility during cholesterol gallstone formation also involves postprandial emptying and refilling phase and the interdigestive (i.e., fasting) rhythmic fluctuations of gallbladder volume. Postprandial refilling requires appropriate gallbladder relaxation promoted by the acid-stimulated duodenal release of vasoactive intestinal peptide and human fibroblast grow factor 19 protein (FGF19; FGF15 in mice) [135]. FGF19 works on the gallbladder epithelium, cholangiocytes [136], and the ileum [137], with concentrations about 23-fold higher in bile than in serum [136]. Increased FGF19 into the portal circulation depends on bile acids which reach the terminal ileum and activate FXR (rank order CDCA > LCA > DCA >> CA). FGF19, in turn, activates the gallbladder fibroblast growth factor receptor 4 (FGFR4) and its co-receptor β-klotho [9▪]. This pathway induces smooth muscle relaxation creating a feedback mechanism that leads to gallbladder refill before the next meal [97,135]. Intraluminal hydrophobic bile acids also act as signaling agents of the G protein-coupled bile acid receptor 1 (GPBAR-1) [138], located in the gallbladder epithelium and smooth muscle [9▪,139] and driving gallbladder relaxation independently on FGF19 [140]. Hydrophobic bile acids inhibit gallbladder smooth muscle contraction via stimulation of GPBAR-1 receptors and activation of ATP-sensitive potassium channel [141]. GPBAR-1 knockout mice display a decreased bile acid pool size, sluggish response to GPBAR-1, and dietary lithogenesis [140,142].
Whether impaired gallbladder refilling (mediated by FGF19 and/or GPBAR-1) contributes to the pathogenesis of gallstones needs to be further addressed. Zhou et al. [143▪] modulated bile acid metabolism by FGF19 in 12-week old Abcb4 knockout mice, which resemble biochemical, histological, and clinical features of human cholangiopathies and cholelithiasis. FGF19 reverses liver injury, decreases hepatic inflammation, attenuates biliary fibrosis, and reduces cholecystolithiasis in Abcb4 knockout mice by inhibiting the hepatic expression of Cyp7a1 and Cyp27a1, encoding enzymes responsible for the rate-limiting steps in the classic and alternate bile acid synthetic pathways, and reducing the bile acid hepatic pool and blood levels.
During fasting, the gallbladder regulates the enterohepatic circulation of bile acids through coordinated neurohormonal mechanisms involving the liver and gut [97,144,145]. Small phasic contractions decrease the gallbladder volume by 20–30% of the fasting volume through vagal-motilin-mediated stimuli at the end of phase II of the migrating myoelectric complexes [146,147]. Cholesterol gallstone patients may have an altered interprandial gallbladder motility [102,148] mainly secondary to less frequent migrating myoelectric complexes cycles and abnormal motilin release compared with healthy control subjects [97–99,148]. The fasting motility defect could increase the direct liver secretion of lithogenic bile to the small intestine with faster recycling of bile acids and increased hydrophobicity of the bile acid pool [149]. This mechanism is another predisposing factor for cholesterol crystallization and stone growth [150].
DIETARY FACTORS AND LIFESTYLES
Lifestyle and dietary factors (Table 3) influence the pathogenesis of gallstone disease. Bertola-Compagnucci et al. [151▪] estimated by specific questionnaire that mean energy intake may be higher in gallstone patients than in control subjects. Thus, diet and lifestyle have a potential role in primary prevention of cholesterol gallstones. The European Society for the Study of the Liver panel concludes that healthy lifestyle and food, regular physical activity, and maintenance of an ideal body weight might prevent cholesterol stones and symptomatic gallstones [10▪▪].
Table 3.
Dietary factors influencing the pathogenesis of cholesterol gallstone disease
| Factors increasing the risk | Factors decreasing the risk |
|---|---|
| Increased energy intake | High consumption of monounsaturated fats and fiber |
| Highly refined sugars and sweet foods | |
| High fructose intake | Olive oil |
| Low fiber consumption | Fish (ω-3 fatty acids) |
| High fat content | Vitamin C supplementation |
| Consumption of fast food | Vegetable proteins |
| Consumption of meat | Fruit consumption |
| Low vitamin C intake | |
Adapted with permission [10▪▪].
CONCLUSION AND FUTURE RESEARCH
Risk factors of cholesterol gallstone share some common pathogenic pathways across major metabolic abnormalities, including insulin resistance, with those in obesity, the metabolic syndrome, and type 2 diabetes. Current research points to some key mechanisms involving the role of LITH genes, nuclear receptors, signaling pathways, gut microbiota, epigenetic factors, and lifestyles in the pathogenesis of cholesterol gallstone disease. Finding modifiable pathogenic factors for cholesterol cholelithiasis will pave the way to primary prevention of cholesterol gallstone disease, a very prevalent hepatobiliary disease worldwide.
