Abstract
Objective:
Affect regulation, eating expectancies, and attention toward food-related cues are interrelated constructs that have been implicated in the maintenance of binge eating. While these processes show considerable temporal variability, the momentary associations between these domains have not been elucidated. This study examined a model that posited momentary fluctuations in affect, eating expectancies, and attention bias (AB) would interact to predict subsequent binge eating.
Method:
Forty women who endorsed recurrent binge eating completed a 10-day ecological momentary assessment protocol with ambulatory measures of AB (i.e., dot-probe task with palatable food and neutral cues) and self-report assessments of positive and negative affect, eating expectancies (i.e., the belief that eating would improve one’s mood), and binge-eating symptoms.
Results:
Generalized linear mixed models indicated higher momentary AB toward palatable food was associated with increased risk of subsequent binge eating, and a two-way interaction showed that moments of higher eating expectancies and negative affect were associated with increased likelihood of subsequent binge eating. Also, a three-way interaction emerged, in that the association between eating expectancies and subsequent binge eating was strongest at lower levels of positive affect and higher AB.
Discussion:
Together, findings partially supported hypotheses and demonstrate meaningful within-person fluctuations in AB that precede binge eating. Further, results demonstrate that the momentary influence of eating expectancies on binge eating depends on both affective state and attentional processes.
Keywords: affect regulation, attention bias, binge eating, eating expectancies, ecological momentary assessment
1 |. INTRODUCTION
Binge eating, defined as consumption of an objectively large amount of food with a sense of loss of control over eating, is a feature of several eating disorders (EDs; American Psychiatric Association, 2013). However, the best available empirically-supported treatments for EDs characterized by binge eating remain only modestly effective (Linardon, 2018; Linardon, & Wade, 2018), which highlights the need to better understand and disrupt the momentary processes that maintain this behavior. As such, ecological momentary assessment (EMA) is a valuable method to study moment-to-moment fluctuations in cognitive and affective factors underlying binge-eating episodes.
Prior EMA work indicates negative affect (NA) increases and positive affect (PA) decreases in the moments leading up to binge-eating episodes, after which affect tends to improve (i.e., NA decreases and PA increases; Engel et al., 2016), thereby lending support for affect regulation theories (Hawkins, 1984; Heatherton & Baumeister, 1991). However, less is known regarding the mechanisms by which affect leads to binge eating and how momentary cognitive factors influence the degree to which PA and NA predict binge eating. While eating expectancies and attention bias (AB) are particularly relevant cognitive processes that have been implicated in affective functioning and binge eating, these variables have not been integrated in a theoretically informed, momentary model.
1.1 |. Eating expectancies and affect
Eating expectancies refer to individuals’ learning history of positive and negative reinforcement from eating (Hohlstein, Smith, & Atlas, 1998). According to expectancy theory, individuals are likely to engage in certain eating behaviors based on anticipated outcomes (Behan, 1953; Tolman & Postman, 1954). This is relevant to affect regulation models of binge eating, as the expectancy of short-term affective improvement after binge eating (i.e., increased PA and/or decreased NA) likely serves to maintain the behavior. This is further supported by research finding associations among eating expectancies and ED psychopathology (e.g., Pearson, Combs, Zapolski, & Smith, 2012).
There is also evidence to suggest that eating expectancies vary from moment-to-moment and influence relationships between affect and eating. A recent EMA study indicated that individuals with obesity were most prone to subsequent binge eating at moments when they reported higher than usual NA, dietary restraint, and expectancies that eating would make them feel better (Pearson et al., 2018). This supports the possibility that momentary changes in affect may activate cognitive expectancies about eating and potentially enhance the anticipatory reward value of food.
1.2 |. AB, eating expectancies, and affect
AB, which refers to the allocation of attention to particular information in the environment (Cisler & Koster, 2010), is posited to contribute to ED behaviors such as binge eating (Stojek et al., 2018). In particular, incentive salience accounts of ED behavior hold that AB toward palatable food cues enhances motivations to eat and the expected reward from food, thereby leading to binge eating (Berridge, 2009). This possibility is also supported by work outside of EDs suggesting that ABs develop via classical conditioning processes. That is, over time, learned associations between eating (i.e., unconditioned stimulus) and eating-related cues (i.e., conditioned stimulus) lead to ABs toward food cues, and the magnitude of such ABs is related to the extent to which cues elicit expectations of reward from the stimulus. In sum, learned eating expectancies may drive AB toward food, in that attention is allocated to salient stimuli associated with expectancies of positive outcomes (Vogt, De Houwer, & Crombez, 2011). Consistent with this framework, research has demonstrated that attention is directed toward stimuli that are associated with higher expectancies of reward (Vogt et al., 2011), and eating expectancies are related to increased AB toward food (Hardman, Scott, Field, & Jones, 2014). Given this evidence, momentary food cue responsivity, and ABs toward food are likely to influence the degree to which affect and cognitive eating expectancies together predict binge eating.
Notably, AB does not appear to be a stable, trait-like construct. Specifically, recent literature suggests that single assessments of AB, such as the dot-probe task, show low reliability as measures of individual (i.e., between-person) differences, which is critically problematic given that the reliability of a measure sets the upper limit on its validity (Price et al., 2015; Rodebaugh et al., 2016). Moreover, AB fluctuates with changing contextual variables such as affect (e.g., Allen, Mason, Stout, & Rokke, 2018), and this within-person variability may be clinically relevant to ED symptoms given that momentary fluctuations in AB been shown to predict other health-related behaviors (Waters & Li, 2008). Thus, assessment of AB is ideally suited for repeated measurements to elucidate the relationship between momentary variability in this construct and eating behavior.
