Abstract
Cholesterol gallstone disease is a major health problem in Westernized countries and depends on a complex interplay between genetic factors, lifestyle and diet, acting on specific pathogenic mechanisms. Overweigh, obesity, dyslipidemia, insulin resistance and altered cholesterol homeostasis have been linked to increased gallstone occurrence, and several studies point to a number of specific nutrients as risk- or protective factors with respect to gallstone formation in humans. There is a rising interest in the identification of common and modifiable dietetic factors that put the patients at risk of gallstones or that are able to prevent gallstone formation and growth. In particular, dietary models characterized by increased energy intake with highly refined sugars and sweet foods, high fructose intake, low fiber contents, high fat, consumption of fast food and low vitamin C intake increase the risk of gallstone formation. On the other hand, high intake of monounsaturated fats and fiber, olive oil and fish (ω−3 fatty acids) consumption, vegetable protein intake, fruit, coffee, moderate alcohol consumption and vitamin C supplementation exert a protective role.
The effect of some confounding factors (e.g., physical activity) cannot be ruled out, but general recommendations about the multiple beneficial effects of diet on cholesterol gallstones must be kept in mind, in particular in groups at high risk of gallstone formation.
Keywords: Caloric intake, diet, fibers, macronutrients, obesity, weight loss
1. INTRODUCTION
Gallstone disease (GSD) is a major health problem in developed countries [1–5] and this trend includes Europe as well [6]. The prevalence of gallstones ranges from 10 to 20% in the adult populations [2, 7–9], which renders cholelithiasis a very common disease. It is estimated that about 6.3 million men and 14.2 million women aged 20 to 74 years suffer from gallbladder disease in the USA [8], and such scenario requires high costs for treating gallstones (about $4–6.2 billion [9, 10]), particularly if surgical complications occur [11].
Pathogenic factors include excessive and fast mobilization of body cholesterol into bile through the liver (with subsequent supersaturation of bile with cholesterol), increased cholesterol crystallization in gallbladder bile, hypersecretion of biliary mucin, and decreased gallbladder motility [12–23]. Genetic factors are certainly of major importance in the risk of cholesterol gallstones, and a series of genes have been identified, which are able to promote gallstone formation and growth [24]. However, the analysis of twin pairs showed that genetic factors are estimated to account for only about 25% of gallstone risk [25], underlying the importance of environmentally related modifiable factors. Several observations have found that a complex genetic basis could play a key role in determining individual predisposition to develop cholesterol gallstones in response to environmental stimuli [26–29], including diet. Dietary habits control quality and quantity of energy intake and play a key role in the onset and further development of metabolic disorders.
In Westernized countries the 75% of gallstones are composed of cholesterol [30–33], and their origin has common pathogenic links with broad metabolic abnormalities characterized by altered cholesterol homeostasis, such as obesity, dyslipidemia, type 2 diabetes [2, 34], and the metabolic syndrome [35–40]. In fact, the majority of components of the metabolic syndrome (visceral adiposity, insulin resistance/diabetes mellitus, dyslipidemia [41–43]) Fig. (1) have been associated with an elevated occurrence of cholesterol gallstones and liver steatosis, which have been recognized as “fellow travelers” with this syndrome [41, 44].
Fig. (1).
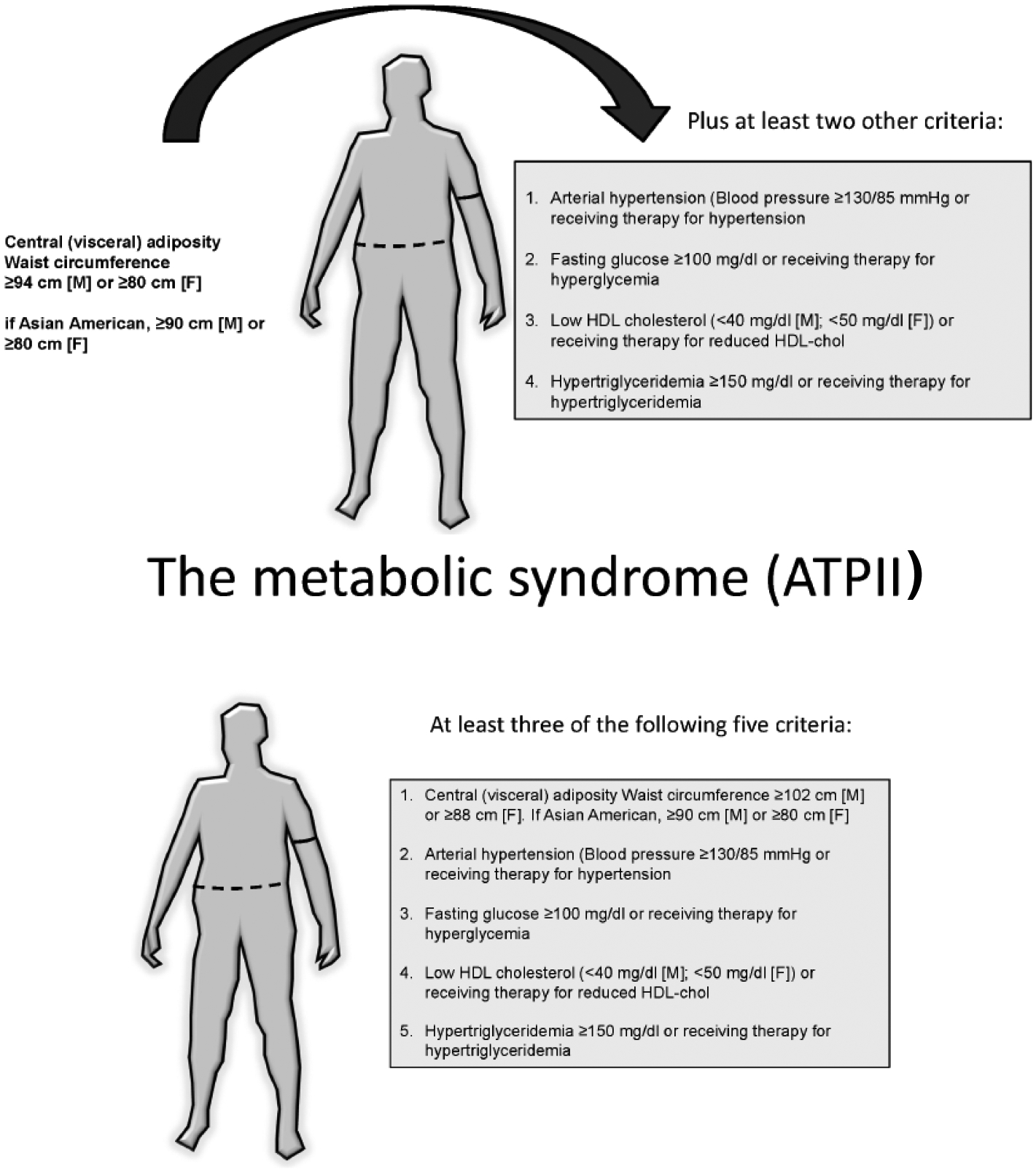
a) Definition of the metabolic syndrome according to the International Diabetes Federation (IDF) [42, 191]. b) Definition of the metabolic syndrome according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) [36].
Increased body size (i.e. BMI equal or greater than 25 kg/m2) due to excessive energy intake could double the risk of symptomatic gallstones, as compared to a BMI of less than 25 kg/m2[45], and obesity (i.e. a BMI equal or greater than 30 kg/m2) strongly predisposes to gallstone formation [46] and increases the rate of cholecystectomy by increasing the risk of symptomatic gallstones [21, 23, 47–55].
Besides the amount of energy intake and metabolic alterations, it is difficult to adequately estimate the exact role played by specific nutrients in large population-based, long-term, prospective epidemiological studies, since gallstone formation is a slow and complex multifactorial process requiring years, and dietary habits are difficult to be described with precision over such a long time span [56]. However, several studies pointed to a number of specific nutrients as risk- or protective factors with respect to gallstone formation in humans (Table 1). This list of factors supports the enormous rising interest in the identification of common and modifiable dietetic factors that put the patients at risk of gallstones or that are able to prevent gallstone formation and growth, particularly in specific high-risk groups [57].
Table 1.
Studies identifying dietary factors potentially able to promote or to prevent the formation of cholesterol gallstone in humans
| PROMOTING FACTORS |
|---|
| Fast food [62] |
| Meat consumption, saturated fat [91, 127] |
| Hypercaloric diet [58, 91] |
| Prolonged fasting [90] |
| Refined sugars, high dietary carbohydrates* [53, 61, 88–95] |
| Low fiber intake [114, 115] |
| Very low calorie diets [12, 14, 16, 75, 76] |
| High fructose intake [87, 101] |
| High bean intake [132, 133] |
| Low vitamin C [61, 122, 128, 129, 131] |
| PREVENTING FACTORS |
| Monounsaturated fats, Polyunsaturated fats, Fiber from cellulose, Nuts [91, 137–140, 155, 188] |
| High-fiber and high-calcium diet [136] |
| Vegetables, fruit [62, 157, 189] |
| Moderate alcohol consumption* [45, 139, 152, 190] |
| Coffee, caffeine* [77, 151, 168–171] |
| Vitamin C supplement [91, 122–126, 130] |
| Regular eating pattern [90, 136] |
| Vegetarian diet* [158–163] |
| Sufficient fat in very-low calorie diet (7–10 g per day) [76, 141–143] |
| Fish, fish oil (PUFA)* [120, 141, 142, 145, 147[127, 150, 151, 153, 155] |
| Monounsaturated fats, Polyunsaturated fats, Fiber from cellulose, Nuts [91, 137–140, 155, 188] |
Asterisk (*) indicates the existence of controversial studies; PUFA, polyunsaturated fatty acids
2. DIET AS A RISK FACTOR FOR GALLSTONE OCCURRENCE
It has been observed that the typical Westernized diet (hypercaloric, with highly refined sugars, low content of fiber, high-lipid) increases the likelihood of gallstone disease [58].
2.1. Energy Intake, Obesity
A nested case-control study by questionnaires showed that increased risks for gallstone disease were associated with high intakes of energy, total fat and saturated and monounsaturated fatty acids [59]. This finding partially confirms previous results from a large cohort study i.e. the Nurses’ Health Study involving 88,837 women aged between 34 to 59 years. The risk of symptomatic gallstones was greater in the highest (1960 kcal/day) than in the lower quintile (1130 kcal/day) of energy intake, and the overall risk of 1.5 increased to 2.1 in lean women [48]. Similar findings have been obtained in a French study showing that the risk of gallstone disease increased in men consuming more than 2500 Kcal per day [60] and in a Spanish study where gallstone patients had a greater consumption of total calories and fats, spent less time walking and slept more than healthy controls [61].
Increased BMI per se acts as a well known risk factor for gallstone disease (particularly in women [62]), with a 7% [62] to 8% [45] rise in the occurrence of symptomatic gallstones with each BMI unit. The risk was 17% in genetically determined BMI [62] and increased with a high waist circumference and central adiposity [45], both factors frequently associated with dyslipidemia (particularly hypertriglyceridemia and low high-density lipoprotein (HDL) concentrations [63]).
These alterations can influence the key steps involved in the pathogenesis of cholesterol gallstones, such as increased biliary cholesterol concentrations [64–66] and hypertriglyceridemia-induced secretion of gallbladder mucin [67]. Furthermore, subjects with overweight and obesity often display larger fasting gallbladder volumes and decreased postprandial gallbladder emptying, i.e. two conditions implying gallbladder stasis, which is a well-established promoting factor for gallstones [68–72]. Altered gallbladder motility is already present in obese children and pre-adolescents, and further deteriorates in obese adults [68, 73], leading to a high risk for gallstones. This trend is similar to other metabolic abnormalities linking childhood to adult obesity [74].
An increased risk of gallstone formation also exists in obese patients during rapid weight loss achieved by very-low-calorie diets containing less than 800 kcal per day [12, 14, 16, 75, 76] or undergoing bariatric surgery (i.e. currently the Roux-en-Y gastric bypass (RYGB) procedure [15, 17, 77–83]. This risk of gallstones is less with low-calorie diet (instead of very-low-calorie diet) [76] because body weight loss is slower, i.e. max. 1.5 kg weekly [84–86].
2.2. Fast food, Westernized Diets, Fructose
The risk of gallstone disease appears to be also dependent on specific dietary components: consumption of fast food, at least once per week [62] and meat consumption [58, 61] have been identified as additional risk factors for symptomatic gallstones. In addition, high intake of refined sugars and sweet foods might represent risk factors for gallstone disease [87] in both genders [53, 61, 88–95]. The mechanism involves increased insulin levels, increased hepatic cholesterol synthesis and hypersecretion of cholesterol into bile [96, 97] leading to increased biliary cholesterol saturation [98]. These are key pathogenic mechanisms involved in cholesterol gallstone formation (1).
