Abstract
Background:
Physical inactivity puts the populations at risk of several health problems, while regular physical activity brings beneficial effects on cardiovascular disease, mortality and other health outcomes, including obesity, glycaemic control and insulin resistance. The hepatobiliary tract is greatly involved in several metabolic aspects which include digestion and absorption of nutrients in concert with intestinal motility, bile acid secretion and flow across the enterohepatic circulation and intestinal microbiota. Several metabolic abnormalities, including nonalcoholic fatty liver as well as cholesterol cholelithiasis, represent two conditions explained by changes of the aforementioned pathways.
Materials and Methods:
This review defines different training modalities and discusses the effects of physical activity in two metabolic disorders, that is nonalcoholic fatty liver disease (NAFLD) and cholelithiasis. Emphasis is given to pathogenic mechanisms involving intestinal bile acids, microbiota and inflammatory status.
Results:
A full definition of physical activity includes the knowledge of aerobic and endurance exercise, metabolic equivalent tasks, duration, frequency and intensity, beneficial and harmful effects. Physical activity influences the hepatobiliary-gut axis at different levels and brings benefits to fat distribution, liver fat and gallbladder disease while interacting with bile acids as signalling molecules, intestinal microbiota and inflammatory changes in the body.
Conclusions:
Several beneficial effects of physical activity are anticipated on metabolic disorders linking liver steatosis, gallstone disease, gut motility, enterohepatic circulation of signalling bile acids in relation to intestinal microbiota and inflammatory changes.
Keywords: bile acids, farnesoid X receptor, G protein–coupled bile acid receptor-1, gallstone disease, gut microbiota, nonalcoholic fatty liver disease
1 |. INTRODUCTION
The beneficial effects of physical activity are shown on several health outcomes, such as cardiovascular disease (CVD) and mortality for all causes.1 Sedentary behaviour, in contrast, affects negatively cardiovascular risk factors2 and represents 23% of the deaths related to chronic diseases.3 Being inactive for several years may lead to increased risks for type 2 diabetes (T2D), CVD and premature mortality.4 The health benefits of physical activity extend to the hepatobiliary and the gastrointestinal tract and involve nonalcoholic fatty liver disease (NAFLD) and cholesterol gallstone disease.
Nonalcoholic fatty liver disease is the most frequent chronic liver disease in developed countries5 with no medication approved as a standard care treatment.6 NAFLD refers to the excess accumulation of hepatic fat (triglycerides) in the liver due to metabolic, rather than alcoholic or viral reasons, and encompasses a wide range of chronic liver abnormalities ranging from simple steatosis (NAFL) to steatohepatitis (NASH), to significant liver fibrosis and cirrhosis, and even hepatocellular carcinoma.7 The estimated prevalence of NAFLD is 20%−30% in Western adults and is strongly associated with other metabolic disorders such as obesity and T2D.8 NAFLD is also linked to a lack of physical activity and improper dietary regimens,9 and patients with NAFLD are more sedentary than their healthy counterparts.10 There are evidences that physical inactivity, low aerobic fitness and overnutrition contribute to NAFLD either separately or in combination.11,12
Cholesterol gallstone disease also shares many metabolic risk factors with NAFLD, such as hyperlipidaemia, obesity and T2D.13 Physical activity has been associated with a decreased progression of gallstone disease at ultrasonography.14 Gallbladder function has also been linked to physical activity.15
The role of physical activity should also be considered with respect to intestinal bile acid (BA) metabolism and intestinal microbiota. BAs are soluble amphiphiles and major lipid components of bile; intestinal BAs contribute to the digestion and absorption of lipids and fat-soluble vitamins as either primary BAs (ie, synthetized in the liver) and secondary/tertiary BAs (ie, biotransformed by the resident colonic microbiota), while re-entering the enterohepatic circulation. Of note, BAs are also signalling molecules which modulate epithelial cell proliferation, gene expression and energy, glucose, lipid and lipoprotein metabolism via activation of intestinal farnesoid X receptor (FXR) and G protein–coupled bile acid receptor-1 (GPBAR-1) found in the intestine, liver Kupffer cells, striated muscle and brown adipose tissue.16
This review discusses current views on the mechanisms relating physical activity to NAFLD, gallbladder disease, bile acids, gut microbiota and metabolic inflammatory changes (Figure 1).
FIGURE 1.
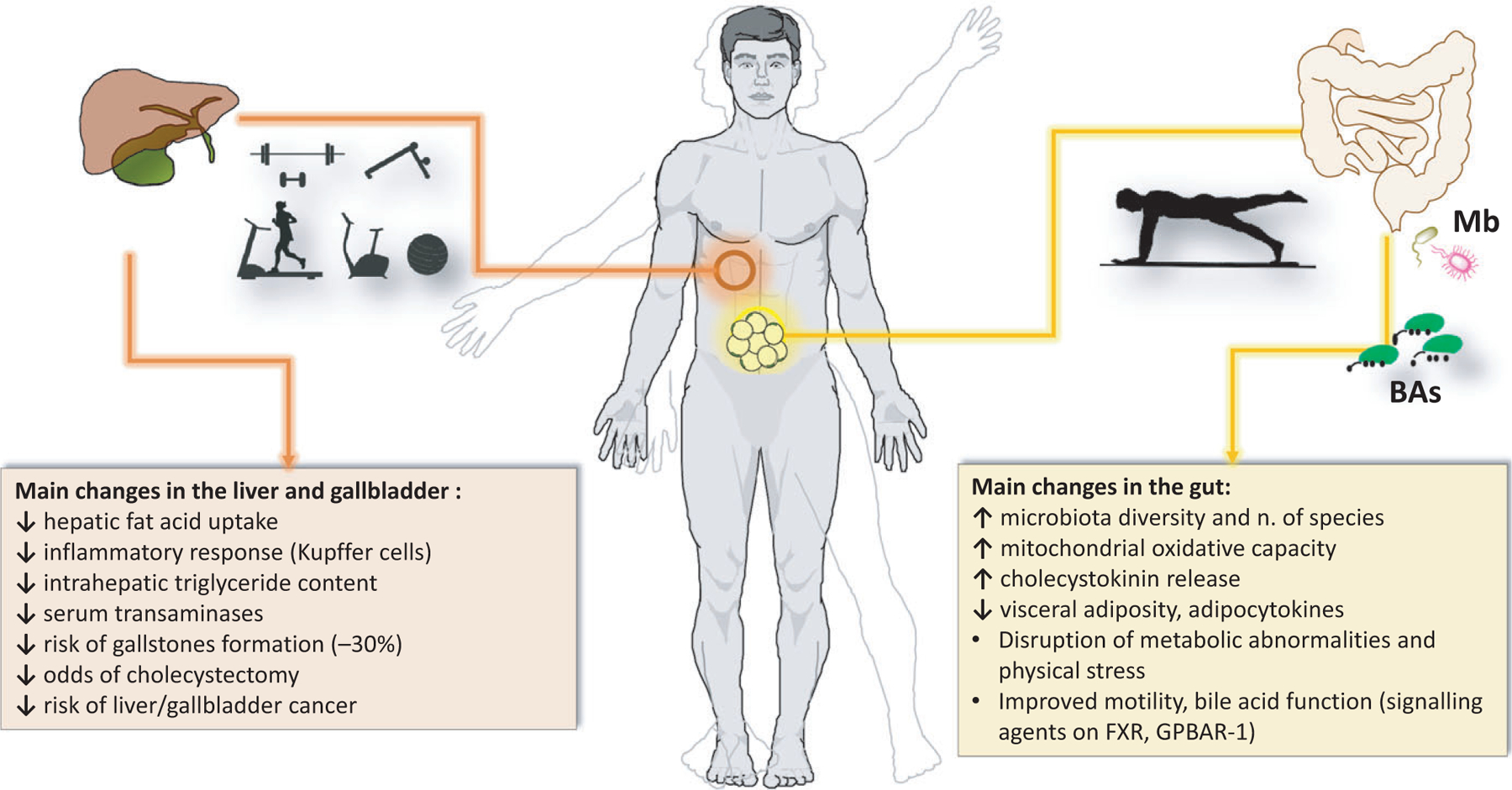
Summary of changes observed in the liver, gallbladder and gut after physical activity. ↑, increased; ↓, decreased; BAs, bile acids; FXR, farnesoid X receptor; GPBAR-1, G protein–coupled bile acid receptor-1; Mb, microbiota
2 |. CLASSIFICATION OF PHYSICAL ACTIVITY
Physical activity is defined as any body movement which uses energy to activate the skeletal muscles, whereas “exercise” defines a subgroup of planned physical activity over time with the main aim of becoming healthier.17 Appropriate understanding of these two terms is needed to avoid misclassification of terminology.18 Sedentary behaviour, in contrast, is defined as sitting, lying down or spending very low energy, reporting a value of 1–1.5 units of metabolic equivalent tasks (MET).19 A MET refers to the resting metabolic rate or its equivalent in oxygen (O2) consumed while resting (3.5 mL/kg/min, which at the same time would equal to burning 1.2 kcal/min for a 70 kg individual in sitting condition).20 A compendium of several physical activities (structured or not) has already been developed in terms of METs equivalent by activity, for example, bathing (1.5), driving a car (2.5) or static cycling (7.0).21 However, the resting metabolic rate of men differs from that of women, as well as within ages, given the changes in body weight and height.22 One classification of physical activity is based on duration, frequency and intensity. The duration refers to the amount of exercise which is performed over time. The normal measurement for physical activity duration is in minutes. Additionally, the term “volume” refers to any indicator of exercising, for example, load, energy expenditure or calories burned,23 or to the product of all duration, frequency, intensity and length of a training programme.24 Frequency is associated with the total number of physical activity sessions during a certain week23 or the number of bouts (times) of physical activity per day25; that is, how often we are exercising for a certain period of time (whereas sessions or bouts per week). Intensity refers to the level of effort perceived in order to perform an activity.24 Intensity can be measured either absolutely (MET) or relatively (maximum heart rate and aerobic capacity).26 Another classification of physical activity is based on aerobic exercise and resistance exercise. Aerobic exercise refers to the use of large amounts of energy, which ultimately leads to a significant increase in heart rate and improvements seen in the cardiovascular system (increase in aerobic capacity, translated into endurance performance). Resistance exercise responds to the adaptations seen in the skeletal muscle after the force generated during muscular contractions, resulting in augmented force production, power and overall strength.27
2.1 |. Beneficial effects of physical activity
The American Heart Association’s guidelines for physical activity in 2013 recommended at least 150 minutes of moderate physical activity, 75 minutes of vigorous physical activity or a combination of both per week.28 Similarly, the American College of Sports Medicine and the American Heart Association prescribes 5 days of moderate aerobic exercise lasting 30 minutes or 3 days of vigorous aerobic exercise for 20 minutes on a weekly basis.29 It is also suggested to maintain or even increase the muscular strength by engaging on endurance and resistance exercise at least twice a week.