1.3 |. The present study
Taken together, previous theoretical and empirical work indicates associations between affect, eating expectancies, and ABs. Each of these constructs is related to ED psychopathology, demonstrates within-person variability, and therefore may have relevance to momentary processes precipitating binge eating. However, these constructs have not been meaningfully integrated in a momentary model to clarify the independent and interactive effects of affect, eating expectancies, and food-related AB on subsequent binge eating. The current research sought to examine a conceptual model (Figure 1) using EMA with ambulatory assessments of AB toward palatable food cues (i.e., dot-probe task). This allows for assessment of within- and between-person variability, as well as evaluation of micro-temporal relationships.
FIGURE 1.
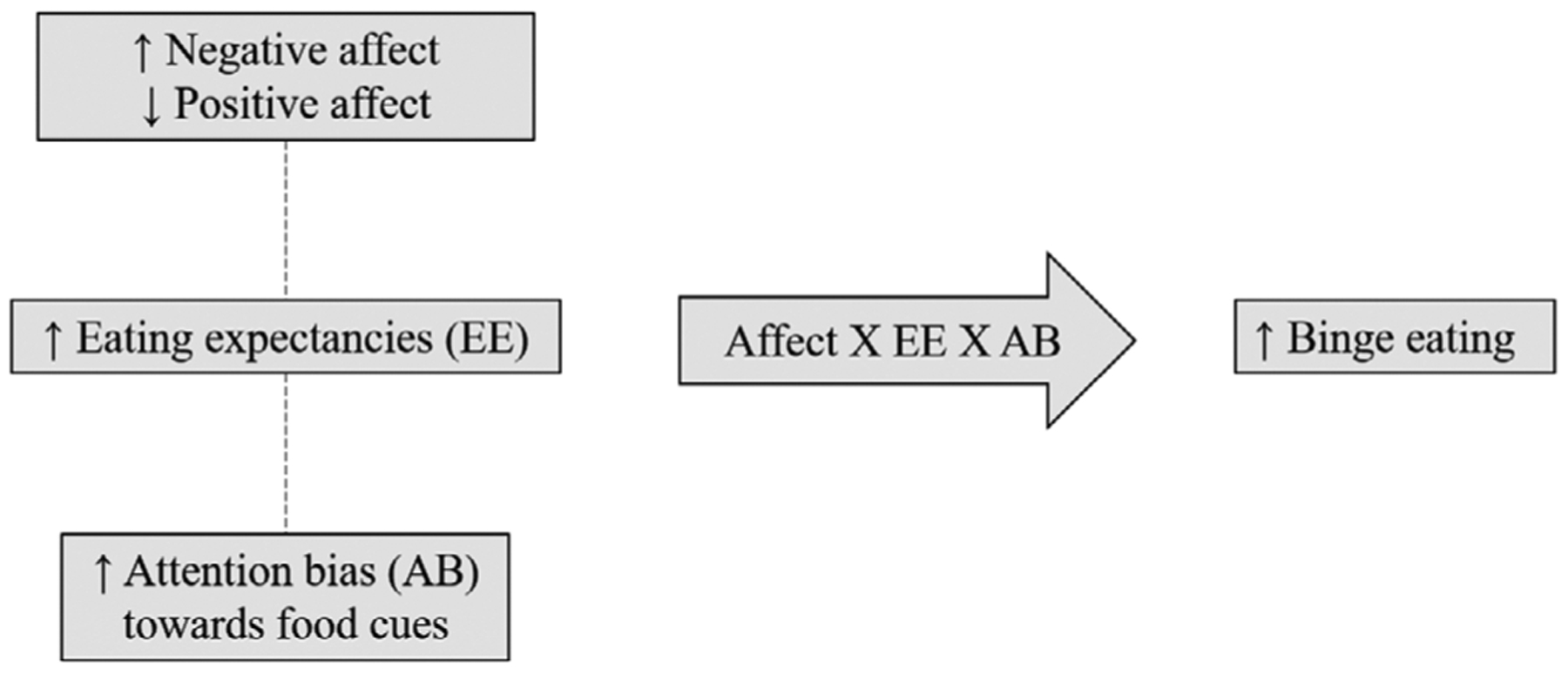
Conceptual momentary model. Dashed lines represent hypothesized associations between variables at the same EMA signal
The model posited that changes in NA and PA activate learned expectancies that eating will improve mood, and in turn predict subsequent binge eating. In addition, affective fluctuations and associated eating expectancies were thought to be related to increased concurrent momentary AB toward palatable food cues, which would strengthen the independent and interactive effects of affect and eating expectancies on subsequent binge eating. Specifically, it was hypothesized that moments at which (a) individuals reported higher NA or lower PA, (b) endorsed the expectancy that eating would lead to improvement in affect, and (c) evidenced increased AB toward cues related to eating reward (i.e., palatable food images), relative to their average levels of these variables, would be related to an increased likelihood of subsequent binge eating. Models were conducted separately for PA and NA given that prior research has indicated these are largely independent constructs (Crawford & Henry, 2004; Merz & Roesch, 2011; Merz et al., 2013; Watson, Clark, & Tellegan, 1988).
2 |. METHODS
2.1 |. Participants
Participants were 40 women (87.5% Caucasian, MBMI = 34.30 ± 9.84 kg/m2; range: 18.21–59.16; Mage = 34.70 ± 15.59; range: 19–64) reporting recurrent binge eating who were recruited from clinical and community settings, which included 29 participants with binge-eating disorder (BED), nine participants with bulimia nervosa (BN), one participant with anorexia nervosa binge-purge subtype (AN-BP), and one participant with other specified feeding or eating disorder (subthreshold BED presentation). Eligibility criteria included endorsement of regular binge eating (i.e., ≥once/week over the past 3 months) as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fifth Edition (DSM-5), Research Version (SCID-5-RV; First, 2015), self-identifying as female, and between the ages of 18 and 65. Participants were excluded for the following reasons: (a) inability to read/speak English, (b) current psychosis, (c) current mania, (d) acute suicidality, (e) medical instability as determined by vital signs and blood pressure at the study visit, (f) severe cognitive impairment or intellectual disability determined by a phone screen, (g) currently pregnant or breastfeeding, (h) inpatient or partial hospitalization in the past 4 weeks, (i) changes to ED treatment in the past 4 weeks, (j) history of bariatric surgery, and (k) body mass index (BMI) < 18.0 kg/m2.