KEY POINTS.
Five primary defects determine the pathogenesis of cholesterol gallstones: genetic background and LITH genes, hepatic hypersecretion of biliary cholesterol (with supersaturated gallbladder bile), rapid phase transitions of cholesterol in bile (with the precipitation of solid cholesterol crystals), gallbladder dysmotility (with the accumulation of mucin gel in the gallbladder lumen and immune-mediated gallbladder inflammation), and intestinal factors (with increased absorption of cholesterol, slow intestinal motility, and dysbiosis).
Pathogenetic pathways link cholesterol gallstones with widely diffused metabolic conditions which include insulin resistance, obesity, the metabolic syndrome, and type 2 diabetes.
The burden of cholesterol gallstones depends on potentially modifiable mechanisms.
Research on cholesterol gallstones should ameliorate the efficiency of current therapies, test novel therapies, and employ appropriate lifestyles for primary prevention.
Financial support and sponsorship
The present chapter is written in the context of the project FOIE GRAS, which has received funding from the European Union’s Horizon 2020 Research and Innovation program under the Marie Sklodowska-Curie Grant Agreement No. 722619.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology 2009; 136: 1134–1144. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep 2005; 7:132–140. [DOI] [PubMed] [Google Scholar]
- 3.Lammert F Gallstone disease: scientific understanding and future treatment. In: Hirschfield G, Adams D, Liaskou E, editors. Biliary disease: from science to clinic. Cham: Springer International Publishing; 2017. 229–241. [Google Scholar]
- 4.▪.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. [DOI] [PubMed] [Google Scholar]; The review deals with basic and clinical aspects of gallstone disease.
- 5.Portincasa P, Wang DQ. Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, et al. , editors. Yamada’s atlas of gastroenterology, 5th ed. UK: Wiley-Blackwell; 2016. 335–353. [Google Scholar]
- 6.Portincasa P, Wang DQ. Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, et al. , editors. Yamada’s textbook of gastroenterology, 6th ed. UK: Wiley-Blackwell; 2015. 1808–1834. [Google Scholar]
- 7.▪.Wang DQ, Neuschwander-Tetri BA, Portincasa P. The biliary system. Colloquium series on integrated systems physiology: from molecule to function. 2nd ed. San Rafael: Morgan & Claypool Life Sciences; 2017; 8:i–178. [Google Scholar]; A compendium of recent progresses in understanding the molecular mechanisms of cholesterol and bile acid metabolism. Essential to understand the pathogenesis of gallstone formation.
- 8.▪.Wang DQH, Portincasa P, editors. Gallstones. Recent advances in epidemiology, pathogenesis, diagnosis and management, 1st ed. New York, NY: Nova Science Publisher Inc.; 2017. 1–676. [Google Scholar]; A comprehensive textbook illustrating major aspects related with cholesterol cholelithiasis.
- 9.▪.Housset C, Chrétien Y, Debray D, Chignard N. Functions of the Gallbladder. Compr Physiol 2016; 6:1549–1577. [DOI] [PubMed] [Google Scholar]; Detailed review on structure and function of the gallbladder in health and disease.
- 10.▪▪.Lammert F, Acalovschi M, Ercolani G, et al. EASL clinical practice guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65:146–181. [DOI] [PubMed] [Google Scholar]; European guidelines addressing the key issues on prevention, diagnosis, medical therapy, surgical therapy of gallbladder stones. Further aspects include diagnosis and endoscopic and surgical therapy of bile duct stones, diagnosis and therapy of intrahepatic stones, and therapy of gallstones during pregnancy.
- 11.Ibiebele I, Schnitzler M, Nippita T, Ford JB. Outcomes of gallstone disease during pregnancy: a population-based data linkage study. Paediatr Perinat Epidemiol 2017; 31:522–530. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence: Clinical Guidelines. In: Developmental Follow-up of Children and Young People Born Preterm. London: National Institute for Health and Care Excellence (UK) National Guidelines (NG72); 2017. [PubMed] [Google Scholar]
- 13.▪.Shabanzadeh DM, Skaaby T, Sorensen LT, et al. Metabolic biomarkers and gallstone disease: a population-based study. Scand J Gastroenterol 2017; 52:1270–1277. [DOI] [PubMed] [Google Scholar]; A population-based study showing that biomarkers of insulin resistance and systemic inflammation are associated with gallstone disease.