Physical activity by different interventions brings a number of health benefits (Table 1). Long-term moderate aerobic exercise, as measured by number of steps, accelerates fat oxidation in the body, improving the lipid profile.30 In a large cohort, Rognmo et al31 found that a unique session of short-duration aerobic exercise reduced the risk of CVD, especially at a higher intensity. Aerobic exercise decreases arterial stiffness by increasing blood flow32 and decreases serum triglyceride levels in obese adults.33 Combining aerobic and resistance exercise increased aerobic capacity and fat mobilization.34 Short-duration anaerobic exercise was effective in reducing blood pressure and heart rate, while increasing aerobic capacity; the effect was also seen in untrained individuals.35
TABLE 1.
Overall benefits from physical activity
| Author | Study | Country | Sample size | Intervention(s) | Benefits |
|---|---|---|---|---|---|
| Sugiura et al30 | Clinical | Japan | 48F | Increasing number of steps | Reduced serum lipid levels |
| Rognmo et al31 | Clinical | Norway | 4846 (3392M, 1454F) | Short-duration aerobic exercise | Reduced risk of CVD |
| Collier et al32 | Clinical | USA | 30 (20M, 10F) | Four weeks of aerobic or resistance training | Reduced arterial stiffness |
| Johnson et al33 | Clinical | Australia | 23 (15M, 8F) | Four weeks of aerobic cycling exercise | Reduced serum triglyceride levels |
| Ho et al34 | Clinical | Australia | 97 (16M, 81F) | Combined aerobic and resistance exercise | Increased aerobic capacity and fat mobilization |
| Zhang et al35 | Clinical | China | 518F | Short-duration anaerobic exercise | Decreased systemic blood pressure and heart rateIncreased aerobic capacity |
| Braith & Stewart36 | Review | USA | N/R | Resistance exercise | Improved insulin sensitivity |
| Meka et al37 | Review | USA | N/R | Resistance exercise | Reduced muscle weakness |
| Patel et al38 | Review | USA | N/R | Aerobic/anaerobic exercise | Improved health markers and cardiovascular function |
| Williams et al39 | Scientific Statement (AHA) | USA | N/R | Resistance exercise | Enhanced muscular strength and endurance and quality of life |
AHA, American Heart Association; CVD, cardiovascular disease; F, females; M, males; N/R, not reported.
Few reviews also support the beneficial effect of physical activity. Resistance exercise improves insulin sensitivity36 and reduces muscle weakness.37 Both aerobic and anaerobic exercise impacts CVD risks by improving the overall health markers and cardiovascular function.38 Furthermore, in the scientific statement of the American Heart Association, resistance exercise was beneficial on muscular strength, endurance and quality of life, regardless of CVD diagnosis.39
2.2 |. Harmful effects of physical activity
Very intense or inappropriate physical activity could lead to harmful consequences on health such as musculoskeletal injury, arrhythmia, sudden cardiac death, myocardial infarction, rhabdomyolysis or bronchoconstriction.40 A form of intensive physical activity is the so-called overtraining syndrome, that is, a maladaptation to excessive exercise without adequate rest.41 Overtraining syndrome may last months, including severe symptoms at the endocrine, as well as immunological, neurological and psychological levels.42,43 Overtraining syndrome-induced impairment of the adrenal glands, for example, leads to adrenal insufficiency and damages in the hypothalamus44 and may increase cortisol levels, resulting in muscle damage.45 Heavy exertion produces perturbations at the immunological level, as seen in the host-pathogen defence and incremented levels of stress hormones, anti-inflammatory cytokines and reactive oxygen species.42 Infections related to the upper respiratory tract have also been associated with overtraining syndrome, with decreased levels of secretory immunoglobulin A, responsible for the mucosal immune defence to external pathogens.46 Similarly and as measured in T and B cell response, immune depression might follow overtraining syndrome.45,47 The main consequence of overtraining syndrome appears to be a decrease in response of neurotransmitters, due to the fatigue deriving from overtraining.43 Decreased reserves of glycogen within the muscle appear to be linked to both fatigue and the immune depression response.41
In athletes, inflammatory cytokines progressively influence the neurotransmitter function as overtraining syndrome develops under strenuous performance, as also shown in neurodegenerative diseases.48 At the level of the central nervous system, the link between nutrition and overtraining syndrome is a matter of research.49 Overtraining and chronic fatigue have shown to induce mood deterioration in elite athletes, causing a decrease in motivation towards training.45 Mood changes could also depend on cardiovascular, endocrine and hormonal factors.50 Overtraining syndrome-related psychological stressors, however, could be also dependent on the social context of the individual.42
3 |. PHYSICAL ACTIVITY AND NONALCOHOLIC FATTY LIVER DISEASE (NAFLD)
The term “lifestyle” consists of structured physical activity and restricted caloric intake.12 Lifestyle factors influence the onset and the natural history of chronic liver diseases51 and unhealthy lifestyles (ie, sedentary behaviour, low physical activity and poor diet) contribute to the development and progression of NAFLD.52 Conversely, increased physical activity was related to decreased risk of liver cancer, independently of body mass index.53 A sedentary behaviour might act as an independent risk factor for NAFLD54 and lower levels of physical activity are found in patients with NAFLD.55,56 Also, reduced physical activity such as longer habitual day napping was independently associated with NAFLD.57
Maintenance of ideal weight or weight loss in overweight/obese patients via lifestyle interventions has become an established strategy also to prevent or treat NAFLD,52,58,59 although the goal might be difficult to achieve in the ambulatory care setting.60 Reducing weight by ≥10% can contribute to the nonalcoholic steatohepatitis (NASH) resolution and fibrosis improvement, while a modest weight loss >5%−7% also produces important benefits on the components of the NAFLD activity score.9,52,61 Thus, gradual weight loss due to caloric restriction, even without increased physical activity, will lead to an improvement in serum liver enzymes, liver fat, degree of hepatic inflammation and fibrosis.52,62 However, when comparing interventions of diet alone vs physical activity alone vs diet plus physical activity, the latter induces the greatest changes in obesity,63,64 insulin resistance65 and NAFLD.62,66,67 The genetic background of patients (ie, PNLPA3 gene polymorphism) is part of the lifestyle response in patients with NAFLD.68,69
Previous individual trials suggest that moderate aerobic exercises is associated with decreased serum levels of liver enzymes.70,71 In a retrospective analysis on 813 adults with biopsy-proven NAFLD, vigorous physical exercise was more beneficial to NAFLD than physical activity in general, with reduced odds of advanced liver fibrosis, especially when exceeding minimum exercise recommendations.72 Resistance exercise reduced insulin resistance and liver fat content,73 with similar effects found in obese patients with NAFLD after 16 weeks of moderate aerobic exercise74,75 and increased cardiorespiratory fitness,76 without changing body composition. Patients with NAFLD performing aerobic or resistance exercise had a similar significant decrease in abdominal, liver and visceral fat,77 serum liver enzymes and diastolic function.71 Indeed, most aerobic exercise interventions reduce liver fat by a small amount, irrespective of weight loss or exercise intensity and volume.78 In a recent randomized clinical trial (RCT) lasting 12 months, both vigorous and moderate aerobic exercise reduced intrahepatic triglyceride content in patients with NAFLD, and vigorous exercise was more effective on weight loss and blood pressure.79
The beneficial effects of physical activity go beyond the weight loss and involve additional pathways. There are concerns, however, regarding the low sample sizes, the insufficient power of several exercise interventions, the variable short duration and modalities of exercise interventions. Moreover, using questionnaires to recall frequency, duration and intensity of physical activity may be inaccurate.61 This context makes the interpretation of hepatic benefits clinically difficult.