2.2 |. Procedure
Participants completed a phone screen to confirm initial eligibility; in total, 98 individuals were screened for initial eligibility. After the screening, participants completed a study visit that included the informed consent process, assessment of vital signs and anthropometric measures, structured interviews, computerized tasks, and questionnaires. At the study visit, participants received training on the EMA protocol and definitions of overeating and loss of control eating. EMA was administered using the Momentary Assessment Tool system (Dvorak, 2018) on Samsung Galaxy tablets (provided by the researchers). During the EMA protocol, participants were asked to make signal-contingent and event-contingent recordings for the next 11 days; the first day was a practice day and was not included in analyses. After the practice day, participants received a call from study staff to answer questions about the protocol; if there were no concerns, participants proceeded to complete the 10-day EMA data collection. Each day, participants received five semi-random signal-contingent prompts distributed around anchor points between 8:30 a.m. and 9:00 p.m. Participants were also instructed to complete event-contingent recordings after eating episodes; if participants forgot to record an episode, they could enter this information at the next semi-random signal. The recordings included self-report questions and ambulatory neurocognitive assessments. At 4 of the 5 semi-random signals (including morning, afternoon, and evening signals), an ambulatory dot-probe task was administered as a measure of AB. Pilot testing confirmed completion of the task and EMA questions at each signal took less than 5 min to complete. After the EMA protocol, participants returned for another study visit to return the tablet and receive payment for participation. This research was reviewed and received approval from the institutional review board.
2.3 |. EMA measures
2.3.1 |. Momentary affect
Momentary NA and PA were assessed at EMA signals by items from the Positive and Negative Affect Schedule Short Form (Thompson, 2007), with the addition of guilt given that this affective state is a particularly relevant facet of NA in EDs (Berg et al., 2013). For each item, participants rated the extent to which they were experiencing the affective state on a scale ranging from 1 (not at all) to 5 (very much). Ratings of NA and PA items were summed to create composite NA and PA scores at each EMA signal (α = .88 and α = .87, respectively).
2.3.2 |. Momentary eating expectancy
Consistent with prior EMA research (Pearson et al., 2018), momentary expectancy of affective benefit from eating was assessed at EMA signals by the following item: Eating would make me feel better right now, which was rated on a scale ranging from 1 (not at all) to 5 (very much).
2.3.3 |. Momentary binge eating
At each eating episode, questions assessed loss of control eating (While you were eating, to what extent did you: feel a sense of loss of control?; feel that you could not stop eating once you started?; feel disconnected [e.g., numb, zoned out, on auto-pilot]?) and overeating (To what extent do you: feel that you overate?; think that others would consider what you ate to be an usual or excessive amount of food?). Items were rated on a scale ranging from 1 (not at all) to 5 (extremely), with 3 corresponding to moderately. Scores on the loss of control and overeating items were averaged to create composite scores (α = .90 and α = .94, respectively); the episode was categorized as a binge-eating episode if the composite ratings were each ≥3.
2.3.4 |. Momentary AB
Momentary AB toward palatable food was assessed by an ambulatory dot-probe task adapted for EMA administration based on a standardized computerized administration (MacLeod, Mathews, & Tata, 1986). The dot-probe task assesses selective attention to varying types of stimuli and was modified in this study to assess attention to palatable food. Palatable food was chosen as the stimulus of interest given evidence in binge-type EDs suggesting ABs for such stimuli (Stojek et al., 2018). Stimuli were food and nonfood images determined as part of a larger study conducted by Juarascio and colleagues (Forman et al., 2019). Conducted through Amazon’s Mechanical Turk, 65 participants (ages, 18–65 years; BMI, M = 25.05 ± 4.73) rated savory and sweet food images on tastiness (“0-not at all” and “5-extremely”). Each image was presented twice in a random order, with 2 and 8 seconds display times, respectively. Images with average ratings ≥4 were included in the final image pool. All images were presented in full color (vs. gray scale) to increase image salience.
Each EMA administration included 60 trials of the task (Figure 2). Trials began with a fixation cross that appeared in the middle of screen for 500 ms, followed by image pairs (i.e., palatable food and neutral) displayed for 500 ms (one on the left and right side of the screen). After 500 ms, the images were replaced by a white screen with a green dot on the left or right side of the screen. The dots were equally distributed between food and neutral images. Participants were instructed to indicate the dot location as quickly as possible by tapping the screen where the dot appeared using their thumb. Participants had 1,000 ms to respond. The inter-trial-interval was randomized, with a new fixation cross appearing between 500 and 1,500 ms following the previous trial completion. Faster response times to the dot when it appeared in the location of food cues reflected greater AB toward these cues. Analyses were limited to trials with a reaction time greater than 100 ms in which there was a correct response. Calculation of AB was counterbalanced for reaction times to left and right dot locations (MacLeod & Mathews, 1988). For each side (right or left), mean reaction times for trials in which the dot location was consistent with the target (i.e., food) were subtracted from reaction times of trials in which the dot and target locations were inconsistent. The sum of these two difference scores was averaged to result in an overall index of AB at each signal, for which higher values indicated greater AB toward palatable food cues (Kujawa et al., 2011; MacLeod & Mathews, 1988).
FIGURE 2.