- 14.Grundy SM. Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr 2004; 80:1–2. [DOI] [PubMed] [Google Scholar]
- 15.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006; 368:230–239. [DOI] [PubMed] [Google Scholar]
- 16.Di Ciaula A, Wang DQ, Bonfrate L, Portincasa P. Current views on genetics and epigenetics of cholesterol gallstone disease. Cholesterol 2013; 2013: 298421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best practice & research. Clin Gastroenterol 2006; 20:981–996. [DOI] [PubMed] [Google Scholar]
- 18.Wittenburg H, Lammert F. Genetic predisposition to gallbladder stones. Semin Liver Dis 2007; 27:109–121. [DOI] [PubMed] [Google Scholar]
- 19.Xie M, Kotecha VR, Andrade JD, et al. Augmented cholesterol absorption and sarcolemmal sterol enrichment slow small intestinal transit in mice, contributing to cholesterol cholelithogenesis. J Physiol 2012; 590:1811–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokes CS, Krawczyk M, Lammert F. Gallstones: environment, lifestyle and genes. Dig Dis 2011; 29:191–201. [DOI] [PubMed] [Google Scholar]
- 21.Portincasa P, Di Ciaula A, Grattagliano I. Preventing a mass disease: the case of gallstones disease: role and competence for family physicians. Korean J Fam Med 2016; 37:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misciagna G, Guerra V, Di Leo A, et al. Insulin and gall stones: a population case control study in southern Italy. Gut 2000; 47:144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin IC, Yang YW, Wu MF, et al. The association of metabolic syndrome and its factors with gallstone disease. BMC Fam Pract 2014; 15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shebl FM, Andreotti G, Meyer TE, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer 2011; 105:1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 2008; 14:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Ciaula A, Garruti G, Wang DQ, Portincasa P. Role of insulin resistance in the formation of cholesterol gallstones. In: Wang DQ-H, Portincasa P, editors. Gallstones: recent advances in epidemiology, pathogenesis, diagnosis and management. New York: Nova Science Publishers; 2017. 357–372. [Google Scholar]
- 27.Sarin SK, Negi VS, Dewan R, et al. High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology 1995; 22: 138–141. [PubMed] [Google Scholar]
- 28.Hsing AW, Bai Y, Andreotti G, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer 2007; 121:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redinger RN, Small DM. Bile composition, bile salt metabolism and gallstones. Arch Intern Med 1972; 130:618–630. [PubMed] [Google Scholar]
- 30.Lammert F, Carey MC, Paigen B. Chromosomal organization of candidate genes involved in cholesterol gallstone formation: a murine gallstone map. Gastroenterology 2001; 120:221–238. [DOI] [PubMed] [Google Scholar]
- 31.Paigen B, Schork NJ, Svenson KL, et al. Quantitative trait loci mapping for cholesterol gallstones in AKR/J and C57L/J strains of mice. Physiol Genomics 2000; 4:59–65. [DOI] [PubMed] [Google Scholar]
- 32.Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology 2006; 131:1943–1970. [DOI] [PubMed] [Google Scholar]
- 33.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lip Res 2009; 50:S406–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buch S, Schafmayer C, Volzke H, et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet 2007; 39:995–999. [DOI] [PubMed] [Google Scholar]
- 35.Goodloe R, Brown-Gentry K, Gillani NB, et al. Lipid trait-associated genetic variation is associated with gallstone disease in the diverse Third National Health and Nutrition Examination Survey (NHANES III). BMC Med Genet 2013; 14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.▪.Joshi AD, Andersson C, Buch S, et al. Four Susceptibility Loci for Gallstone Disease Identified in a Meta-analysis of Genome-Wide Association Studies. Gastroenterology 2016; 151:351.e28–363.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]; GWAS study in European citizens (8720 cases and 55 152 controls) identifying four loci in genes that have putative functions in cholesterol metabolism and transport, and sulfonylation of bile acids or hydoxysteroids.