Moreover, insulin levels act as independent risk factor for gallstone formations, as shown in a large study on Italian subjects without diabetes. Serum insulin levels were associated with gallstones in multiple logistic regression analysis, when controlling for confounders (sex, age, body mass index and serum glucose concentrations) [99]. Of note, the risk of gallstones was more than doubled (2.66, 95%CI 1.04–6.72) in subjects falling in the highest quintile of serum insulin [99]. Insulin resistance has an independent effect on the risk of gallstone formation: in a large cohort of non-diabetic subjects the prevalence of elevated HOMA index was higher in patients with gallstones as compared to those without stones; only age and HOMA were independent predictors of gallstones, regardless of obesity by multiple logistic regression analysis [100].
The effects of a high carbohydrate and fructose intake on gallstone risk have been confirmed by a large ultrasonographic study in pregnant women [101]. Women were assessed for dietary habits and the risk of incident biliary sludge/gallstones during pregnancy was significantly higher among women in the highest quartile of total carbohydrate intake versus women in the lowest quartile. High intake of fructose (but not sucrose, lactose or galactose) was associated with an increased risk of incident sludge/gallstones, and this association was independent from total carbohydrate intake [101]. The lithogenic effect of fructose appears to depend from several concurrent mechanisms, as induction of insulin resistance, visceral adiposity, metabolic syndrome [102–111], fatty liver secondary to accumulation of triglycerides [106, 112], and gallbladder stasis [87]. The deleterious effects of excess fructose consumption can cause gastrointestinal symptoms due to intolerance and intestinal fermentation by resident intestinal microbiota [113], and can affect several liver metabolic pathways (i.e. gluconeogenesis, synthesis of glycerol which is the backbone of triglycerides, and de novo lipogenesis, where fatty acids are provided for triglyceride assembly [64]) Fig. (2).
Fig. (2).
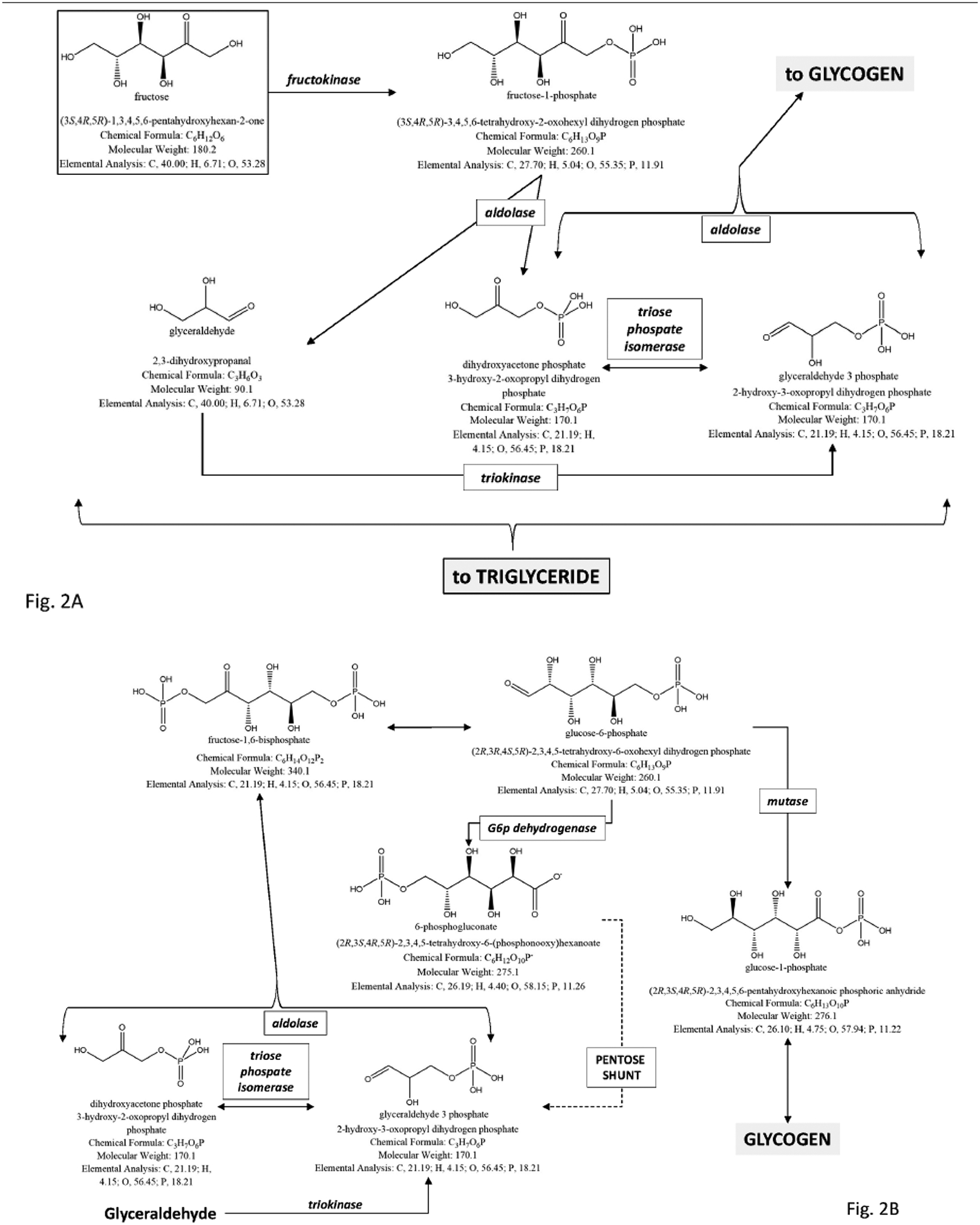
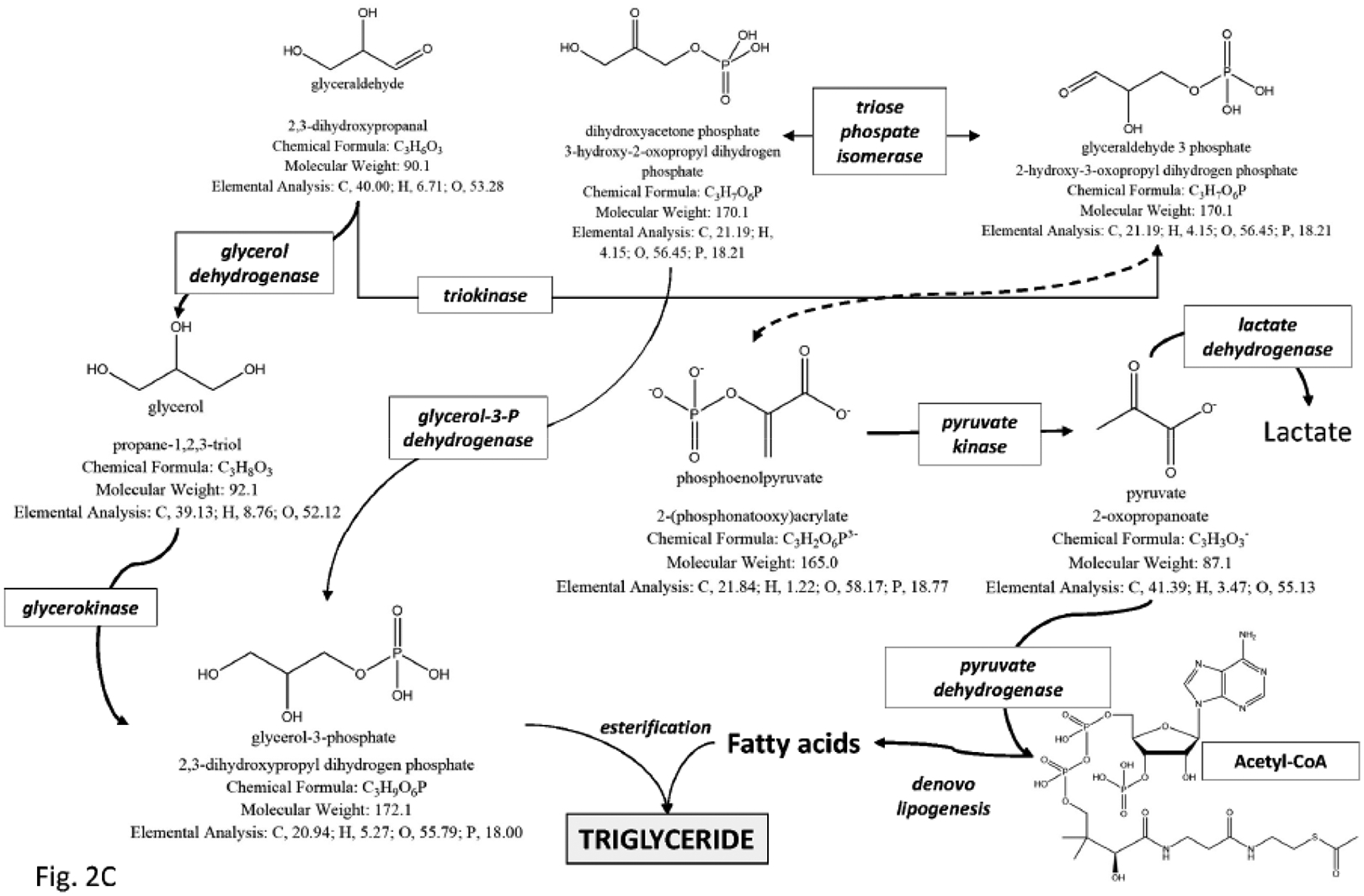
The potential deleterious metabolic effects of excess fructose consumption in diet are shown by the study of metabolic pathways of fructose in the liver. a) A first step is the conversion of fructose to fructose-1-phosphate and then to glyceraldehyde, dihydroxyacetone phosphate, and glyceraldehyde-3-phosphate. This first important step paves the way to the synthesis of glycogen and the synthesis of triglycerides, this latter pathway, a potential risk for liver steatosis. b) The conversion of fructose to glycogen is shown in the liver, anticipated by gluconeogenic precursors. Once liver glycogen is accumulated, the following pathway re-direct the fructose intermediates towards the synthesis of triglyceride. c) Conversion of fructose to triglyceride in the liver. Especially in the presence of excess fructose intake, the steps leading to glycerol synthesis and pyruvate synthesis are followed by construction of the backbone of triglyceride.
2.3. Low Fiber, Trans-Fats, Low Vitamin C
Gallstone patients consume less fiber than controls [61], and low fiber intake might increase the risk of cholesterol gallstones. The mechanism involves a negative effect on colonic motility, and increased production of secondary (lithogenic) bile acids, i.e. deoxycholic acid and lithocholic acid [114, 115] Fig. (3). A negative effect is also attributed to the consumption of fats of animal origin. Eating all visible fat on the meat and using butter were positively associated with cholelithiasis [116]. In French patients, a direct relationship between total and saturated fat intake and gallstone disease has been described [60].
Fig. (3).