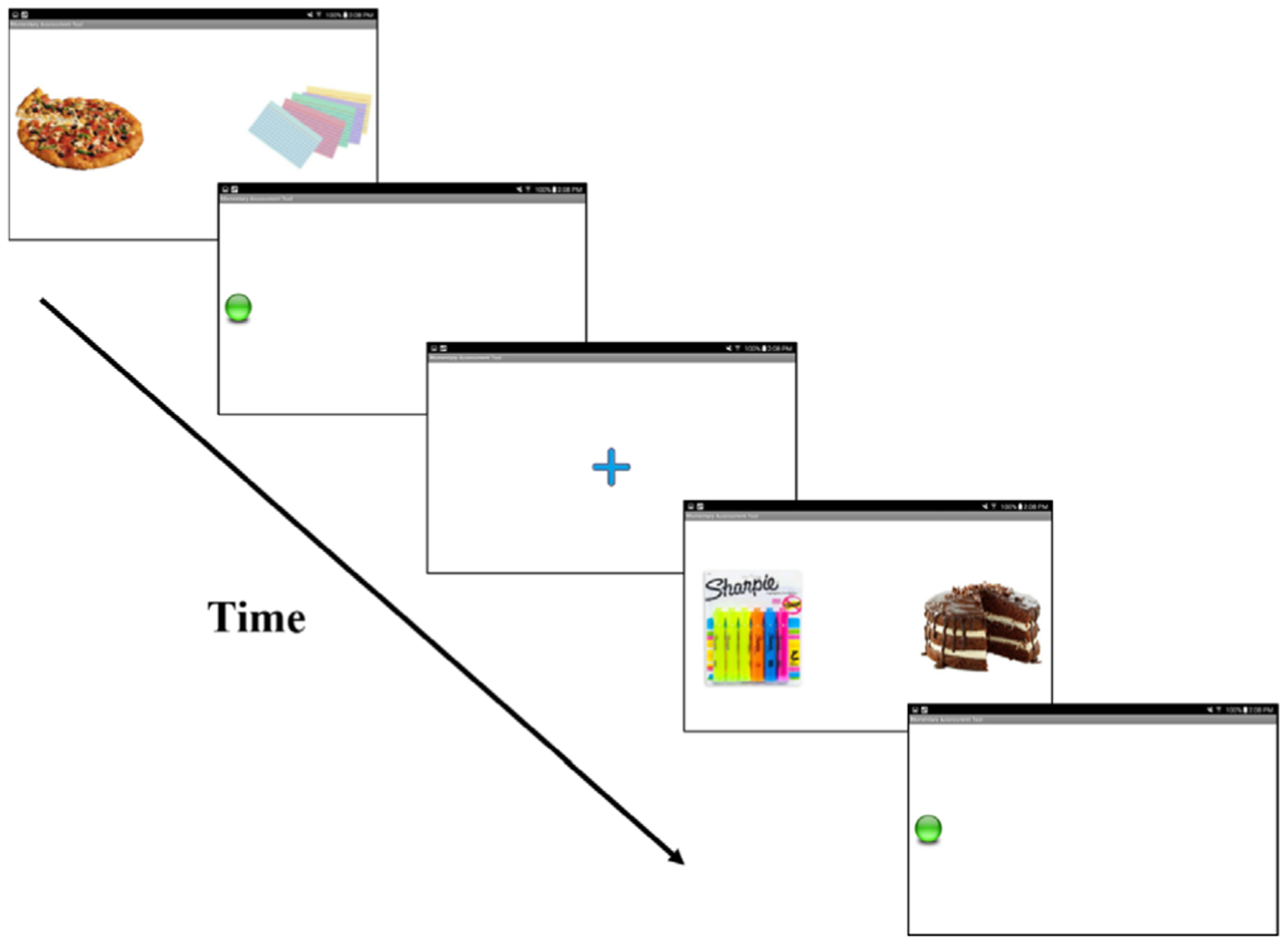
Ambulatory dot-probe task administered on smart tablets
2.4 |. Statistical analyses
Between-person reliability of AB scores was assessed by the intra-class correlation coefficient (ICC). The single measure ICC reflects the expected correlation between two randomly sampled AB measurements within the same person, whereas the average ICC indicates the reliability across all AB measurements. Two generalized linear mixed models (GLMMs) were conducted (i.e., one for PA and one for NA) without data imputation. Models examined the independent and interactive effects of momentary affect, AB, and eating expectancy on likelihood of subsequent binge eating (i.e., measured at the next EMA signal). GLMMs included a random intercept effect, an AR1 serial autocorrelation to account for dependences within the nested data, and a binary logistic function given dichotomous coding of binge-eating episodes.
GLMMs included within- and between-person effects of independent variables (i.e., NA/PA, eating expectancy, and AB). To obtain within-person effects, variables were person mean-centered, reflecting the degree to which an individual’s momentary value of a variable differs from their average level. To obtain between-person effects, variables were averaged across EMA and grand mean-centered, reflecting the degree to which an individual’s average level of a variable differs from the total sample mean. To examine the temporal relationship between within-person independent variables and subsequent binge eating, independent variables were lagged from the previous EMA signal but not lagged across individuals or days. In addition to the main effects of within- and between-person components of independent variables, two- and three-way interaction terms of within-person affect, expectancy, and AB predicting subsequent binge eating were examined. Age and BMI were included as covariates (i.e., grand mean-centered), as well as the presence of regular compensatory behaviors. The presence of regular compensatory behaviors was coded as a dichotomous variable based on ED diagnosis (i.e., BED/subclinical BED vs. BN/AN-BP). The conditional R2 statistic (reflecting variance explained by random and fixed effects) was calculated according to procedures outlined by Nakagawa and Schielzeth (2013) for GLMMs with binary outcome data. Analyses were conducted using SPSS version 25. IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
3 |. RESULTS
A total of 2,239 EMA signals were completed (90.3% compliance for signal-contingent recordings). Mean loss of control and overeating composite scores (aggregated within each person) were 1.99 ± 0.62 and 2.09 ± 0.68, respectively. The mean number of binge-eating episodes reported per participant was 5.82 ± 5.56 (Md = 4.00; range, 0–22). Single measure ICCs for NA, PA, and expectancies were 0.63, 0.34, and 0.54, respectively. Average measure ICCs for NA, PA, and expectancies were all >0.99. The single measure and average ICCs of AB scores were 0.16 and >0.99, respectively, which indicate the assessments captured substantial within-person variability as well as reliable between-person differences when aggregating scores.