- 37.Grunhage F, Acalovschi M, Tirziu S, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology 2007; 46:793–801. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Jiang ZY, Fei J, et al. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin Chim Acta 2007; 384:80–85. [DOI] [PubMed] [Google Scholar]
- 39.Jiang ZY, Parini P, Eggertsen G, et al. Increased expression of LXR alpha, ABCG5, ABCG8, and SR-BI in the liver from normolipidemic, nonobese Chinese gallstone patients. J Lipid Res 2008; 49:464–472. [DOI] [PubMed] [Google Scholar]
- 40.Kuo KK, Shin SJ, Chen ZC, et al. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br J Surg 2008; 95:1005–1011. [DOI] [PubMed] [Google Scholar]
- 41.Rudkowska I, Jones PJ. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr Rev 2008; 66:343–348. [DOI] [PubMed] [Google Scholar]
- 42.von Kampen O, Buch S, Nothnagel M, et al. Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology 2013; 57:2407–2417. [DOI] [PubMed] [Google Scholar]
- 43.von Schonfels W, Buch S, Wolk M, et al. Recurrence of gallstones after cholecystectomy is associated with ABCG5/8 genotype. J Gastroenterol 2013; 48:391–396. [DOI] [PubMed] [Google Scholar]
- 44.Xu HL, Cheng JR, Andreotti G, et al. Cholesterol metabolism gene polymorphisms and the risk of biliary tract cancers and stones: a population-based case-control study in Shanghai, China. Carcinogenesis 2011; 32: 58–62. [DOI] [PubMed] [Google Scholar]
- 45.Hirobe-Jahn S, Harsch S, Renner O, et al. Association of FXR gene variants with cholelithiasis. Clin Res Hepatol Gastroenterol 2015; 39:68–79. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Lopez E, Curiel-Lopez F, Hernandez-Nazara A, et al. Influence of ApoE and FABP2 polymorphisms and environmental factors in the susceptibility to gallstone disease. Ann Hepatol 2015; 14:515–523. [PubMed] [Google Scholar]
- 47.Chuang SC, Hsi E, Lee KT. Mucin genes in gallstone disease. Clin Chim Acta 2012; 413:1466–1471. [DOI] [PubMed] [Google Scholar]
- 48.Chen Q, Li WJ, Wan YY, et al. Fibroblast growth factor receptor 4 Gly388Arg polymorphism associated with severity of gallstone disease in a Chinese population. Genet Mol Res 2012; 11:548–555. [DOI] [PubMed] [Google Scholar]
- 49.Chuang SC, Hsi E, Wang SN, et al. Polymorphism at the mucin-like protocadherin gene influences susceptibility to gallstone disease. Clin Chim Acta 2011; 412:2089–2093. [DOI] [PubMed] [Google Scholar]
- 50.Graf GA, Yu L, Li WP, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem 2003; 278:48275–48282. [DOI] [PubMed] [Google Scholar]
- 51.Poupon R, Rosmorduc O, Boelle PY, et al. Genotype-phenotype relationships in the low-phospholipid-associated cholelithiasis syndrome: a study of 156 consecutive patients. Hepatology 2013; 58:1105–1110. [DOI] [PubMed] [Google Scholar]
- 52.Lammert F, Wang DQ, Hillebrandt S, et al. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid-associated cholelithiasis. Hepatology 2004; 39:117–128. [DOI] [PubMed] [Google Scholar]
- 53.▪.Rodriguez S, Gaunt TR, Guo Y, et al. Lipids, obesity and gallbladder disease in women: insights from genetic studies using the cardiovascular gene-centric 50K SNP array. Eur J Hum Genet 2016; 24:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]; The largest gallbladder disease genetic study to date (15 241 women of European ancestry from three cohorts, including 3216 with gallbladder disease). The authors show that specific, mainly hepatocyte centered, components of lipid metabolism are important to gallbladder disease risk in women.
- 54.Nakeeb A, Comuzzie AG, Martin L, et al. Gallstones: genetics versus environment. Ann Surg 2002; 235:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katsika D, Grjibovski A, Einarsson C, et al. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology 2005; 41:1138–1143. [DOI] [PubMed] [Google Scholar]
- 56.Di Ciaula A, Portincasa P. Fat, epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med 2014; 25:865–873. [DOI] [PubMed] [Google Scholar]
- 57.Moore KJ, Rayner KJ, Suarez Y, Fernandez-Hernando C. microRNAs and cholesterol metabolism. Trends Endocrinol Metab 2010; 21:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang B, Liu B, Bi P, et al. An integrated analysis of differential miRNA and mRNA expressions in human gallstones. Mol Biosyst 2015; 11:1004–1011. [DOI] [PubMed] [Google Scholar]
- 59.Wang R, Hong J, Cao Y, et al. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur J Endocrinol 2015; 172:291–300. [DOI] [PubMed] [Google Scholar]
- 60.▪.Singh TD, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic profiling of gallbladder cancer and gall stone diseases: Evaluation of role of tumour associated genes. Gene 2016; 576:743–752. [DOI] [PubMed] [Google Scholar]; A study from India found that accumulation of epigenetic alterations increases poor prognosis of patient with gallbladder patients, but not in gallstone disease.