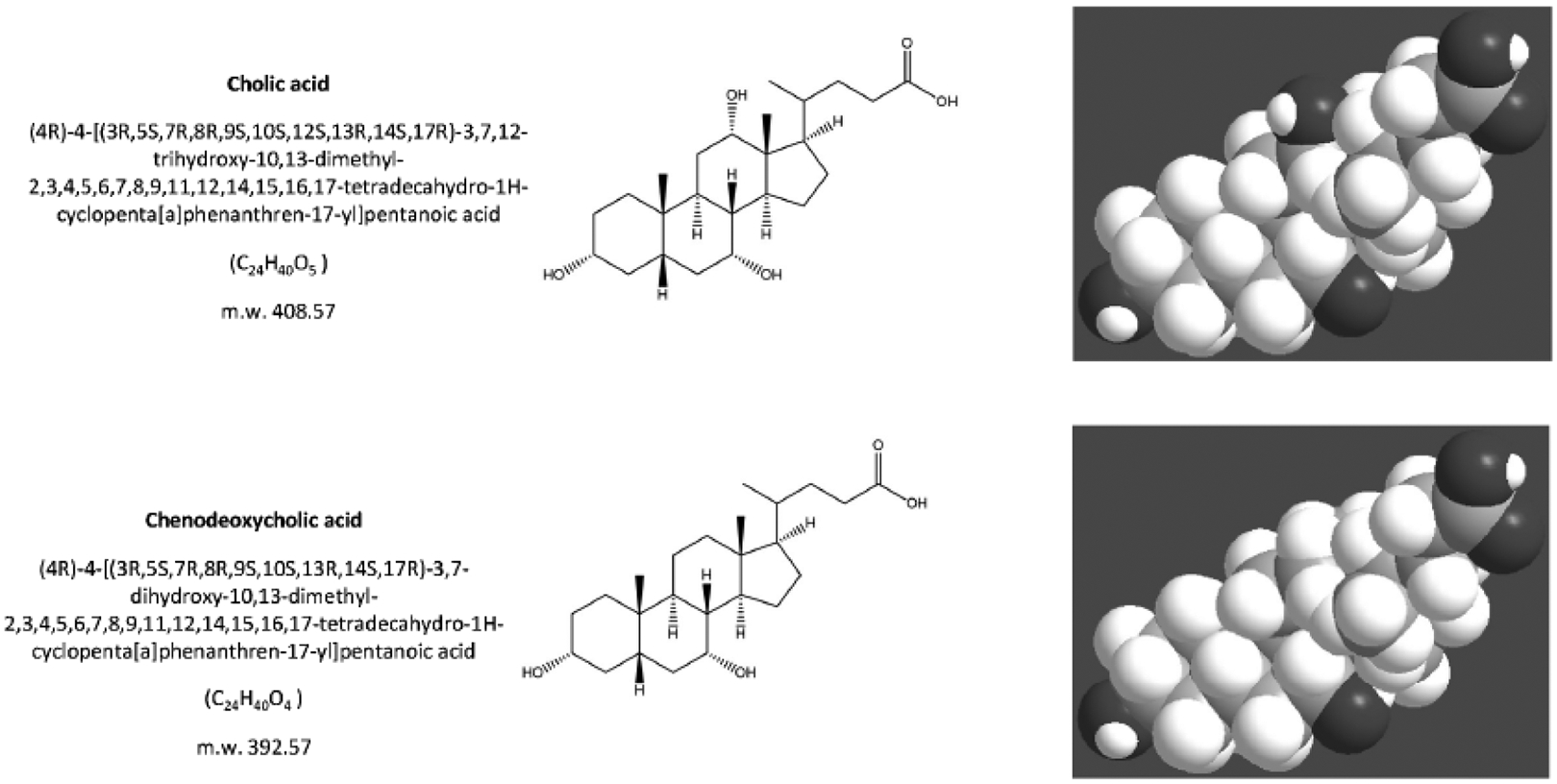
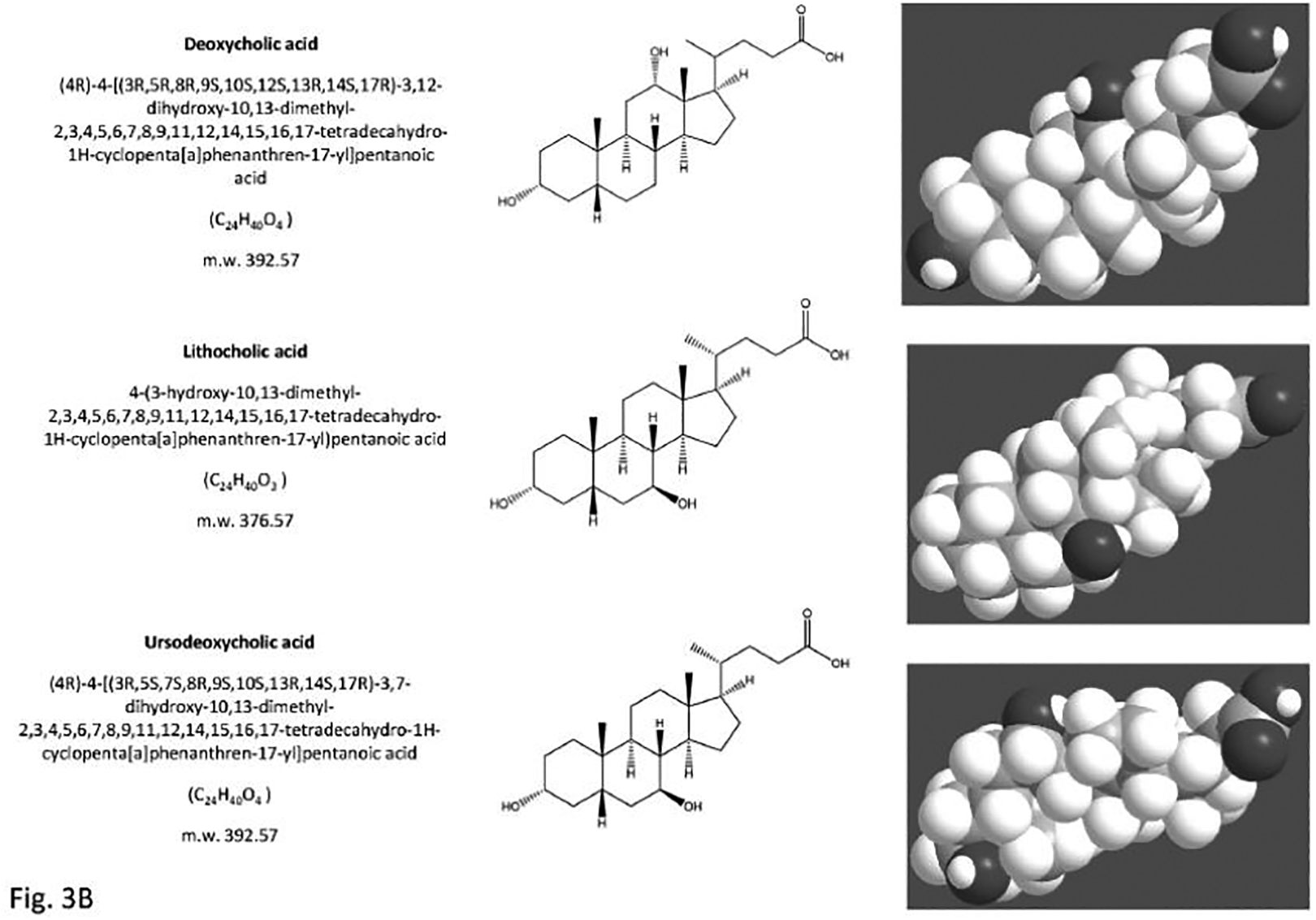
The structure of the primary bile acids, secondary bile acids and “tertiary” bile acid ursodeoxycholic acid. Bile acids are synthesized from cholesterol in the liver as soluble amphiphiles. The biliary bile acid pool in humans is mainly made of the primary bile acids, i.e. the 3,7,12-trihydroxy cholic acid and the 3,7-dihydroxy chenodeoxyholic acid. After being secreted in bile and entering the recirculation in the intestine, the primary bile acids are biotransformed by colonic bacteria into secondary bile acids, i.e. the 3,12-dihydroxy deoxycholic acid (from cholic acid) and the3- monohydroxy lithocholic acid (from chenodeoxycholic acid). “Tertiary” bile acids are the result of modification of secondary bile acids by intestinal flora or hepatocytes. These are the sulfate ester of lithocholic acid and the 3,7-dihydroxy ursodeoxycholic acid (UDCA), and the 7β-epimer of chenodeoxycholic acid. Bile acids are highly soluble, detergent-like amphiphilic molecules; the hydrophilic (polar) areas of bile acids are the hydroxyl groups and conjugation side chain of either glycine or taurine and their hydrophobic (nonpolar) area is the ringed steroid nucleus [64, 192].
An high intake of saturated fats and refined sugars has been also documented in subjects from Southern Italy with incident gallstones (detected by ultrasound) as compared to controls [91]. It has been reported that diet with high content of trans fatty acids might increase the cardiovascular risk [117, 118] and might also predispose to the formation of cholesterol gallstones [119].
Dietary vitamin C might also play a key role, since vitamin C modulates the hepatic and biliary pathways of cholesterol homeostasis by promoting the conversion of cholesterol into bile acids through liver 7α-hydroxylation [120, 121]. A deficiency of vitamin C has been associated with an increased risk of cholesterol gallstone formation [122]. Vitamin C deficiency promotes gallstone formation while vitamin C supplementation prevents lithogenesis in the animal model [123–126]. Clinical surveys found a positive relation between low vitamin C consumption and risk of gallstone formation, gallbladder disease [122, 127, 128], and cholecystectomy [129]. The supplementation of diet with vitamin C (2 g per day over 2 weeks) in humans prolongs the cholesterol crystallization time due to qualitative changes of bile acid composition and increased phospholipid concentrations in bile [130]. An observational study by ultrasonography showed that gallstone prevalence was half in subjects with regular intake of vitamin C (powder, tablets or capsules) as compared to those not taking the vitamin [131].
2.4. High Legume Intake
Some populations such as Amerindians Mapuche and Pima Indians consume a diet based on high legume intake (beans) which represents a risk factor for cholesterol gallstones [56, 132, 133]. The mechanism implies the decrease of total and very low density lipoprotein cholesterol concentrations in serum, and a high biliary cholesterol output [134]. Beans, in fact, contain the plant steroids saponins, which are able to increase biliary cholesterol secretion and crystallization [135].
3. DIET AS A PROTECTIVE FACTOR FOR GALLSTONE OCCURRENCE
3.1. Meal Patterns
Frequent meals and avoidance of prolonged fasting periods act as protective factors against the formation of gallstones [90]. A regular gallbladder emptying follows meal stimulation and this physiological neurohormonal response decreases prolonged gallbladder stasis (a key factor involved in gallstone pathogenesis [136]).
3.2. Fats, Fish Oil, n-3 PUFA
High intake of monounsaturated fats and fibers from cellulose is inversely associated with the risk of gallstone formation [91]. Long-term intake of cisunsaturated and monounsaturated fats had a protective effect in men studied in a prospective population-based study [137]. Nuts can also be protective against gallstone disease [138, 139]. Monounsaturated fat appear to increase gallbladder motility avoiding bile stasis in the gallbladder [140]. The mechanism might involve the fat-dependent stimulation of galbladder contraction, since an appropriate content of fat (at least 7–10 g per day) in a very-low-calorie diet is able to improve gallbladder motility and prevent bile stasis [76, 141–143]. Regular consumption of olive oil which is enriched with monounsaturated plus polyunsaturated omega-6 fatty acids appears also to protect against gallstones [116]. In a small study from Spain, dietary supplementation with virgin olive oil (40 g per day of monounsaturated fat in 9 gallstone patients) or sunflower oil (20 g per day of polyunsaturated fat in 9 gallstone patients) for one month did not affect cholesterol saturation or bile acid species in the gallbladder bile. However, the cholesterol saturation index of hepatic bile decreased significantly in the postprandial period in patients given the olive oil diet but not in the group supplemented with sunflower oil, suggesting that type of dietary fat can influence bile composition in humans [144].
Low HDL cholesterol and high triglyceride concentrations have been associated with a high incidence of gallstones either in both genders [145] or in men only [146]. Dietary habits promoting an increase in serum HDL levels can therefore protect against gallstones, since they are able to increase the hepatic synthesis of primary bile salts (i.e., cholic acid and chenodeoxycholic acid) and, in turn, to ameliorate biliary cholesterol solubilization [147, 148].
Impaired gallbladder emptying is found in patients with hypertriglyceridemia, likely due to decreased sensitivity to the endogenous gastrointestinal hormone cholecystokinin [149] (Fig. (4) Fish consumption might be inversely correlated with the occurrence of cholesterol gallstones [127, 150, 151], although not all studies are consistent [152]. In a study from Netherlands, fish oil supplementation was given to patients with hypertriglyceridemia for seven weeks (total 5 g per day ω−3 fatty acids mainly as 1.9 g of eicosapentaenoic acid C20:5 and 1.1 g of docosahexaenoic acid C22:6) (Fig. (5). Fish oil treatment decreased hypertriglyceridemia and was associated with improved gallbladder motility in response to exogenous infusion of cholecystokin and postprandially, without adversely affecting biliary cholesterol saturation. The results of this study suggest amelioration of gallbladder sensitivity to cholecystokinin upon fish oil supplementation [149]. Biliary enrichment in phospholipids and (less hydrophobic) ω−3 fatty acids might also play a role [153]. In fact, dietary supplementation with 1.5 g ω−3 fatty acids per day over six weeks decreases biliary cholesterol saturation and lithogenicity, without modifying phospholipid and bile acid composition. A similar effect was noted in gallstone patients supplemented with dietary fish oil n-3 polyunsaturated fatty acids (PUFA), in spite of unchanged cholesterol crystallization time [154]. Furthermore, oral supplementation with 11 g n-3 PUFA per day for 6 weeks has been demonstrated to ameliorate bile composition and to maintain cholesterol saturation index and cholesterol crystallization time of women undergoing rapid weight loss on hypocaloric diet (1,200 kcal per day) [155]. The beneficial effects of n-3 PUFA might involve fatty acid composition in bile and enrichment with the above mentioned eicosapentaenoic acid- and docosahexaenoic acid-containing phospholipids at the expenses of linoleic- and arachidonic acid-containing species. A fat-dependent effect on gallbladder contraction is also likely [155].