GLMM results are shown in Table 1. For the GLMM with NA, there were main effects of within-person NA, and within-person AB scores, with a nonsignificant (p = .053) effect of between-person eating expectancies. That is, moments of higher NA and greater AB toward food cues (relative to an individual’s average level), were related to an increased likelihood of binge eating at the next EMA signal. In addition, there was a significant two-way interaction between NA and expectancies at the within-person level, such that momentary increases in NA, in conjunction with momentary increases in eating expectancies, were related to increased likelihood of binge eating (Figure 3). The NA GLMM conditional R2 was .3635, indicating the fixed effects in this model explained 36.35% of the variance in binge eating.
TABLE 1.
Generalized linear mixed model results predicting binge-eating episodes
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Negative affect | B | SE | t | p | Lower | Upper | Exponentiated coefficient |
| Intercept | −1.90 | 0.53 | −3.56 | <0.001 | −2.95 | −0.85 | 0.15 |
| Age | −0.01 | 0.02 | −0.35 | 0.726 | −0.04 | 0.03 | 0.99 |
| BMI | <−0.01 | 0.03 | −0.11 | 0.910 | −0.06 | 0.05 | 1.00 |
| Compensatory behavior | −0.14 | 0.62 | −0.23 | 0.819 | −1.36 | 1.07 | 0.87 |
| NA (between) | −0.02 | 0.08 | −0.19 | 0.849 | −0.18 | 0.15 | 0.98 |
| EE (between) | 0.69 | 0.36 | 1.94 | 0.053 | −0.01 | 1.39 | 2.00 |
| AB (between) | −0.01 | 0.02 | −0.56 | 0.575 | −0.04 | 0.02 | 0.99 |
| NA (within) | 0.10 | 0.04 | 2.68 | 0.008 | 0.03 | 0.17 | 1.10 |
| EE (within) | 0.12 | 0.13 | 0.94 | 0.348 | −0.13 | 0.37 | 1.13 |
| AB (within) | 0.01 | 0.00 | 2.49 | 0.013 | <0.01 | 0.02 | 1.01 |
| NA × EE (within) | 0.08 | 0.04 | 2.11 | 0.036 | 0.01 | 0.15 | 1.08 |
| NA × AB (within) | <0.01 | <0.01 | 0.53 | 0.595 | <−0.01 | <0.01 | 1.00 |
| EE × AB (within) | 0.01 | <0.01 | 1.82 | 0.069 | <−0.01 | 0.02 | 1.01 |
| NA × EE × AB (within) | <0.01 | <0.01 | 1.07 | 0.286 | <−0.01 | <0.01 | 1.00 |
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Negative affect | B | SE | Z | p | Lower | Upper |
| Random intercept variance | 1.41 | 0.49 | 2.91 | 0.004 | 0.72 | 2.77 |
| AR1 Diagonala | 0.79 | 0.05 | 17.63 | <0.001 | 0.71 | 0.88 |
| AR1 Rhob | −0.34 | 0.17 | −2.05 | 0.040 | −0.62 | 0.01 |
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Positive affect | B | SE | t | p | Lower | Upper | Exponentiated coefficient |
| Intercept | −1.76 | 0.48 | −3.65 | <0.001 | −2.70 | −0.81 | 0.17 |
| Age | 0.01 | 0.02 | 0.33 | 0.739 | −0.03 | 0.04 | 1.01 |
| BMI | −0.01 | 0.02 | −0.39 | 0.696 | −0.06 | 0.04 | 0.99 |
| Compensatory behavior | −0.24 | 0.56 | −0.44 | 0.663 | −1.34 | 0.85 | 0.78 |
| PA (between) | −0.17 | 0.10 | −1.74 | 0.082 | −0.35 | 0.02 | 0.85 |
| EE (between) | 0.54 | 0.25 | 2.11 | 0.035 | 0.04 | 1.04 | 1.71 |
| AB (between) | <−0.01 | 0.01 | −0.12 | 0.906 | −0.03 | 0.03 | 1.00 |
| PA (within) | −0.08 | 0.03 | −2.36 | 0.018 | −0.14 | −0.01 | 0.93 |
| EE (within) | 0.18 | 0.13 | 1.36 | 0.174 | −0.08 | 0.43 | 1.19 |
| AB (within) | 0.01 | <0.01 | 2.35 | 0.019 | <0.01 | 0.01 | 1.01 |
| PA × EE (within) | −0.03 | 0.04 | −0.65 | 0.516 | −0.10 | 0.05 | 0.97 |
| PA × AB (within) | <−0.01 | <0.01 | −0.21 | 0.837 | <−0.01 | <0.01 | 1.00 |
| EE × AB (within) | 0.01 | <0.01 | 2.67 | 0.008 | <0.01 | 0.02 | 1.01 |
| PA × EE × AB (within) | <0.01 | <0.01 | 2.11 | 0.035 | <0.01 | <0.01 | 1.00 |
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Positive affect | B | SE | Z | p | Lower | Upper |
| Random intercept variance | 1.10 | 0.21 | 2.69 | 0.007 | 0.53 | 2.27 |
| AR1 Diagonala | 0.81 | 0.05 | 17.68 | <0.001 | 0.72 | 0.90 |
| AR1 Rhob | −0.28 | 0.21 | −1.34 | 0.180 | −0.62 | 0.15 |
Abbreviations: CI, confidence interval; BMI, body mass index; NA, negative affect; PA, positive affect; EE, eating expectancy; AB, attention bias score; SE, standard error; between, grand-mean centered variable; within, person-mean centered variable; compensatory behavior, presence or absence of compensatory behaviors (reference category = compensatory behavior group). Within-person variables were lagged from the previous ecological momentary assessment signal. Negative affect model summary statistics: Akaike Information Criterion = 3,545.89; Bayesian Information
Criterion = 3,558.71; −2 log likelihood = 3,539.25. Positive affect model summary statistics: Akaike Information Criterion = 3,546.39; Bayesian Information
Criterion = 3,559.82; −2 log likelihood = 3,540.35. Exponentiated coefficients are similar to odds ratios.