Few exhaustive reviews and meta-analyses have more precisely addressed the benefits of physical activity in NAFLD61,80–84 (Table 2). Magkos80 reviewed the extreme variability of results linking physical exercise and liver fat accumulation (ie, intrahepatic triglycerides). In general, habitual physical activity and cardiorespiratory fitness are inversely associated with liver fat. Although independent on age and body size, intra-abdominal obesity appears to govern the exercise-induced reduction in liver fat and visceral fat, and the effect is specific in the male sex. Both hypocaloric diet and exercise appear interchangeable and significantly reduce liver fat (≈35%−45%) acting through an even moderate (≈10%) weight loss (ie, institution of a negative energy balance). Exercise training with decreased visceral fat but without weight loss had either no effect85,86 or decreased liver fat in obese adults,33 obese adolescents87 and elderly subjects.88 Resistance (anaerobic) exercise training, at variance with evidences in few animal studies, could not affect intrahepatic fat content, at least in obese adolescents.89
TABLE 2.
Benefits of physical activity in nonalcoholic fatty liver disease (NAFLD) according to recent reviews, systematic reviews and meta-analyses
| Author | Study | Country | Sample size | Intervention | Benefits |
|---|---|---|---|---|---|
| Magkos80 | Review | USA | - |
|
Variability in results. Increased cardiorespiratory fitness or reduced intra-abdominal obesity after training not necessarily accompanied by intrahepatic fat depletion33,85–89 |
| Keating et al81 | Systematic review and meta-analysis on 12 studies33,73,85,90,97 | Australia | 439 (241M, 177F, 21 N/R) Mean age 36–68 y |
|
|
| Musso et al83 | Systematic review and meta-analysis on 78 RCTs | Italy | 38 RCTs NASH 40 RCTs NAFLD | ||
| Orci et al84 | Meta-analysis and Metaregression on 28 RCTs33,55,74,78,85,90,91,93,94–111 | Switzerland | Patients with NAFLD or patients with obesity, T2D or metabolic syndrome (with established or likely NAFLD) |
|
|
| Katsagoni et al82 | Meta-analysis on 20 RCTs71,73,74,76,77,79,103,110–122 | Greece | 1073 exactly characterized NAFLD patients |
|
|
| Romero-Gomez et al61 | Review | Spain | - | Discussion on role of physical activity in NAFLD
|
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; F, females; HIIT, high-intensity interval training; M, males; MIT, medium-intensity interval training; MRS, magnetic resonance spectroscopy; NAFLD, nonalcoholic fatty liver; NASH, nonalcoholic steatohepatitis; N/R not reported; RCT, randomized clinical trial; T2D, type 2 diabetes; VLDL, very low-density lipoprotein.
A systematic review and meta-analysis by Keating et al81 also pointed to the scarcity of data and poor meaningful effect in the majority of current literature. However, by focusing on 12 (out of 16 822) relevant studies (n = 439 adult participants, 11 randomized studies33,73,85,90–97), the authors confirm a benefit of exercise therapy (with minimal or no weight loss) on liver fat but not on serum alanine aminotransferase. The gain is achieved with exercise levels below current exercise recommendations for obesity management and, together with studies in adolescents87 and elderly subjects,88 suggest a lifespan effect of physical exercise per se.
Musso et al83 in his systematic review and meta-analysis on 78 RCTs on NAFLD (n = 38 RCTs) and NASH patients (n = 40 RCTs) listed five studies assessing the effect of moderate-intensity aerobic exercise alone in NAFLD.33,55,73,97,98 Physical exercise improved the liver steatosis assessed by magnetic resonance spectroscopy (MRS) as well as serum level of liver aspartate aminotransferase (ALT), while in the only available study liver histology was unchanged.55
Orci et al84 studied 28 RCTs33,55,74,78,85,90,91,93,94,99–111 focusing on patients with NAFLD or patients with metabolic abnormalities (with established or likely NAFLD). Interventions consisted of exercise alone vs no exercise and exercise + diet vs diet alone, looking at the effect on intrahepatic lipid content, and serum transaminases ALT and aspartate aminotransferase (AST). There was a significant exercise-induced reduction in intrahepatic lipid content assessed by MRS, computed tomography, ultrasonography, liver histology, AST and ALT, independently from dietary change. Also, individuals with increasing body mass index are more likely to benefit from the intervention, while the intensity of the intervention is less important.
Katsagoni et al82 examined a total of 20 RCTs71,73,74,76,77,79,103,110–122 and confirmed that aerobic and resistance exercise alone or with dietary intervention improve serum levels of liver enzymes and liver fat or histology. Again, the beneficial effects of exercise liver fat are seen even in the absence of weight loss. Continuous moderate-to-high volume moderate-intensity training is superior to continuous low-to-moderate volume moderate-intensity training or high-intensity interval training exercise protocols.