Fig. (4).
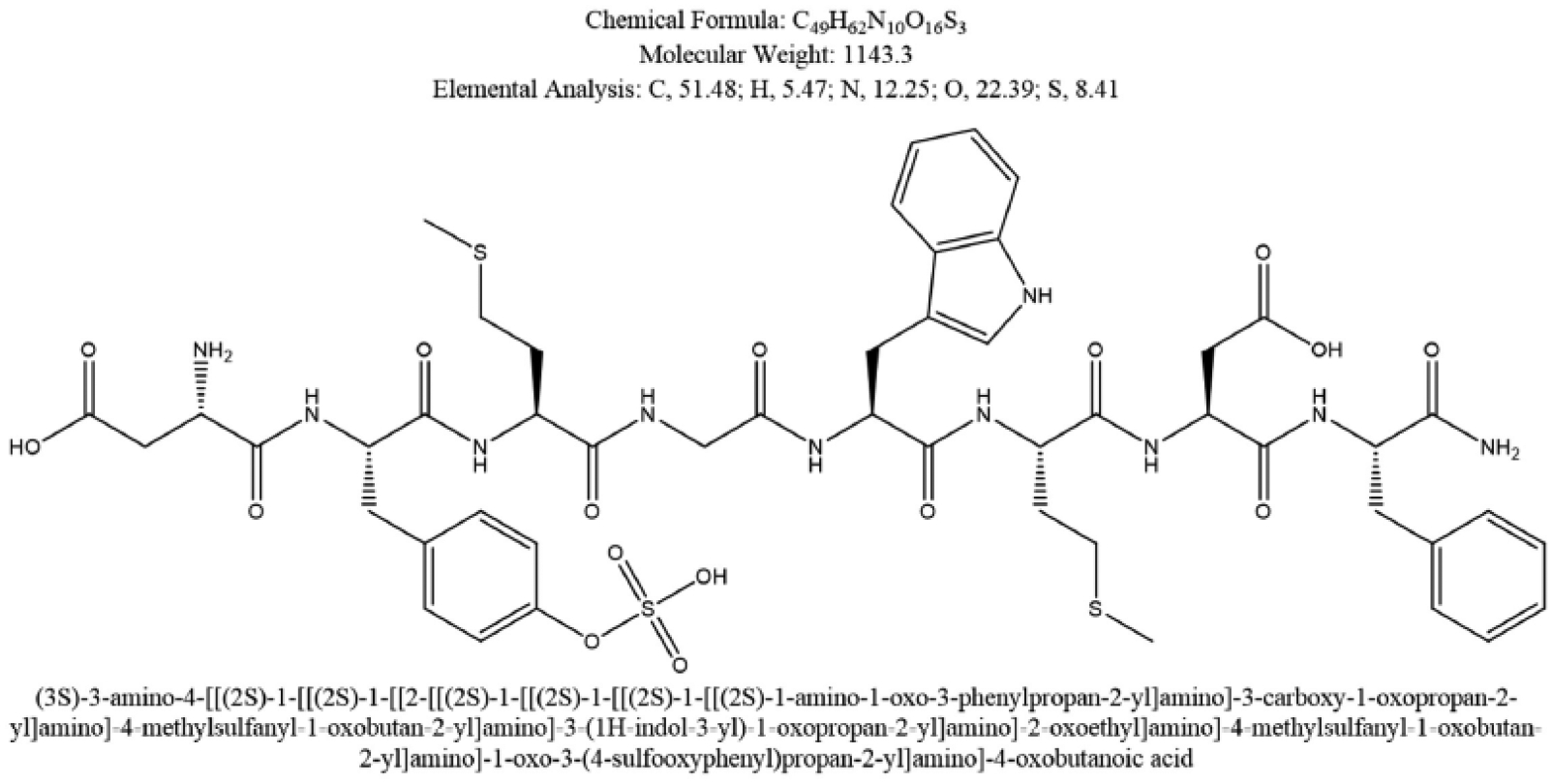
The structure of the potent gastrointestinal hormone cholecystokinin (CCK) acting on the smooth muscle contractility at the level of the gallbladder, upon fat stimulation in food [2, 193].The figure depicts the 8-amino acid C-terminal fragment of cholecystokinin, and also known as CCK-8. From National Center for Biotechnology Information. PubChem Compound Database; CID=9833444, https://pubchem.ncbi.nlm.nih.gov/compound/9833444 (accessed May 12, 2016).
Fig. (5).
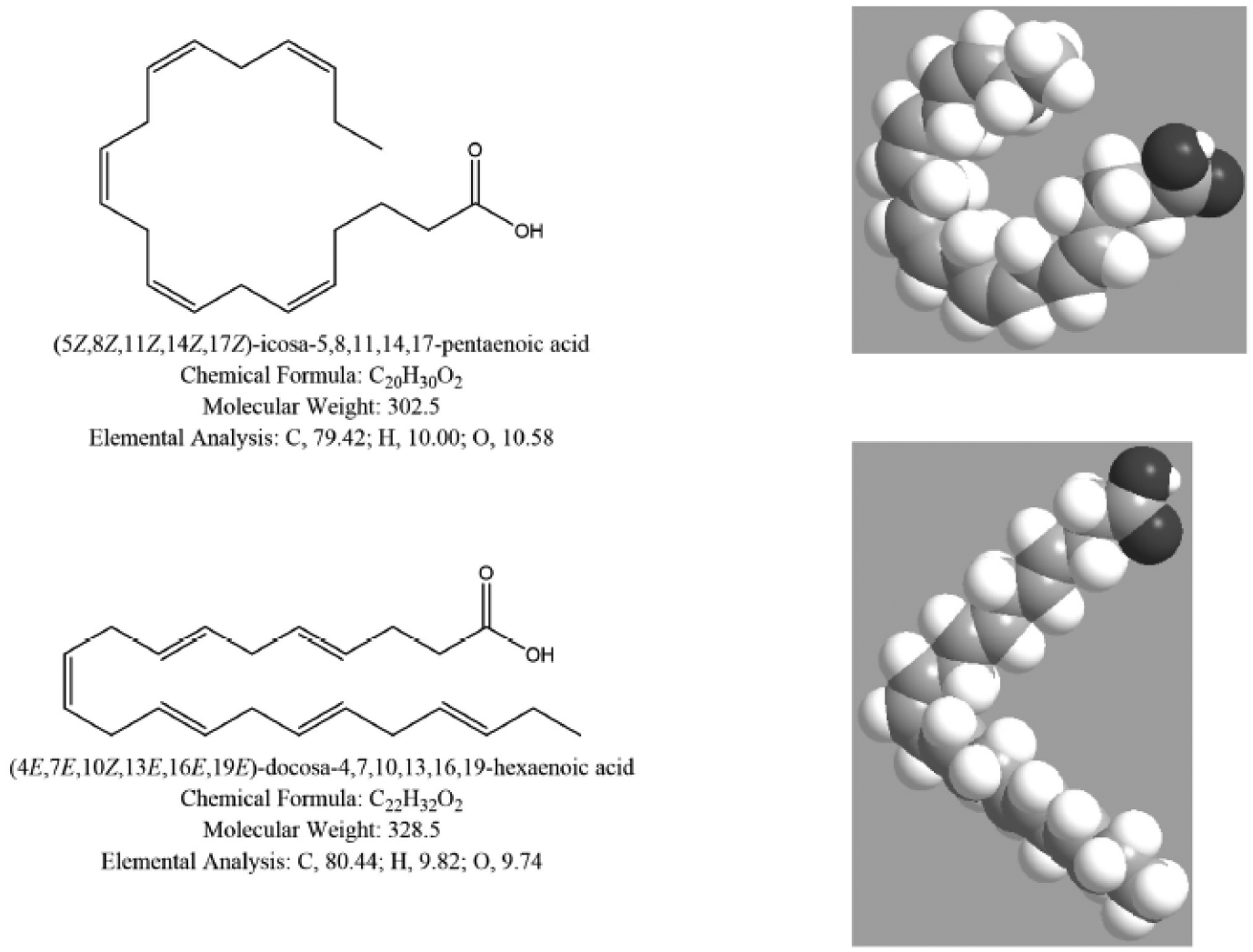
The structure of ω−3 fatty acids eicosapentaenoic acid (top) and docosahexaenoic acid (bottom).
3.3. Vegetables, Vegetarian Diets
Vegetable protein intake might also be beneficial: the Women’s Health Initiative was a large observational study in postmenopausal women and showed that subjects in the highest quintile of energy-adjusted vegetable protein intake (>24 g/d) had a lower risk of gallbladder disease, as compared with those in the lowest quintile (<16.3g/d) [156]. The Nurses’ Health Study reported a decreased risk for cholecystectomy for fruits and vegetables in general as well as for individual groups of green leafy vegetables, citrus fruit and vitamin C-rich fruits and vegetables [157]. The vegetarian diets might also be beneficial because of a secondary effect inducing a lower BMI [158]. Previous studies in different populations, however, showed either protective [159–163] or no effect of vegetarian diets on gallstones [133, 152, 164]. A recent study reported an 8% prevalence of gallstone disease in 1,721 Taiwanese vegetarians of both sexes. Risk factors predicting gallstone disease in vegetarians were age and total bilirubin level in men, and age, BMI, and alcohol consumption in women. Thus, many previously identified risk factors for general population does arecthe observation that dietary fiber supplementation in obese patients undergoing diet-induced weight loss is able to prevent gallstone formation [166]. On the other hand, a reduced intake of fiber has been identified as a risk factor during weight loss in a study with 171 obese patients undergoing bariatric surgery, not taking ursodeoxycholic acid and monitored for 180 days. After surgery, patients who developed gallstones had a significantly (P<0.001) lower consumption of fiber (4.8 ± 3.2 g/day), as compared with gallstone-free subjects (6.9 ± 3.4 g/day) [167].
3.4. Coffee
The effect of caffeine is still controversial, since some [77, 151, 168–171] but not all [150, 164, 172] studies suggest a potentially protective effect on gallstone formation. The effect is mainly mediated by the decreased hepatic synthesis and secretion of cholesterol [173, 174] and a positive effect on gallbladder [175, 176] and intestinal [177] motility. Contradictory results, however, might be related to different drinking patterns of coffee and other caffeinated soft drinks in different countries. A sex hormone-related effect is also possible, since a prospective study in a large cohort of Swedish subjects demonstrated an inverse association between coffee consumption and the risk of cholecystectomy in premenopausal women and in women who used hormone replacement therapy, but neither in other women nor in men [178].
3.5. Alcohol
Alcohol intake influences cholesterol homeostasis and beneficial effect on the risk of cholesterol gallstones are possible. Alcohol inhibits the cholesteryl ester transfer protein (CETP)-mediated conversion of HDL into low-density lipoprotein (LDL)-cholesterol [179]. This step is followed by increased HDL cholesterol concentrations [147, 180, 181] which are inversely correlated with biliary cholesterol saturation [147, 148]. Small doses of alcohol also stimulate gallbladder contractility through increased cholecystokinin release [182, 183].
Such general pathophysiological effect of alcohol on biliary function need clinical confirmation. The Nurses’ Health Study cohort [48] suggests that regular alcohol consumption (as compared with abstention) might have a protective effect on gallstones; indeed an alcohol intake larger than 5 g per day was associated with decreased incidence of symptomatic gallstones. Later studies demonstrated an inverse relation between consumption of different alcoholic beverages (wine, beer, liquors) and the risk of cholecystectomy (76), while a prospective study found that frequent and moderate alcohol intake reduces the risk for symptomatic gallstones, as compared to infrequent or episodic alcohol intake (194). Furthermore, a protective effect of small doses of alcohol (particularly in men) was documented in the cohort from the European Prospective Investigation into Cancer-Norfolk (EPIC-Norfolk). In this study, every unit of alcohol consumed per week decreased the risk of symptomatic gallstones by 3% [45].
Findings from the EMIL study also showed a protective effect, starting from a consumption of 21–40 g alcohol per day [164]. The topic, however, is controversial in many respects: not all studies have confirmed the protective effect of alcohol on gallstone disease [160, 172, 184], while others find some protection with elevated doses of alcohol consumption [139, 164, 185]. Excessive alcohol consumption, by contrast, increases the risk of chronic liver injury and cirrhosis, a condition associated with an increased risk of (pigment) gallstones [186, 187].