- 61.Nepokroeff CM, Lakshmanan MR, Ness GC, et al. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys 1974; 160:387–396. [DOI] [PubMed] [Google Scholar]
- 62.Palasciano G, Portincasa P, Belfiore A, et al. Gallbladder volume and emptying in diabetics: the role of neuropathy and obesity. J Intern Med 1992; 231:123–127. [DOI] [PubMed] [Google Scholar]
- 63.de Bari O, Wang TY, Liu M, et al. Estrogen induces two distinct cholesterol crystallization pathways by activating ERalpha and GPR30 in female mice. J Lipid Res 2015; 56:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HH, Liu M, Clegg DJ, et al. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim Biophys Acta 2009; 1791:1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 2004; 10:1352–1358. [DOI] [PubMed] [Google Scholar]
- 66.Aguilar-Olivos NE, Carrillo-Cordova D, Oria-Hernandez J, et al. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in nonalcoholic fatty liver disease. Ann Hepatol 2015; 14:487–493. [PubMed] [Google Scholar]
- 67.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recep Signal 2010; 8:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uppal H, Zhai Y, Gangopadhyay A, et al. Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology 2008; 47:1331–1342. [DOI] [PubMed] [Google Scholar]
- 69.Katsika D, Magnusson P, Krawczyk M, et al. Gallstone disease in Swedish twins: risk is associated with ABCG8 D19H genotype. J Intern Med 2010; 268:279–285. [DOI] [PubMed] [Google Scholar]
- 70.Gylling H, Hallikainen M, Pihlajamaki J, et al. Polymorphisms in the ABCG5 and ABCG8 genes associate with cholesterol absorption and insulin sensitivity. J Lipid Res 2004; 45:1660–1665. [DOI] [PubMed] [Google Scholar]
- 71.▪.Portincasa P, Di Ciaula A, de Bari O, et al. Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol 2016; 10:93–112. [DOI] [PubMed] [Google Scholar]; A review on recent advances in the clinical management of gallstone disease according to established guidelines. Indications for ‘prophylactic’ cholecystectomy are discussed.
- 72.▪.Xie Y, Cifarelli V, Pietka T, et al. Cd36 knockout mice are protected against lithogenic diet-induced gallstones. J Lipid Res 2017; 58:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]; Germline Cd36 knockout mice are protected against diet-induced gallstones compared with congenic (C57BL6/J) controls. Tensiometric changes of gallbladder smooth muscle strips in response to methacholine and KCl improve.
- 73.van Erpecum KJ, Wang DQ, Moschetta A, et al. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res 2006; 47:32–41. [DOI] [PubMed] [Google Scholar]
- 74.▪.Asai Y, Yamada T, Tsukita S, et al. Activation of the hypoxia inducible factor 1α subunit pathway in steatotic liver contributes to formation of cholesterol gallstones. Gastroenterology 2017; 152:1521.e8–1535.e8. [DOI] [PubMed] [Google Scholar]; In steatotic livers of mice, hypoxia upregulates expression of HIF1A, which reduces expression of AQP8 and concentrates biliary lipids via suppression of water secretion from hepatocytes. This promotes cholesterol gallstone formation. Livers from patients with NAFLD and gallstones express higher levels of HIF1A than livers from patients with NAFLD without gallstones.
- 75.Kern F Jr. Effects of dietary cholesterol on cholesterol and bile acids homeostasis in patients with cholesterol gallstones. J Clin Invest 1994; 93: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang DQ. Regulation of intestinal cholesterol absorption. Ann Rev Physiol 2007; 69:221–248. [DOI] [PubMed] [Google Scholar]
- 77.Kesaniemi YA, Ehnholm C, Miettinen TA. Intestinal cholesterol absorption efficiency is related to apoprotein E phenotype. J Clin Invest 1987; 80: 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosner MS, Lange LG, Stenson WF, Ostlund RE Jr. Percentage cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res 1999; 40:302–308. [PubMed] [Google Scholar]
- 79.Wang DQ, Lee SP. Physical chemistry of intestinal absorption of biliary cholesterol in mice. Hepatology 2008; 48:177–185. [DOI] [PubMed] [Google Scholar]
- 80.Wang DQ, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta 2005; 1733:90–99. [DOI] [PubMed] [Google Scholar]