Romero-Gomez et al61 pointed to the role of sedentary life, unstructured physical activity and structured physical activity (exercise) in NAFLD. Exercise, even without weight loss, is associated with a 20%−30% relative reduction in intrahepatic lipid, and the benefits are reached using different modalities of exercise, that is moderate- and high-intensity aerobic and resistance exercise.81,123,124 No additional benefit on liver fat occurs by more vigorous aerobic exercise.78,79
While many studies have a short duration, that is 2–4 months of physical exercise,71,73,78 recent data show that the favourable effects of continued exercise can last up to 1 year79,125 but that benefits disappear upon exercise discontinuation.126
Despite lifestyle interventions are fully recommended in patients with NAFLD, cognitive and behavioural functioning might influence the ultimate outcome.127 A recent exhaustive systematic review128 suggests that NAFLD encompasses a cognitive-behavioural disease and confirms that lifestyle changes (ie, diet and exercise) are the most effective approaches. However, despite the intrinsic importance of exercise is understood, the confidence to exercise is poor because of fear of falling in older patients with NAFLD, an aspect which makes more difficult to engage in constant structured physical activity.129 Also, ratings of perceived exertion (a measure to monitor and prescribe exercise intensity) in patients with NAFLD were related to a metabolic factor (fasting glucose level) and level of physical activity in adulthood.130 Patients with NAFLD appear poorly motivated towards dietary and physical activity changes.131 Patients with NAFLD also have disrupted levels of physical activity as daily activities of physical functioning.132
4 |. PHYSICAL ACTIVITY AND GALLBLADDER DISEASE
Cholesterol gallstone disease is one of the most prevalent and costly digestive diseases in the USA. About 20 million Americans (10%−15% of adults in Europe and the USA) suffer from gallstones,133,134 and the prevalence of gallstones is increasing because of the obesity epidemic.135 Gallstones in Western countries are composed mainly of cholesterol in 75%−80% of cases and often associated with systemic metabolic abnormalities.136 The pathogenesis of cholesterol gallstones is determined by interaction of five primary defects which include (i) lithogenic (LITH) genes and genetic factors, (ii) hepatic hypersecretion of cholesterol contributing to supersaturated gallbladder bile, (iii) rapid phase transitions of cholesterol in bile leading to precipitation of solid cholesterol crystals, (iv) impaired gallbladder motility with hypersecretion and accumulation of mucin gel in the gallbladder lumen and immune-mediated gallbladder inflammation, and (v) intestinal factors involving absorption of cholesterol, slow intestinal motility and altered gut microbiota.137
Physical activity acts as a protective agent against gallstones formation, as recently underscored by the European Association for the Study of the Liver (EASL) guidelines.135 Physical activity is inversely related to gallstone occurrence, as found in an American Indian population,4 but rapid weight loss results in increased gallstone formation in about 30% of the individuals.138 Both physical inactivity and overnutrition lead to increased body mass index and hepatic cholesterol synthesis rate, acting as precursors of gallstones formation.138 The chances of developing gallstones are associated with the degree of obesity at baseline as well as weight loss.139 Central obesity is linked to gastrointestinal morbidity and mortality, including gallstone disease, similar to the effect induced by tobacco smoking or aspirin intake.140
A list of benefits of physical activity in gallstone disease is anticipated (Table 3). Vigorous aerobic exercise (measured by history of running distance) and cardiorespiratory fitness (an independent predictor of liver fat141) are both inversely related to gallstone disease risk.142 Indeed, the highest level of physical activity achieved after 5 years may be associated with a 70% decrease of suffering from symptomatic gallstones.143 Recreational physical activity is inversely related to risk of asymptomatic gallstones in adult women.144 Also in women, vigorous exercise is associated with a decreased rate of cholecystectomy,145 an additional potential risk factor for NAFLD.146 Of note, an increment of cardiorespiratory fitness by one MET reduces the odds of suffering from gallstone disease by 8% in men and 13% in women.147 Performance of vigorous physical activity has been inversely associated with risk of gallstone disease in comparison with inactivity,148 and regular aerobic exercise may potentially decrease the risk of gallstones formation and gallbladder cancer.149 The intensity response seems however to be stronger for vigorous vs nonvigorous physical activity in gallbladder disease.150
TABLE 3.
Benefits of physical activity in gallbladder disease
| Author | Study | Country | Sample Size | Intervention | Benefits |
|---|---|---|---|---|---|
| Williams142 | Clinical | USA | 278 (166M; 112F), mean age 39–50 y | Vigorous aerobic exercise reported by baseline cardiorespiratory fitness (see Table 4) | Decreased gallbladder risk in relation to higher cardiorespiratory fitness and speed:
|
| Banim et al143 | Clinical | UK | 24 201 (11 133M; 13 068F), mean age 40–74 y |
|
|
| Henao-Moran et al144 | Clinical | Mexico | 4953F, age 17–94 y |
|
|
| Talseth et al145 | Clinical | Norway | 63 249 (29 982M; 33 267F), age ≥20 y | Hard physical activity (h/wk) | ≥1 h/wk of physical activity reduced risk of cholecystectomy |
| Li et al147 | Clinical | USA | 54 734 (41 528M; 13 206F), age 20–90 y | Cardiorespiratory fitness (see Table 4) |
|
| Figuereido et al148 | Clinical | USA | 144 409 (64 901M; 79 508F), age 45–75 y | Vigorous physical activity (h/d) |
|
| Shephard149 | Review | Canada | N/R | General physical activity (questionnaires) | Likely decrease in gallstones formation and gallbladder cancer |
| Aune et al150 | Review | UK | 218 204 | General physical activity | Higher levels of physical activity inversely relates to gallbladder disease |
aOR, adjusted odd ratio; F, females; GBD, gallbladder disease; h/d, hours per day; M, males; MET, metabolic equivalent task; N/R, not reported.
The main mechanism behind physical activity effect in gallstone disease might be linked to the release of the upper gastrointestinal hormone cholecystokinin (CCK),151 with a prokinetic effect.152 Suppression of hunger has also been identified with increased levels of CCK after acute exercise (ranging from 30 to 120 minutes).153 Similarly, smooth muscle activation enhances gallbladder emptying and refilling processes, which influence the pathogenesis of cholesterol gallstones.154
Conversely, excessive physical activity may have a gallbladder-related injury effect, as seen by liver transaminases and high-density lipoprotein cholesterol levels, measured 2 and 9 days after a 24 hours ultra-marathon performance in 11 athletes.155 In mice, however, plasma cholesterol and triglyceride concentrations did not change after 12 weeks of endurance exercise. Changes in gene expression could explain the inhibition of gallstone formation by hepatic cholesterol clearance.156 More objective measurements (accelerometers and physical activity questionnaires) are still required to come with a clear association between gallstone disease and physical activity levels.14
5 |. PHYSICAL ACTIVITY, MICROBIOTA, BILE ACIDS AND INFLAMMATION
The effect of physical activity on the hepatobiliary tract should also consider additional factors, namely intestinal microbiota, the enterohepatic circulation of BAs acting as signalling molecules on metabolic function and with anti-inflammatory properties.
5.1 |. Microbiota
A relationship occurs between intestinal digestion/absorption and physical activity-induced catabolic state.157 Physical activity alters gut bacteria composition and diversity.158 The relationship is mostly beneficial, but also relies on dietary patterns.159 The effects of physical activity on microbiota are very wide, from increasing commensal bacteria to enriching microflora diversity and to augmenting the number of microbial species.160 The influence of physical activity on microbiota selection and richness/diversity might also be mediated by changes in expression of inter-leukin-6 and tumour necrosis factor (TNF)-α cytokines, as shown in athletes compared with control group.161 A modality of vigorous physical activity, high-intensity interval training, improved the microbiota of obese mice, countering the changes following a high-fat diet.162 High degree of physical conditioning, as seen in elite athletes, results in a distinctive microbiota and more metabolic and inflammatory profiles.163 Especially in endurance athletes, mitochondrial oxidative capacity is increased as mediated by mitochondrial regulation of inflammasomes; however, the effects of overtraining on the intestinal tract results in a major production of stressors, which facilitates the entrance of pathogens.164 In line with the previous study, the effect of long-term aerobic exercise (over 90 minutes or 60% of the individual’s aerobic capacity) may disrupt the metabolic homeostasis and lead to physical stress, being more significant as exercise intensity increases. This stressful event is comparable to food deprivation or psychological stress in athletes during the precompetition periods.165 They all lead to the triggering of the hypothalamic-pituitary-adrenal axis, and together with stress, it may change the gut microbiota composition.166 Contrarily, moderate-to-vigorous physical activity can improve gastrointestinal symptoms (gas clearance/transit and abdominal bloating/distension), provide a feeling of relaxation as well as decrease the severity of symptoms in patients with irritable bowel syndrome.167 Furthermore, in rugby athletes, gut microbiota was more diverse when compared to sedentary subjects.159 This major variation on microbiota composition is positively associated with the metabolic status.168 It has also been hypothesized that a higher training regime plus a higher intake of protein (as in many endurance athletes) may negatively influence the microbiota composition.164 In a mouse model, an increase in the ratio of Bacteroidetes (Gram−)/Firmicutes (Gram+) phyla is proportional to an increase in running distance.169 In mice, there is a significant relationship between myocardial infarction and gut microbiota composition after physical exercise.170 Other studies, however, found only moderate changes to gut microbiota composition and no significant changes reported in the inflammatory profile, after a moderate aerobic exercise intervention in mice.171 Observations, therefore, need to be translated to a more meaningful clinical setting.