CONCLUSION
The formation of cholesterol gallstones derives from a complex interplay of genetic factors with environmental stimuli, through well-known pathogenic mechanisms. The role of genes, however, seem to be of lower epidemiologic importance, as compared with modifiable environmental factors affecting potentially modifiable mechanisms as cholesterol synthesis by the liver and secretion into bile, bile composition, cholesterol aggregation in the gallbladder, fasting and postprandial gallbladder motility. Lifestyle and dietary factors, in particular, might either indirectly (e.g., inducing overweight, obesity, insulin resistance and the metabolic syndrome) or directly (e.g. dietary content in fiber and specific macronutrients, vitamin C) interfere with the pathogenesis of cholesterol gallstones acting on common pathogenic pathways (Fig. 6).
Fig. (6).
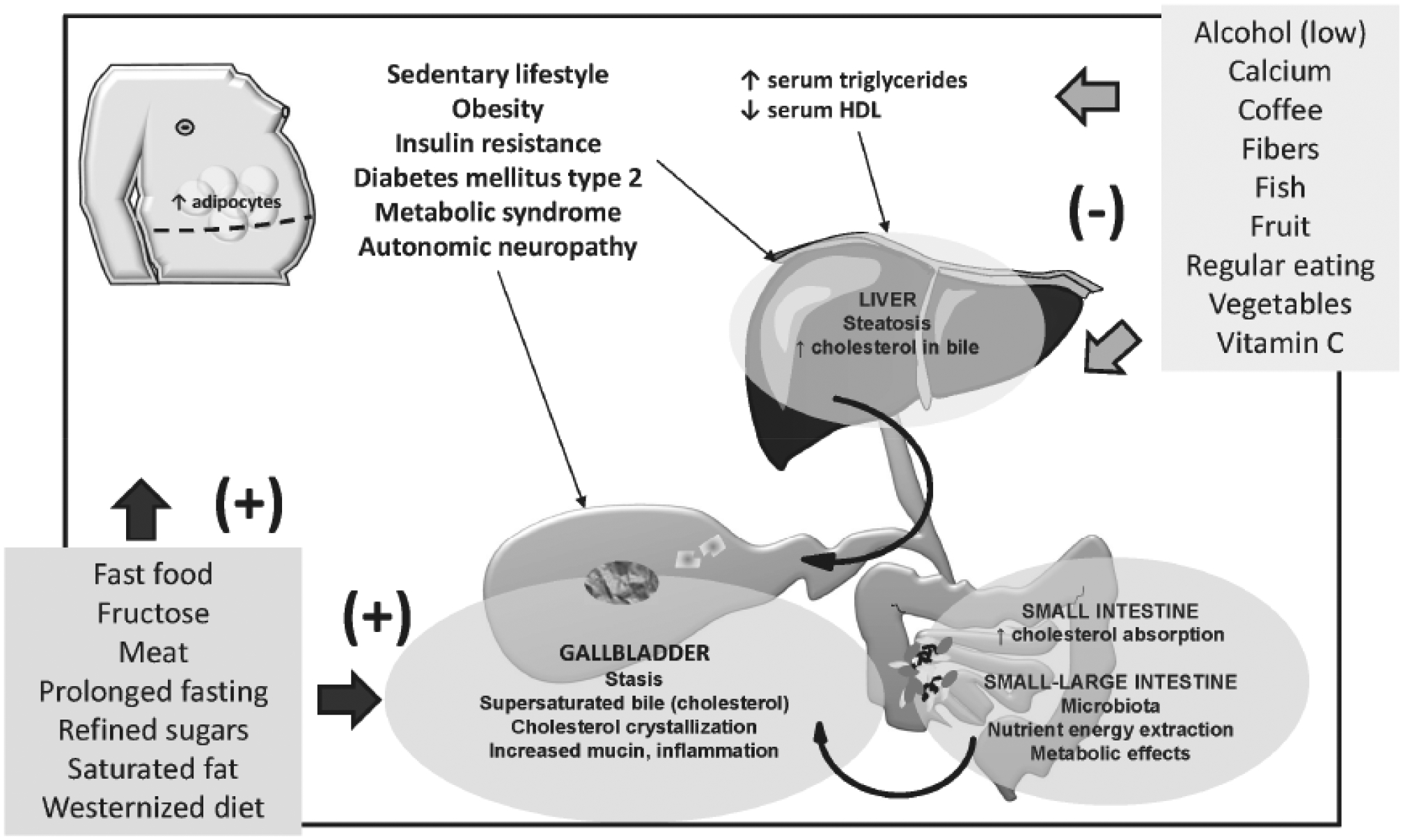
Dietary factors and lifestyles may act as risk factors (+) or protective factors (−) on typical pathogenic factors of cholesterol cholelithiasis, involving the liver, the intestine and the gallbladder.
Factors linking dietary habits to gallstone disease have been broadly investigated and open the way to interventions acting on modifiable factors able to prevent gallstone formation, particularly in risk groups. Maintenance of ideal body weight by adequate energy intake and lifestyle interventions, appropriate weight loss among overweight and obese individuals and possible selection of specific nutrients might significantly lower the risk of gallstone disease also in the general population.
The ultimate efficacy of single dietary components or specific dietary regimens is prone to the effect of a number of confounding factors (i.e. genetic factors, physical activity, extra-dietary environmental influences). Thus, further studies are needed in order to better explore the role of diet as a whole, of dietary single components and on the role of gene/environmental interaction in the multifactorial pathogenesis of cholesterol gallstones. However, recommendations on beneficial effects of diet should be considered, particularly in high-risk groups and considering that cholesterol cholelithiasis is often part of the broader scenario linked with the metabolic syndrome [44].
ABBREVIATIONS
- GSD
Gallstone disease
- BMI
Body mass index
- HDL
High-density lipoprotein
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
REFERENCES
- [1].Attili AF; Capocaccia R; Carulli N; Festi D; Roda E; Barbara L; Capocaccia L; Menotti A; Okolicsanyi L; Ricci G; Lalloni L; Mariotti S; Sama C; Scafato E Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology, 1997, 26, 809–818. [DOI] [PubMed] [Google Scholar]
- [2].Portincasa P; Moschetta A; Palasciano G Cholesterol gallstone disease. Lancet, 2006, 368, 230–239. [DOI] [PubMed] [Google Scholar]
- [3].Everhart JE; Ruhl CE Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology, 2009, 136, 376–386. [DOI] [PubMed] [Google Scholar]
- [4].Portincasa P; Wang DQH Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, editors. Yamada’s Textbook of Gastroenterology. 6th ed. UK: Wiley-Blackwell; 2015. p. 1808–1834. [Google Scholar]
- [5].Portincasa P; Wang DQH Gallstones. In: Podolsky KD, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, editors. Yamada’s Atlas of Gastroenterology. 5th ed. UK: Wiley-Blackwell; 2016. p. 335–353. [Google Scholar]
- [6].Farthing M; Roberts SE; Samuel DG; Williams JG; Thorne K; Morrison-Rees S; John A; Akbari A; Williams JC Survey of digestive health across Europe: Final report. Part 1: The burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United European Gastroenterol J, 2014, 2, 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang DQ; Afdhal NH Genetic analysis of cholesterol gallstone formation: searching for Lith (gallstone) genes. Curr Gastroenterol Rep, 2004, 6, 140–150. [DOI] [PubMed] [Google Scholar]
- [8].Everhart JE; Khare M; Hill M; Maurer KR Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology, 1999, 117, 632–639. [DOI] [PubMed] [Google Scholar]
- [9].Sandler RS; Everhart JE; Donowitz M; Adams E; Cronin K; Goodman C; Gemmen E; Shah S; Avdic A; Rubin R The burden of selected digestive diseases in the United States. Gastroenterology, 2002, 122, 1500–1511. [DOI] [PubMed] [Google Scholar]
- [10].Everhart JE; Ruhl CE Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology, 2009, 136, 1134–1144. [DOI] [PubMed] [Google Scholar]
- [11].Shaffer EA Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr.Gastroenterol.Rep, 2005, 7, 132–140. [DOI] [PubMed] [Google Scholar]
- [12].Shiffman ML; Kaplan GD; Brinkman-Kaplan V; Vickers FF Prophylaxis against gallstone formation with ursodeoxycholic acid in patients participating in a very-lowcalorie diet program. Ann Intern Med, 1995, 122, 899–905. [DOI] [PubMed] [Google Scholar]
- [13].Li VK; Pulido N; Fajnwaks P; Szomstein S; Rosenthal R; Martinez-Duartez P Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc, 2009, 23, 1640–1644. [DOI] [PubMed] [Google Scholar]
- [14].Kamrath RO; Plummer LJ; Sadur CN; Adler MA; Strader WJ; Young RL; Weinstein RL Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr, 1992, 56, 255S–257S. [DOI] [PubMed] [Google Scholar]
- [15].Yang H; Petersen GM; Roth MP; Schoenfield LJ; Marks JW Risk factors for gallstone formation during rapid loss of weight. Dig Dis Sci, 1992, 37, 912–918. [DOI] [PubMed] [Google Scholar]
- [16].Liddle RA; Goldstein RB; Saxton J Gallstone formation during weight-reduction dieting. Arch Intern Med, 1989, 149, 1750–1753. [PubMed] [Google Scholar]
- [17].Broomfield PH; Chopra R; Sheinbaum RC; Bonorris GG; Silverman A; Schoenfield LJ; Marks JW Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight [see comments]. N.Engl.J.Med, 1988, 319, 1567–1572. [DOI] [PubMed] [Google Scholar]
- [18].Shiffman ML; Sugerman HJ; Kellum JM; Moore EW Changes in gallbladder bile composition following gallstone formation and weight reduction. Gastroenterology, 1992, 103, 214–221. [DOI] [PubMed] [Google Scholar]
- [19].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Weight cycling and risk of gallstone disease in men. Arch Intern Med, 2006, 166, 2369–2374. [DOI] [PubMed] [Google Scholar]
- [20].Syngal S; Coakley EH; Willett WC; Byers T; Williamson DF; Colditz GA Long-term weight patterns and risk for cholecystectomy in women. Ann Intern Med, 1999, 130, 471–477. [DOI] [PubMed] [Google Scholar]
- [21].Amaral JF; Thompson WR Gallbladder disease in the morbidly obese. Am J Surg, 1985, 149, 551–557. [DOI] [PubMed] [Google Scholar]
- [22].Shiffman ML; Sugerman HJ; Kellum JM; Brewer WH; Moore EW Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol, 1991, 86, 1000–1005. [PubMed] [Google Scholar]
- [23].Lammert F; Gurusamy K; Ko CW; Miquel JF; Mendez-Sanchez N; Portincasa P; van Erpecum KJ; van Laarhoven CJ; Wang DQ Gallstones. Nat Rev Dis Prim, 2016, 2, 16024. [DOI] [PubMed] [Google Scholar]
- [24].Wang DQ; Cohen DE; Carey MC Biliary lipids and cholesterol gallstone disease. J.Lip.Res, 2009, 50 Suppl, S406–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Katsika D; Grjibovski A; Einarsson C; Lammert F; Lichtenstein P; Marschall HU Genetic and environmental influences on symptomatic gallstone disease: A Swedish study of 43,141 twin pairs. Hepatology, 2005, 41, 1138–1143. [DOI] [PubMed] [Google Scholar]
- [26].Lammert F; Miquel JF Gallstone disease: from genes to evidence-based therapy. J Hepatol, 2008, 48 Suppl 1, S124–S135. [DOI] [PubMed] [Google Scholar]
- [27].Lammert F; Sauerbruch T Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat.Clin.Pract.Gastroenterol.Hepatol, 2005, 2, 423–433. [DOI] [PubMed] [Google Scholar]
- [28].Wang DQH; Zhang L; Wang HH High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim.Biophys.Acta, 2005, 1733, 90–99. [DOI] [PubMed] [Google Scholar]
- [29].Wittenburg H; Lammert F Genetic predisposition to gallbladder stones. Semin.Liver Dis, 2007, 27, 109–121. [DOI] [PubMed] [Google Scholar]
- [30].Diehl AK Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am, 1991, 20, 1–19. [PubMed] [Google Scholar]
- [31].Sherlock S; Dooley J Diseases of the liver and biliary system. Oxford: Blackwell Science; 2002. [Google Scholar]
- [32].Trotman BW; Ostrow JD; Soloway RD Pigment vs cholesterol cholelithiasis: comparison of stone type and bile composition. Am J Dig Dis, 1974, 19, 585–590. [DOI] [PubMed] [Google Scholar]
- [33].Attili AF; Carulli N; Roda E; Barbara B; Capocaccia L; Menotti A; Okoliksanyi L; Ricci G; Capocaccia R; Festi D; et al. Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.). Am J Epidemiol, 1995, 141, 158–165. [DOI] [PubMed] [Google Scholar]
- [34].Stinton LM; Myers RP; Shaffer EA Epidemiology of gallstones. Gastroenterol Clin North Am, 2010, 39, 157–169, vii. [DOI] [PubMed] [Google Scholar]
- [35].Grundy SM; Barnett JP Metabolic and health complications of obesity. Dis Mon., 1990, 36, 641–731. [PubMed] [Google Scholar]
- [36].Grundy SM Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler.Thromb.Vasc.Biol, 2005, 25, 2243–2244. [DOI] [PubMed] [Google Scholar]
- [37].Grundy SM; Cleeman JI; Daniels SR; Donato KA; Eckel RH; Franklin BA; Gordon DJ; Krauss RM; Savage PJ; Smith SC Jr.; Spertus JA; Costa F Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 2005, 112, 2735–2752. [DOI] [PubMed] [Google Scholar]
- [38].Eckel RH; Grundy SM; Zimmet PZ The metabolic syndrome. Lancet, 2005, 365, 1415–1428. [DOI] [PubMed] [Google Scholar]
- [39].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr, 2004, 80, 38–44. [DOI] [PubMed] [Google Scholar]
- [40].Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation, 2002, 106, 3143–3421. [PubMed] [Google Scholar]
- [41].Grundy SM A constellation of complications: the metabolic syndrome. Clin Cornerstone, 2005, 7, 36–45. [DOI] [PubMed] [Google Scholar]
- [42].Zimmet P; Magliano D; Matsuzawa Y; Alberti G; Shaw J The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb, 2005, 12, 295–300. [DOI] [PubMed] [Google Scholar]
- [43].Alberti KG; Zimmet P; Shaw J Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med, 2006, 23, 469–480. [DOI] [PubMed] [Google Scholar]
- [44].Grundy SM Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr, 2004, 80, 1–2. [DOI] [PubMed] [Google Scholar]
- [45].Banim PJ; Luben RN; Bulluck H; Sharp SJ; Wareham NJ; Khaw KT; Hart AR The aetiology of symptomatic gallstones quantification of the effects of obesity, alcohol and serum lipids on risk. Epidemiological and biomarker data from a UK prospective cohort study (EPIC-Norfolk). Eur J Gastroenterol Hepatol, 2011, 23, 733–740. [DOI] [PubMed] [Google Scholar]