- 81.Wang LJ, Wang J, Li N, et al. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J Biol Chem 2011; 286:7397–7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang HH, Portincasa P, de Bari O, et al. Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest 2013; 43:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuniga S, Molina H, Azocar L, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int 2008; 28:935–947. [DOI] [PubMed] [Google Scholar]
- 84.Wang HH, Portincasa P, Mendez-Sanchez N, et al. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008; 134:2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krawczyk M, Lutjohann D, Schirin-Sokhan R, et al. Phytosterol and cholesterol precursor levels indicate increased cholesterol excretion and biosynthesis in gallstone disease. Hepatology 2012; 55:1507–1517. [DOI] [PubMed] [Google Scholar]
- 86.Renner O, Lutjohann D, Richter D, et al. Role of the ABCG8 19H risk allele in cholesterol absorption and gallstone disease. BMC Gastroenterol 2013; 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paramsothy P, Knopp RH, Kahn SE, et al. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am J Clin Nutr 2011; 94:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gylling H, Hallikainen M, Pihlajamaki J, et al. Insulin sensitivity regulates cholesterol metabolism to a greater extent than obesity: lessons from the METSIM Study. J Lipid Res 2010; 51:2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takemoto M, Tada K, Nakatsuka K, et al. Effects of aging and hyperlipidemia on plasma osteopontin level. Nihon Ronen Igakkai zasshi 1999; 36: 799–802. [DOI] [PubMed] [Google Scholar]
- 90.▪.Lin J, Shao WQ, Chen QZ, et al. Osteopontin deficiency protects mice from cholesterol gallstone formation by reducing expression of intestinal NPC1L1. Mol Med Rep 2017; 16:1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]; OPN gene knockout mice are protected vs. 8 weeks dietary-induced cholelithogenesis because of reduced expression of intestinal NPC1L1 protein and intestinal cholesterol absorption.
- 91.Wu T, Zhang Z, Liu B, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics 2013; 14:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas LA, Veysey MJ, Murphy GM, et al. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut 2005; 54:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keren N, Konikoff FM, Paitan Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep 2015; 7:874–880. [DOI] [PubMed] [Google Scholar]
- 94.▪.Wang Q, Jiao L, He C, et al. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol 2017; 17:74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gut microbiota dysbiosis might play an important role in the pathogenesis of cholesterol gallstone formation in mice.
- 95.▪▪.Liu Q, Shao W, Zhang C, et al. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ Pollut 2017; 226:268–276. [DOI] [PubMed] [Google Scholar]; The authors show that abnormal gut microbiota occurs after 8 week exposure to organochlorine pesticides. Changes include bile acid composition, enhanced hydrophobicity, decreased expression of genes regulating bile acid reabsorption in the terminal ileum, and compensatory increased expression of genes involved in the synthesis of hepatic bile acids.
- 96.Portincasa P, Di Ciaula A, Palmieri VO, et al. Ultrasonographic study of gallbladder and gastric dynamics in obese people after oral cholestyramine. Dordrecht: Kluwer Academic Publisher; 1994. 323–327. [Google Scholar]
- 97.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008; 47: 2112–2126. [DOI] [PubMed] [Google Scholar]
- 98.Portincasa P, Di Ciaula A, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol 1994; 21:430–440. [DOI] [PubMed] [Google Scholar]
- 99.Stolk MF, van Erpecum KJ, Renooij W, et al. Gallbladder emptying in vivo, bile composition, and nucleation of cholesterol crystals in patients with cholesterol gallstones. Gastroenterology 1995; 108:1882–1888. [DOI] [PubMed] [Google Scholar]
- 100.van Erpecum KJ, van Berge Henegouwen GP, Stolk MF, et al. Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J Hepatol 1992; 14:194–202. [DOI] [PubMed] [Google Scholar]