5.2 |. Bile acids
Primary BAs (namely cholic acid and chenodeoxycholic acid) are synthetized from cholesterol in hepatocytes, conjugated to taurine and glycine, secreted in bile and biotransformed into secondary BAs (namely deoxycholic acid and lithocholic acid), and tertiary ursodeoxycholic acid, upon contact with the resident colonic microbiota. Approximately 50% of secondary BAs are reabsorbed in the terminal ileum and colon and return to the liver via the portal vein across the enterohepatic circulation.16 BAs contribute to digestion and absorption of fat, cholesterol and fat-soluble vitamins, but also act as signalling molecules and display antimicrobial and anti-inflammatory functions.16
Physical activity might ameliorate BAs circulation and their pleiotropic functions because of improved gastrointestinal motility and peristalsis, but studies show conflicting results.172 Previous animals studies found that moderate physical activity increased bile acid excretion.173–175 BAs activate intestinal farnesoid X receptor (FXR) which, in turn, increases the expression of the human enterokine fibroblast growth factor 19 (FGF19) leading to activation of the hepatic FGF4 receptor/β-clotho and subsequent small heterodimer-mediated inhibition of BA synthesis.16 Thus, one might speculate that increased intestinal motility would also increase BA flow and therefore FXR expression, with ultimate inhibition of BA synthesis. However, a recent study in mice found that physical activity indeed stimulated BA secretion and faecal output; a mechanism likely mediated by increased reverse cholesterol transport and is independent on upregulation of genes involved in BA synthesis and FXR-FGF15 (FGF19 in humans) feedback.176 Metabolic post-transcriptional mechanisms (ie, increased fatty acid absorption) might be involved, instead. The situation might differ in humans: both faecal and serum concentrations of BAs were significantly decreased in runners,177,178 with less mutagenic secondary BAs.178 FXR pathways, however, were not investigated in these studies.
Bile acids also interact with the GPBAR-1 receptor in the intestine and in the liver Kupffer cells. Intestinal GPBAR-1 governs metabolically relevant hormonal pathways (ie, release of peptide YY and glucagon-like peptides GLP-1 and GLP-2) with effects on appetite, glucose and insulin metabolism via the tissue GPBAR-1 receptors located in the cells of brown adipose tissue and skeletal muscle.16,179 Whether physical exercise will induce additional BA-mediated metabolic or anti-inflammatory effects (see below) is unclear so far, due to the poor translational value of animal studies (see above).
5.3 |. Inflammation
Physical activity might also induce inflammatory effects in the body, and BAs as well as additional mechanisms might play a role. In the liver Kupffer cells, GPBAR-1 activation induced by circulating BAs might promote an anti-inflammatory effect.180 Physical activity might partly mediate this effect.181,182 Moreover, BA-induced activation of GPBAR-1, also expressed in other immune cells such as macrophages, monocytes and dendritic cells183 might modulate the inflammatory changes by inhibiting the NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome, with protective mechanism against lipopolysaccharide-induced inflammation and atherosclerosis.184 Change in gut microbiota during physical exercise might also induce additional anti-inflammatory effects. Gut microbiota interacts with BAs in the enterohepatic circulation by converting primary to secondary BAs which, in turn, regulate microbiota by exerting antimicrobial effects.185 Ursodeoxycholic acid and lithocholic acid have been recently shown to have anti-inflammatory properties by decreasing the release of proinflammatory cytokines while increasing macrophage release of anti-inflammatory cytokines.185
Regular physical activity also leads to increased vagal tone186,187 and decreased expression of inflammatory markers,187 although recent studies provide conflicting results or suggest reduction in circulatory apoptosis marker112,188, an effect correlating with high cardiorespiratory fitness.189 This scenario might also involve a neurohormonal mechanism with vagal-mediated gallbladder emptying before exercise.149,190
6 |. FUTURE DIRECTIONS
The benefits of physical activity extend beyond the typical effect on risk factors for cardiovascular disease. The knowledge about the interaction between physical activity and the hepatobiliary-gut axis is gaining importance but protocols employed so far show nonstandardized approaches and wide variability (Table 4). Overall, several benefits of physical activity are anticipated in the hepatobiliary tract in conditions such as liver steatosis, gallbladder disease and also gut motility, enterohepatic recirculation of signalling BAs, intestinal microbiota and metabolic inflammatory changes. More studies are urgently required in this field to dissect the role of type, duration, intensity of exercise alone and in relation to gender, diet, weight loss and starting health status. Clinicians should not underestimate the relationship between the gut and the liver when addressing physical activity recommendations to the patients: “Just do it, keep on doing it, don’t stop it!”.58,126
TABLE 4.
Summary of most popular physical activity protocols used in patients with hepatobiliary disease
| Duration | Frequency | Intensity | Context References | ||
|---|---|---|---|---|---|
| AEROBIC | |||||
| Brisk walking, jogging or rhythm aerobic | 45 min/session (at least) | 5x/wk (at least), minimum follow- up of 3 mo | Moderate (achieve 60%−70% of their MHR) | NAFLD70 | |
Baseline cardiorespiratory fitness
|
N/R | History of participants’ previous 5 y | Vigorous (anaerobic event) | Gallbladder disease142 |
|
| Brisk walking on a treadmill | 30–60 min/session | 5x/wk for 16 wk | 45%−55% of their VO2 peak | NAFLD74 | |
Psychological support to increase physical activity
|
3 h/wk | 1x/2 wk, 3–4 mo total duration | >20 METs/wk (or 3 h/wk of moderate aerobic exercise) | NAFLD75 | |
| Continuous cycling on ergometer plus brisk walking at home vs stretching, self-massage and fitball programme (placebo group) | HI:LO & LO:LO: 90–135 min/wk LO:HI: 180–240 min/wk PLA: 5 min cycle | HI:LO & LO:LO: 2x/wk cycling + 1x/wk brisk walking LO:HI: 3x/wk cycling + 1x/wk brisk walking Placebo: 1x/2 wk 8 wk total duration |
HI:LO: 60%−70% of their VO2 peak (from 50% to 70%) LO:HI & LO:LO: 50% of their VO2 peak PLA: 30W (cycling) |
NAFLD78 | |
Aerobic exercise
|
30–45 min/session (progressively) | 3–5x/wk (progressively) for 16 wk | 30%−60% heart rate reserve (progressively) | NAFLD76 | |
| Jogging and brisk walking | V-M: 150 min/wk jogging (6 mo) + 150 min/wk brisk walking (6 mo) M: 150 min/wk brisk walking (12 mo) |
5x/wk, 12 mo of duration | V-M: 65%−80% of their MHR (8–10 METs) M: 45%−55% of their MHR (120 steps/min) |
NAFLD79 | |
Cardiorespiratory fitness by maximal treadmill exercise (modified Balkeprotocol)
|
Minimum of 25 min | N/R | First 25 min: speed 88 m/min, with a grade of 0% (1st min), a grade of 2% 2nd min) and an increase of 1% each min thereafter. After 25 min: grade did not change, but speed increased to 5.4 m/min till the end. Participants are encouraged to do a maximal effort to reach at least 85% of their MHR. |
Gallbladder disease147 |
|
| RESISTANCE | |||||
Eight exercises
|
45–60 min/session + 10 min warm-up at the beginning and at the end | 3x/wk for 8 wk | Moderate (50%−70% of their 1RM, 60% of their MHR for the warm-up) | NAFLD73 | |
| AEROBIC AND RESISTANCE | |||||
Four-level physical activity index
|
N/R | Participants’ previous year | N/R | Gallbladder disease143 |
|
19 recreational activities
|
Min/wk | N/R | Moderate and vigorous (METs/min/wk) | NAFLD72 | |
Aerobic and resistance training combined
|
60 min/session and 3 series of 10 repetitions + 1 min recovery in between | 3x/wk for 4 mo | 60%−65% of their heart rate reserve and 70%- 80% of their 1RM | NAFLD77 | |
Aerobic and resistance training combined
|
5 min warm-up + 5 intervals (2 min each) interspersed with 3 min recovery + 3 min cool-down +10s added to each interval/wk |
3x/wk on nonconsecutive days for 12 wks | Warm-up: 9–13 rating of perceived exertion Intervals: 16–17 rating of perceived exertion |
NAFLD71 | |
16 recreational physical activity items
|
Hours or days/wk | Weekly time over last year | Classified by METs/h/wk | Gallbladder disease144 | |
| Report of hard physical activity | Less or more than 1 h/wk | Median follow-up of 0.6–16.4 y | Hard (intense) | Gallbladder disease145 | |
| Data on general physical activity | Hours/day using quartile distributions | Median follow-up of 10.7 y | Vigorous | Gallbladder disease148 | |
1RM, one-repetition maximum; HI:LO, high-intensity low-volume aerobic exercise; LO:HI, low-to-moderate intensity high-volume aerobic exercise; LO:LO, low-to-moderate intensity low-volume aerobic exercise; M, moderate; MET, metabolic equivalent task; MHR, maximal heart rate; N/R, not reported; V-M, vigorous-moderate; VO2 peak, peak oxygen consumption.