- [46].Friedman GD; Kannel WB; Dawber TR The epidemiology of gallbladder disease: observations in the Framingham Study. J Chronic Dis, 1966, 19, 273–292. [DOI] [PubMed] [Google Scholar]
- [47].Volzke H; Baumeister SE; Alte D; Hoffmann W; Schwahn C; Simon P; John U; Lerch MM Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion, 2005, 71, 97–105. [DOI] [PubMed] [Google Scholar]
- [48].Maclure KM; Hayes KC; Colditz GA; Stampfer MJ; Speizer FE; Willett WC Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med, 1989, 321, 563–569. [DOI] [PubMed] [Google Scholar]
- [49].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Central adiposity, regional fat distribution, and the risk of cholecystectomy in women. Gut, 2006, 55, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Klein S; Wadden T; Sugerman HJ AGA technical review on obesity. Gastroenterology, 2002, 123, 882–932. [DOI] [PubMed] [Google Scholar]
- [51].Willett WC; Dietz WH; Colditz GA Guidelines for healthy weight. N Engl J Med, 1999, 341, 427–434. [DOI] [PubMed] [Google Scholar]
- [52].The epidemiology of gallstone disease in Rome, Italy. Part I. Prevalence data in men. The Rome Group for Epidemiology and Prevention of Cholelithiasis (GREPCO). Hepatology, 1988, 8, 904–906. [PubMed] [Google Scholar]
- [53].Scragg RK; McMichael AJ; Baghurst PA Diet, alcohol, and relative weight in gall stone disease: a case-control study. Br Med J (Clin Res Ed), 1984, 288, 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jorgensen T Prevalence of gallstones in a Danish population. Am J Epidemiol, 1987, 126, 912–921. [DOI] [PubMed] [Google Scholar]
- [55].Stampfer MJ; Maclure KM; Colditz GA; Manson JE; Willett WC Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr, 1992, 55, 652–658. [DOI] [PubMed] [Google Scholar]
- [56].Cuevas A; Miquel JF; Reyes MS; Zanlungo S; Nervi F Diet as a risk factor for cholesterol gallstone disease. J Am.Coll.Nutr, 2004, 23, 187–196. [DOI] [PubMed] [Google Scholar]
- [57].Hofmann AF Primary and secondary prevention of gallstone disease: implications for patient management and research priorities. Am J Surg, 1993, 165, 541–548. [DOI] [PubMed] [Google Scholar]
- [58].Tsunoda K; Shirai Y; Hatakeyama K Prevalence of cholesterol gallstones positively correlates with per capita daily calorie intake. Hepatogastroenterology, 2004, 51, 1271–1274. [PubMed] [Google Scholar]
- [59].Bertola Compagnucci A; Perroud HA; Batalles SM; Villavicencio R; Brasca A; Berli D; Pezzotto SM A nested case-control study on dietary fat consumption and the risk for gallstone disease. J Hum Nutr Diet, 2016, 29, 338–344. [DOI] [PubMed] [Google Scholar]
- [60].Caroli-Bosc FX; Deveau C; Peten EP; Delabre B; Zanaldi H; Hebuterne X; Hastier P; Viudes F; Belanger F; Caroli-Bosc C; Harris A; Hardion M; Rampal P; Delmont JP Cholelithiasis and dietary risk factors: an epidemiologic investigation in Vidauban, Southeast France. General Practitioner’s Group of Vidauban. Dig Dis Sci, 1998, 43, 2131–2137. [DOI] [PubMed] [Google Scholar]
- [61].Ortega RM; Fernandez-Azuela M; Encinas-Sotillos A; Andres P; Lopez-Sobaler AM Differences in diet and food habits between patients with gallstones and controls. J Am Coll Nutr, 1997, 16, 88–95. [DOI] [PubMed] [Google Scholar]
- [62].Stender S; Nordestgaard BG; Tybjaerg-Hansen A Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology, 2013, 58, 2133–2141. [DOI] [PubMed] [Google Scholar]
- [63].Jensen MD; Ryan DH; Apovian CM; Ard JD; Comuzzie AG; Donato KA; Hu FB; Hubbard VS; Jakicic JM; Kushner RF; Loria CM; Millen BE; Nonas CA; Pi-Sunyer FX; Stevens J; Stevens VJ; Wadden TA; Wolfe BM; Yanovski SZ; Jordan HS; Kendall KA; Lux LJ; Mentor-Marcel R; Morgan LC; Trisolini MG; Wnek J; Anderson JL; Halperin JL; Albert NM; Bozkurt B; Brindis RG; Curtis LH; DeMets D; Hochman JS; Kovacs RJ; Ohman EM; Pressler SJ; Sellke FW; Shen WK; Smith SC Jr.; Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice, G.; Obesity S 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 2014, 129, S102–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang DQH; Neuschwander-Tetri BA; Portincasa P The Biliary System. Colloquium Series on Integrated Systems Physiology: From Molecule to Function, 2012, 4, 1–148. [Google Scholar]
- [65].Ahlberg J; Angelin B; Einarsson K; Hellstrom K; Leijd B Biliary lipid composition in normo-and hyperlipoproteinemia. Gastroenterology, 1980, 79, 90–94. [PubMed] [Google Scholar]
- [66].Stahlberg D; Rudling M; Angelin B; Bjorkhem I; Forsell P; Nilsell K; Einarsson K Hepatic cholesterol metabolism in human obesity. Hepatology, 1997, 25, 1447–1450. [DOI] [PubMed] [Google Scholar]
- [67].Mingrone G; Greco AV; Finotti E; Passi S Free fatty acids: a stimulus for mucin hypersecretion in cholesterol gallstone biles. Biochim Biophys Acta, 1988, 958, 52–59. [DOI] [PubMed] [Google Scholar]
- [68].Di Ciaula A; Wang DQ; Portincasa P Gallbladder and gastric motility in obese newborns, pre-adolescents and adults. J Gastroenterol Hepatol, 2012, 27, 1298–1305. [DOI] [PubMed] [Google Scholar]
- [69].Portincasa P; Di Ciaula A; Palmieri VO; Palasciano G Acute and chronic effects of oral cholestyramine on gallbladder and antral motiliy in obese and lean individuals. Gut, 1993, 34(Suppl.3), S31. [Google Scholar]
- [70].Vezina WC; Paradis RL; Grace DM; Zimmer RA; Lamont DD; Rycroft KM; King ME; Hutton LC; Chey WY Increased volume and decreased emptying of the gallbladder in large (morbidly obese, tall normal, and muscular normal) people. Gastroenterology, 1990, 98, 1000–1007. [DOI] [PubMed] [Google Scholar]
- [71].Portincasa P; Di Ciaula A; Wang HH; Palasciano G; Van Erpecum KJ; Moschetta A; Wang DQH Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology, 2008, 47, 2112–2126. [DOI] [PubMed] [Google Scholar]
- [72].Mathus-Vliegen EM; Van Ierland-Van Leeuwen ML; Terpstra A Determinants of gallbladder kinetics in obesity. Dig Dis Sci, 2004, 49, 9–16. [DOI] [PubMed] [Google Scholar]
- [73].Palasciano G; Portincasa P; Vinciguerra V; Velardi A; Tardi S; Baldassarre G; Albano O Gallstone prevalence and gallbladder volume in children and adolescents: an epidemiological ultrasonographic survey and relationship to body mass index. Am J Gastroenterol, 1989, 84, 1378–1382. [PubMed] [Google Scholar]
- [74].Faienza MF; Wang DQ; Fruhbeck G; Garruti G; Portincasa P The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med, 2016. [DOI] [PubMed] [Google Scholar]
- [75].Tsai AG; Wadden TA Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med, 2005, 142, 56–66. [DOI] [PubMed] [Google Scholar]
- [76].Johansson K; Sundstrom J; Marcus C; Hemmingsson E; Neovius M Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond), 2014, 38, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].La Vecchia C; Negri E; D’Avanzo B; Franceschi S; Boyle P Risk factors for gallstone disease requiring surgery. Int J Epidemiol, 1991, 20, 209–215. [DOI] [PubMed] [Google Scholar]
- [78].Williams C; Gowan R; Perey BJ A Double-Blind Placebo-controlled Trial of Ursodeoxycholic Acid in the Prevention of Gallstones during Weight Loss after Vertical Banded Gastroplasty. Obes Surg, 1993, 3, 257–259. [DOI] [PubMed] [Google Scholar]
- [79].Worobetz LJ; Inglis FG; Shaffer EA The effect of ursodeoxycholic acid therapy on gallstone formation in the morbidly obese during rapid weight loss. American Journal of Gastroenterology, 1993, 88, 1705–1710. [PubMed] [Google Scholar]
- [80].Sugerman HJ; Brewer WH; Shiffman ML; Brolin RE; Fobi MA; Linner JH; MacDonald KG; MacGregor AM; Martin LF; Oram-Smith JC; et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg, 1995, 169, 91–96; discussion 96–97. [DOI] [PubMed] [Google Scholar]
- [81].Wudel LJ Jr.; Wright JK; Debelak JP; Allos TM; Shyr Y; Chapman WC Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res, 2002, 102, 50–56. [DOI] [PubMed] [Google Scholar]
- [82].Miller K; Hell E; Lang B; Lengauer E Gallstone formation prophylaxis after gastric restrictive procedures for weight loss: a randomized double-blind placebocontrolled trial. Ann Surg, 2003, 238, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schauer PR; Burguera B; Ikramuddin S; Cottam D; Gourash W; Hamad G; Eid GM; Mattar S; Ramanathan R; Barinas-Mitchel E; Rao RH; Kuller L; Kelley D Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg, 2003, 238, 467–484; discussion 484–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Weinsier RL; Wilson LJ; Lee J Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med, 1995, 98, 115–117. [DOI] [PubMed] [Google Scholar]
- [85].Heshka S; Spitz A; Nunez C; Fittante AM; Heymsfield SB; Pi-Sunyer FX Obesity and risk of gallstone development on a 1200 kcal/d (5025 Kj/d) regular food diet. Int J Obes Relat Metab Disord, 1996, 20, 450–454. [PubMed] [Google Scholar]
- [86].Al-Jiffry BO; Shaffer EA; Saccone GT; Downey P; Kow L; Toouli J Changes in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obestity. Can J Gastroenterol, 2003, 17, 169–174. [DOI] [PubMed] [Google Scholar]
- [87].Mathur A; Megan M; Al-Azzawi HH; Lu D; Swartz-Basile DA; Nakeeb A; Pitt HA High dietary carbohydrates decrease gallbladder volume and enhance cholesterol crystal formation. Surgery, 2007, 141, 654–659. [DOI] [PubMed] [Google Scholar]
- [88].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut, 2005, 54, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Glycemic load, glycemic index, and carbohydrate intake in relation to risk of cholecystectomy in women. Gastroenterology, 2005, 129, 105–112. [DOI] [PubMed] [Google Scholar]
- [90].Attili AF; Scafato E; Marchioli R; Marfisi RM; Festi D; and Unit… Operativa B; Albano O; Palasciano G; Calo’ Gabrieli G; Portincasa P; Tardi S; Vendemiale G; Velardi AL; Baldassarre G; Belfiore A; Morelli N; Origlia C; Palmieri VO; Vinciguerra V Diet and gallstones in Italy: the cross-sectional MICOL results. Hepatology, 1998, 27, 1492–1498. [DOI] [PubMed] [Google Scholar]
- [91].Misciagna G; Centonze S; Leoci C; Guerra V; Cisternino AM; Ceo R; Trevisan M Diet, physical activity, and gallstones--a population-based, case- control study in southern Italy. Am.J.Clin.Nutr, 1999, 69, 120–126. [DOI] [PubMed] [Google Scholar]
- [92].Tandon RK; Saraya A; Paul S; Kapur B Dietary habits of gallstone patients in Northern India: a case control study. J Clin Gastroenterol, 1996, 22, 23–27. [DOI] [PubMed] [Google Scholar]
- [93].Diehl AK; Haffner SM; Knapp JA; Hazuda HP; Stern MP Dietary intake and the prevalence of gallbladder disease in Mexican Americans. Gastroenterology, 1989, 97, 1527–1533. [DOI] [PubMed] [Google Scholar]