Reflects the residual variance at each measurement.
Reflects the residual correlation between consecutive measurements.
FIGURE 3.
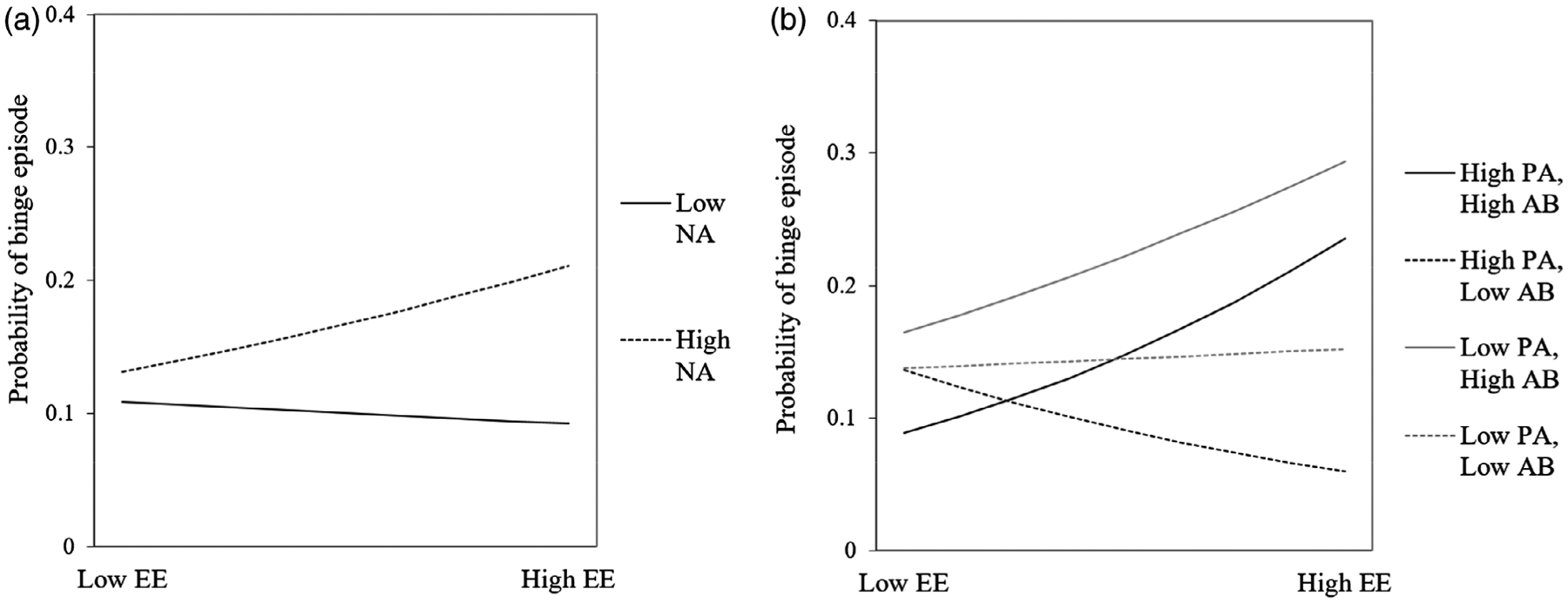
(a) Two-way interaction between momentary NA and EE predicting binge eating at the next EMA signal. (b) Three-way interaction between momentary PA, AB toward palatable food cues, and EE predicting binge eating at the next EMA signal. High and low values reflect 1 SD above and below means, respectively. AB, attention bias; EE, eating expectancies; EMA, ecological momentary assessment; NA, negative affect; PA, positive affect; SD, standard deviation
With respect to PA, there was a similar main effect of within-person AB scores, in addition to a significant main effect of between-person eating expectancies, which indicated participants with higher overall eating expectancies reported higher levels of binge eating during the EMA protocol. There was also a main effect of within-person PA, a two-way interaction of within-person eating expectancies and AB scores, as well as a three-way interaction of within-person PA, eating expectancies, and AB scores predicting subsequent binge eating. As shown in Figure 3b, higher momentary AB scores strengthened the association between eating expectancies and subsequent binge eating at low and high PA levels, with the strongest effect for low PA. That is, when individuals reported lower or higher PA, had a greater expectancy that eating would make them feel better, and evidenced higher AB (relative to their own averages), they were more likely to report binge eating at the next EMA signal. Conversely, when participants reported higher PA, higher eating expectancies, and lower AB scores, the likelihood of subsequent binge eating was reduced. Eating expectancies were not related to subsequent binge eating when both PA and AB were low. The PA GLMM conditional R2 was .3388, indicating the fixed effects in this model explained 33.88% of the variance in binge eating.
4 |. DISCUSSION
The current study examined the extent to which momentary affect, eating expectancies, and AB toward palatable food independently and interactively predict binge eating. The hypothesis was partially confirmed, as there was a three-way interaction among within-person PA, expectancy that eating would improve mood, and AB to food cues in predicting subsequent binge eating. Although previous AB research has focused on between-person associations between AB and binge eating (Stojek et al., 2018), this is the first EMA study to find that momentary AB toward food cues predicts binge eating, and further explicates the nuanced conditions in which this occurs. In both models, within-person effects for eating expectancy also depended on levels of PA or NA, and in the model with PA, the effect also depended on momentary AB. Therefore, momentary eating expectancies appear to precipitate binge eating only in combination with other momentary risk factors, which is consistent with other EMA research (e.g., Pearson et al., 2018).
For NA, there was a significant two-way interaction between within-person NA and expectancies predicting binge eating, such that there was increased likelihood of binge eating at moments when NA and expectancies were higher than one’s average. In other words, if individuals believed that eating would make them feel better when they experienced negative emotional states, they were more likely to engage in disinhibited eating, likely in order to regulate their affect. However, AB did not interact with NA or expectancies, which is contrary to prior evidence demonstrating that women with ED symptoms display more AB to ED-relevant cues in states of NA (Allen et al., 2018). As such, it may be the case that there was little variability in AB at high states of NA. In addition, other factors such as dietary restraint may be more salient in exacerbating the risk of binge eating in the context of heightened NA and eating expectancies (Pearson et al., 2018).