ACKNOWLEDGEMENTS
The present chapter is written in the context of the project FOIE GRAS, which has received funding from the European Union’s Horizon 2020 Research and Innovation programme under the Marie Sklodowska-Curie Grant Agreement No. 722619. EMM and RLB are recipients of Foie Gras Early Research Training Grant.
Footnotes
CONFLICT OF INTEREST
We declare that we have no conflict of interests.
REFERENCES
- 1.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz JR, Labayen I, Ortega FB, et al. Physical activity, sedentary time, and liver enzymes in adolescents: the HELENA study. Pediatr Res. 2014;75:798–802. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31:S663–S667. [DOI] [PubMed] [Google Scholar]
- 4.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. [DOI] [PubMed] [Google Scholar]
- 6.Cole BK, Feaver RE, Wamhoff BR, Dash A. Non-alcoholic fatty liver disease (NAFLD) models in drug discovery. Expert Opin Drug Discov. 2018;13:193–205. [DOI] [PubMed] [Google Scholar]
- 7.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 9.Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9338–9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silva HE, Arendt BM, Noureldin SA, Therapondos G, Guindi M, Allard JP. A cross-sectional study assessing dietary intake and physical activity in Canadian patients with nonalcoholic fatty liver disease vs healthy controls. J Acad Nutr Diet. 2014;114:1181–1194. [DOI] [PubMed] [Google Scholar]
- 11.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol (1985). 2011;111:1828–1835. [DOI] [PubMed] [Google Scholar]
- 12.Rector RS, Uptergrove GM, Morris EM, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300:G874–G883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loria P, Lonardo A, Lombardini S, et al. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176–1184. [DOI] [PubMed] [Google Scholar]
- 14.Kriska AM, Brach JS, Jarvis BJ, et al. Physical activity and gallbladder disease determined by ultrasonography. Med Sci Sports Exerc. 2007;39:1927–1932. [DOI] [PubMed] [Google Scholar]
- 15.Utter AC, Whitcomb DC, Nieman DC, Butterworth DE, Vermillion SS. Effects of exercise training on gallbladder function in an obese female population. Med Sci Sports Exerc. 2000;32:41–45. [DOI] [PubMed] [Google Scholar]
- 16.Di Ciaula A, Garruti G, Lunardi Baccetto R, et al. Bile acid physiology. Ann Hepatol. 2017;16:s4–s14. [DOI] [PubMed] [Google Scholar]
- 17.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 18.Ceria-Ulep CD, Tse AM, Serafica RC. Defining exercise in contrast to physical activity. Issues Ment Health Nurs. 2011;32:476–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 22.Melzer K, Heydenreich J, Schutz Y, Renaud A, Kayser B, Mader U. Metabolic equivalent in adolescents, active adults and pregnant women. Nutrients. 2016;8:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courneya KS, McAuley E. Are there different determinants of the frequency, intensity, and duration of physical activity? Behav Med. 1994;20:84–90. [DOI] [PubMed] [Google Scholar]
- 24.Kay MC, Carroll DD, Carlson SA, Fulton JE. Awareness and knowledge of the 2008 Physical Activity Guidelines for Americans. J Phys Act Health. 2014;11:693–698. [DOI] [PubMed] [Google Scholar]
- 25.Brooke HL, Atkin AJ, Corder K, Brage S, van Sluijs EM. Frequency and duration of physical activity bouts in school-aged children: a comparison within and between days. Prev Med Rep. 2016;4:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. http://www.who.int/iris/handle/10665/44399 [PubMed]
- 27.Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33:S364–S369; discussion S419–20. [DOI] [PubMed] [Google Scholar]
- 28.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. [DOI] [PubMed] [Google Scholar]
- 29.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura H, Sugiura H, Kajima K, Mirbod SM, Iwata H, Matsuoka T. Effects of long-term moderate exercise and increase in number of daily steps on serum lipids in women: randomised controlled trial [ISRCTN21921919]. BMC Womens Health. 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high-versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126:1436–1440. [DOI] [PubMed] [Google Scholar]
- 32.Collier SR, Kanaley JA, Carhart R Jr, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–686. [DOI] [PubMed] [Google Scholar]
- 33.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. [DOI] [PubMed] [Google Scholar]
- 34.Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Xu L, Zhang X, Yao Y, Sun Y, Qi L. Effects of different durations of aerobic exercise intervention on the cardiovascular health in untrained women: a meta-analysis and meta-regression. J Sports Med Phys Fitness. 2017. 10.23736/s0022-4707.17.07029-3 [DOI] [PubMed] [Google Scholar]
- 36.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113:2642–2650. [DOI] [PubMed] [Google Scholar]
- 37.Meka N, Katragadda S, Cherian B, Arora RR. Endurance exercise and resistance training in cardiovascular disease. Ther Adv Cardiovasc Dis. 2008;2:115–121. [DOI] [PubMed] [Google Scholar]
- 38.Patel H, Alkhawam H, Madanieh R, Shah N, Kosmas CE, Vittorio TJ. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J Cardiol. 2017;9:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. [DOI] [PubMed] [Google Scholar]
- 40.Physical activity guidelines for Americans. Okla Nurse. 2008;53:25. [PubMed] [Google Scholar]
- 41.Kreher JB, Schwartz JB. Overtraining syndrome: a practical guide. Sports Health. 2012;4:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meeusen R, Duclos M, Foster C, et al. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013;45:186–205. [DOI] [PubMed] [Google Scholar]
- 43.Fry AC, Kraemer WJ. Resistance exercise overtraining and over-reaching. Neuroendocrine responses. Sports Med. 1997;23:106–129. [DOI] [PubMed] [Google Scholar]
- 44.Brooks K, Carter J. Overtraining, exercise, and adrenal insufficiency. J Nov Physiother. 2013;3. 10.4172/2165-7025.1000125 [DOI] [PubMed] [Google Scholar]
- 45.Budgett R. Fatigue and underperformance in athletes: the overtraining syndrome. Br J Sports Med. 1998;32:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackinnon LT, Hooper S. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int J Sports Med. 1994;15(suppl 3):S179–S183. [DOI] [PubMed] [Google Scholar]
- 47.Keast D, Cameron K, Morton AR. Exercise and the immune response. Sports Med. 1988;5:248–267. [DOI] [PubMed] [Google Scholar]
- 48.Angeli A, Minetto M, Dovio A, Paccotti P. The overtraining syndrome in athletes: a stress-related disorder. J Endocrinol Invest. 2004;27:603–612. [DOI] [PubMed] [Google Scholar]
- 49.Shephard RJ, Shek PN. Heavy exercise, nutrition and immune function: is there a connection? Int J Sports Med. 1995;16:491–497. [DOI] [PubMed] [Google Scholar]
- 50.Morgan WP, Brown DR, Raglin JS, O’Connor PJ, Ellickson KA. Psychological monitoring of overtraining and staleness. Br J Sports Med. 1987;21:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. 2016;63:1026–1040. [DOI] [PubMed] [Google Scholar]
- 52.European Association for the Study of the L, European Association for the Study of D and European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 53.Behrens G, Matthews CE, Moore SC, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. Eur J Epidemiol. 2013;28:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryu S, Chang Y, Jung HS, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–1237. [DOI] [PubMed] [Google Scholar]
- 55.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. [DOI] [PubMed] [Google Scholar]
- 56.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–1798. [DOI] [PubMed] [Google Scholar]
- 57.Qu H, Wang H, Deng M, Wei H, Deng H. Associations between longer habitual day napping and non-alcoholic fatty liver disease in an elderly Chinese population. PLoS ONE. 2014;9:e105583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keating SE, Adams LA. Exercise in NAFLD: just do it. J Hepatol. 2016;65:671–673. [DOI] [PubMed] [Google Scholar]
- 59.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. [DOI] [PubMed] [Google Scholar]
- 60.Dudekula A, Rachakonda V, Shaik B, Behari J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS ONE. 2014;9:e111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–846. [DOI] [PubMed] [Google Scholar]
- 62.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. e5; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 63.Okura T, Nakata Y, Lee DJ, Ohkawara K, Tanaka K. Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int J Obes (Lond). 2005;29:1259–1266. [DOI] [PubMed] [Google Scholar]
- 64.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313–323. [DOI] [PubMed] [Google Scholar]