- [94].Jorgensen T; Jorgensen LM Gallstones and diet in a Danish population. Scand J Gastroenterol, 1989, 24, 821–826. [DOI] [PubMed] [Google Scholar]
- [95].Moerman CJ; Smeets FWM; Kromhout D Dietary risk factors for clinically diagnosed gallstones in middle-aged men A 25-year follow-up study (The Zutphen study). Ann Epidemiol, 1994, 4, 248–254. [DOI] [PubMed] [Google Scholar]
- [96].Bennion LJ; Grundy SM Risk factors for the development of cholelithiasis in man (second of two parts). N.Engl.J Med, 1978, 299, 1221–1227. [DOI] [PubMed] [Google Scholar]
- [97].Scragg RK; Calvert GD; Oliver JR Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J (Clin Res Ed), 1984, 289, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Thornton JR; Emmett PM; Heaton KW Diet and gall stones: effects of refined and unrefined carbohydrate diets on bile cholesterol saturation and bile acid metabolism. Gut, 1983, 24, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Misciagna G; Guerra V; Di Leo A; Correale M; Trevisan M Insulin and gall stones: a population case control study in southern Italy. Gut, 2000, 47, 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chang Y; Sung E; Ryu S; Park YW; Jang YM; Park M Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci, 2008, 23, 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wong AC; Ko CW Carbohydrate intake as a risk factor for biliary sludge and stones during pregnancy. J Clin Gastroenterol, 2013, 47, 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Faeh D; Minehira K; Schwarz JM; Periasamy R; Park S; Tappy L Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes, 2005, 54, 1907–1913. [DOI] [PubMed] [Google Scholar]
- [103].Dirlewanger M; Schneiter P; Jequier E; Tappy L Effects of fructose on hepatic glucose metabolism in humans. Am J Physiol Endocrinol Metab, 2000, 279, E907–911. [DOI] [PubMed] [Google Scholar]
- [104].Biddinger SB; Haas JT; Yu BB; Bezy O; Jing E; Zhang W; Unterman TG; Carey MC; Kahn CR Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med, 2008, 14, 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tappy L; Le KA; Tran C; Paquot N Fructose and metabolic diseases: new findings, new questions. Nutrition, 2010, 26, 1044–1049. [DOI] [PubMed] [Google Scholar]
- [106].Tetri LH; Basaranoglu M; Brunt EM; Yerian LM; Neuschwander-Tetri BA Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol, 2008, 295, G987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Havel PJ Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev, 2005, 63, 133–157. [DOI] [PubMed] [Google Scholar]
- [108].Stanhope KL; Bremer AA; Medici V; Nakajima K; Ito Y; Nakano T; Chen G; Fong TH; Lee V; Menorca RI; Keim NL; Havel PJ Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab, 2011, 96, E1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Stanhope KL; Schwarz JM; Keim NL; Griffen SC; Bremer AA; Graham JL; Hatcher B; Cox CL; Dyachenko A; Zhang W Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest, 2009, 119, 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Vila L; Roglans N; Alegret M; Sanchez RM; Vazquez-Carrera M; Laguna JC Suppressor of cytokine signaling-3 (SOCS-3) and a deficit of serine/threonine (Ser/Thr) phosphoproteins involved in leptin transduction mediate the effect of fructose on rat liver lipid metabolism. Hepatology, 2008, 48, 1506–1516. [DOI] [PubMed] [Google Scholar]
- [111].Ko CW; Beresford SA; Schulte SJ; Matsumoto AM; Lee SP Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology, 2005, 41, 359–365. [DOI] [PubMed] [Google Scholar]
- [112].Lim JS; Mietus-Snyder M; Valente A; Schwarz JM; Lustig RH The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol, 2010, 7, 251–264. [DOI] [PubMed] [Google Scholar]
- [113].Bonfrate L; Krawczyk M; Lembo A; Grattagliano I; Lammert F; Portincasa P Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur J Gastroenterol Hepatol, 2015, 27, 785–796. [DOI] [PubMed] [Google Scholar]
- [114].Heaton KW; Emmett PM; Symes CL; Braddon FEM An explanation for gallstones in normal-weight woman: slow intestinal transit. Lancet, 1993, 341, 8–10. [DOI] [PubMed] [Google Scholar]
- [115].Marcus SN; Heaton KW Intestinal transit, deoxycholic acid and the cholesterol saturation of bile: three inter-related factors. Gut, 1986, 27, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Linos AD; Daras V; Linos DA; Kekis V; Tsoukas MM; Golematis V Dietary and Other Risk Factors in The Aetiology of Cholelithiasis: A Case Control Study. HPB Surgery, 1989, 1, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kummerow FA Dietary effects of trans fatty acids. J Environ Pathol Toxicol Oncol, 1986, 6, 123–149. [PubMed] [Google Scholar]
- [118].Oomen CM; Ocke MC; Feskens EJM; van Erp-Baart MAJ; Kok FJ; Kromhout D Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet, 2001, 357, 746–751. [DOI] [PubMed] [Google Scholar]
- [119].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Long-term intake of trans-fatty acids and risk of gallstone disease in men. Arch.Intern.Med, 2005, 165, 1011–1015. [DOI] [PubMed] [Google Scholar]
- [120].Ginter E Cholesterol: vitamin C controls its transformation to bile acids. Science, 1973, 179, 702–704. [DOI] [PubMed] [Google Scholar]
- [121].Ginter E Chenodeoxycholic acid, gallstones and vitamin C. N Engl J Med, 1976, 295, 1260–1261. [DOI] [PubMed] [Google Scholar]
- [122].Simon JA; Hudes ES Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III). Arch.Intern.Med, 2000, 160, 931–936. [DOI] [PubMed] [Google Scholar]
- [123].Bjorkhem I; Kallner A Hepatic 7alpha-hydroxylation of cholesterol in ascorbate-deficient and ascorbate-supplemented guinea pigs. J Lipid Res, 1976, 17, 360–365. [PubMed] [Google Scholar]
- [124].Bergman F; Curstedt T; Eriksson H; van der Linden W; Sjovall J Gallstone formation in guinea pigs under different dietary conditions. Effect of vitamin C on bile acid pattern. Med Biol, 1981, 59, 92–98. [PubMed] [Google Scholar]
- [125].Jenkins SA Biliary lipids, bile acids and gallstone formation in hypovitaminotic C guinea-pigs. Br J Nutr, 1978, 40, 317–322. [DOI] [PubMed] [Google Scholar]
- [126].Jenkins SA Hypovitaminosis C and cholelithiasis in guinea pigs. Biochem Biophys Res Commun, 1977, 77, 1030–1035. [DOI] [PubMed] [Google Scholar]
- [127].Ortega RM; Fern ndez-Azuela M; Encinas-Sotillos A; Andr‚s P; L¢pez-Sobaler AM Differences in diet and food habits between patients with gallstones and controls. Journal of the American College of Nutrition, 1997, 16, 88–95. [DOI] [PubMed] [Google Scholar]
- [128].Simon JA; Hudes ES Serum ascorbic acid and other correlates of gallbladder disease among US adults. Am J Public Health, 1998, 88, 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Simon JA; Grady D; Snabes MC; Fong J; Hunninghake DB Ascorbic acid supplement use and the prevalence of gallbladder disease. Heart & Estrogen-Progestin Replacement Study (HERS) Research Group. J Clin Epidemiol, 1998, 51, 257–265. [DOI] [PubMed] [Google Scholar]
- [130].Gustafsson U; Wang FH; Axelson M; Kallner A; Sahlin S; Einarsson K The effect of vitamin C in high doses on plasma and biliary lipid composition in patients with cholesterol gallstones: prolongation of the nucleation time. Eur J Clin Invest, 1997, 27, 387–391. [DOI] [PubMed] [Google Scholar]
- [131].Walcher T; Haenle MM; Kron M; Hay B; Mason RA; Walcher D; Steinbach G; Kern P; Piechotowski I; Adler G; Boehm BO; Koenig W; Kratzer W; group Es. Vitamin C supplement use may protect against gallstones: an observational study on a randomly selected population. BMC Gastroenterol, 2009, 9, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rigotti A; Marzolo MP; Ulloa N; Gonzalez O; Nervi F Effect of bean intake on biliary lipid secretion and on hepatic cholesterol metabolism in the rat. J Lipid Res, 1989, 30, 1041–1048. [PubMed] [Google Scholar]
- [133].Nervi F; Covarrubias C; Bravo P; Velasco N; Ulloa N; Cruz F; Fava M; Severin C; Del Pozo R; Antezana C; et al. Influence of legume intake on biliary lipids and cholesterol saturation in young Chilean men. Identification of a dietary risk factor for cholesterol gallstone formation in a highly prevalent area. Gastroenterology, 1989, 96, 825–830. [PubMed] [Google Scholar]
- [134].Duane WC Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res, 1997, 38, 1120–1128. [PubMed] [Google Scholar]
- [135].Mendez-Sanchez N; Zamora-Valdes D; Chavez-Tapia NC; Uribe M Role of diet in cholesterol gallstone formation. Clin Chim Acta, 2007, 376, 1–8. [DOI] [PubMed] [Google Scholar]
- [136].Leitzmann MF; Giovannucci EL; Rimm EB; Stampfer MJ; Spiegelman D; Wing AL; Willett WC The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med, 1998, 128, 417–425. [DOI] [PubMed] [Google Scholar]
- [137].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med, 2004, 141, 514–522. [DOI] [PubMed] [Google Scholar]
- [138].Tsai CJ; Leitzmann MF; Hu FB; Willett WC; Giovannucci EL Frequent nut consumption and decreased risk of cholecystectomy in women. Am J Clin Nutr, 2004, 80, 76–81. [DOI] [PubMed] [Google Scholar]
- [139].Leitzmann MF; Tsai CJ; Stampfer MJ; Rimm EB; Colditz GA; Willett WC; Giovannucci EL Alcohol consumption in relation to risk of cholecystectomy in women. Am J Clin Nutr, 2003, 78, 339–347. [DOI] [PubMed] [Google Scholar]
- [140].Froehlich F; Gonvers JJ; Fried M Role of nutrient fat and cholecystokinin in regulation of gallbladder emptying in man. Dig Dis Sci, 1995, 40, 529–533. [DOI] [PubMed] [Google Scholar]
- [141].Festi D; Colecchia A; Orsini M; Sangermano A; Sottili S; Simoni P; Mazzella G; Villanova N; Bazzoli F; Lapenna D; Petroni ML; Pavesi S; Neri M; Roda E Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int.J.Obes.Relat.Metab.Disord, 1998, 22, 592–600. [DOI] [PubMed] [Google Scholar]
- [142].Gebhard RL; Prigge WF; Ansel HJ; Schlasner L; Ketover SR; Sande D; Holtmeier K; Peterson FJ The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology, 1996, 24, 544–548. [DOI] [PubMed] [Google Scholar]
- [143].Marks JW; Bonorris GG; Schoenfield LJ Effect of ursodiol or ibuprofen on contraction of gallbladder and bile among obese patients during weight loss. Dig Dis Sci, 1996, 41, 242–249. [DOI] [PubMed] [Google Scholar]
- [144].Yago MD; Gonzalez V; Serrano P; Calpena R; Martinez MA; Martinez-Victoria E; Manas M Effect of the type of dietary fat on biliary lipid composition and bile lithogenicity in humans with cholesterol gallstone disease. Nutrition, 2005, 21, 339–347. [DOI] [PubMed] [Google Scholar]
- [145].Boland LL; Folsom AR; Rosamond WD; Atherosclerosis Risk in Communities Study, I. Hyperinsulinemia, dyslipidemia, and obesity as risk factors for hospitalized gallbladder disease. A prospective study. Ann Epidemiol, 2002, 12, 131–140. [DOI] [PubMed] [Google Scholar]