- 101.Pomeranz IS, Davison JS, Shaffer EA. The effects of prosthetic gallstones on gallbladder function and bile composition. J Surg Res 1986; 41: 47–52. [DOI] [PubMed] [Google Scholar]
- 102.Portincasa P, Di Ciaula A, vanBerge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep 2004; 6:151–162. [DOI] [PubMed] [Google Scholar]
- 103.Colecchia A, Sandri L, Bacchi-Reggiani ML, et al. Is it possible to predict the clinical course of gallstone disease? Usefulness of gallbladder motility evaluation in a clinical setting. Am J Gastroenterol 2006; 101:2576–2581. [DOI] [PubMed] [Google Scholar]
- 104.Jennings LJ, Xu QW, Firth TA, et al. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol 1999; 277:G1017–G1026. [DOI] [PubMed] [Google Scholar]
- 105.Tharp KM, Khalifeh-Soltani A, Park HM, et al. Prevention of gallbladder hypomotility via FATP2 inhibition protects from lithogenic diet-induced cholelithiasis. Am J Physiol Gastrointest Liver Physiol 2016; 310: G855–G864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Conter RL, Roslyn JJ, Porter-Fink V, DenBesten L. Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg 1986; 151:184–191. [DOI] [PubMed] [Google Scholar]
- 107.Roslyn JJ, Doty J, Pitt HA, et al. Enhanced gallbladder absorption during gallstone formation: the roles of cholesterol saturated bile and gallbladder stasis. Am J Med Sci 1986; 292:75–80. [DOI] [PubMed] [Google Scholar]
- 108.Ginanni Corradini S, Elisei W, Giovannelli L, et al. Impaired human gallbladder lipid absorption in cholesterol gallstone disease and its effect on cholesterol solubility in bile. Gastroenterology 2000; 118:912–920. [DOI] [PubMed] [Google Scholar]
- 109.Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology 2001; 120:506–511. [DOI] [PubMed] [Google Scholar]
- 110.Chen Q, Amaral J, Oh S, et al. Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology 1997; 113:930–937. [DOI] [PubMed] [Google Scholar]
- 111.Wang HH, Portincasa P, Wang DQ. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci 2008; 13:401–423. [DOI] [PubMed] [Google Scholar]
- 112.Lavoie B, Nausch B, Zane EA, et al. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil 2012; 24:e313–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Portincasa P, van Erpecum KJ, van De Meeberg PC, et al. Apolipoprotein E4 genotype and gallbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology 1996; 24:580–587. [DOI] [PubMed] [Google Scholar]
- 114.Pauletzki J, Althaus R, Holl J, et al. Gallbladder emptying and gallstone formation: a prospective study on gallstone recurrence. Gastroenterology 1996; 111:765–771. [DOI] [PubMed] [Google Scholar]
- 115.Niebergall-Roth E, Teyssen S, Singer MV. Neurohormonal control of gallbladder motility. Scand J Gastroenterol 1997; 32:737–750. [DOI] [PubMed] [Google Scholar]
- 116.Otsuki M Pathophysiological role of cholecystokinin in humans. J Gastroenterol Hepatol 2000; 15:D71–D83. [DOI] [PubMed] [Google Scholar]
- 117.Beglinger C Effect of cholecystokinin on gastric motility in humans. Ann N Y Acad Sci 1994; 713:219–225. [DOI] [PubMed] [Google Scholar]
- 118.Maselli MA, Piepoli AL, Pezzolla F, et al. Effect of three nonpeptide cholecystokinin antagonists on human isolated gallbladder. Dig Dis Sci 2001; 46:2773–2778. [DOI] [PubMed] [Google Scholar]
- 119.▪.Wang HH, Liu M, Portincasa P, et al. Lack of endogenous cholecystokinin promotes cholelithogenesis in mice. Neurogastroenterol Motil 2016; 28:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]; CCK knockout mice lack endogenous CCK, have impaired gallbladder contraction, increased susceptibility to gallstone formation, prolonged small intestinal transit time, and increased intestinal absorption of cholesterol.
- 120.▪.Wang HH, Portincasa P, Wang DQ. The cholecystokinin-1 receptor antagonist devazepide increases cholesterol cholelithogenesis in mice. Eur J Clin Invest 2016; 46:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]; The potent CCK-1R antagonist devazepide increases susceptibility to gallstone formation by impairing gallbladder emptying function, disrupting biliary cholesterol metabolism, and enhancing intestinal cholesterol absorption in mice.