- 65.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. [DOI] [PubMed] [Google Scholar]
- 66.Oh S, Tanaka K, Tsujimoto T, So R, Shida T, Shoda J. Regular exercise coupled to diet regimen accelerates reduction of hepatic steatosis and associated pathological conditions in nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2014;12:290–298. [DOI] [PubMed] [Google Scholar]
- 67.Gelli C, Tarocchi M, Abenavoli L, Di Renzo L, Galli A, De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23:3150–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2015;30:139–146. [DOI] [PubMed] [Google Scholar]
- 69.Krawczyk M, Jimenez-Aguero R, Alustiza JM, et al. PNPLA3 p. I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg Obes Relat Dis. 2016;12:1838–1846. [DOI] [PubMed] [Google Scholar]
- 70.Sreenivasa BC, Alexander G, Kalyani B, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191–198. [DOI] [PubMed] [Google Scholar]
- 71.Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond). 2015;129:1097–1105. [DOI] [PubMed] [Google Scholar]
- 72.Kistler KD, Brunt EM, Clark JM, et al. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–468; quiz 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montesi L, Caselli C, Centis E, et al. Physical activity support or weight loss counseling for nonalcoholic fatty liver disease? World J Gastroenterol. 2014;20:10128–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130:93–104. [DOI] [PubMed] [Google Scholar]
- 77.Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58:1287–1295. [DOI] [PubMed] [Google Scholar]
- 78.Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. [DOI] [PubMed] [Google Scholar]
- 79.Zhang HJ, He J, Pan LL, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176:1074–1082. [DOI] [PubMed] [Google Scholar]
- 80.Magkos F. Exercise and fat accumulation in the human liver. Curr Opin Lipidol. 2010;21:507–517. [DOI] [PubMed] [Google Scholar]
- 81.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. [DOI] [PubMed] [Google Scholar]
- 82.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. [DOI] [PubMed] [Google Scholar]
- 83.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. [DOI] [PubMed] [Google Scholar]
- 84.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. [DOI] [PubMed] [Google Scholar]
- 85.Shojaee-Moradie F, Baynes KC, Pentecost C, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–413. [DOI] [PubMed] [Google Scholar]
- 86.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring). 2008;16:2281–2288. [DOI] [PubMed] [Google Scholar]
- 87.van der Heijden GJ, Wang ZJ, Chu ZD, et al. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity (Silver Spring). 2010;18:384–390. [DOI] [PubMed] [Google Scholar]
- 88.Finucane FM, Sharp SJ, Purslow LR, et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia. 2010;53:624–631. [DOI] [PubMed] [Google Scholar]
- 89.Van Der Heijden GJ, Wang ZJ, Chu Z, et al. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc. 2010;42:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thompson D, Markovitch D, Betts JA, Mazzatti D, Turner J, Tyrrell RM. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol. 2009;108:769–779. [DOI] [PubMed] [Google Scholar]
- 91.Tamura Y, Tanaka Y, Sato F, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan S, Kirk EP, Patterson B, Klein S. Effect of endurance exercise on non-alcoholic fatty liver disease. Gastroenterology. 2011;140:S-700. [Google Scholar]
- 93.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring). 2009;17:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levinger I, Goodman C, Peake J, et al. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet Med. 2009;26:220–227. [DOI] [PubMed] [Google Scholar]
- 95.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and car-diometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen SM, Liu CY, Li SR, Huang HT, Tsai CY, Jou HJ. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc. 2008;71:551–558. [DOI] [PubMed] [Google Scholar]
- 97.Bonekamp S, Barone BB, Clark J, Stewart KJ. The effect of an exercise training intervention on hepatic steatosis. Hepatology, JOHN WILEY & SONS INC 111 RIVER ST, HOBOKEN, NJ 07030 USA. 2008;48:806A-A. [Google Scholar]
- 98.Hayward RS, Wensel RH, Kibsey P. Relapsing Clostridium difficile colitis and Reiter’s syndrome. Am J Gastroenterol. 1990;85:752–756. [PubMed] [Google Scholar]
- 99.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring). 2008;16:1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305:E1222–E1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pugh CJ, Cuthbertson DJ, Sprung VS, et al. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2013;305:E50–E58. [DOI] [PubMed] [Google Scholar]
- 105.Savoye M, Caprio S, Dziura J, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301: E1033–E1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Straznicky N, Lambert E, Grima M, et al. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabetes Obes Metab. 2012;14:139–148. [DOI] [PubMed] [Google Scholar]
- 108.Valizadeh R, Nikbakht M, Davodi M, Khodadoost M. The effect of eight weeks elected aerobic exercise on the levels of (AST, ALT) enzymes of men patients with have fat liver. Procedia Soc Behav Sci. 2011;15:3362–3365. [Google Scholar]
- 109.Wang CL, Liang L, Fu JF, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. [DOI] [PubMed] [Google Scholar]
- 111.Zelber-Sagi S, Buch A, Yeshua H, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20:4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15:96–102. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pugh CJ, Spring VS, Kemp GJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298–H1306. [DOI] [PubMed] [Google Scholar]
- 114.Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in iranian men with nonalcoholic fatty liver disease. Hepat Mon. 2015;15:e31434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shojaee-Moradie F, Cuthbertson D, Barrett M, et al. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J Clin Endocrinol Metab. 2016;101:4219–4228. [DOI] [PubMed] [Google Scholar]
- 116.Eckard C, Cole R, Lockwood J, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, Ashmawy EM. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci. 2013;13:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rezende RE, Duarte SM, Stefano JT, et al. Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause. 2016;23:876–883. [DOI] [PubMed] [Google Scholar]
- 119.Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. [DOI] [PubMed] [Google Scholar]
- 120.Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. 2016;10:486–492. [PubMed] [Google Scholar]
- 121.Kani AH, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition. 2014;30:814–821. [DOI] [PubMed] [Google Scholar]
- 122.Arefhosseini SR, Ebrahimi-Mameghani M, Naeimi AF, Khosh-baten M, Rashid J. Lifestyle modification through dietary intervention: health promotion of patients with non-alcoholic fatty liver disease. Health Promot Perspect. 2011;1:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. [DOI] [PubMed] [Google Scholar]
- 124.Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. [DOI] [PubMed] [Google Scholar]
- 125.Zhang HJ, Pan LL, Ma ZM, et al. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: a 1-year follow-up study. Diabetes Obes Metab. 2017;19:284–289. [DOI] [PubMed] [Google Scholar]
- 126.Pugh CJ, Sprung V, Jones H, et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes (Lond). 2016;40:1927. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen V, George J. Nonalcoholic fatty liver disease management: dietary and lifestyle modifications. Semin Liver Dis. 2015;35:318–337. [DOI] [PubMed] [Google Scholar]
- 128.Macavei B, Baban A, Dumitrascu DL. Psychological factors associated with NAFLD/NASH: a systematic review. Eur Rev Med Pharmacol Sci. 2016;20:5081–5097. [PubMed] [Google Scholar]
- 129.Frith J, Day CP, Robinson L, Elliott C, Jones DE, Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol. 2010;52:112–116. [DOI] [PubMed] [Google Scholar]
- 130.Weinstein AA, Escheik C, Oe B, Price JK, Gerber LH, Younossi ZM. Perception of effort during activity in patients with chronic hepatitis C and nonalcoholic fatty liver disease. PM R. 2016;8:28–34. [DOI] [PubMed] [Google Scholar]
- 131.Centis E, Marzocchi R, Suppini A, et al. The role of lifestyle change in the prevention and treatment of NAFLD. Curr Pharm Des. 2013;19:5270–5279. [PubMed] [Google Scholar]
- 132.Elliott C, Frith J, Day CP, Jones DE, Newton JL. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig Dis Sci. 2013;58:2383–2391. [DOI] [PubMed] [Google Scholar]
- 133.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. [DOI] [PubMed] [Google Scholar]