The three-way interaction of within-person PA, eating expectancies, and AB indicated that elevated momentary AB to food cues strengthened the association between momentary eating expectancies and likelihood of subsequent binge eating, and this effect was strongest at moments of low PA. This is consistent with the hypothesis that low mood may activate expectancies that eating will improve affect, which in turn increases attention toward cues that are associated with expectations of food-related reward. Given that these variables were measured concurrently in the present study, future research employing shorter EMA intervals would be useful to examine temporal order of effects.
Nevertheless, it is notable that higher momentary PA was still associated with subsequent binge eating in the context of higher eating expectancies and AB. An earlier experimental study of individuals with binge-eating disorder by Dingemans et al. (2009) also found that individuals with higher positive eating expectancies (i.e., expectancy that food is pleasurable and useful as a reward) evidenced greater caloric intake after a positive mood induction. Thus, NA and PA may trigger different types of expected reward from eating, in that NA may activate beliefs about negative reinforcement from eating (i.e., eating will reduce aversive emotional states), while PA may activate beliefs about positive reinforcement from eating (i.e., eating will increase pleasurable emotional states). While this study utilized a single item measure of eating expectancies, future EMA research is warranted to explore the influence of different types of eating expectancies. Conversely, the relationship between eating expectancies and binge eating was not apparent at low levels of PA and AB. However, moments of higher eating expectancies and PA were related to a lower likelihood of binge eating at lower levels of AB. Thus, it appears that states of low AB may mitigate overall risk for binge eating, potentially signaling an increased ability to disengage or distract oneself from food-related thoughts and behavior.
In addition, women who reported higher overall (i.e., between-person) expectancies that eating would improve their mood engaged in more binge eating across EMA. This is consistent with previous research that has identified affect regulation eating expectancies as an important trait-based factor associated with binge eating (Smith, Mason, Peterson, & Pearson, 2018). This is also important in light of recent theoretical work suggesting transitions from emotionally-driven to habit-based binge eating (Pearson, Wonderlich, & Smith, 2015). That is, individuals who more strongly endorse the belief that eating alleviates negative mood will, over time, become prone to eat in a more compulsive, habitual way, even if it no longer serves to regulate affect.
It is also worth noting that the reliability indices of AB scores, as measured by the dot-probe task, strongly highlight the relevance of assessing AB in an ambulatory fashion. First, the single measure ICC in the present study (0.164) was consistent with prior work showing low single measure ICCs of dot-probe indices in laboratory settings (i.e., <0.20; Price et al., 2015), which suggests that there is substantial variability in AB within persons. Second, the average measure ICC in the present study (>0.99) indicates that the reliability of the dot-probe task as an individual difference measure is dramatically improved when repeated administrations are employed. This result is consistent with EMA research using other ambulatory neurocognitive tasks, which shows similar average measure ICC values across all EMA time points (i.e., >0.97; Sliwinski et al., 2018). Collectively, these findings demonstrate the potential utility of integrating momentary assessments of neurocognitive functioning such as AB into EMA research, which allows for more reliable individual difference indices and simultaneously captures meaningful fluctuations from moment-to-moment.
Limitations of the study should be noted. The sample size was modest, which necessitates replication in larger studies. The sample mostly consisted of women with binge-eating disorder, and thus, more research is needed to determine if these findings are similar in other ED presentations. That is, it is possible that food-related AB and eating expectancies differ across EDs (e.g., Stojek et al., 2018). While we adjusted for BMI and presence of regular compensatory behaviors in the present study to mitigate some differences related to diagnostics status, it is important for future studies to compare effects across ED diagnoses in samples with larger groups of each diagnostic category. Participants were adult women and primarily Caucasian, which limits generalizability across other demographic groups. Eating expectancies were assessed with a single item; while this is consistent with previous research and minimized burden, a more rigorous assessment of different types of expectancies should be considered in future studies. It cannot be determined whether the AB reflects a bias toward palatable food specifically or food generally, and additional research comparing different types of food stimuli (e.g., high vs. low calorie) may be useful. In addition, the dot-probe results cannot discern whether the AB indicates faster initial orienting to food cues and/or difficulties disengaging from food cues; as such, future research could include a neutral condition (i.e., trials in which a nonfood stimulus appears at both locations) to distinguish these effects.