- 121.Yu P, Chen Q, Xiao Z, et al. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am J Physiol 1998; 275: G203–G211. [DOI] [PubMed] [Google Scholar]
- 122.Xiao ZL, Chen Q, Amaral J, et al. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol 1999; 276:G1401–G1407. [DOI] [PubMed] [Google Scholar]
- 123.Cong P, Pricolo V, Biancani P, Behar J. Effects of cholesterol on CCK-1 receptors and caveolin-3 proteins recycling in human gallbladder muscle. Am J Physiol Gastrointest Liver Physiol 2010; 299:G742–G750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu P, De Petris G, Biancani P, et al. Cholecystokinin-coupled intracellular signaling in human gallbladder muscle. Gastroenterology 1994; 106: 763–770. [DOI] [PubMed] [Google Scholar]
- 125.Yu P, Harnett KM, Biancani P, et al. Interaction between signal transduction pathways contributing to gallbladder tonic contraction. Am J Physiol 1994; 265:1082–G1089. [DOI] [PubMed] [Google Scholar]
- 126.Yu P, Chen Q, Harnett KM, et al. Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am J Physiol 1995; 269:G659–G665. [DOI] [PubMed] [Google Scholar]
- 127.Miyasaka K, Takata Y, Funakoshi A. Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism. J Gastroenterol 2002; 37(Suppl 14):102–106. [DOI] [PubMed] [Google Scholar]
- 128.Zhu J, Han TQ, Chen S, et al. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol 2005; 11:1685–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakeeb A, Comuzzie AG, Al-Azzawi H, et al. Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg 2006; 10:940–948. [DOI] [PubMed] [Google Scholar]
- 130.Ding X, Lu CY, Mei Y, et al. Correlation between gene expression of CCK-A receptor and emptying dysfunction of the gallbladder in patients with gallstones and diabetes mellitus. Hepatobiliary Pancreat Dis Int 2005; 4:295–298. [PubMed] [Google Scholar]
- 131.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Isik S, Ozcan HN, Ozuguz U, et al. Impaired gallbladder motility and the effect of metformin therapy in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012; 76:373–378. [DOI] [PubMed] [Google Scholar]
- 133.▪.Villanacci V, Del Sordo R, Salemme M, et al. The enteric nervous system in patients with calculous and acalculous gallbladder. Dig Liver Dis 2016; 48:792–795. [DOI] [PubMed] [Google Scholar]; Gallstones patients showed decrease of neurons, enteric glial cells and mast cells compared with gallstone-free study participants. The ICC were extremely few and only found in two patients, one for each group. The results show that intrinsic innervations of the gallbladder is abnormal in gallstone patients.
- 134.Tan YY, Ji ZL, Zhao G, et al. Decreased SCF/c-kit signaling pathway contributes to loss of interstitial cells of Cajal in gallstone disease. Int J Clin Exp Med 2014; 7:4099–4106. [PMC free article] [PubMed] [Google Scholar]
- 135.Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med 2006; 12:1253–1255. [DOI] [PubMed] [Google Scholar]
- 136.Barrera F, Azocar L, Molina H, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol 2015; 14:710–721. [PubMed] [Google Scholar]
- 137.Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 2012; 55:575–583. [DOI] [PubMed] [Google Scholar]
- 138.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 2002; 298:714–719. [DOI] [PubMed] [Google Scholar]
- 139.Keitel V, Cupisti K, Ullmer C, et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 2009; 50:861–870. [DOI] [PubMed] [Google Scholar]
- 140.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 2011; 25:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lavoie B, Balemba OB, Godfrey C, et al. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol 2010; 588:3295–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vassileva G, Golovko A, Markowitz L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 2006; 398:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.▪.Zhou M, Learned RM, Rossi SJ, et al. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology 2016; 63:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]; Potential for treating cholangiopathy by safely harnessing FGF19 biology to suppress bile acid synthesis.
- 144.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest 1977; 59:828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ryan JP. Motility of the gallbladder and biliary tree. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1987. 695–722. [Google Scholar]
- 146.Luiking YC, Peeters TL, Stolk MF, et al. Motilin induces gall bladder emptying and antral contractions in the fasted state in humans. Gut 1998; 42: 830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Portincasa P, Peeters TL, van Berge-Henegouwen GP, et al. Acute intra-duodenal bile salt depletion leads to strong gallbladder contraction, altered antroduodenal motility and high plasma motilin levels in humans. Neurogastroenterol Motil 2000; 12:421–430. [DOI] [PubMed] [Google Scholar]
- 148.Stolk MF, Van Erpecum KJ, Peeters TL, et al. Interdigestive gallbladder emptying, antroduodenal motility, and motilin release patterns are altered in cholesterol gallstone patients. Dig Dis Sci 2001; 46:1328–1334. [DOI] [PubMed] [Google Scholar]
- 149.vanBerge-Henegouwen GP, Venneman NG, Portincasa P, et al. Relevance of hereditary defects in lipid transport proteins for the pathogenesis of cholesterol gallstone disease. Scand J Gastroenterol Suppl 2004; 60–69. [DOI] [PubMed] [Google Scholar]
- 150.van Erpecum KJ, Portincasa P, Gadellaa M, et al. Effects of bile salt hydrophobicity on nucleation behaviour of cholesterol crystals in model bile. Eur J Clin Invest 1996; 26:602–608. [DOI] [PubMed] [Google Scholar]
- 151.▪.Bertola-Compagnucci A, Perroud HA, Batalles SM, et al. A nested case-control study on dietary fat consumption and the risk for gallstone disease. J Hum Nutr Diet 2016; 29:338–344. [DOI] [PubMed] [Google Scholar]; Studied were 49 cases and 65 controls. The mean energy intake was higher in cases than in controls, and significant differences were found for dietary fat consumption. Total fat, saturated and monounsaturated fatty acids high intakes are associated with increased gallstone disease risk.