- [146].Festi D; Dormi A; Capodicasa S; Staniscia T; Attili AF; Loria P; Pazzi P; Mazzella G; Sama C; Roda E; Colecchia A Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol, 2008, 14, 5282–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Thornton J; Symes C; Heaton K Moderate alcohol intake reduces bile cholesterol saturation and raises HDL cholesterol. Lancet, 1983, 2, 819–822. [DOI] [PubMed] [Google Scholar]
- [148].Thornton JR; Heaton KW; Macfarlane DG A relation between high-density-lipoprotein cholesterol and bile cholesterol saturation. Br Med J (Clin Res Ed), 1981, 283, 1352–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Jonkers IJ; Smelt AH; Ledeboer M; Hollum ME; Biemond I; Kuipers F; Stellaard F; Boverhof R; Meinders AE; Lamers CH; Masclee AA Gall bladder dysmotility: a risk factor for gall stone formation in hypertriglyceridaemia and reversal on triglyceride lowering therapy by bezafibrate and fish oil. Gut, 2003, 52, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pastides H; Tzonou A; Trichopoulos D; Katsouyanni K; Trichopoulou A; Kefalogiannis N; Manousos O A Case-Control Study of the Relationship between Smoking, Diet, and Gallbladder-Disease. Arch Intern Med, 1990, 150, 1409–1412. [PubMed] [Google Scholar]
- [151].Misciagna G; Leoci C; Guerra V; Chiloiro M; Elba S; Petruzzi J; Mossa A; Noviello MR; Coviello A; Minutolo MC; Mangini V; Messa C; Cavallini A; De Michele G; Giorgio I Epidemiology of cholelithiasis in southern Italy. Part II: Risk factors. Eur J Gastroenterol Hepatol, 1996, 8, 585–593. [DOI] [PubMed] [Google Scholar]
- [152].Tseng M; DeVellis RF; Maurer KR; Khare M; Kohlmeier L; Everhart JE; Sandler RS Food intake patterns and gallbladder disease in Mexican Americans. Public Health Nutr, 2000, 3, 233–243. [DOI] [PubMed] [Google Scholar]
- [153].Scobey MW; Johnson FL; Parks JS; Rudel LL Dietary fish oil effects on biliary lipid secretion and cholesterol gallstone formation in the African green monkey. Hepatology, 1991, 14, 679–684. [DOI] [PubMed] [Google Scholar]
- [154].Berr F; Holl J; Jungst D; Fischer S; Richter WO; Seifferth B; Paumgartner G Dietary N-3 polyunsaturated fatty acids decrease biliary cholesterol saturation in gallstone disease. Hepatology, 1992, 16, 960–967. [DOI] [PubMed] [Google Scholar]
- [155].Mendez-Sanchez N; Gonzalez V; Aguayo P; Sanchez JM; Tanimoto MA; Elizondo J; Uribe M Fish oil (n-3) polyunsaturated fatty acids beneficially affect biliary cholesterol nucleation time in obese women losing weight. J Nutr, 2001, 131, 2300–2303. [DOI] [PubMed] [Google Scholar]
- [156].Lander EM; Wertheim BC; Koch SM; Chen Z; Hsu CH; Thomson CA Vegetable protein intake is associated with lower gallbladder disease risk: Findings from the Women’s Health Initiative prospective cohort. Prev Med, 2016, 88, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Tsai CJ; Leitzmann MF; Willett WC; Giovannucci EL Fruit and vegetable consumption and risk of cholecystectomy in women. Am J Med, 2006, 119, 760–767. [DOI] [PubMed] [Google Scholar]
- [158].Key TJ; Davey GK; Appleby PN Health benefits of a vegetarian diet. Proc Nutr Soc, 1999, 58, 271–275. [DOI] [PubMed] [Google Scholar]
- [159].Biss K; Ho KJ; Mikkelson B; Lewis L; Taylor CB Some unique biologic characteristics of the Masai of East Africa. N Engl J Med, 1971, 284, 694–699. [DOI] [PubMed] [Google Scholar]
- [160].Kratzer W; Kron M; Hay B; Pfeiffer MM; Kachele V [Prevalence of cholecystolithiasis in South Germany--an ultrasound study of 2,498 persons of a rural population]. Z Gastroenterol, 1999, 37, 1157–1162. [PubMed] [Google Scholar]
- [161].Kratzer W; Kachele V; Mason RA; Hill V; Hay B; Haug C; Adler G; Beckh K; Muche R Gallstone prevalence in Germany: the Ulm Gallbladder Stone Study. Dig Dis Sci, 1998, 43, 1285–1291. [DOI] [PubMed] [Google Scholar]
- [162].Pixley F; Mann J Dietary factors in the aetiology of gall stones: a case control study. Gut, 1988, 29, 1511–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Pixley F; Wilson D; McPherson K; Mann J Effect of vegetarianism on development of gall stones in women. Br Med J (Clin Res Ed), 1985, 291, 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Walcher T; Haenle MM; Mason RA; Koenig W; Imhof A; Kratzer W; Group ES The effect of alcohol, tobacco and caffeine consumption and vegetarian diet on gallstone prevalence. Eur J Gastroenterol Hepatol, 2010, 22, 1345–1351. [DOI] [PubMed] [Google Scholar]
- [165].Chen YC; Chiou C; Lin MN; Lin CL The prevalence and risk factors for gallstone disease in taiwanese vegetarians. PLoS One, 2014, 9, e115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Moran S; Uribe M; Prado ME; de la Mora G; Munoz RM; Perez MF; Milke P; Blancas JM; Dehesa M [Effects of fiber administration in the prevention of gallstones in obese patients on a reducing diet. A clinical trial]. Rev Gastroenterol Mex, 1997, 62, 266–272. [PubMed] [Google Scholar]
- [167].Festi D; Villanova N; Colecchia A Risk Factors for Gallstone Formation During Weight Loss. Clinical Gastroenterology and Hepatology, 2015, 13, 613. [DOI] [PubMed] [Google Scholar]
- [168].Leitzmann MF; Willett WC; Rimm EB; Stampfer MJ; Spiegelman D; Colditz GA; Giovannucci E A prospective study of coffee consumption and the risk of symptomatic gallstone disease in men. JAMA, 1999, 281, 2106–2112. [DOI] [PubMed] [Google Scholar]
- [169].Leitzmann MF; Stampfer MJ; Willett WC; Spiegelman D; Colditz GA; Giovannucci EL Coffee intake is associated with lower risk of symptomatic gallstone disease in women. Gastroenterology, 2002, 123, 1823–1830. [DOI] [PubMed] [Google Scholar]
- [170].Jorgensen T Gall stones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut, 1989, 30, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ruhl CE; Everhart JE Association of coffee consumption with gallbladder disease. Am J Epidemiol, 2000, 152, 1034–1038. [DOI] [PubMed] [Google Scholar]
- [172].Kratzer W; Kachele V; Mason RA; Muche R; Hay B; Wiesneth M; Hill V; Beckh K; Adler G Gallstone prevalence in relation to smoking, alcohol, coffee consumption, and nutrition. The Ulm Gallstone Study. Scand J Gastroenterol, 1997, 32, 953–958. [DOI] [PubMed] [Google Scholar]
- [173].Halvorsen B; Ranheim T; Nenseter MS; Huggett AC; Drevon CA Effect of a coffee lipid (cafestol) on cholesterol metabolism in human skin fibroblasts. J Lipid Res, 1998, 39, 901–912. [PubMed] [Google Scholar]
- [174].Lillemoe KD; Magnuson TH; High RC; Peoples GE; Pitt HA Caffeine prevents cholesterol gallstone formation. Surgery, 1989, 106, 400–406. [PubMed] [Google Scholar]
- [175].Douglas BR; Jansen JBMJ; Tham RTO; Lamers CBHW Coffee stimulation of cholecystokinin release and gallbladder contraction in humans. Am J Clin Nutr, 1990, 52, 553–556. [DOI] [PubMed] [Google Scholar]
- [176].Magnuson TH; Zarkin BA; Lillemoe KD; May CA; Bastidas JA; Pitt HA Caffeine inhibits gallbladder absorption. Curr Surg, 1989, 46, 477–479. [PubMed] [Google Scholar]
- [177].Brown SR; Cann PA; Read NW Effect of coffee on distal colon function. Gut, 1990, 31, 450–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Nordenvall C; Oskarsson V; Wolk A Inverse association between coffee consumption and risk of cholecystectomy in women but not in men. Clin Gastroenterol Hepatol, 2015, 13, 1096–1102 e1091. [DOI] [PubMed] [Google Scholar]
- [179].Hannuksela ML; Rantala M; Kesaniemi YA; Savolainen MJ Ethanol-induced redistribution of cholesteryl ester transfer protein (CETP) between lipoproteins. Arterioscler Thromb Vasc Biol, 1996, 16, 213–221. [DOI] [PubMed] [Google Scholar]
- [180].Gaziano JM; Buring JE; Breslow JL; Goldhaber SZ; Rosner B; VanDenburgh M; Willett W; Hennekens CH Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med, 1993, 329, 1829–1834. [DOI] [PubMed] [Google Scholar]
- [181].Haskell WL; Camargo C Jr.; Williams PT; Vranizan KM; Krauss RM; Lindgren FT; Wood PD The effect of cessation and resumption of moderate alcohol intake on serum high-density-lipoprotein subfractions. A controlled study. N Engl J Med, 1984, 310, 805–810. [DOI] [PubMed] [Google Scholar]
- [182].Saluja AK; Bhagat L Pathophysiology of alcohol-induced pancreatic injury. Pancreas, 2003, 27, 327–331. [DOI] [PubMed] [Google Scholar]
- [183].Probert C; Emmett P; Heaton K Some determinants of whole-gut transit time: a population-based study. QJM, 1995, 88, 311–315. [PubMed] [Google Scholar]
- [184].Basso L; McCollum PT; Darling MR; Tocchi A; Tanner WA A descriptive study of pregnant women with gallstones. Relation to dietary and social habits, education, physical activity, height, and weight. Eur J Epidemiol, 1992, 8, 629–633. [DOI] [PubMed] [Google Scholar]
- [185].La Vecchia C; Decarli A; Ferraroni M; Negri E Alcohol drinking and prevalence of self-reported gallstone disease in the 1983 Italian National Health Survey. Epidemiology, 1994, 5, 533–536. [PubMed] [Google Scholar]
- [186].Fornari F; Imberti D; Squillante MM; Squassante L; Civardi G; Buscarini E; Cavanna L; Caturelli E; Buscarini L Incidence of gallstones in a population of patients with cirrhosis. J Hepatol, 1994, 20, 797–801. [DOI] [PubMed] [Google Scholar]
- [187].Conte D; Fraquelli M; Fornari F; Lodi L; Bodini P; Buscarini L Close relation between cirrhosis and gallstones: cross-sectional and longitudinal survey. Arch Intern Med, 1999, 159, 49–52. [DOI] [PubMed] [Google Scholar]
- [188].Mensink RP; Katan MB Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb, 1992, 12, 911–919. [DOI] [PubMed] [Google Scholar]
- [189].Maclure KM; Hayes KC; Colditz GA; Stampfer MJ; Willett WC Dietary predictors of symptom-associated gallstones in middle-aged women. Am J Clin Nutr, 1990, 52, 916–922. [DOI] [PubMed] [Google Scholar]
- [190].Leitzmann MF; Giovannucci EL; Stampfer MJ; Spiegelman D; Colditz GA; Willett WC; Rimm EB Prospective study of alcohol consumption patterns in relation to symptomatic gallstone disease in men. Alcohol Clin Exp Res, 1999, 23, 835–841. [PubMed] [Google Scholar]
- [191].Alberti KG; Zimmet P; Shaw J The metabolic syndrome--a new worldwide definition. Lancet, 2005, 366, 1059–1062. [DOI] [PubMed] [Google Scholar]
- [192].Portincasa P; Di Ciaula A; Wang HH; Moschetta A; Wang DQ Medicinal treatments of cholesterol gallstones: old, current and new perspectives. Curr.Med.Chem, 2009, 16, 1531–1542. [DOI] [PubMed] [Google Scholar]
- [193].Portincasa P; Di Ciaula A; Wang HH; Palasciano G; van Erpecum KJ; Moschetta A; Wang DQ Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology, 2008, 47, 2112–2126. [DOI] [PubMed] [Google Scholar]