In conclusion, results extend theoretical frameworks on affect, AB, and expectancies by elucidating the interactive role of momentary affect, AB, and expectancies on binge eating. The findings also offer insight into novel targets for interventions, including just-in-time adaptive interventions that are designed to intervene on moments of risk across the day (Nahum-Shani, Hekler, & Spruijt-Metz, 2015). While affect regulation treatments have been developed for EDs (e.g., Wonderlich, Peterson, & Smith, 2015), more work is warranted to explore strategies to target momentary food-related ABs and cognitive expectancies surrounding binge eating. For instance, attention bias modification has shown promise in anxiety treatment (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015), though less is known regarding applications to EDs. Results further highlight the importance of considering the interplay of multiple intraindividual factors and elucidate exact states in which AB and expectancies are associated with symptoms in daily life, which can increase the precision of novel interventions (e.g., attention bias modification). Based on this study, moments of higher AB in combination with higher eating expectancies and lower PA may be key intervention points.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Mental Health (T32MH082761).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Allen JL, Mason TB, Stout DM, & Rokke PD (2018). Emotion specific effects on attentional bias among women with shape and weight concerns. Cognitive Therapy and Research, 42(5), 612–621. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. Washington, DC: Author. [Google Scholar]
- Behan RA (1953). Expectancies and Hullian theory. Psychological Review, 60(4), 252–256. 10.1037/h0059102 [DOI] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, & Wonderlich SA (2013). Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. Journal of Abnormal Psychology, 122 (1), 111–118. 10.1037/a0029703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2009). ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiology & Behavior, 97(5), 537–550. 10.1016/j.physbeh.2009.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, & Koster EH (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30(2), 203–216. 10.1016/j.cpr.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, & Henry JD (2004). The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 43(3), 245–265. [DOI] [PubMed] [Google Scholar]
- Dingemans AE, Martijn C, van Furth EF, & Jansen AT (2009). Expectations, mood, and eating behavior in binge eating disorder. Beware of the bright side. Appetite, 53(2), 166–173. 10.1016/j.appet.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Dvorak RD (2018). Momentary assessment tool (MAT): An integrated mobile platform for ecological assessment and intervention [computer software]. Orlando, FL: The University of Central Florida. [Google Scholar]
- Engel SG, Crosby RD, Thomas G, Bond D, Lavender JM, Mason T, … Wonderlich SA (2016). Ecological momentary assessment in eating disorder and obesity research: A review of the recent literature. Current Psychiatry Reports, 18(4), 37. 10.1007/s11920-016-0672-7 [DOI] [PubMed] [Google Scholar]
- First M, Williams JBW, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Forman EM, Manasse SM, Dallal DH, Crochiere RJ, Loyka CM, Butryn ML, … Houben K (2019). Computerized neurocognitive training for improving dietary health and facilitating weight loss. Journal of Behavioral Medicine, 42, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CA, Scott J, Field M, & Jones A (2014). To eat or not to eat. The effects of expectancy on reactivity to food cues. Appetite, 76, 153–160. 10.1016/j.appet.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Hawkins R, & Clement EE (1984). Measurement problems and a conceptual model. In Hawkins W & Hawkins PE (Eds.), The binge purge syndrome: Diagnosis, treatment and research (pp. 229–251). New York: Pergamon Press. [Google Scholar]
- Heatherton TF, & Baumeister RF (1991). Binge eating as escape from self-awareness. Psychological Bulletin, 110(1), 86–108. [DOI] [PubMed] [Google Scholar]
- Hohlstein LA, Smith GT, & Atlas JG (1998). An application of expectancy theory to eating disorders: Development and validation of measures of eating and dieting expectancies. Psychological Assessment, 10 (1), 49–58. [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, & Klein DN (2011). Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology, 39(1), 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardon J (2018). Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. The International Journal of Eating Disorders, 51(8), 785–797. 10.1002/eat.22897 [DOI] [PubMed] [Google Scholar]
- Linardon J, & Wade TD (2018). How many individuals achieve symptom abstinence following psychological treatments for bulimia nervosa? A meta-analytic review. International Journal of Eating Disorders, 51(8), 785–797. [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, & Bar-Haim Y (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety, 32(6), 383–391. 10.1002/da.22344 [DOI] [PubMed] [Google Scholar]
- MacLeod C, & Mathews A (1988). Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology Section A, 40(4), 653–670. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, & Tata P (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Merz EL, Malcarne VL, Roesch SC, Ko CM, Emerson M, Roma VG, & Sadler GR (2013). Psychometric properties of positive and negative affect schedule (PANAS) original and short forms in an African American community sample. Journal of Affective Disorders, 151(3), 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EL, & Roesch SC (2011). Modeling trait and state variation using multilevel factor analysis with PANAS daily diary data. Journal of Research in Personality, 45(1), 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Hekler EB, & Spruijt-Metz D (2015). Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychology, 34S, 1209–1219. 10.1037/hea0000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, & Schielzeth H (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution, 4(2), 133–142. [Google Scholar]
- Pearson CM, Combs JL, Zapolski TC, & Smith GT (2012). A longitudinal transactional risk model for early eating disorder onset. Journal of Abnormal Psychology, 121(3), 707–718. 10.1037/a0027567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Mason TB, Cao L, Goldschmidt AB, Lavender JM, Crosby RD, … Peterson CB (2018). A test of a state-based, self-control theory of binge eating in adults with obesity. Eating Disorders, 26(1), 26–38. 10.1080/10640266.2018.1418358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Wonderlich SA, & Smith GT (2015). A risk and maintenance model for bulimia nervosa: From impulsive action to compulsive behavior. Psychological Review, 122(3), 516–535. 10.1037/a0039268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, … Amir N (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment, 27(2), 365–376. 10.1037/pas0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, … Lenze EJ (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology, 125(6), 840–851. 10.1037/abn0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, & Lipton RB (2018). Reliability and validity of ambulatory cognitive assessments. Assessment, 25(1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Peterson CB, & Pearson CM (2018). Relationships between eating disorder-specific and transdiagnostic risk factors for binge eating: An integrative moderated mediation model of emotion regulation, anticipatory reward, and expectancy. Eating Behaviors, 31, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojek M, Shank LM, Vannucci A, Bongiorno DM, Nelson EE, Waters AJ, … Tanofsky-Kraff M (2018). A systematic review of attentional biases in disorders involving binge eating. Appetite, 123, 367–389. 10.1016/j.appet.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ER (2007). Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS). Journal of Cross-Cultural Psychology, 38(2), 227–242. [Google Scholar]
- Tolman EC, & Postman L (1954). Learning. Annual Review of Psychology, 5, 27–56. 10.1146/annurev.ps.05.020154.000331 [DOI] [PubMed] [Google Scholar]
- Vogt J, De Houwer J, & Crombez G (2011). Multiple goal management starts with attention: Goal prioritizing affects the allocation of spatial attention to goal-relevant events. Experimental Psychology, 58(1), 55–61. 10.1027/1618-3169/a000066 [DOI] [PubMed] [Google Scholar]
- Waters AJ, & Li Y (2008). Evaluating the utility of administering a reaction time task in an ecological momentary assessment study. Psychopharmacology, 197(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Peterson CB, & Smith TL (2015). Integrative cognitive-affective therapy for bulimia nervosa: A treatment manual. New York, NY: Guilford Publications. [Google Scholar]