- 134.Wang DQH, Portincasa P, eds. Gallstones. Recent advances in epidemiology, pathogenesis, diagnosis and management, 1st edn. New York, NY: Nova Science Publisher Inc.; 2017:1–676. [Google Scholar]
- 135.Lammert F, Acalovschi M, Ercolani G, van Erpecum KJ, Gurusamy KS, van Laarhoven CJ, Portincasa P. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146–181. [DOI] [PubMed] [Google Scholar]
- 136.Grundy SM. Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr. 2004;80:1–2. [DOI] [PubMed] [Google Scholar]
- 137.Wang DQH, Neuschwander-Tetri BA, Portincasa P. The biliary system, second edition. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2016;8:i–178. [Google Scholar]
- 138.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [DOI] [PubMed] [Google Scholar]
- 139.Heida A, Koot BG, vd Baan-Slootweg OH, et al. Gallstone disease in severely obese children participating in a lifestyle intervention program: incidence and risk factors. Int J Obes (Lond). 2014;38:950–953. [DOI] [PubMed] [Google Scholar]
- 140.Farrell GC. The liver and the waistline: fifty years of growth. J Gastroenterol Hepatol. 2009;24(suppl 3):S105–S118. [DOI] [PubMed] [Google Scholar]
- 141.Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. [DOI] [PubMed] [Google Scholar]
- 142.Williams PT. Independent effects of cardiorespiratory fitness, vigorous physical activity, and body mass index on clinical gallbladder disease risk. Am J Gastroenterol. 2008;103:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Banim PJ, Luben RN, Wareham NJ, Sharp SJ, Khaw KT, Hart AR. Physical activity reduces the risk of symptomatic gallstones: a prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:983–988. [DOI] [PubMed] [Google Scholar]
- 144.Henao-Moran S, Denova-Gutierrez E, Moran S, et al. Recreational physical activity is inversely associated with asymptomatic gallstones in adult Mexican women. Ann Hepatol. 2014;13:810–818. [PubMed] [Google Scholar]
- 145.Talseth A, Ness-Jensen E, Edna TH, Hveem K. Risk factors for requiring cholecystectomy for gallstone disease in a prospective population-based cohort study. Br J Surg. 2016;103:1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwak MS, Kim D, Chung GE, Kim W, Kim YJ, Yoon JH. Cholecystectomy is independently associated with nonalcoholic fatty liver disease in an Asian population. World J Gastroenterol. 2015;21:6287–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li C, Mikus C, Ahmed A, et al. A cross-sectional study of cardiorespiratory fitness and gallbladder disease. Ann Epidemiol. 2017;27:269–273. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Figueiredo JC, Haiman C, Porcel J, et al. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol. 2017;17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shephard RJ. Physical activity and the biliary tract in health and disease. Sports Med. 2015;45:1295–1309. [DOI] [PubMed] [Google Scholar]
- 150.Aune D, Leitzmann M, Vatten LJ. Physical activity and the risk of gallbladder disease: a systematic review and meta-analysis of cohort studies. J Phys Act Health. 2016;13:788–795. [DOI] [PubMed] [Google Scholar]
- 151.Utter A, Goss F. Exercise and gall bladder function. Sports Med. 1997;23:218–227. [DOI] [PubMed] [Google Scholar]
- 152.Krishnamurthy S, Krishnamurthy GT. Biliary dyskinesia: role of the sphincter of Oddi, gallbladder and cholecystokinin. J Nucl Med. 1997;38:1824–1830. [PubMed] [Google Scholar]
- 153.Schubert MM, Desbrow B, Sabapathy S, Leveritt M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite. 2013;63:92–104. [DOI] [PubMed] [Google Scholar]
- 154.Portincasa P, Di Ciaula A, van Berge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. 2004;6:151–162. [DOI] [PubMed] [Google Scholar]
- 155.Wu HJ, Chen KT, Shee BW, Chang HC, Huang YJ, Yang RS. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J Gastroenterol. 2004;10:2711–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wilund KR, Feeney LA, Tomayko EJ, Chung HR, Kim K. Endurance exercise training reduces gallstone development in mice. J Appl Physiol (1985). 2008;104:761–765. [DOI] [PubMed] [Google Scholar]
- 157.Fandriks L. Roles of the gut in the metabolic syndrome: an overview. J Intern Med. 2017;281:319–336. [DOI] [PubMed] [Google Scholar]
- 158.Queipo-Ortuno MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE. 2013;8:e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. [DOI] [PubMed] [Google Scholar]
- 160.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017:3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hold GL. The gut microbiota, dietary extremes and exercise. Gut. 2014;63:1838–1839. [DOI] [PubMed] [Google Scholar]
- 162.Denou E, Marcinko K, Surette MG, Steinberg GR, Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310:E982–E993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cronin O, O’Sullivan O, Barton W, Cotter PD, Molloy MG, Shanahan F. Gut microbiota: implications for sports and exercise medicine. Br J Sports Med. 2017;51:700–701. [DOI] [PubMed] [Google Scholar]
- 164.Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 2017;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cerda B, Perez M, Perez-Santiago JD, Tornero-Aguilera JF, Gonzalez-Soltero R, Larrosa M. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health? Front Physiol. 2016;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70–79. [PubMed] [Google Scholar]
- 167.Johannesson E, Simren M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106:915–922. [DOI] [PubMed] [Google Scholar]
- 168.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. [DOI] [PubMed] [Google Scholar]
- 169.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE. 2014;9:e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Z, Liu HY, Zhou H, et al. Moderate-intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol. 2017;8:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lamoureux EV, Grandy SA, Langille MGI. Moderate exercise has limited but distinguishable effects on the mouse microbiome. mSystems. 2017;2. 10.1128/msystems.00006-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yiamouyiannis CA, Martin BJ, Watkins JB 3rd. Chronic physical activity alters hepatobiliary excretory function in rats. J Pharmacol Exp Ther. 1993;265:321–327. [PubMed] [Google Scholar]
- 174.Watkins JB 3rd, Crawford ST, Sanders RA. Chronic voluntary exercise may alter hepatobiliary clearance of endogenous and exogenous chemicals in rats. Drug Metab Dispos. 1994;22:537–543. [PubMed] [Google Scholar]
- 175.Bouchard G, Carrillo MC, Tuchweber B, et al. Moderate long-term physical activity improves the age-related decline in bile formation and bile salt secretion in rats. Proc Soc Exp Biol Med. 1994;206:409–415. [DOI] [PubMed] [Google Scholar]
- 176.Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. 2011;218:323–329. [DOI] [PubMed] [Google Scholar]
- 177.Sutherland WH, Nye ER, Macfarlane DJ, Robertson MC, Williamson SA. Fecal bile acid concentration in distance runners. Int J Sports Med. 1991;12:533–536. [DOI] [PubMed] [Google Scholar]
- 178.Danese E, Salvagno GL, Tarperi C, et al. Middle-distance running acutely influences the concentration and composition of serum bile acids: potential implications for cancer risk? Onco-target. 2017;8:52775–52782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brighton CA, Rievaj J, Kuhre RE, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156:3961–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oh S, So R, Shida T, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. 2017;7:43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Komine S, Akiyama K, Warabi E, et al. Exercise training enhances in vivo clearance of endotoxin and attenuates inflammatory responses by potentiating Kupffer cell phagocytosis. Sci Rep. 2017;7:11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lewis ND, Patnaude LA, Pelletier J, et al. A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS ONE. 2014;9:e100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694.e3. [DOI] [PubMed] [Google Scholar]
- 185.Ward JBJ, Lajczak NK, Kelly OB, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol. 2017;312:G550–G558. [DOI] [PubMed] [Google Scholar]
- 186.Kai S, Nagino K, Ito T, et al. Effectiveness of moderate intensity interval training as an index of autonomic nervous activity. Rehabil Res Pract. 2016;2016:6209671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fealy CE, Haus JM, Solomon TP, et al. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol (1985). 2012;113:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Philipp E, Wilckens T, Friess E, Platte P, Pirke KM. Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides. 1992;13:125–128. [DOI] [PubMed] [Google Scholar]
- 191.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]


